Seasonal Variation in Fecal Glucocorticoid Levels and Their Relationship to Reproductive Success in Captive Populations of an Endangered Parrot
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Reproductive Information
2.3. Pair Selection and Breeding Stages
2.4. Assay Validation
2.5. Fecal Sampling and Analysis
2.6. Statistical Analysis
3. Results
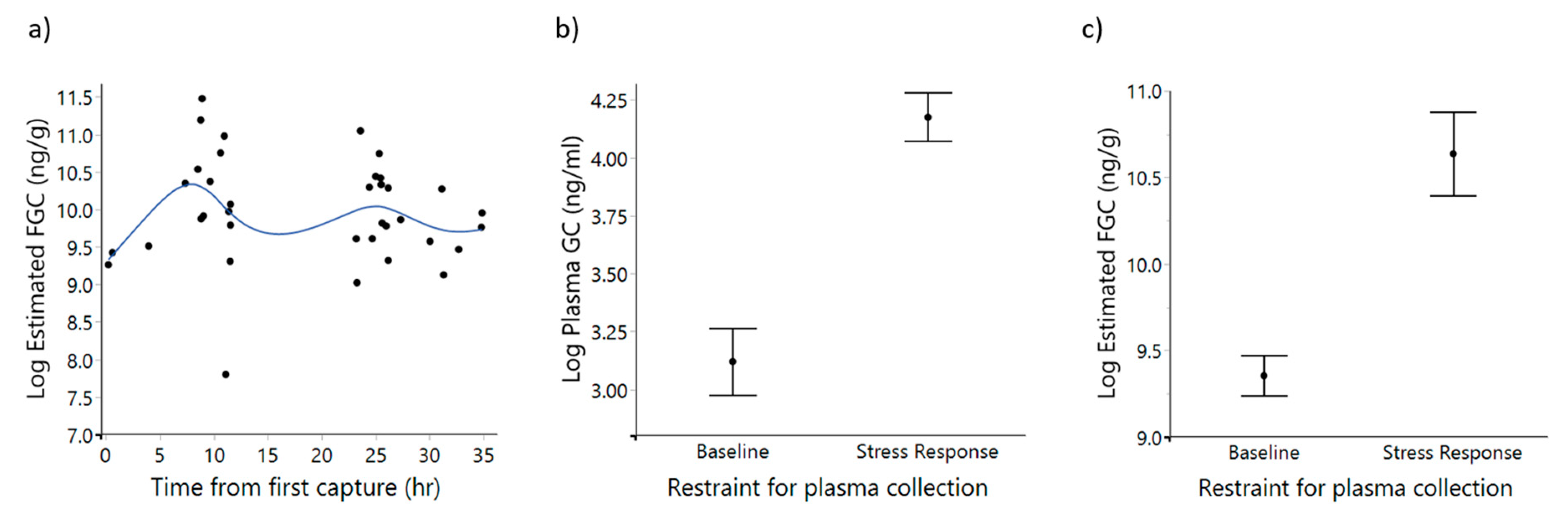
3.1. Validation of Corticosterone Assay
3.2. Glucocorticoid Metabolite Levels and Measures of Reproductive Success
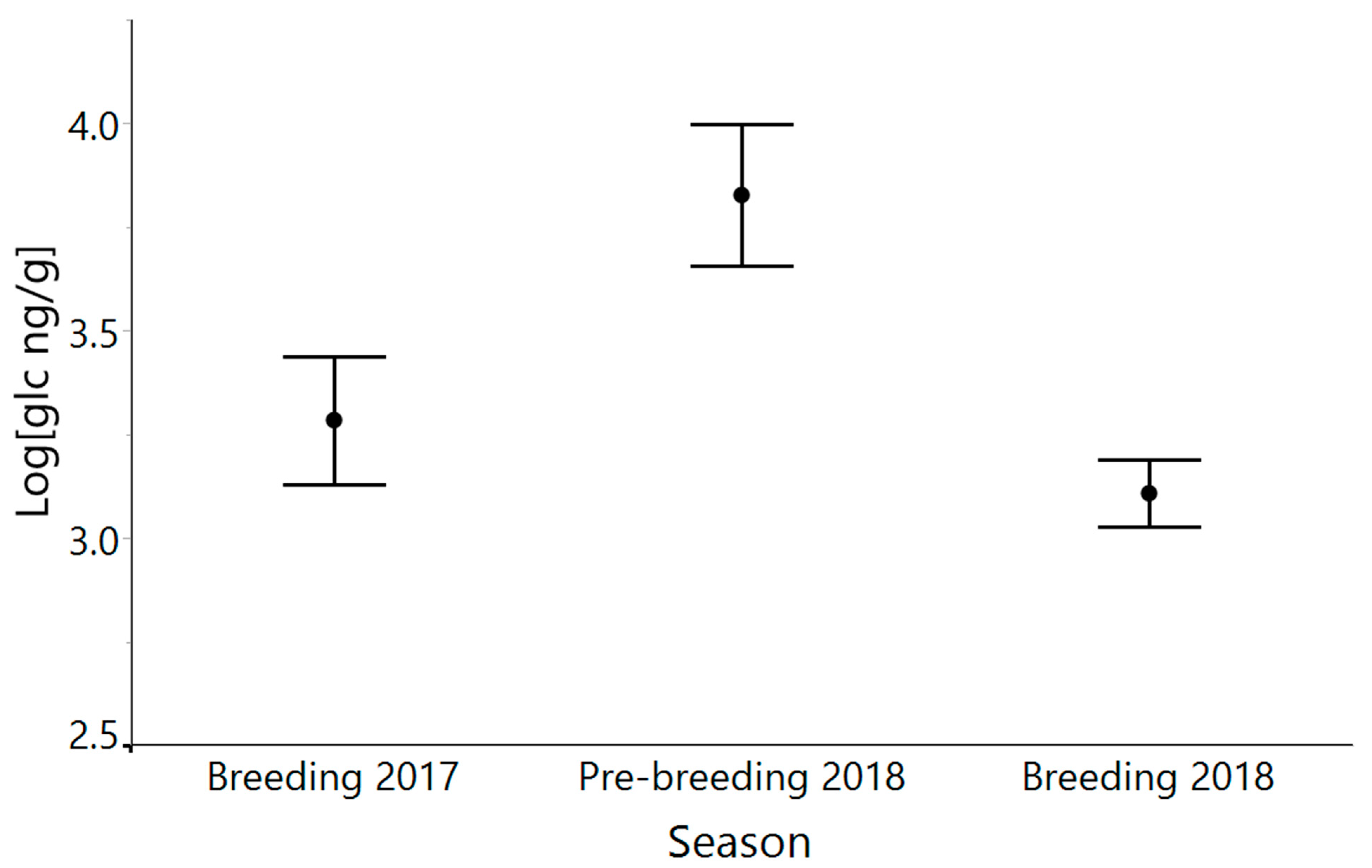
3.3. Hurricane Maria and Seasonal Variation
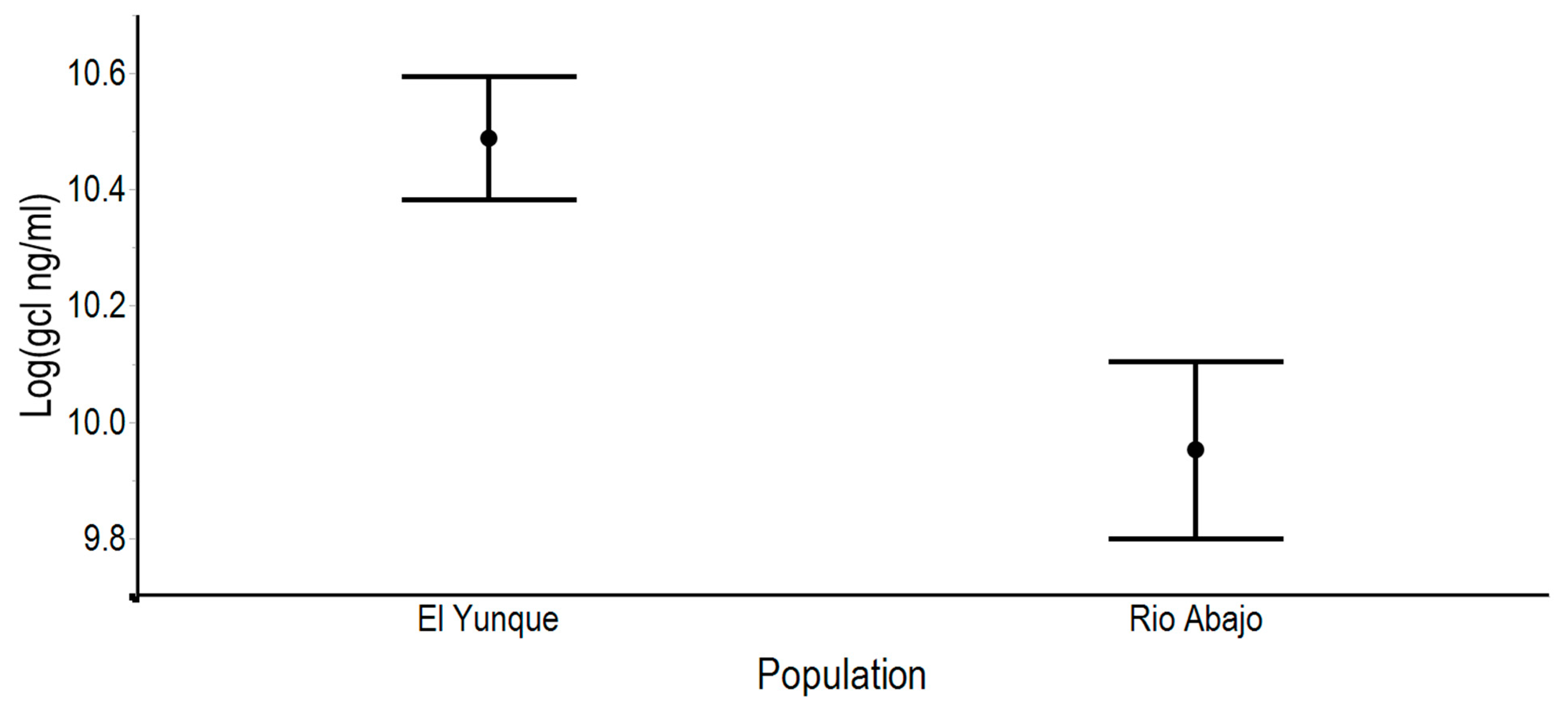
3.4. Glucocorticoid Metabolite Levels Differed between Sexes and Populations
4. Discussion
4.1. Fecal Glucocorticoid Metabolite Levels and Reproductive Success
4.2. Seasonal Variation
4.3. Glucocorticoid Levels Do Not Differ between Breeding Seasons, despite Hurricane Maria
4.4. Males Show Higher Fecal Glucocorticoid Metabolite Levels than Females
4.5. Differences between Captive Populations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Permits and Approval Statement
References
- Romero, L.M.; Wingfield, J.C. Tempest, Poxes, and Predators, and People: Stress in Wild Animals and How They Cope; Oxford University Press: Oxford, UK, 2016; Volume 1. [Google Scholar]
- Boonstra, R. Reality as the leading cause of stress: Rethinking the impact of chronic stress in nature. Funct. Ecol. 2013, 27, 11–23. [Google Scholar] [CrossRef]
- Romero, L.M.; Reed, J.M.; Wingfield, J.C. Effects of weather on corticosterone responses in wild free-living passerine birds. Gen. Comp. Endocrinol. 2000, 118, 113–122. [Google Scholar] [CrossRef]
- Busch, D.S.; Hayward, L.S. Stress in a conservation context: A discussion of glucocorticoid actions and how levels change with conservation-relevant variables. Biol. Conserv. 2009, 142, 2844–2853. [Google Scholar] [CrossRef]
- McEwen, B.S.; Wingfield, J.C. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003, 43, 2–15. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef]
- Thiel, D.; Jenni-Eiermann, S.; Palme, R.; Jenni, L. Winter tourism increases stress hormone levels in the Capercaillie Tetrao urogallus. Ibis 2011, 153, 122–133. [Google Scholar] [CrossRef]
- Angelier, F.; Clément-Chastel, C.; Welcker, J.; Gabrielsen, G.W.; Chastel, O. How does corticosterone affect parental behaviour and reproductive success? A study of prolactin in black-legged kittiwakes. Funct. Ecol. 2009, 23, 784–793. [Google Scholar] [CrossRef]
- Love, O.P.; Breuner, C.W.; Vézina, F.; Williams, T.D. Mediation of a corticosterone-induced reproductive conflict. Horm. Behav. 2004, 46, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Bowers, E.K.; Thompson, C.F.; Bowden, R.M.; Sakaluk, S.K. Posthatching Parental Care and Offspring Growth Vary with Maternal Corticosterone Level in a Wild Bird Population. Physiol. Biochem. Zool. 2019, 92, 496–504. [Google Scholar] [CrossRef]
- Lin, H.; Decuypere, E.; Buyse, J. Oxidative stress induced by corticosterone administration in broiler chickens (Gallus gallus domesticus): 1. Chronic exposure. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004, 139, 737–744. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, J.; Lai, Z.; Tian, Y.; Fang, L.; Wu, M.; Xiong, J.; Qin, X.; Luo, A.; Wang, S. Long-term moderate oxidative stress decreased ovarian reproductive function by reducing follicle quality and progesterone production. PLoS ONE 2016, 11, e0162194. [Google Scholar] [CrossRef] [PubMed]
- Earnhardt, J.M. The Role of Captive Populations in Reintroduction Programs. In Wild Mammals in Captivity: Principles and Techniques for Zoo Management; The University of Chicago Press: Chicago, IL, USA, 2010; pp. 268–280. [Google Scholar]
- Earnhardt, J.; Vélez-Valentín, J.; Valentin, R.; Long, S.; Lynch, C.; Schowe, K. The puerto rican parrot reintroduction program: Sustainable management of the aviary population. Zoo Biol. 2014, 33, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Gilby, A.J.; Mainwaring, M.C.; Rollins, L.A.; Griffith, S.C. Parental care in wild and captive zebra finches: Measuring food delivery to quantify parental effort. Anim. Behav. 2011, 81, 289–295. [Google Scholar] [CrossRef]
- Mason, G.J. Species differences in responses to captivity: Stress, welfare and the comparative method. Trends Ecol. Evol. 2010, 25, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, S.; Carrete, M.; Tella, J.L.; Marchant, T.A.; Bortolotti, G.R. Differences in acute stress responses between wild-caught and captive-bred birds: A physiological mechanism contributing to current avian invasions? Biol. Invasions 2013, 15, 521–527. [Google Scholar] [CrossRef][Green Version]
- Adkins-Regan, E. Do hormonal control systems produce evolutionary inertia? Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1599–1609. [Google Scholar] [CrossRef]
- Ball, G.F.; Balthazart, J. Individual variation and the endocrine regulation of behaviour and physiology in birds: A cellular/molecular perspective. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1699–1710. [Google Scholar] [CrossRef]
- Dawson, A. Control of the annual cycle in birds: Endocrine constraints and plasticity in response to ecological variability. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1621–1633. [Google Scholar] [CrossRef]
- Nelson, B.F.; Daunt, F.; Monaghan, P.; Wanless, S.; Butler, A.; Heidinger, B.J.; Newell, M.; Dawson, A. Protracted treatment with corticosterone reduces breeding success in a long-lived bird. Gen. Comp. Endocrinol. 2015, 210, 38–45. [Google Scholar] [CrossRef]
- Wingfield, J.C. The concept of allostasis: Coping with a capricious environment. J. Mammal. 2005, 86, 248–254. [Google Scholar] [CrossRef]
- Wingfield, J.C. Environmental endocrinology: Insights into the diversity of regulatory mechanisms in life cycles. Integr. Comp. Biol. 2018, 58, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Lance, V.A.; Elsey, R.M.; Butterstein, G.; Trosclair, P.L.; Merchant, M. The effects of hurricane Rita and subsequent drought on alligators in Southwest Louisiana. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2010, 313, 106–113. [Google Scholar] [CrossRef]
- Waide, R.B. The effect of hurricane Hugo on bird populations in the Luquillo Experimental Forest, Puerto Rico. Biotropica 1991, 23, 475–480. [Google Scholar] [CrossRef]
- Wunderle, J.M. Pre- and Post-Hurricane fruit availability: Implications for Puerto Rican Parrots in the Luquillo Mountains. Caribb. J. Sci. 1999, 35, 249–264. [Google Scholar]
- Anestis, S.F. Urinary cortisol responses to unusual events in captive chimpanzees (Pan troglodytes). Stress 2009, 12, 49–57. [Google Scholar] [CrossRef] [PubMed]
- IUCN. The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/search/stats?taxonomies=22672853&searchType=species (accessed on 10 August 2020).
- Berkunsky, I.; Quillfeldt, P.; Brightsmith, D.J.; Abbud, M.C.; Aguilar, J.M.R.E.; Alemán-Zelaya, U.; Aramburú, R.M.; Arce Arias, A.; Balas McNab, R.; Balsby, T.J.S.; et al. Current threats faced by Neotropical parrot populations. Biol. Conserv. 2017, 214, 278–287. [Google Scholar] [CrossRef]
- Dahlin, C.R.; Blake, C.; Rising, J.; Wright, T.F. Long-term monitoring of Yellow-naped Amazons (Amazona auropalliata) in Costa Rica: Breeding biology, duetting, and the negative impact of poaching. J. Field Ornithol. 2018, 89, 1–10. [Google Scholar] [CrossRef]
- Olah, G.; Butchart, S.H.M.; Symes, A.; Guzmán, I.M.; Cunningham, R.; Brightsmith, D.J.; Heinsohn, R. Ecological and socio-economic factors affecting extinction risk in parrots. Biodivers. Conserv. 2016, 25, 205–223. [Google Scholar] [CrossRef]
- Grajal, A. The Neotropics (Americas). In Parrots. Status Surveys and Conservation Action Plan 2000–2004; IUCN: Gland, Switzerland; Cambridge, UK, 2000; pp. 98–151. ISBN 2831705045. [Google Scholar]
- Wright, T.F.; Toft, C.A.; Enkerlin-Hoeflich, E.; Gonzalez-Elizondo, J.; Albornoz, M.; Rodríguez-Ferraro, A.; Rojas-Suárez, F.; Sanz, V.; Trujillo, A.; Beissinger, S.R.; et al. Nest poaching in Neotropical parrots. Conserv. Biol. 2001, 15, 710–720. [Google Scholar] [CrossRef]
- Snyder, N.F.R.; Wiley, J.W.; Kepler, C.B. The Parrots of Luquillo: Natural History and Conservation of the Puerto Rican Parrot; Western Foundation of Vertebrate Zoology: Los Angeles, CA, USA, 1987. [Google Scholar]
- Beissinger, S.R.; Wunderle, J.M.; Meyers, J.M.; Saether, B.E.; Engen, S. Anatomy of a bottleneck: Diagnosing factors limiting population growth in the puerto rican parrot. Ecol. Monogr. 2008, 78, 185–203. [Google Scholar] [CrossRef]
- Snyder, N.F.R.; Derrickson, S.R.; Beissinger, S.R.; Wiley, J.W.; Smith, T.B.; Toone, W.D.; Miller, B. Limitations of captive breeding in endangered species recovery. Conserv. Biol. 1996, 10, 338–348. [Google Scholar] [CrossRef]
- Wilson, M.H.; Kepler, C.B.; Snyder, N.F.R.; Derrickson, S.R.; Josh, F.; Wiley, J.W.; Wunderle, J.M.; Lugo, A.E.; Graham, D.L.; Toone, D. Puerto Rican Parrots of the Potential Approach Metapopulation Species Conservation. Conserv. Biol. 1994, 8, 114–123. [Google Scholar] [CrossRef]
- Mohlman, J.L.; Navara, K.J.; Sheriff, M.J.; Terhune, T.M.; Martin, J.A. Validation of a noninvasive technique to quantify stress in northern bobwhite (Colinus virginianus). Conserv. Physiol. 2020, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Washburn, B.E.; Millspaugh, J.J. Effects of simulated environmental conditions on glucocorticoid metabolite measurements in white-tailed deer feces. Gen. Comp. Endocrinol. 2002, 127, 217–222. [Google Scholar] [CrossRef]
- Terio, K.A.; Brown, J.L.; Moreland, R.; Munson, L. Comparison of different drying and storage methods on quantifiable concentrations of fecal steroids in the cheetah. Zoo Biol. 2002, 21, 215–222. [Google Scholar] [CrossRef]
- McGlothlin, J.W.; Jawor, J.M.; Greives, T.J.; Casto, J.M.; Phillips, J.L.; Ketterson, E.D. Hormones and honest signals: Males with larger ornaments elevate testosterone more when challenged. J. Evol. Biol. 2008, 21, 39–48. [Google Scholar] [CrossRef]
- Jawor, J.M.; McGlothlin, J.W.; Casto, J.M.; Greives, T.J.; Snajdr, E.A.; Bentley, G.E.; Ketterson, E.D. Seasonal and individual variation in response to GnRH challenge in male dark-eyed juncos (Junco hyemalis). Gen. Comp. Endocrinol. 2006, 149, 182–189. [Google Scholar] [CrossRef]
- McGlothlin, J.W.; Jawor, J.M.; Ketterson, E.D. Natural variation in a testosterone-mediated trade-off between mating effort and parental effort. Am. Nat. 2007, 170, 864–875. [Google Scholar] [CrossRef]
- DeVries, M.S.; Winters, C.P.; Jawor, J.M. Testosterone elevation and response to gonadotropin-releasing hormone challenge by male Northern Cardinals (Cardinalis cardinalis) following aggressive behavior. Horm. Behav. 2012, 62, 99–105. [Google Scholar] [CrossRef]
- DeVries, M.S.; Jawor, J.M. Natural variation in circulating testosterone does not predict nestling provisioning rates in the northern cardinal, Cardinalis cardinalis. Anim. Behav. 2013, 85, 957–965. [Google Scholar] [CrossRef]
- Susan Devries, M.; Holbrook, A.L.; Winters, C.P.; Jawor, J.M. Non-breeding gonadal testosterone production of male and female Northern Cardinals (Cardinalis cardinalis) following GnRH challenge. Gen. Comp. Endocrinol. 2011, 174, 370–378. [Google Scholar] [CrossRef]
- Duckworth, B.M.; Jawor, J.M. Corticosterone profiles in northern cardinals (Cardinalis cardinalis): Do levels vary through life history stages? Gen. Comp. Endocrinol. 2018, 263, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Clubb, S.; Velez, J.; Garner, M.M.; Zaias, J.; Cray, C. Health and Reproductive Assessment of Selected Puerto Rican Parrots (Amazona vittata) in Captivity. J. Avian Med. Surg. 2015, 29, 313–325. [Google Scholar] [CrossRef]
- Johnson, B.H.; Welsh, T.H.; Juniewicz, P.E. Suppression of luteinizing hormone and testosterone secretion in bulls following adrenocorticotropin hormone treatment. Biol. Reprod. 1982, 26, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Orr, T.E.; Mann, D.R. Role of glucocorticoids in the stress-induced suppression of testicular steroidogenesis in adult male rats. Horm. Behav. 1992, 26, 350–363. [Google Scholar] [CrossRef]
- Deviche, P.; Gao, S.; Davies, S.; Sharp, P.J.; Dawson, A. Rapid stress-induced inhibition of plasma testosterone in free-ranging male rufous-winged sparrows, Peucaea carpalis: Characterization, time course, and recovery. Gen. Comp. Endocrinol. 2012, 177, 1–8. [Google Scholar] [CrossRef]
- Bowers, E.K.; Bowden, R.M.; Sakaluk, S.K.; Thompson, C.F. Immune activation generates corticosterone-mediated terminal reproductive investment in a wild bird. Am. Nat. 2015, 185, 769–783. [Google Scholar] [CrossRef]
- Breuner, C.W. Stress and Reproduction in Birds. In Hormones and Reproduction of Vertebrates; Academic Press: Cambridge, MA, USA, 2011; Volume 4, pp. 129–151. ISBN 9780123749291. [Google Scholar]
- Young, A.M.; Hobson, E.A.; Lackey, L.B.; Wright, T.F. Survival on the ark: Life-history trends in captive parrots. Anim. Conserv. 2012, 15, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Lattin, C.R.; Romero, L.M. Seasonal variation in corticosterone receptor binding in brain, hippocampus, and gonads in House Sparrows (Passer domesticus). Auk 2013, 130, 591–598. [Google Scholar] [CrossRef]
- Li, D.; Wang, G.; Wingfield, J.C.; Zhang, Z.; Ding, C.; Lei, F. Seasonal changes in adrenocortical responses to acute stress in Eurasian tree sparrow (Passer montanus) on the Tibetan Plateau: Comparison with house sparrow (P. domesticus) in North America and with the migratory P. domesticus in Qinghai Province. Gen. Comp. Endocrinol. 2008, 158, 47–53. [Google Scholar] [CrossRef]
- Romero, L.M. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen. Comp. Endocrinol. 2002, 128, 1–24. [Google Scholar] [CrossRef]
- Romero, L.M.; Cyr, N.E.; Romero, R.C. Corticosterone responses change seasonally in free-living house sparrows (Passer domesticus). Gen. Comp. Endocrinol. 2006, 149, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Romero, L.M.; Meister, C.J.; Cyr, N.E.; Kenagy, G.J.; Wingfield, J.C. Seasonal glucocorticoid responses to capture in wild free-living mammals. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 294, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Popp, L.G.; Serafini, P.P.; Reghelin, A.L.S.; Spercoski, K.M.; Roper, J.J.; Morais, R.N. Annual pattern of fecal corticoid excretion in captive Red-tailed parrots (Amazona brasiliensis). J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2008, 178, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Dickens, M.J.; Bentley, G.E. Stress, captivity, and reproduction in a wild bird species. Horm. Behav. 2014, 66, 685–693. [Google Scholar] [CrossRef]
- White, T.H.; Collazo, J.A.; Vilella, F.J.; Guerrero, S.A. Effects of Hurricane Georges on habitat use by captive-reared Hispaniolan Parrots (Amazona ventralis) released in the Dominican Republic. Ornitol. Neotrop. 2005, 16, 405–417. [Google Scholar]
- Helfenstein, F.; Wagner, R.H.; Danchin, E.; Rossi, J.M. Functions of courtship feeding in black-legged kittiwakes: Natural and sexual selection. Anim. Behav. 2003, 65, 1027–1033. [Google Scholar] [CrossRef]
- Seymour, R.M.; Sozou, P.D. Duration of courtship effort as a costly signal. J. Theor. Biol. 2009, 256, 1–13. [Google Scholar] [CrossRef]
- MacLeod, K.J.; Sheriff, M.J.; Ensminger, D.C.; Owen, D.A.S.; Langkilde, T. Survival and reproductive costs of repeated acute glucocorticoid elevations in a captive, wild animal. Gen. Comp. Endocrinol. 2018, 268, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cyr, N.E.; Romero, L.M. Fecal glucocorticoid metabolites of experimentally stressed captive and free-living starlings: Implications for conservation research. Gen. Comp. Endocrinol. 2008, 158, 20–28. [Google Scholar] [CrossRef]
- Vidal, A.C.; Roldan, M.; Christofoletti, M.D.; Tanaka, Y.; Galindo, D.J.; Duarte, J.M.B. Stress in captive Blue-fronted parrots (Amazona aestiva): The animalists’tale. Conserv. Physiol. 2019, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Macchi, E.; Valle, E.; De Marco, M.; Nucera, D.M.; Gasco, L.; Schiavone, A. An association between feather damaging behavior and corticosterone metabolite excretion in captive African grey parrots (Psittacus erithacus). PeerJ 2016, 2016, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jill Heatley, J.; Oliver, J.W.; Hosgood, G.; Columbini, S.; Tully, T.N. Serum corticosterone concentrations in response to restraint, anesthesia, and skin testing in hispaniolan amazon parrots (Amazona ventralis). J. Avian Med. Surg. 2000, 14, 172–176. [Google Scholar] [CrossRef]
- Owen, D.J.; Lane, J.M. High levels of corticosterone in feather-plucking parrots (Psittacus erithacus). Vet. Rec. 2006, 158, 804–805. [Google Scholar] [CrossRef] [PubMed]
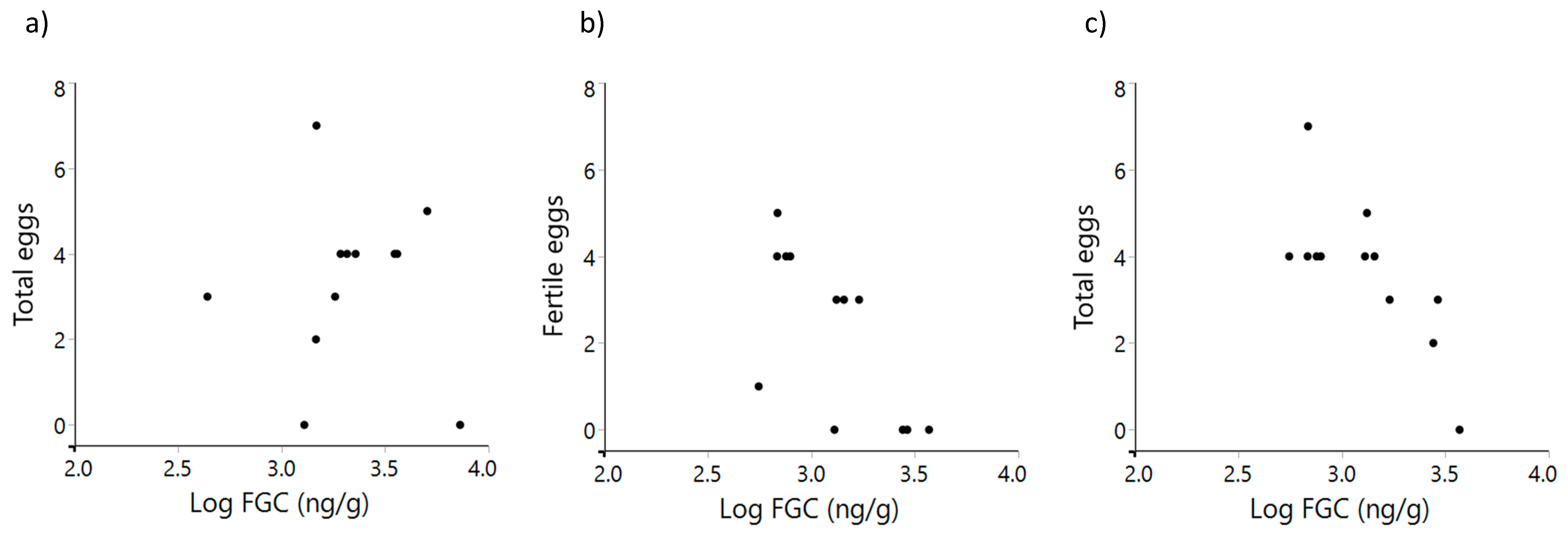

| Reproductive Success | Season | n | r | p |
|---|---|---|---|---|
| Total eggs | Breeding 2017 | 12 | 0.2587 | 0.4169 |
| Pre-breeding 2018 | 10 | 0.4411 | 0.2019 | |
| Breeding 2018 | 12 | −0.7255 | 0.0076 | |
| Fertile eggs | Breeding 2017 | 12 | −0.0431 | 0.8942 |
| Pre-breeding 2018 | 10 | −0.0187 | 0.9591 | |
| Breeding 2018 | 12 | −0.6538 | 0.0211 | |
| Chicks | Breeding 2017 | 12 | −0.4139 | 0.1810 |
| Pre-breeding 2018 | 10 | 0.0781 | 0.8301 | |
| Breeding 2018 | 12 | −0.3897 | 0.2105 | |
| Fledglings | Breeding 2017 | 12 | −0.4193 | 0.1810 |
| Pre-breeding 2018 | 10 | 0.0855 | 0.8143 | |
| Breeding 2018 | 12 | −0.3528 | 0.2607 |
| Reproductive Success | n | r | p |
|---|---|---|---|
| Total eggs | 11 | 0.6404 | 0.0338 |
| Fertile eggs | 11 | 0.3202 | 0.3371 |
| Chicks | 11 | 0.2741 | 0.4147 |
| Fledglings | 11 | 0.2513 | 0.4561 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Güivas, B.; Jawor, J.M.; Wright, T.F. Seasonal Variation in Fecal Glucocorticoid Levels and Their Relationship to Reproductive Success in Captive Populations of an Endangered Parrot. Diversity 2021, 13, 617. https://doi.org/10.3390/d13120617
Ramos-Güivas B, Jawor JM, Wright TF. Seasonal Variation in Fecal Glucocorticoid Levels and Their Relationship to Reproductive Success in Captive Populations of an Endangered Parrot. Diversity. 2021; 13(12):617. https://doi.org/10.3390/d13120617
Chicago/Turabian StyleRamos-Güivas, Brian, Jodie M. Jawor, and Timothy F. Wright. 2021. "Seasonal Variation in Fecal Glucocorticoid Levels and Their Relationship to Reproductive Success in Captive Populations of an Endangered Parrot" Diversity 13, no. 12: 617. https://doi.org/10.3390/d13120617
APA StyleRamos-Güivas, B., Jawor, J. M., & Wright, T. F. (2021). Seasonal Variation in Fecal Glucocorticoid Levels and Their Relationship to Reproductive Success in Captive Populations of an Endangered Parrot. Diversity, 13(12), 617. https://doi.org/10.3390/d13120617






