Preference for Artificial Refugia over Natural Refugia in an Endangered Fish
Abstract
:1. Introduction
2. Materials and Methods
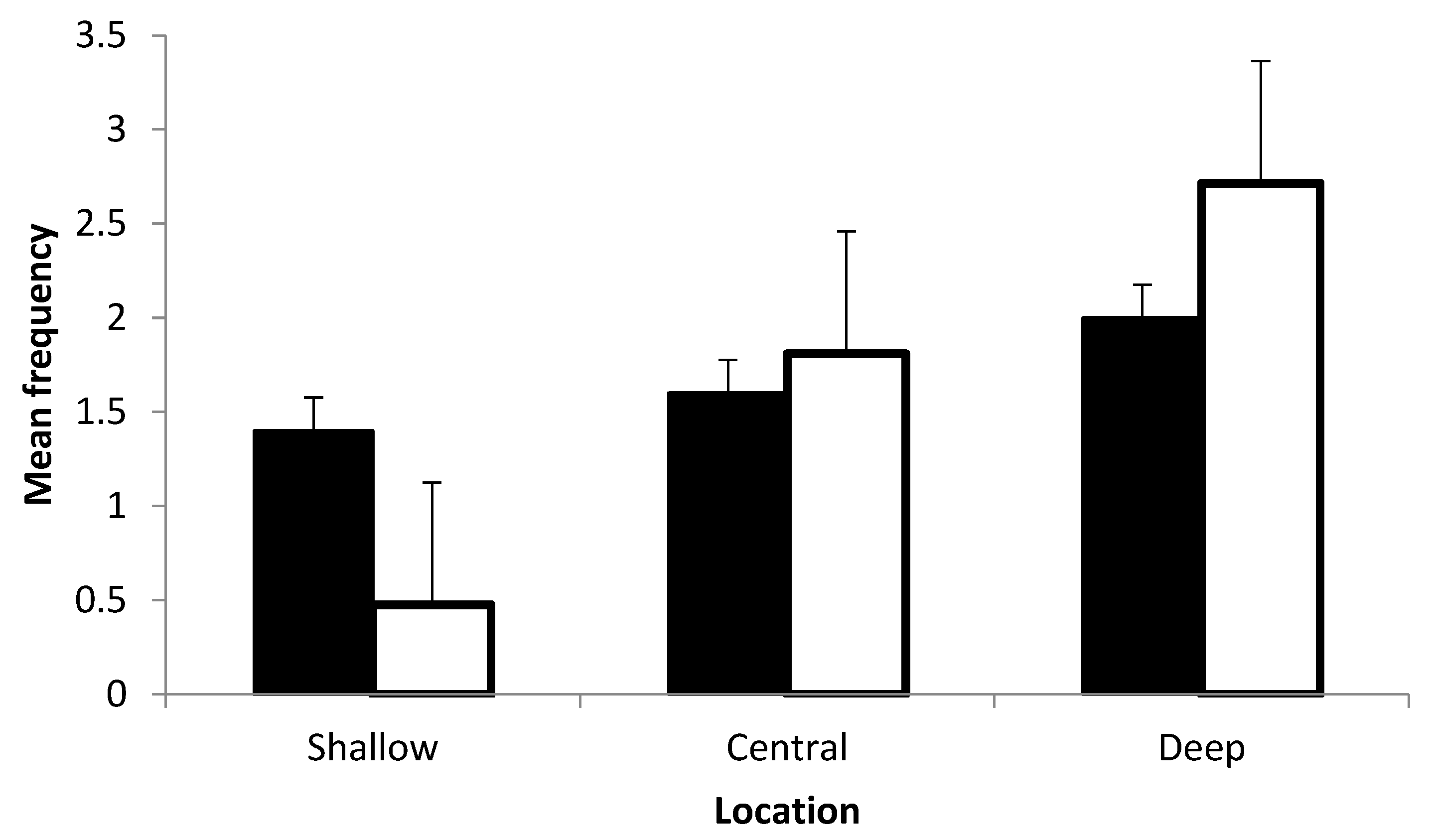
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar]
- Geist, J. Integrative freshwater ecology and biodiversity conservation. Ecol. Indic. 2011, 11, 1507–1516. [Google Scholar] [CrossRef]
- Dudgeon, D. Prospects for sustaining freshwater biodiversity in the 21st century: Linking ecosystem structure and function. Curr. Opin. Environ. Sustain. 2010, 2, 422–430. [Google Scholar] [CrossRef]
- Matsuzaki, S.S.; Sakamoto, M.; Kawabe, K.; Takamura, N. A laboratory study of the effects of shelter availability and invasive crayfish on the growth of native stream fishes. Freshw. Biol. 2012, 57, 874–882. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin, F.S., III; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global Biodiversity Scenarios for the Year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Santos, L.N.; Araújo, F.G.; Brotto, D.S. Artificial structures as tools for fish habitat rehabilitation in a neotropical reservoir. Aquat. Cons. Mar. Freshw. Ecosyst. 2008, 18, 896–908. [Google Scholar] [CrossRef]
- Selwood, K.E.; Zimmer, H.C. Refuges for biodiversity conservation: A review of the evidence. Biol. Cons. 2020, 245, 108502. [Google Scholar] [CrossRef]
- Pander, J.; Geist, J. Seasonal and spatial bank habitat use by fish in highly altered rivers—A comparison of four different restoration measures. Ecol. Freshw. Fish 2010, 19, 127–138. [Google Scholar] [CrossRef]
- Magoulick, D.D.; Kobza, R.M. The role of refugia for fishes during drought: A review and synthesis. Freshw. Biol. 2003, 48, 1186–1198. [Google Scholar] [CrossRef]
- Cole, N.C.; Jones, C.G.; Harris, S. The need for enemy-free space: The impact of an invasive gecko on island endemics. Biol. Conserv. 2005, 125, 467–474. [Google Scholar] [CrossRef]
- Magellan, K.; García-Berthou, E. Experimental evidence for the use of artificial refugia to ameliorate the impacts of invasive Gambusia holbrooki on an endangered fish. Biol. Inv. 2016, 18, 873–882. [Google Scholar] [CrossRef]
- Persson, L.; Eklöv, P. Prey refuges affecting interactions between piscivorous perch and juvenile perch and roach. Ecology 1995, 76, 70–81. [Google Scholar] [CrossRef]
- Nagayama, S.; Nakamura, F. Fish habitat rehabilitation using wood in the world. Landsc. Ecol. Eng. 2010, 6, 289–305. [Google Scholar] [CrossRef]
- Davey, A.J.; Kelly, D.J.; Biggs, B.J. Refuge-use strategies of stream fishes in response to extreme low flows. J. Fish Biol. 2006, 69, 1047–1059. [Google Scholar] [CrossRef]
- Wedderburn, S.D.; Hillyard, K.A.; Shiel, R.J. Zooplankton response to flooding of a drought refuge and implications for the endangered fish species Craterocephalus fluviatilis cohabiting with alien Gambusia holbrooki. Aquat. Ecol. 2013, 47, 263–275. [Google Scholar] [CrossRef]
- Hughes, A.R. A neighboring plant species creates associational refuge for consumer and host. Ecology 2012, 93, 1411–1420. [Google Scholar] [CrossRef]
- Westhoff, J.T.; Watts, A.V.; Mattingly, H.T. Efficacy of artificial refugia to enhance survival of young Barrens topminnows exposed to western mosquitofish. Aquat. Cons. Mar. Freshw. Ecosyst. 2013, 23, 56–76. [Google Scholar] [CrossRef]
- Ellender, B.R.; Weyl, O.L.; Swartz, E. Invasion of a headwater stream by non-native fishes in the Swartkops River system, South Africa. Afr. Zool. 2011, 46, 39–46. [Google Scholar] [CrossRef]
- IUCN. IUCN Red List of Threatened Species, Version 2015.1. Available online: http://www.iucnredlist.org (accessed on 30 June 2021).
- Kadye, W.T.; Booth, A.J. Inter-seasonal persistence and size-structuring of two minnow species within headwater streams in the Eastern Cape, South Africa. J. Appl. Ichthy. 2012, 28, 791–799. [Google Scholar] [CrossRef]
- Kadye, W.T.; Booth, A.J. Alternative responses to predation in two headwater stream minnows is reflected in their contrasting diel activity patterns. PLoS ONE 2014, 9, e93666. [Google Scholar]
- Gasith, A.; Resh, V.H. Streams in Mediterranean climate regions: Abiotic influences and biotic responses to predictable seasonal events. Ann. Rev. Ecol. Syst. 1999, 30, 51–81. [Google Scholar] [CrossRef] [Green Version]
- Hughes, D.A.; Mantel, S.K. Estimating the uncertainty in simulating the impacts of small farm dams on streamflow regimes in South Africa. Hydrol. Sci. J. 2010, 55, 578–592. [Google Scholar] [CrossRef]
- Scharler, U.M.; Baird, D. The nutrient status of the agriculturally impacted Gamtoos Estuary, South Africa, with special reference to the river-estuarine interface region (REI). Aquat. Cons. Mar. Freshw. Ecosyst. 2003, 13, 99–119. [Google Scholar] [CrossRef]
- Magellan, K.; Booth, A.J.; Weyl, O.L. Innate responses to conspecific and heterospecific alarm cues in the Endangered Eastern Cape Redfin, Pseudobarbus afer. J. Fish Biol. 2020, 96, 1284–1290. [Google Scholar] [CrossRef]
- Magellan, K.; García-Berthou, E. Influences of size and sex on invasive species aggression and native species vulnerability: A case for modern regression techniques. Rev. Fish Biol. Fish. 2015, 25, 537–549. [Google Scholar] [CrossRef]
- Baird, I.G.; Flaherty, M.S. Mekong River fish conservation zones in southern laos: Assessing effectiveness using local ecological knowledge. Environ. Manag. 2005, 36, 439–454. [Google Scholar] [CrossRef]
- Pereira, P.H.; Rodrigues Macedo, C.H.; de Lima, G.V.; de Jesus Benevides, L. Effects of depth on reef fish flight initiation distance: Implications of deeper reefs conservation. Environ. Biol. Fish. 2020, 103, 1247–1256. [Google Scholar] [CrossRef]
- Magellan, K.; García-Berthou, E. Prioritizing sex recognition over learned species recognition: Hierarchical mate recognition in an invasive fish. Front. Ecol. Evol. 2021, 9, 646357. [Google Scholar] [CrossRef]
- Magellan, K.; Swartz, E.R. Crypsis in a heterogeneous environment: Relationships between changeable polymorphic colour patterns and behaviour in a galaxiid fish. Freshw. Biol. 2013, 58, 793–799. [Google Scholar] [CrossRef]
- Magellan, K.; Bonebrake, T.C.; Dudgeon, D. Temperature effects on exploratory behaviour and learning ability of invasive mosquitofish. Aquat. Inv. 2019, 14, 502–517. [Google Scholar] [CrossRef]
- Arthington, A.H.; Dulvy, N.; Gladstone, W.; Winfield, I.J. Fish conservation in freshwater and marine realms: Status, threats and management Aquatic Conserv. Mar. Freshw. Ecosyst. 2016, 26, 838–857. [Google Scholar] [CrossRef] [Green Version]
- Manjarrés-Hernández, A.; Guisande, C.; García-Roselló, E.; Heine, J.; Pelayo-Villamil, P.; Pérez-Costas, E.; González-Vilas, L.; González-Dacosta, J.; Duque, S.R.; Granado-Lorencio, C.; et al. Predicting the effects of climate change on future freshwater fish diversity at global scale. Nat. Conserv. 2021, 43, 1–24. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Quiñones, R.M.; Clements, S.; Letcher, B.H. Managing climate refugia for freshwater fishes under an expanding human footprint. Front. Ecol. Environ. 2020, 18, 271–280. [Google Scholar] [CrossRef]
- Chakona, A.; Skelton, P.H. A review of the Pseudobarbus afer (Peters, 1864) species complex (Teleostei, Cyprinidae) in the Eastern Cape Fold ecoregion of South Africa. ZooKeys 2017, 657, 109–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magellan, K.; Weyl, O.L.F.; Booth, A.J. Preference for Artificial Refugia over Natural Refugia in an Endangered Fish. Diversity 2021, 13, 635. https://doi.org/10.3390/d13120635
Magellan K, Weyl OLF, Booth AJ. Preference for Artificial Refugia over Natural Refugia in an Endangered Fish. Diversity. 2021; 13(12):635. https://doi.org/10.3390/d13120635
Chicago/Turabian StyleMagellan, Kit, Olaf L. F. Weyl, and Anthony J. Booth. 2021. "Preference for Artificial Refugia over Natural Refugia in an Endangered Fish" Diversity 13, no. 12: 635. https://doi.org/10.3390/d13120635
APA StyleMagellan, K., Weyl, O. L. F., & Booth, A. J. (2021). Preference for Artificial Refugia over Natural Refugia in an Endangered Fish. Diversity, 13(12), 635. https://doi.org/10.3390/d13120635





