Range Shifts in the Worldwide Expansion of Oenothera drummondii subsp. drummondii, a Plant Species of Coastal Dunes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Species Occurrence Data
2.2. Environmental Analysis and Climatic Profile
2.3. Species Distribution Modeling
2.4. Evaluation of the Models
3. Results
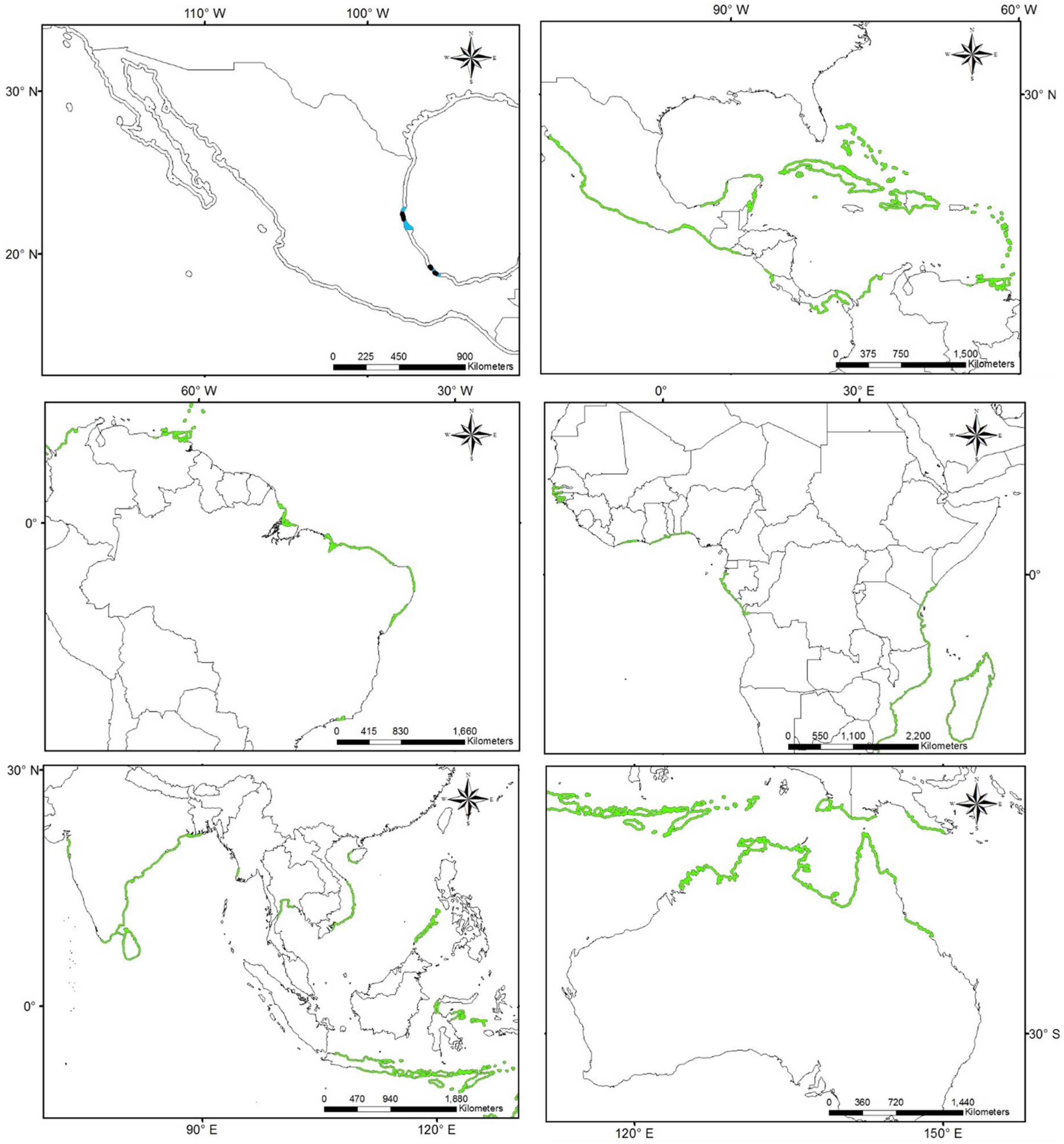
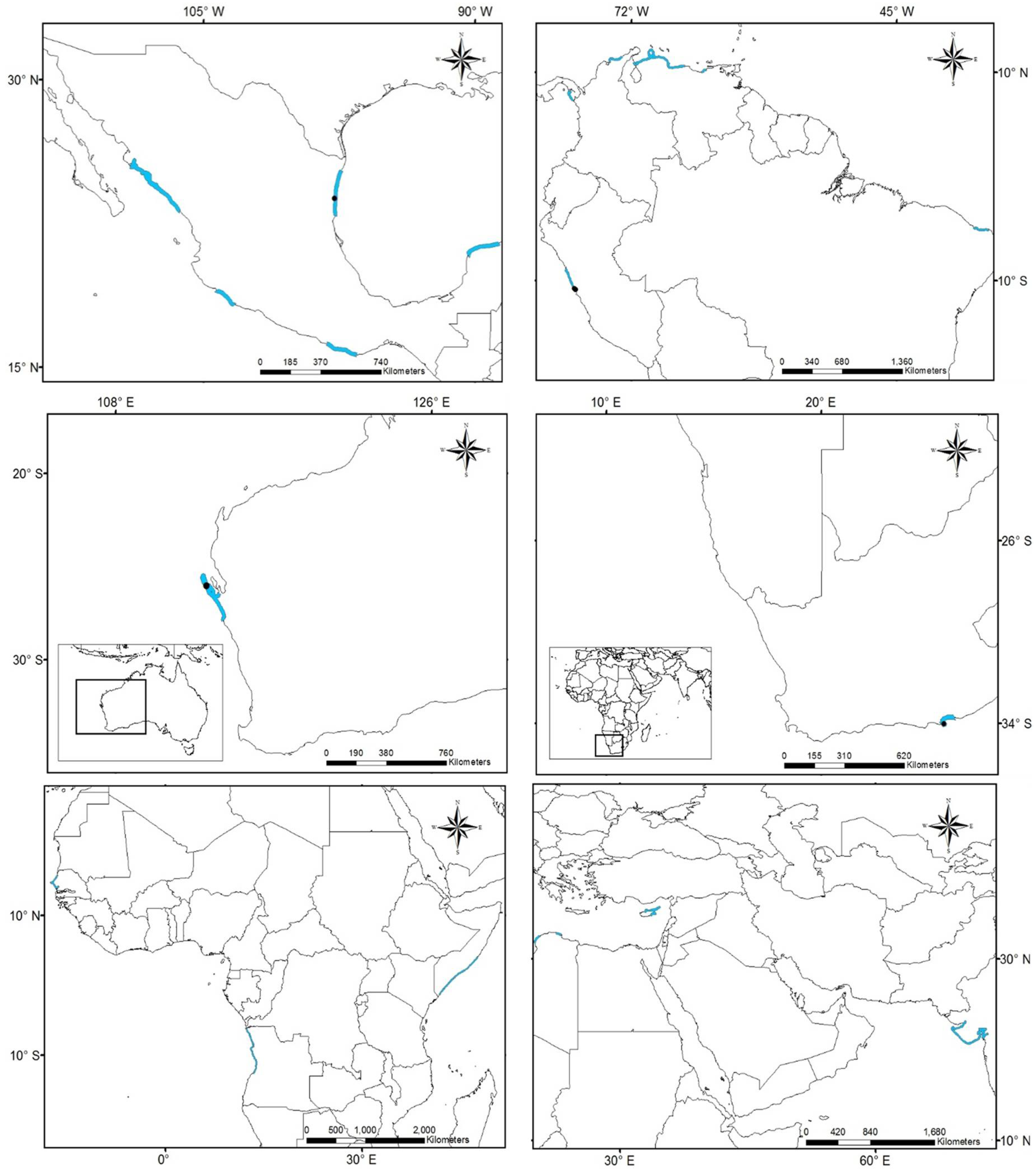
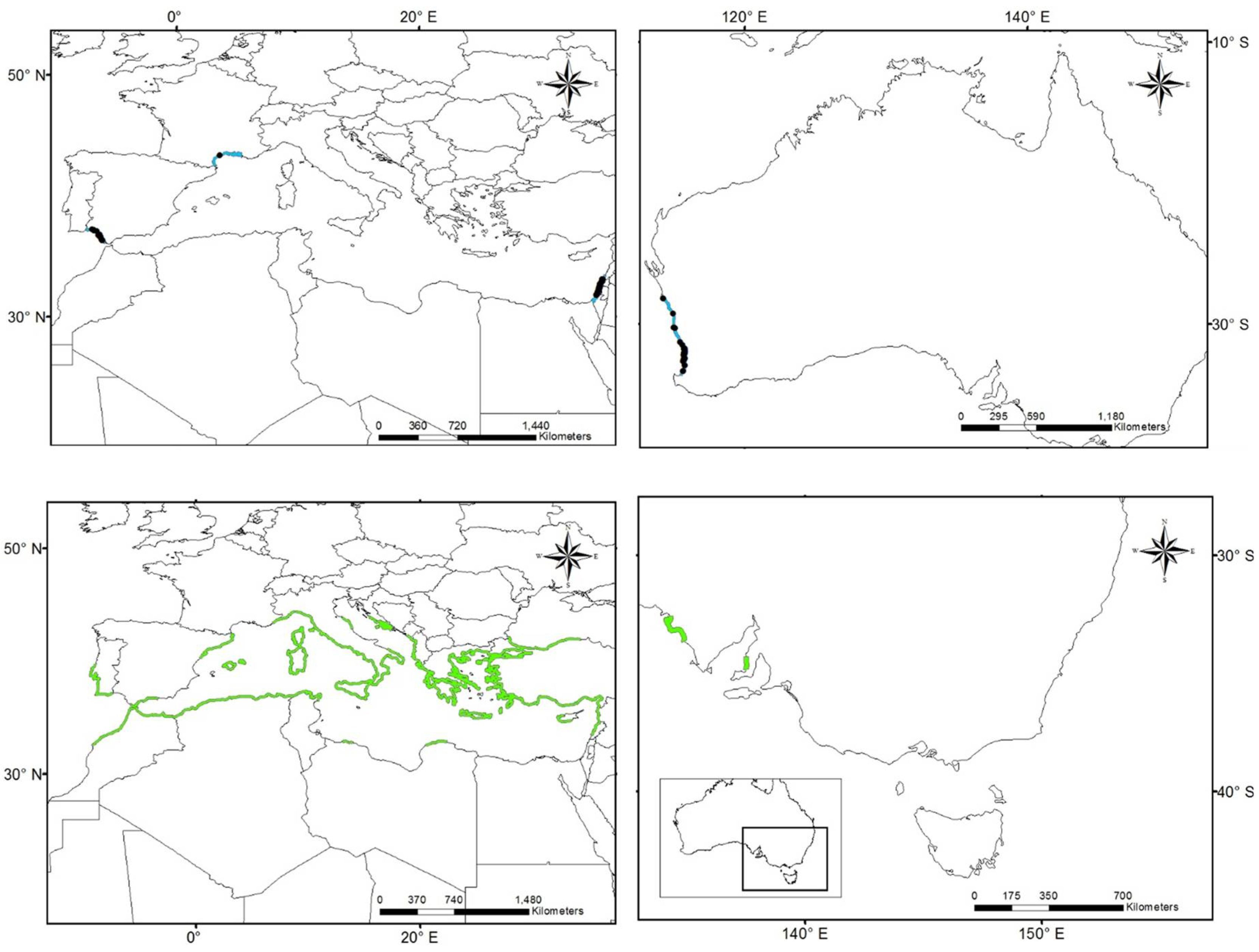
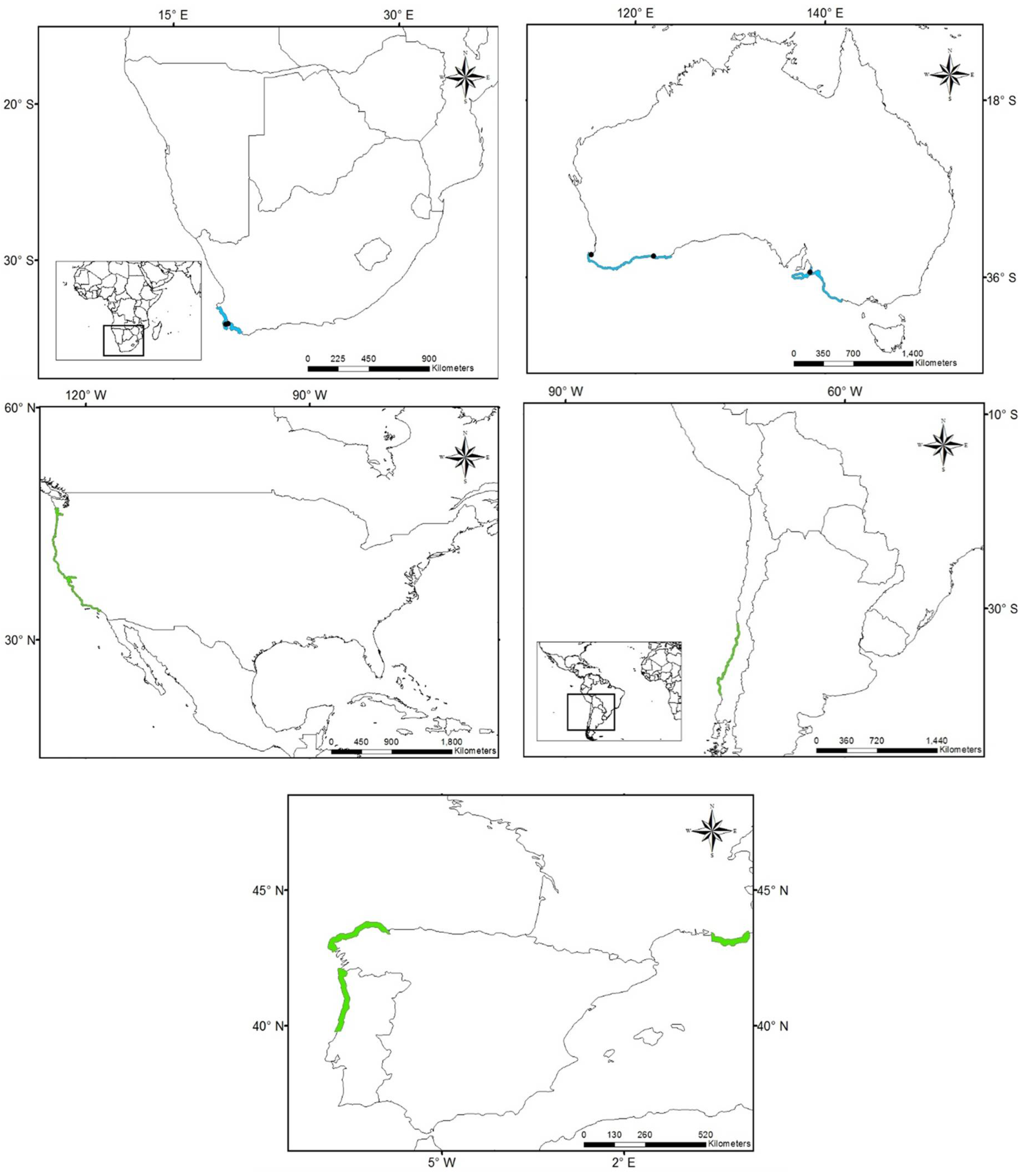
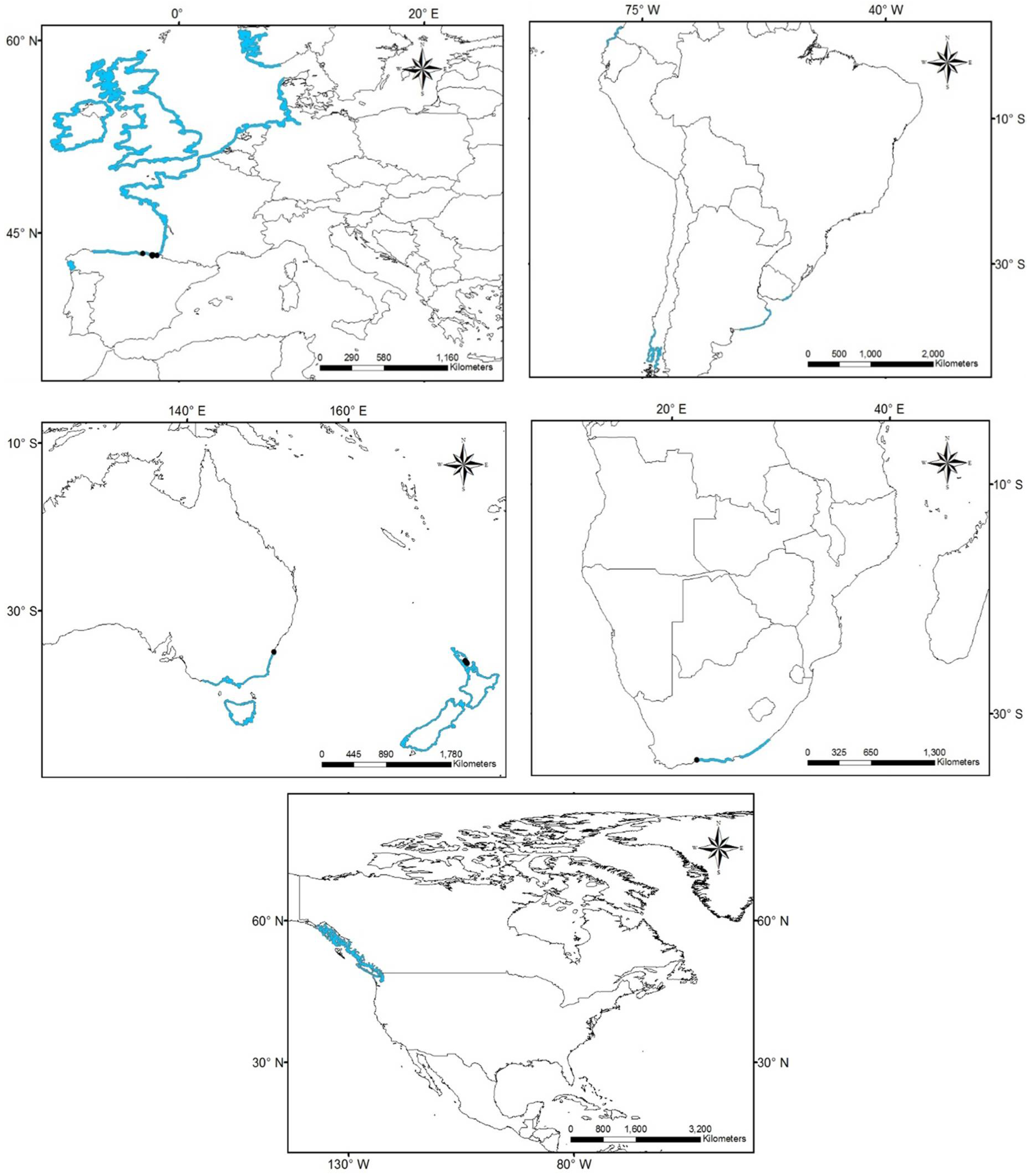
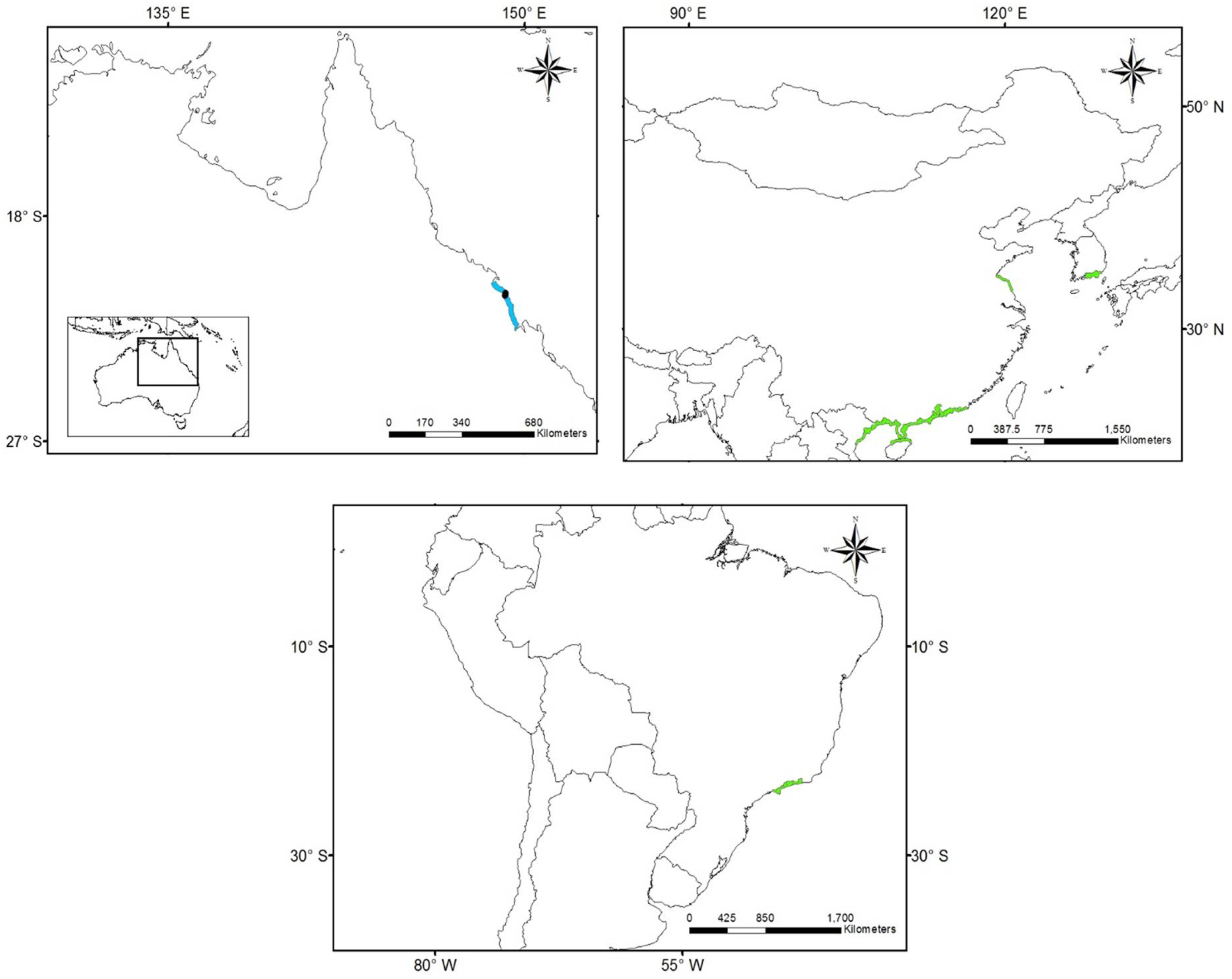
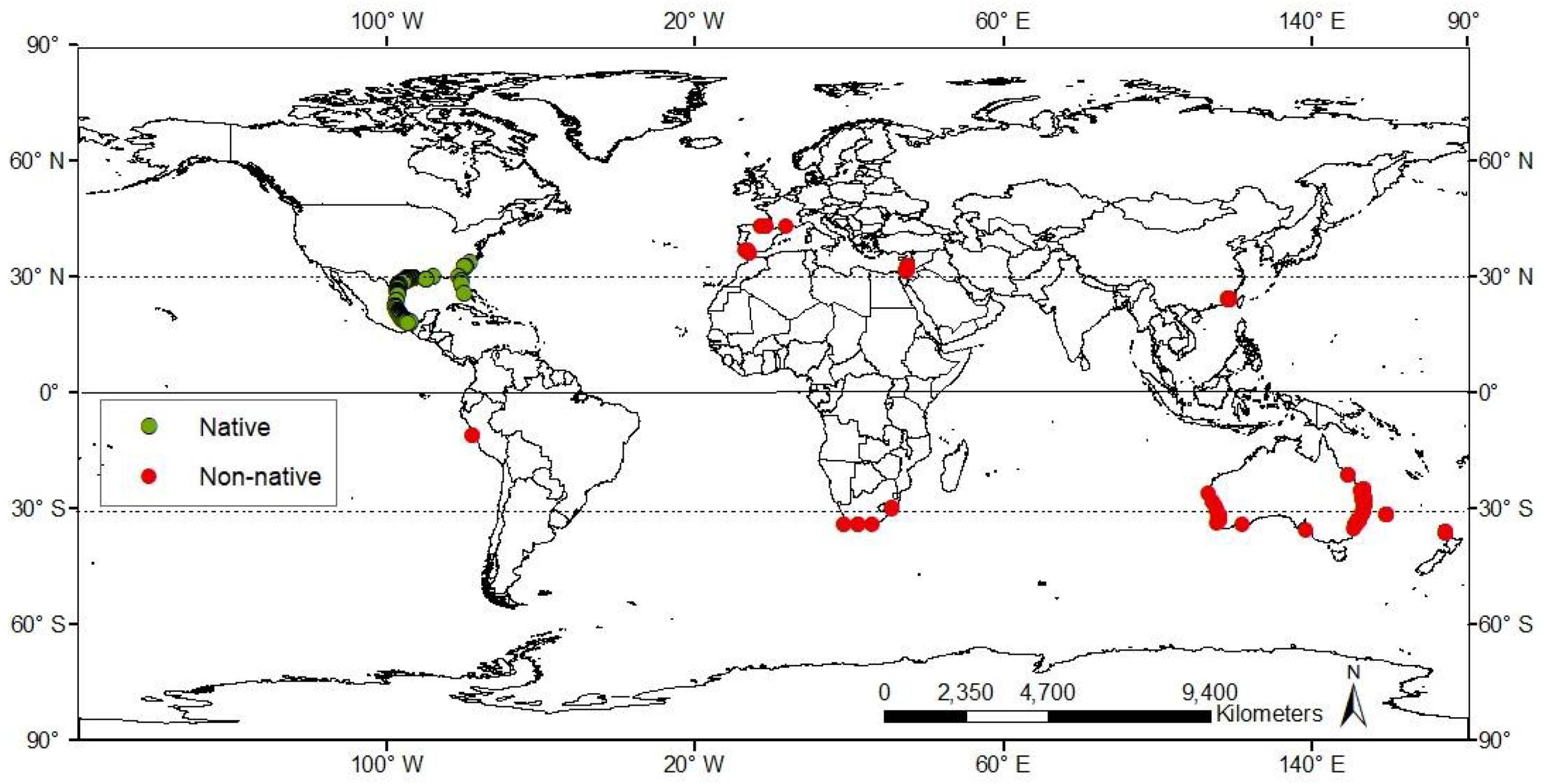
3.1. Worldwide Current Distribution
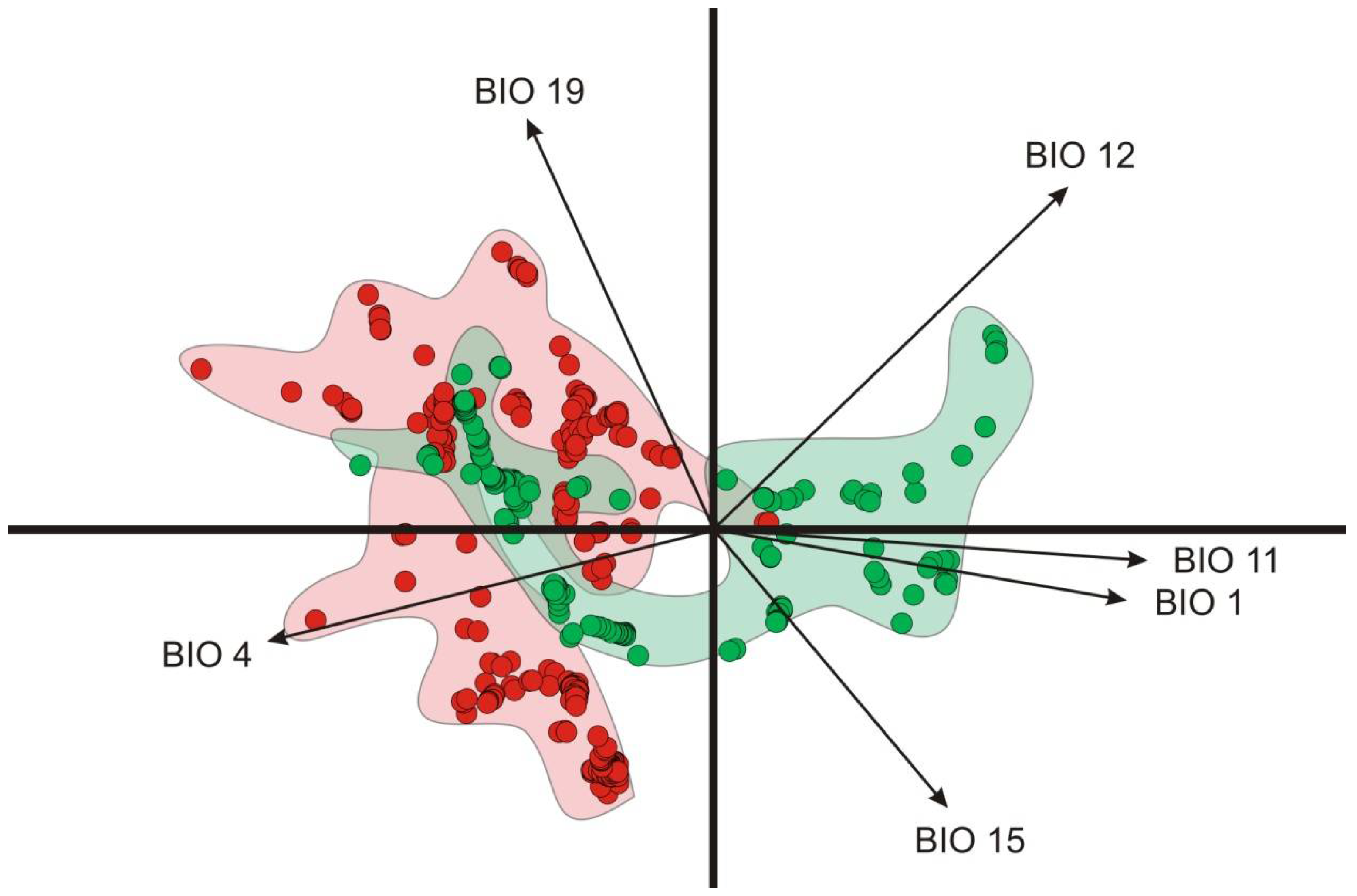
3.2. Environmental Analysis and Climatic Profile in Native and Non-Native Distribution
3.3. Potential of Invasion
3.4. Model Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Synthesis; Island Press: Washington, DC, USA, 2005; ISBN 978-1-59726-040-4. [Google Scholar]
- Mozdzer, T.J.; Zieman, J.C. Ecophysiological Differences between Genetic Lineages Facilitate the Invasion of Non-Native Phragmites Australis in North American Atlantic Coast Wetlands. J. Ecol. 2010, 98, 451–458. [Google Scholar] [CrossRef]
- Novoa, A.; González, L.; Moravcová, L.; Pyšek, P. Constraints to Native Plant Species Establishment in Coastal Dune Communities Invaded by Carpobrotus Edulis: Implications for Restoration. Biol. Conserv. 2013, 164, 1–9. [Google Scholar] [CrossRef]
- Paini, D.; Sheppard, A.; Cook, D.; Barro, P.; Worner, S.; Thomas, M. Global Threat to Agriculture from Invasive Species. Proc. Natl. Acad. Sci. USA 2016, 113, 7575–7579. [Google Scholar] [CrossRef] [Green Version]
- Castro-Díez, P.; Godoy, O.; Alonso, A.; Gallardo, A.; Saldaña, A. What Explains Variation in the Impacts of Exotic Plant Invasions on the Nitrogen Cycle? A Meta-Analysis. Ecol. Lett. 2014, 17, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrenfeld, J.G. Ecosystem Consequences of Biological Invasions. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 59–80. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.; Peng, R.; Luo, Y.; Zhou, X.; Wu, X.; Fang, C.; Chen, J.; Li, B. Altered Ecosystem Carbon and Nitrogen Cycles by Plant Invasion: A Meta-Analysis. New Phytol. 2008, 177, 706–714. [Google Scholar] [CrossRef]
- Pyšek, P.; Jarošík, V.; Hulme, P.E.; Pergl, J.; Hejda, M.; Schaffner, U.; Vilà, M. A Global Assessment of Invasive Plant Impacts on Resident Species, Communities and Ecosystems: The Interaction of Impact Measures, Invading Species’ Traits and Environment. Glob. Chang. Biol. 2012, 18, 1725–1737. [Google Scholar] [CrossRef]
- Pimentel, D.; McNair, S.; Janecka, J.; Wightman, J.; Simmonds, C.; O’Connell, C.; Wong, E.; Russel, L.; Zern, J.; Aquino, T.; et al. Economic and Environmental Threats of Alien Plant, Animal and Microbe Invasions. Agric. Ecosyst. 2001, 84, 1–20. [Google Scholar] [CrossRef]
- Elorza, M.; Sánchez, E.; Eduardo, S. Atlas de Las Plantas Alóctonas Invasoras En España; Gobierno de España: Madrid, Spain, 2004. [Google Scholar]
- Lomas, J.; Fernández-Carrillo, L.; Saavedra, M.; Mangas, L.; Rodríguez, C.; Gullón, E.; Martínez, E. Invasión de Oenothera drummondii Hook (Onagraceae) En El Paraje Natural Marismas Del Odiel (Huelva, S España). Bases Para La Gestión de Una Invasión Avanzada. Rev. Soc. Gaditana Hist. Nat. 2015, 9, 41–50. [Google Scholar]
- Hulme, P.E. Trade, Transport and Trouble: Managing Invasive Species Pathways in an Era of Globalization. J. Appl. Ecol. 2009, 46, 10–18. [Google Scholar] [CrossRef]
- Gallego-Fernández, J.; Martínez, M.; Garcia-Franco, J.G.; Zunzunegui, M. The Impact on Plant Communities of an Invasive Alien Herb, Oenothera Drummondii, Varies along the Beach-Coastal Dune Gradient. Flora 2019, 260, 151466. [Google Scholar] [CrossRef]
- Carboni, M.; Santoro, R.; Acosta, A.T.R. Are Some Communities of the Coastal Dune Zonation More Susceptible to Alien Plant Invasion? J. Plant Ecol. 2010, 3, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Antunes, C.; Pereira, A.J.; Fernandes, P.; Ramos, M.; Ascensão, L.; Correia, O.; Máguas, C. Understanding Plant Drought Resistance in a Mediterranean Coastal Sand Dune Ecosystem: Differences between Native and Exotic Invasive Species. J. Plant Ecol. 2018, 11, 26–38. [Google Scholar] [CrossRef] [Green Version]
- Maun, M.A. The Biology of Coastal Sand Dunes; Oxford University Press: Oxford, UK, 2009; ISBN 978-0-19-857035-6. [Google Scholar]
- Nentwig, W. Biological Invasions; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-3-540-36919-6. [Google Scholar]
- Lowry, E.; Rollinson, E.; Laybourn, A.; Scott, T.; Aiello-Lammens, M.; Gray, S.; Mickley, J.; Gurevitch, J. Biological Invasions: A Field Synopsis, Systematic Review, and Database of the Literature. Ecol. Evol. 2013, 3, 182–196. [Google Scholar] [CrossRef]
- Reyes, J.; Martínez, D. La Plasticidad de Las Plantas. Elem. Cienc. Cult. 2001, 8, 39–43. [Google Scholar]
- Villasenor, J.L.; Espinosa-García, F.J. The alien flowering plants of Mexico. Divers. Distrib. 2004, 10, 113–123. [Google Scholar] [CrossRef]
- Peterson, A.T. Predicting the Geography of Species’ Invasions via Ecological Niche Modeling. Q. Rev. Biol. 2003, 78, 419–433. [Google Scholar] [CrossRef] [Green Version]
- Peterson, A. Ecological Niches and Geographic Distributions; Princeton University Press: Princeton, NJ, USA, 2011; ISBN 978-0-691-13688-2. [Google Scholar]
- Peterson, A.; Papes, M.; Soberón, J. Rethinking Receiver Operating Characteristic Analysis Applications in Ecological Niche Modeling. Ecol. Model. 2008, 213, 63–72. [Google Scholar] [CrossRef]
- Richardson, D.M.; Thuiller, W. Home Away from Home—Objective Mapping of High-Risk Source Areas for Plant Introductions. Divers. Distrib. 2007, 13, 299–312. [Google Scholar] [CrossRef]
- Ibáñez, I.; Silander, J.A., Jr.; Wilson, A.M.; LaFleur, N.; Tanaka, N.; Tsuyama, I. Multivariate Forecasts of Potential Distributions of Invasive Plant Species. Ecol. Appl. 2009, 19, 359–375. [Google Scholar] [CrossRef] [PubMed]
- Zalba, S.M.; Sonaglioni, M.I.; Compagnoni, C.A.; Belenguer, C.J. Using a Habitat Model to Assess the Risk of Invasion by an Exotic Plant. Biol. Conserv. 2000, 93, 203–208. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting Species Distribution: Offering More than Simple Habitat Models. Ecol. Lett. 2005, 9, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- Broennimann, O.; Treier, U.; Müller-Schärer, H.; Thuiller, W.; Peterson, A.; Guisan, A. Evidence of Climatic Niche Shift during Biological Invasion. Ecol. Lett. 2007, 10, 701–709. [Google Scholar] [CrossRef] [Green Version]
- Gallien, L.; Münkemüller, T.; Albert, C.; Boulangeat, I.; Thuiller, W. Predicting Potential Distributions of Invasive Species: Where to Go from Here? Divers. Distrib. 2010, 16, 331–342. [Google Scholar] [CrossRef]
- Qiao, H.; Soberon, J.; Peterson, A.T. No silver bullets in correlative ecological niche modelling: Insights from testing among many potential algorithms for niche estimation. Methods Ecol. Evol. 2015, 6, 1126–1136. [Google Scholar] [CrossRef]
- Phillips, S.J. GUÍA DIDÁCTICA SOBRE MAXENT, AT&T RESEARCH, POR STEVEN PHILLIPS. Available online: https://www.calameo.com/books/006723252d12f9d7c4d17 (accessed on 27 September 2021).
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Hutchinson, E. Concluding Remarks. Biology 1957, 22, 415–427. [Google Scholar] [CrossRef]
- Soberón, J. Grinnellian and Eltonian Niches and Geographic Distributions of Species. Ecol. Lett. 2008, 10, 1115–1123. [Google Scholar] [CrossRef]
- Soberón, J.; Peterson, A. Interpretation of Models of Fundamental Ecological Niches and Species’ Distributional Areas. Biodivers. Inform. 2005, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, W.; Wagner, W.L. Systematics of Oenothera Section Oenothera Subsection Raimannia and Subsection Nutantigemma (Onagraceae). Syst. Bot. Monogr. 1988, 24, 1–91. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef] [Green Version]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger Climate Classification Updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Gallego-Fernández, J.B.; García-Franco, J.G. Floral traits variation in Oenothera drummondii subsp. drummondii across a wide latitudinal range of native and non-native populations. Flora 2021, 280, 151851. [Google Scholar] [CrossRef]
- Gallego-Fernández, J.B.; García-Franco, J.G. Self-Compatibility and Reproductive Success of Oenothera drummondii Subsp. drummondii: Is It Similar between Native and Non-Native Populations? Diversity 2021, 13, 431. [Google Scholar] [CrossRef]
- Gallego-Fernández, J.B.; Martínez, M.L.; García-Franco, J.G.; Zunzunegui, M. Multiple seed dispersal modes of an invasive plant species on coastal dunes. Biol. Invasions 2020, 23, 111–127. [Google Scholar] [CrossRef]
- Dufour-Dror, J.-M. Alien Invasive Plants in Israel; The Middle East Nature Conservation Promotion Association: Jerusalem, Israel, 2012. [Google Scholar]
- Xu, H.; Qiang, S.; Genovesi, P.; Ding, H.; Wu, J.; Meng, L.; Han, Z.; Miao, J.; Hu, B.; Guo, J.; et al. An Inventory of Invasive Alien Species in China. NeoBiota 2012, 15, 1–26. [Google Scholar] [CrossRef]
- Shaltout, K.; Hosni, H.A.; El-Kady, H.F.; El-Beheiry, M.A.; Shaltout, S.K. Composition and pattern of alien species in the Egyptian flora. Flora 2016, 222, 104–110. [Google Scholar] [CrossRef]
- Frean, M.; Balkwill, K.; Anderson, C.G.; Burt, S. The Expanding Distributions and Invasiveness of Oenothera in Southern Africa. S. Afr. J. Bot. 1997, 63, 449–458. [Google Scholar] [CrossRef] [Green Version]
- Heenan, P.B.; De Lange, P.J.; Cameron, E.K.; Champion, P.D. Checklist of dicotyledons, gymnosperms, and pteridophytes naturalised or casual in New Zealand: Additional records 1999–2000. N. Z. J. Bot. 2002, 40, 155–174. [Google Scholar] [CrossRef]
- Heyligers, P.C. Flora of the Stockton and Port Hunter Sandy Foreshores with Comments on Fifteen Notable Introduced Species. Cunninghamia 2009, 10, 493–511. [Google Scholar]
- Qu, J.; Han, Q.; Dong, G.; Zhang, K.; Zu, R. A study of the characteristics of aeolian sand activity and the effects of a comprehensive protective system in a coastal dune area in southern China. Coast. Eng. 2013, 77, 28–39. [Google Scholar] [CrossRef]
- GBIF. Available online: https://www.gbif.org/ (accessed on 27 September 2021).
- Anthos. Sistema de Información Sobre Las Plantas de España. Available online: http://www.anthos.es/ (accessed on 27 September 2021).
- BioGIS—Israel Biodiversity Web Site. Available online: https://biogis.huji.ac.il/ (accessed on 27 September 2021).
- Australasian Virtual Herbarium. Available online: https://avh.chah.org.au/https//avh.chah.org.au/ (accessed on 27 September 2021).
- Tropicos. Available online: https://www.tropicos.org/home (accessed on 27 September 2021).
- Dietrich, W.; Wagner, W.L. New Taxa of Oenothera L. Sect. Oenothera (Onagraceae). Ann. Mo. Bot. Gard. 1987, 74, 144. [Google Scholar] [CrossRef]
- Webber, B.L.; Yates, C.J.; Le Maitre, D.C.; Scott, J.K.; Kriticos, D.J.; Ota, N.; McNeill, A.; Le Roux, J.J.; Midgley, G.F. Modelling horses for novel climate courses: Insights from projecting potential distributions of native and alien Australian acacias with correlative and mechanistic models. Divers. Distrib. 2011, 17, 978–1000. [Google Scholar] [CrossRef]
- WorldClim. Available online: https://www.worldclim.org/ (accessed on 27 September 2021).
- Hijmans, R.; Cameron, S.; Parra, J.; Jones, P.; Jarvis, A. Very High Resolution Interpolated Climate Surfaces of Global Land Areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Mackey, B.; Lindenmayer, D. Towards a Hierarchical Framework for Modeling the Spatial Distribution of Animals. J. Biogeogr. 2001, 28, 1147–1166. [Google Scholar] [CrossRef]
- Synes, N.; Osborne, P.E. Choice of predictor variables as a source of uncertainty in continental-scale species distribution modelling under climate change. Glob. Ecol. Biogeogr. 2011, 20, 904–914. [Google Scholar] [CrossRef]
- Low, B.W.; Zeng, Y.; Tan, H.H.; Yeo, D.C. Predictor complexity and feature selection affect Maxent model transferability: Evidence from global freshwater invasive species. Divers. Distrib. 2020, 27, 497–511. [Google Scholar] [CrossRef]
- Groom, D. Dale Groom’s Texas Gardening Guide; Springer: Nashville, TN, USA, 2002; ISBN 978-1-930604-39-1. [Google Scholar]
- Ajilvsgi, G. Wildflowers of Texas; Shearer Publishing: Fredericksburg, TX, USA, 2003; ISBN 978-0-940672-73-4. [Google Scholar]
- SPSS Statistics 24.0. Available online: https://www.ibm.com/support/pages/downloading-ibm-spss-statistics-24 (accessed on 6 October 2021).
- Peterson, A.; Papes, M.; Eaton, M. Transferability and Model Evaluation in Ecological Niche Modeling: A Comparison of GARP and Maxent. Ecography 2007, 30, 550–560. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Jarnevich, C.S.; Young, N. Using the MAXENT program for species distribution modelling to assess invasion risk. In Pest Risk Modelling and Mapping for Invasive Alien Species; CAB International: Wallingford, UK, 2015; pp. 65–81. [Google Scholar] [CrossRef]
- West, A.M.; Kumar, S.; Brown, C.S.; Stohlgren, T.J.; Bromberg, J. Field validation of an invasive species Maxent model. Ecol. Informatics 2016, 36, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Papeş, M.; Havel, J.E.; Zanden, J.V. Using maximum entropy to predict the potential distribution of an invasive freshwater snail. Freshw. Biol. 2016, 61, 457–471. [Google Scholar] [CrossRef]
- Zhang, H.; Song, J.; Zhao, H.; Li, M.; Han, W. Predicting the Distribution of the Invasive Species Leptocybe invasa: Combining MaxEnt and Geodetector Models. Insects 2021, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Spaulding, S.; Stohlgren, T.; Hermann, K.; Schmidt, T.; Bahls, L. Potential habitat distribution for the freshwater diatom Didymosphenia geminata in the continental US. Front. Ecol. Environ. 2009, 7, 415–420. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Mendoza-González, G.; Martínez, M.; Rojas-Soto, O.; Téllez-Valdés, O.; Arias-Del Razo, I. Priority Areas for Conservation of Beach and Dune Vegetation of the Mexican Atlantic Coast. J. Nat. Conserv. 2016, 33, 25–34. [Google Scholar] [CrossRef]
- Tool for Partial-ROC, Ver. 1.0. Available online: https://scholar.google.com/citations?view_op=view_citation&hl=es&user=10Ojgh0AAAAJ&citation_for_view=10Ojgh0AAAAJ:-f6ydRqryjwC (accessed on 27 September 2021).
- Hammada, S.; Linares, L.; Cortes, J. Biodiversité floristique des dunes littorales de l’Oued El Maleh (Martil) et du bas Tahaddart: Résultats préliminaires. Trav. Instit. Scient. Rabat 2009, 6, 45–50. [Google Scholar]
- Atlas of Living Australia. Available online: https://www.ala.org.au (accessed on 28 September 2021).
- Tela Botanica. Available online: https://www.tela-botanica.org/ (accessed on 28 September 2021).
- Campos, J.A.; Herrera, M. Diagnosis de La Flora Alóctona Invasora de La CAPV; Departamento de Medio Ambiente y Ordenación del territorio: Bilbao, Spain, 2009. [Google Scholar]
- Silvestre, S. Notas Breves. 15. Oenothera drummondii. Lagascalia 1980, 9, 244–245. [Google Scholar]
- Dietrich, W.; Wagner, W.L.; Raven, P.H. Systematics of Oenothera Section Oenothera Subsection Oenothera (Onagraceae). Syst. Bot. Monogr. 1997, 50, 1–234. [Google Scholar] [CrossRef]
- Moreno-Casasola, P. Patterns of Plant Species Distribution on Coastal Dunes Along the Gulf of Mexico. J. Biogeogr. 1988, 15, 787–806. [Google Scholar] [CrossRef]
- Gallego-Fernández, J.B.; Martínez, M.L. Environmental Filtering and Plant Functional Types on Mexican Foredunes along the Gulf of Mexico. Écoscience 2011, 18, 52–62. [Google Scholar] [CrossRef]
- Gallagher, R.V.; Beaumont, L.J.; Hughes, L.; Leishman, M.R. Evidence for Climatic Niche and Biome Shifts between Native and Novel Ranges in Plant Species Introduced to Australia. J. Ecol. 2010, 98, 790–799. [Google Scholar] [CrossRef]
- Zunzunegui, M.; Ruíz-Valdepeñas, E.; Sert, M.; Díaz, M.; Gallego-Fernández, J. Field Comparison of Ecophysiological Traits between an Invader and a Native Species in a Mediterranean Coastal Dune. Plant Physiol. Biochem. 2019, 146, 278–286. [Google Scholar] [CrossRef]
- Díaz-Barradas, M.C.; Gallego-Fernández, J.B.; Zunzunegui, M. Plant Response to Water Stress of Native and Non-Native Oenothera drummondii Populations. Plant Physiol. Biochem. 2020, 154, 219–228. [Google Scholar] [CrossRef]
- Liu, C.; Wolter, C.; Xian, W.; Jeschke, J.M. Most invasive species largely conserve their climatic niche. Proc. Natl. Acad. Sci. USA 2020, 117, 23643–23651. [Google Scholar] [CrossRef]
- Hernández-Espinosa, R.; González-Astorga, J.; Espinosa de los Monteros, A.; Cabrera-Toledo, D.; Gallego-Fernández, J.B. Transferability of Microsatellite Markers Developed in Oenothera Spp. to the Invasive Species Oenothera drummondii Hook. (Onagraceae). Diversity 2020, 12, 387. [Google Scholar] [CrossRef]
- Zunzunegui, M.; Morales Sánchez, J.Á.; Díaz Barradas, M.C.; Gallego-Fernández, J.B. Different Tolerance to Salinity of Two Populations of Oenothera drummondii with Contrasted Biogeographical Origin. Plant Physiol. Biochem. 2021, 162, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Jutila, H. Seed Bank and Emergent Vascular Flora of Ballast Areas in Reposaari, Finland. Ann. Bot. Fenn. 1996, 33, 165–182. [Google Scholar]
- Ridley, H.N. The Dispersal of Plants throughout the World; L. Reeve & Co., Ltd.: Ashford, UK, 1930. [Google Scholar]
- Ouren, T. The Impact of the Old Shipyards on the Invasion of Alien Plants to Norway. Nor. Geogr. Tidsskr. Nor. J. Geogr. 1980, 34, 145–152. [Google Scholar] [CrossRef]
- Castroviejo, S. Flora Iberica. Vol. 8. Haloragaceae-Euphorbiaceae. Available online: https://www.biodiversitylibrary.org/bibliography/71304 (accessed on 28 September 2021).
- NaturaLista. Available online: https://www.naturalista.mx/ (accessed on 6 October 2021).
- The Catalogue of Life. Available online: https://www.catalogueoflife.org/ (accessed on 6 October 2021).
- Integrated Taxonomic Information System. Available online: https://www.itis.gov/ (accessed on 6 October 2021).
- Consejería de Medio Ambiente y Ordenación del Territorio. Memoria de Actividades y Resultados 2012. Espacio Natural Doñana; Portal Ambiental de Andalucía: Seville, Spain, 2013. [Google Scholar]
- Consejería de Medio Ambiente y Ordenación del Territorio. Memoria de Actividades y Resultados 2015. Espacio Natural Doñana; Portal Ambiental de Andalucía: Seville, Spain, 2016. [Google Scholar]
- Lomas, J.; Fernández-Carrillo, L.; Saavedra, C.; Dana, E.; Rodríguez, C.; Martínez, E. Feasibility of Using Glyphosate to Control Beach Evening Primrose Oenothera drummondii in Heavily Invaded Coastal Dunes, Odiel Marshes, Spain. Conserv. Evid. 2016, 13, 72–78. [Google Scholar]
- Ministerio de Medio Ambiente y Medio Rural y Marino; Dirección General de Sostenibilidad de la Costa y del Mar. Plan de Control y Eliminación de Especies Vegetales Invasoras de Sistemas Dunares; Instituto de Ecología Litoral: Campello, Spain, 2011. [Google Scholar]

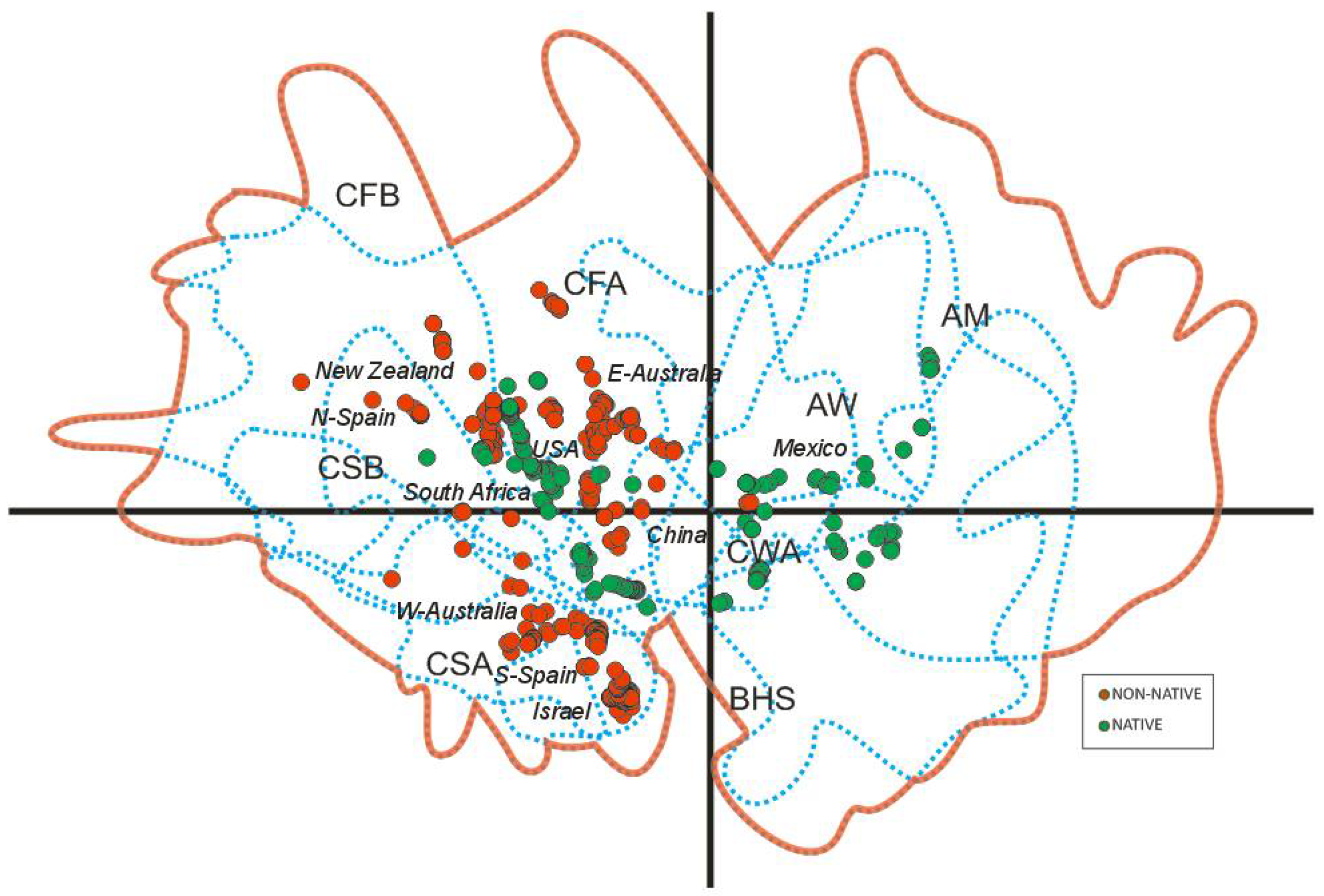
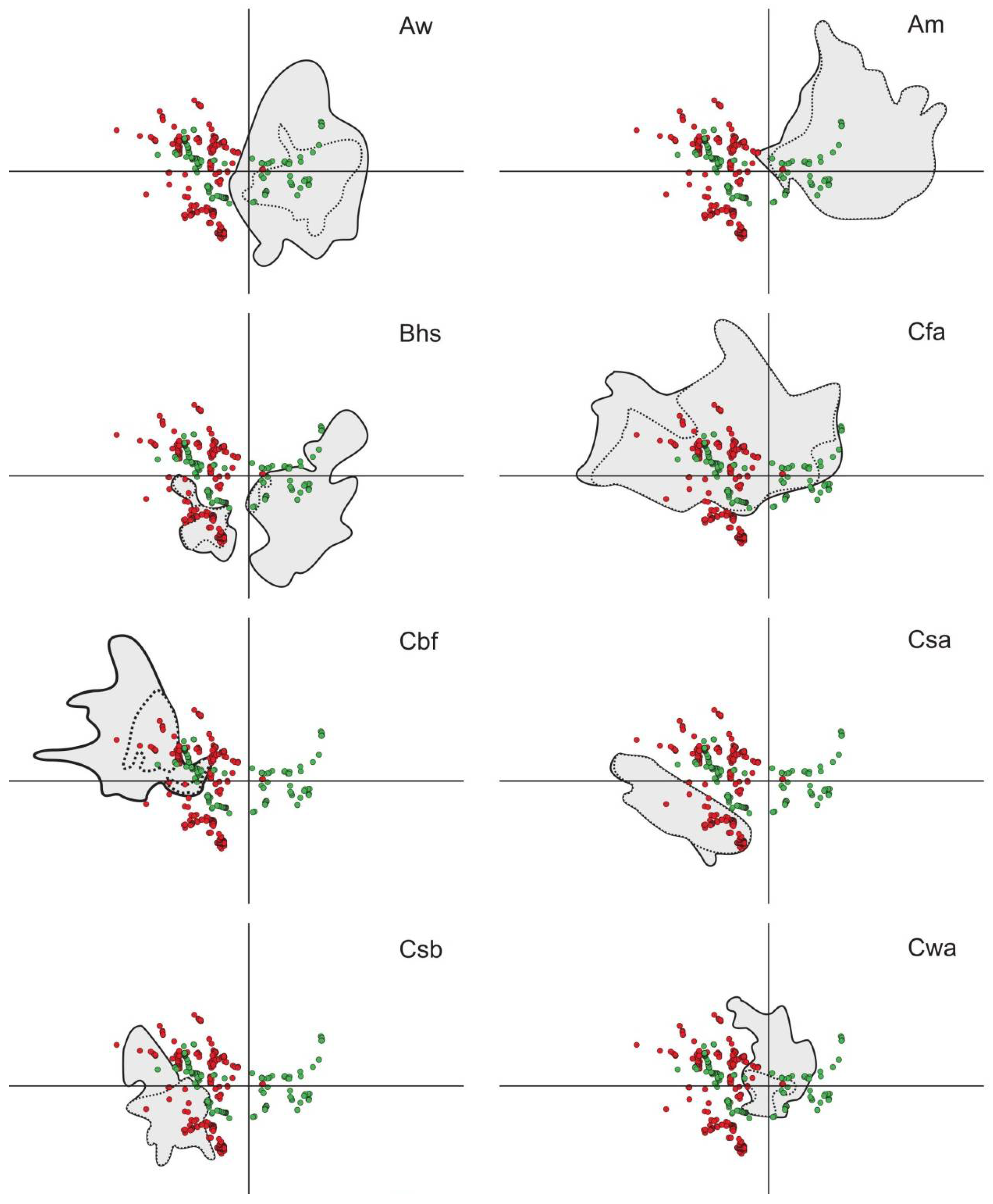
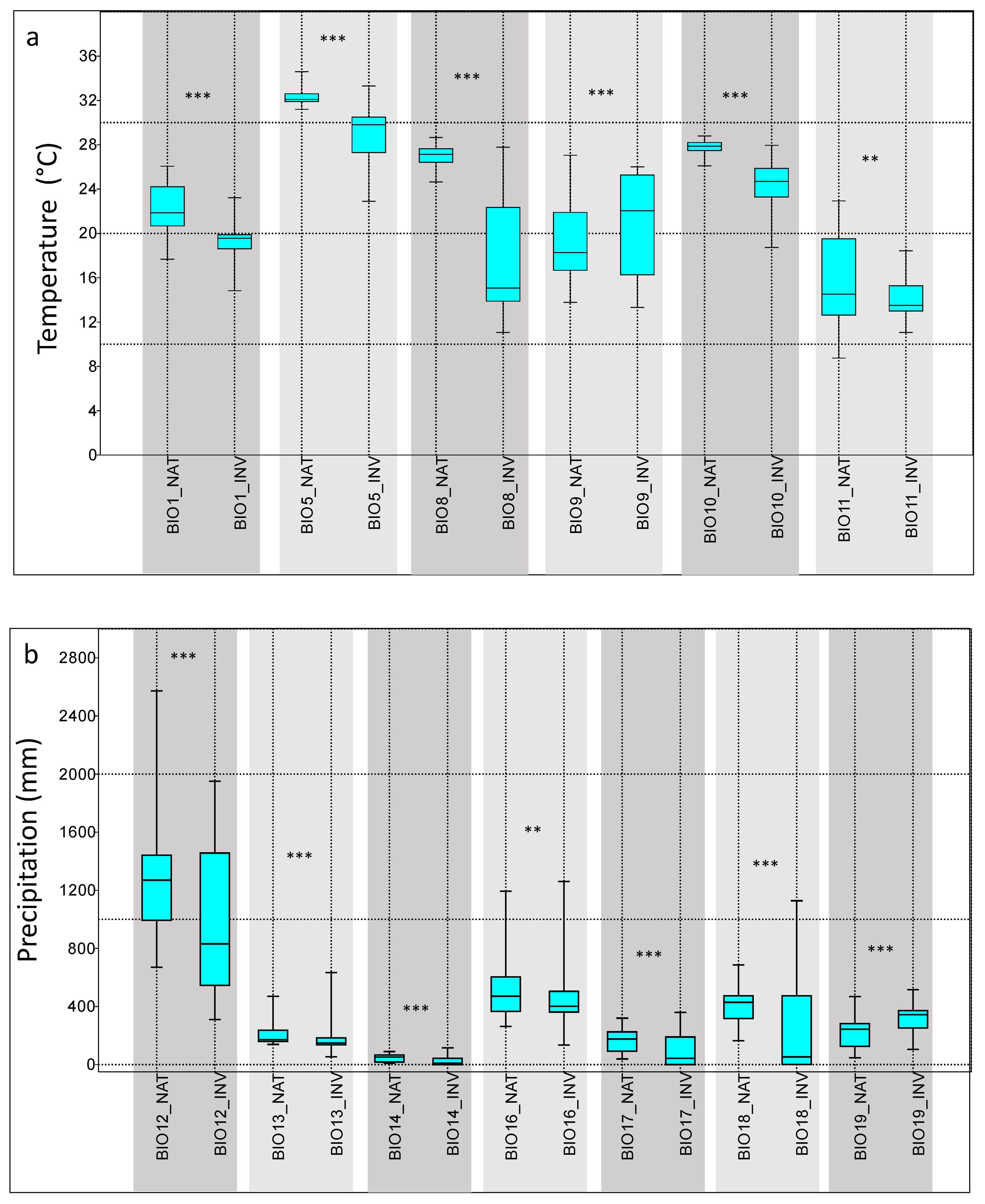

| Distribution | Native | Non-Native | Non-Native | Non-Native | Non-Native |
|---|---|---|---|---|---|
| Köppen–Geiger Climatic Regions | Am, Aw, Bsh, Cfa | Csa | Csb | Cfb | Cwa |
| BIO1 | X | X | X | X | |
| BIO 2 | |||||
| BIO3 | X | ||||
| BIO4 | X | X | X | X | X |
| BIO5 | X | ||||
| BIO6 | |||||
| BIO7 | |||||
| BIO8 | X | X | X | ||
| BIO9 | X | X | X | X | X |
| BIO10 | X | X | |||
| BIO11 | X | X | X | X | |
| BIO12 | X | ||||
| BIO13 | X | X | X | X | X |
| BIO14 | X | X | X | X | |
| BIO15 | X | X | X | X | X |
| BIO16 | X | ||||
| BIO17 | X | ||||
| BIO18 | X | X | X | X | X |
| BIO19 | X | X | X | X |
| Köppen–Geiger Climatic Region | Distribution | # Ocurrences Records | %Validation | # Bioclimatic Variables | Figure in Appendix A |
|---|---|---|---|---|---|
| Am | N | 19 | 15% | 10 | A1 |
| Aw | N | 23 | 15% | 10 | A2 |
| Bsh | N | 8 | 15% | 10 | A3 |
| Cfa | N | 324 | 15% | 10 | A4 |
| Csa | NonN | 229 | 15% | 10 | A5 |
| Csb | NonN | 5 | 0% | 10 | A6 |
| Cfb | NonN | 40 | 15% | 10 | A7 |
| Cwa | NonN | 3 | 0% | 11 | A8 |
| Country | Region/Location | Köppen–Geiger Climatic Regions | Number of Records | First Record | Current Status | References |
|---|---|---|---|---|---|---|
| Australia | Queensland | Cwa | 222 | 1924 | N | [76] |
| Cfa | 1924 | N | ||||
| South New Wales | Cfa, Cfb | 1929 | N | |||
| Southwestern Australia | Bsh, Csa, Csb, | 1947 | N | |||
| Lord Howe Island | Cfa | 1962 | N | |||
| Adelaida | Csb | 1989 | N | |||
| China | Fujian | Cfa | 10 | 1923 | I | [43] |
| Egypt | Mediterranean coast | BWh | - | 1871 | N | [44] |
| France | Marseillan | Csa | 1 | 2014 | N | [77] |
| Israel | Tel Aviv district Central district Meridional district | Csa | 150 | 1902 | I | [42] |
| Morocco | Tetouan | Csa | - | 1930 | I | [75] |
| Spain (North) | Basque Country—Zarautz | Cfb | 22 | 1915 | N | [13,39,11,78] |
| New Zealand | Auckland | Cfb | 12 | 1997 | N | [46] |
| Peru | Supe | Bsh | 3 | 1938 | N | [11] |
| Spain (South) | Gulf of Cadiz | Csa | 21 | 1957 | I | [13,39,11,79] |
| South Africa | Western Cape | Csb | 8 | 1912 | N | [45] |
| Eastern Cape | Cfb | 1912 | ||||
| Kawa Zulu-Natal | Cfa | 1912 | ||||
| Mexico | Tamaulipas | Aw, Bsh, Cfa | 76 | 1898 | NAT | [36,80,81,82] |
| Veracruz | Aw, Am | 1903 | NAT | |||
| Tabasco | Am | 1983 | NAT | |||
| USA | South Carolina, Georgia, East Florida | Cfa | 126 | 1880 | NAT | [36,80,81,82] |
| Gulf of Mexico states | Cfa | 1843 | NAT |
| Aw | Am | BSh | Cfa | Cfb | Csa | Csb | Cwa | TOTAL | |
|---|---|---|---|---|---|---|---|---|---|
| Presence | 15,230 | 80,268 | 2118 | 39,212 | 14,571 | 49,161 | 10,395 | 4686 | 215,641 |
| Absence | 203,185 | 20,762 | 21,838 | 66,884 | 228,329 | 26,560 | 16,770 | 16,668 | 600,996 |
| TOTAL (km) | 218,415 | 101,030 | 23,956 | 106,096 | 242,900 | 75,721 | 27,165 | 21,354 | 816,637 |
| % presence | 7 | 37.2 | 0.9 | 18.1 | 6.7 | 22.7 | 4.8 | 2.1 | 100 |
| % absence | 33.8 | 3.4 | 3.6 | 11.12 | 37.9 | 4.4 | 2.7 | 2.7 | 100 |
| Köppen–Geiger Climatic Region | Distribution | ROC | Partial ROC | p-Value | |
|---|---|---|---|---|---|
| Am | Tropical monsoon climate | Native | 0.967 | NA | NA |
| Aw | Tropical savanna climate with dry-winter characteristics | Native | 0.933 | 1.836 | <0.001 |
| Bsh | Hot semi-arid climate | Native | 0.923 | NA | NA |
| Cfa | Humid subtropical climate | Native | 0.939 | 1.717 | <0.001 |
| Csa | Mediterranean hot summer climates | Non-native | 0.819 | 0.989 | >0.05 |
| Csb | Mediterranean warm/cool summer climates | Non-native | NA | NA | NA |
| Cfb | Temperate oceanic climate | Non-native | 0.998 | 1.994 | <0.001 |
| Cwa | Dry-winter humid subtropical climate | Non-native | NA | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo-Infante, F.R.; Mendoza-González, G.; Rioja-Nieto, R.; Gallego-Fernández, J.B. Range Shifts in the Worldwide Expansion of Oenothera drummondii subsp. drummondii, a Plant Species of Coastal Dunes. Diversity 2021, 13, 603. https://doi.org/10.3390/d13110603
Castillo-Infante FR, Mendoza-González G, Rioja-Nieto R, Gallego-Fernández JB. Range Shifts in the Worldwide Expansion of Oenothera drummondii subsp. drummondii, a Plant Species of Coastal Dunes. Diversity. 2021; 13(11):603. https://doi.org/10.3390/d13110603
Chicago/Turabian StyleCastillo-Infante, Frida R., Gabriela Mendoza-González, Rodolfo Rioja-Nieto, and Juan B. Gallego-Fernández. 2021. "Range Shifts in the Worldwide Expansion of Oenothera drummondii subsp. drummondii, a Plant Species of Coastal Dunes" Diversity 13, no. 11: 603. https://doi.org/10.3390/d13110603
APA StyleCastillo-Infante, F. R., Mendoza-González, G., Rioja-Nieto, R., & Gallego-Fernández, J. B. (2021). Range Shifts in the Worldwide Expansion of Oenothera drummondii subsp. drummondii, a Plant Species of Coastal Dunes. Diversity, 13(11), 603. https://doi.org/10.3390/d13110603







