Assessing Suitable Habitats for Treefrog Species after Previous Declines in Costa Rica
Abstract
1. Introduction
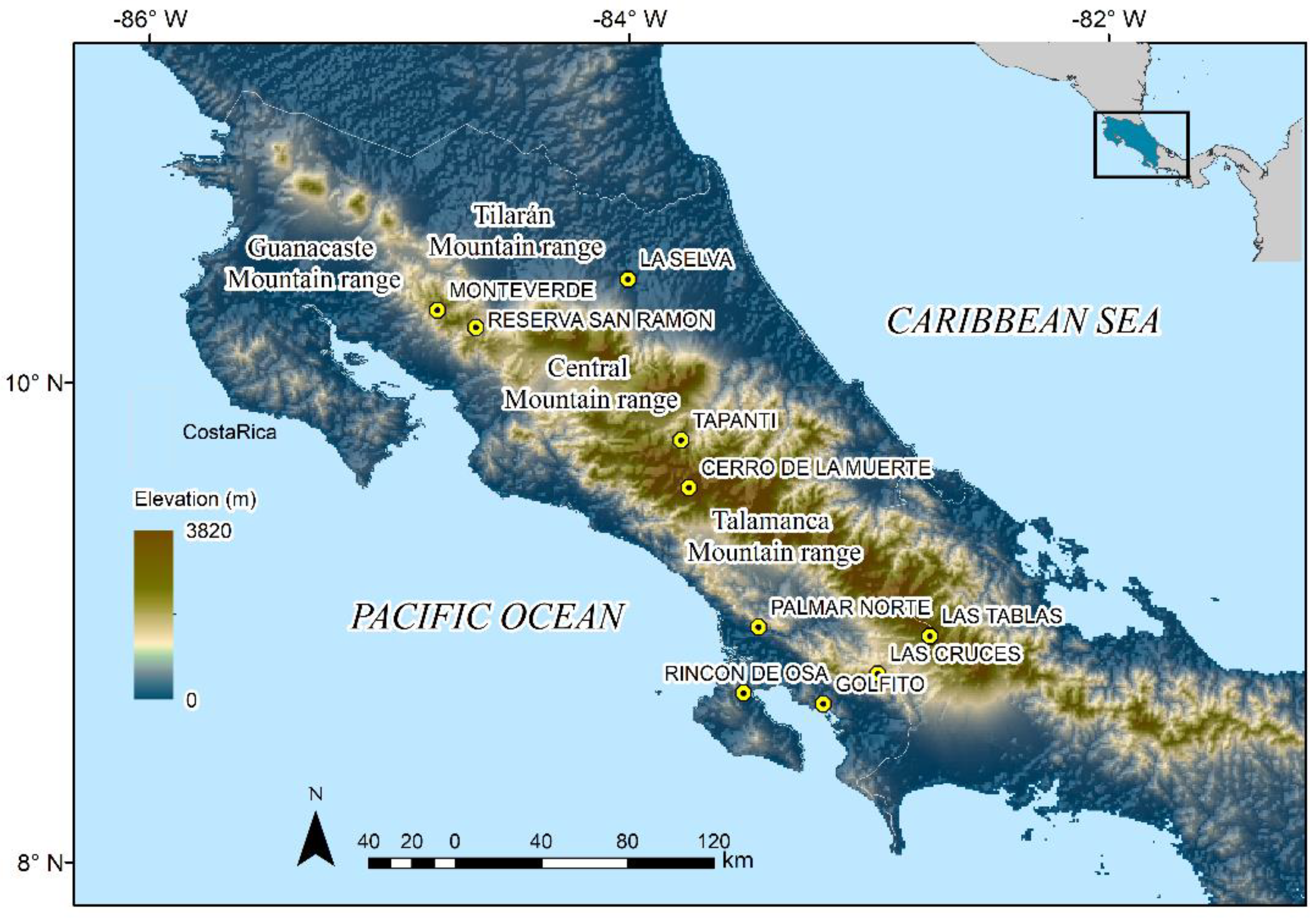
History of Amphibian Monitoring in Costa Rica
2. Materials and Methods
2.1. Species Assessment
2.2. Study Species
2.3. Datasets and Abiotic Data
2.4. Species-Range Predictions
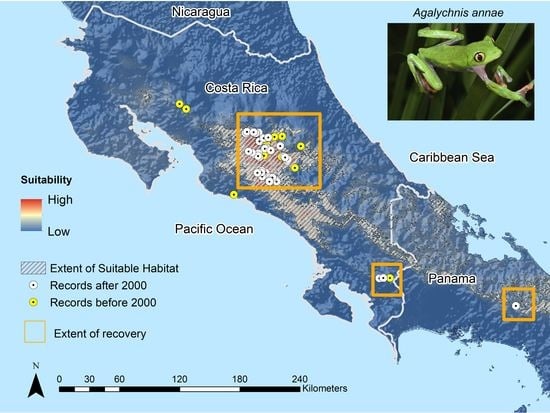
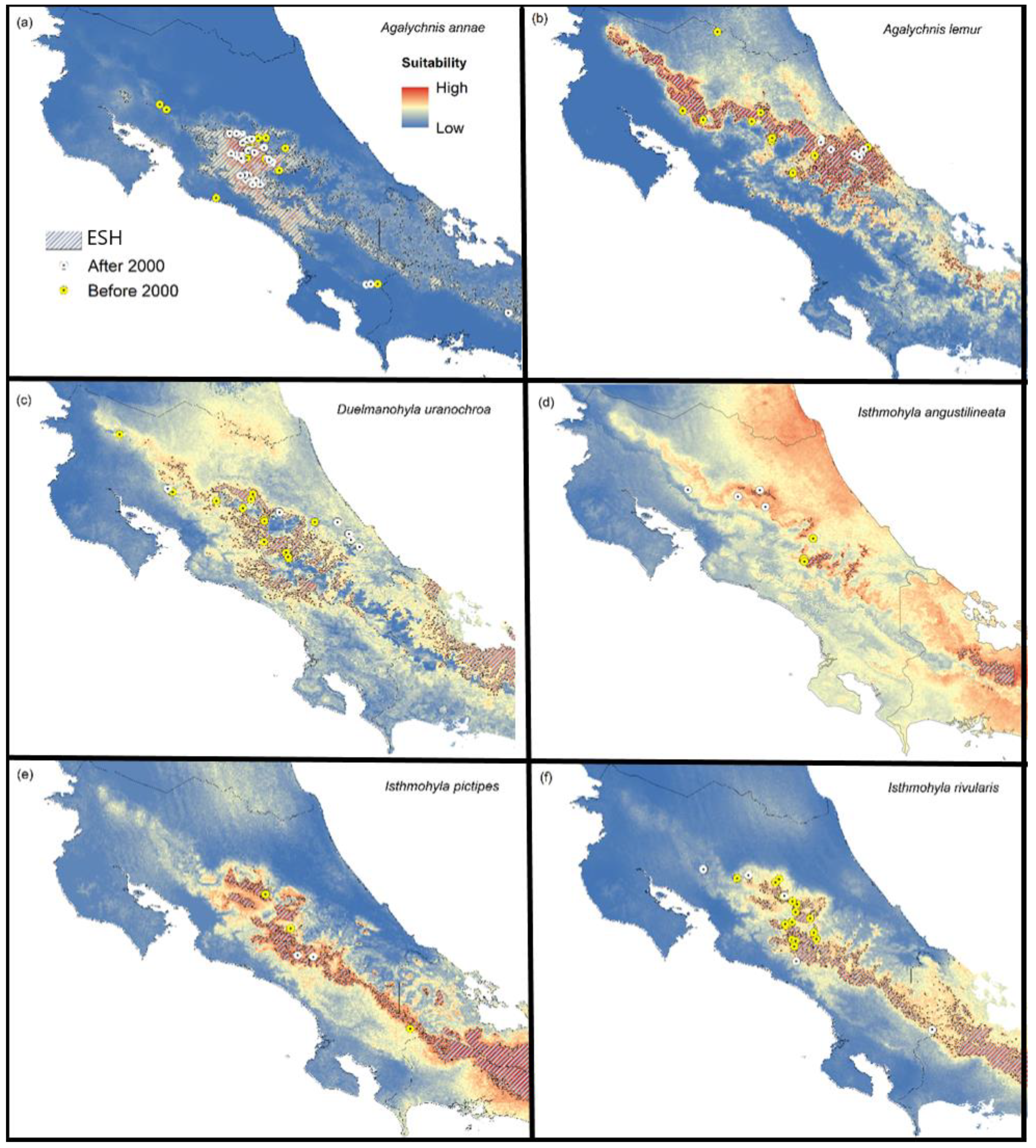
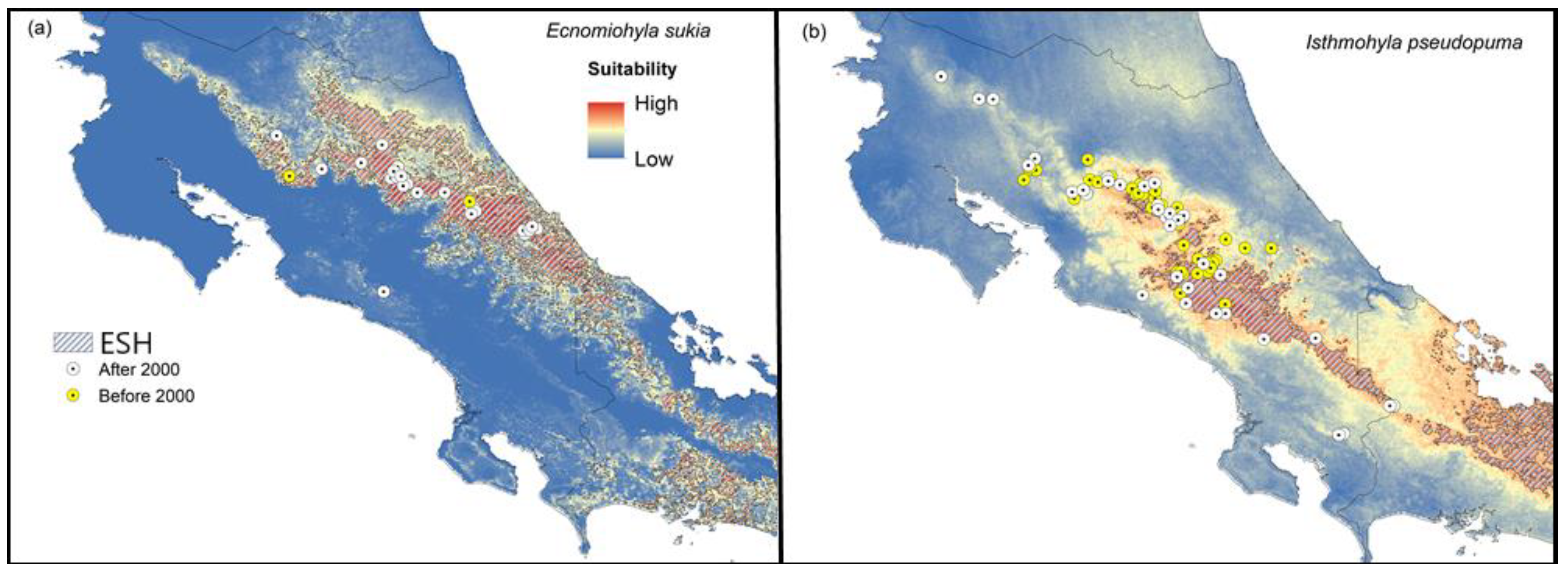
3. Results
3.1. Species Assessment
3.2. Suitable Habitat
3.3. Suitable Habitat
4. Discussion
4.1. Species Assessment
4.2. Spread Patterns
4.2.1. Species Recovery
4.2.2. Rapid Spread
4.2.3. Widespread Uncommon Species
4.3. Study Limitations
4.4. Implications for Conservation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frost, D.R. Amphibian Species of the World: An Online Reference. Version 6.1. 2021. Available online: https://amphibiansoftheworld.amnh.org/ (accessed on 1 September 2021).
- Pyron, R.A.; Wiens, J.J. Large-Scale Phylogenetic Analyses Reveal the Causes of High Tropical Amphibian Diversity. Proc. R. Soc. B Biol. Sci. 2013, 280, 20131622. [Google Scholar] [CrossRef]
- Sewell, D.; Griffiths, R.A. Can a Single Amphibian Species Be a Good Biodiversity Indicator? Diversity 2009, 1, 102. [Google Scholar] [CrossRef]
- Hocking, D.J.; Babbitt, K.J. Amphibian Contributions to Ecosystem Services. Herpetol. Conserv. Biol. 2014, 9, 1–17. [Google Scholar]
- Whiles, M.R.; Lips, K.R.; Pringle, C.M.; Kilham, S.S.; Bixby, R.J.; Brenes, R.; Connelly, S.; Colon-Gaud, J.C.; Hunte-Brown, M.; Huryn, A.D.; et al. The Effects of Amphibian Population Declines on the Structure and Function of Neotropical Stream Ecosystems. Front. Ecol. Environ. 2006, 4, 27–34. [Google Scholar] [CrossRef]
- Wiens, J.J. Global Patterns of Diversification and Species Richness in Amphibians. Am. Nat. 2007, 170, S86–S106. [Google Scholar] [CrossRef] [PubMed]
- Duellman, W.E. The Hylid Frogs of Middle America; Natural History Museum, University of Kansas: Lawrence, KS, USA, 1970. [Google Scholar]
- Wiens, J.J.; Graham, C.H.; Moen, D.S.; Smith, S.A.; Reeder, T.W. Evolutionary and Ecological Causes of the Latitudinal Diversity Gradient in Hylid Frogs: Treefrog Trees Unearth the Roots of High Tropical Diversity. Am. Nat. 2006, 168, 579–596. [Google Scholar] [CrossRef]
- Savage, J.M. The Amphibians and Reptiles of Costa Rica: A Herpetofauna between Two Continents, between Two Seas; The University of Chicago Press: Chicago, IL, USA, 2002. [Google Scholar]
- Becker, C.G.; Fonseca, C.R.; Haddad, C.F.B.; Prado, P.I. Habitat Split as a Cause of Local Population Declines of Amphibians with Aquatic Larvae. Conserv. Biol. 2010, 24, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Catenazzi, A. State of the World’s Amphibians. Annu. Rev. Environ. Resour. 2015, 40, 91–119. [Google Scholar] [CrossRef]
- Longcore, J.E.; Pessier, A.P.; Nichols, D.K. Batrachochytrium dendrobatidis Gen. et Sp. Nov., a Chytrid Pathogenic to Amphibians. Mycologia 1999, 91, 219–227. [Google Scholar] [CrossRef]
- Berger, L.; Speare, R.; Daszak, P.; Green, D.E.; Cunningham, A.A.; Goggin, C.L.; Slocombe, R.; Ragan, M.A.; Hyatt, A.D.; McDonald, K.R.; et al. Chytridiomycosis Causes Amphibian Mortality Associated with Population Declines in the Rain Forests of Australia and Central America. Proc. Natl. Acad. Sci. USA 1998, 95, 9031–9036. [Google Scholar] [CrossRef] [PubMed]
- Voyles, J.; Young, S.; Berger, L.; Campbell, C.; Voyles, W.F.; Dinudom, A.; Cook, D.; Webb, R.; Alford, R.A.; Skerratt, L.F.; et al. Pathogenesis of Chytridiomycosis, a Cause of Catastrophic Amphibian Declines. Science 2009, 326, 582–585. [Google Scholar] [CrossRef]
- Lips, K.R.; Reeve, J.D.; Witters, L.R. Ecological Traits Predicting Amphibian Population Declines in Central America. Conserv. Biol. 2003, 17, 1078–1088. [Google Scholar] [CrossRef]
- Ranvestel, A.W.; Lips, K.R.; Pringle, C.M.; Whiles, M.R.; Bixby, R.J. Neotropical Tadpoles Influence Stream Benthos: Evidence for the Ecological Consequences of Decline in Amphibian Populations. Freshw. Biol. 2004, 49, 274–285. [Google Scholar] [CrossRef]
- Scheele, B.C.; Pasmans, F.; Skerratt, L.F.; Berger, L.; Martel, A.; Beukema, W.; Acevedo, A.A.; Burrowes, P.A.; Carvalho, T.; Catenazzi, A.; et al. Amphibian Fungal Panzootic Causes Catastrophic and Ongoing Loss of Biodiversity. Science 2019, 363, 1459–1463. [Google Scholar] [CrossRef]
- Lambert, M.R.; Womack, M.C.; Byrne, A.Q.; Hernández-Gómez, O.; Noss, C.F.; Rothstein, A.P.; Blackburn, D.C.; Collins, J.P.; Crump, M.L.; Koo, M.S.; et al. Comment on “Amphibian Fungal Panzootic Causes Catastrophic and Ongoing Loss of Biodiversity. ” Science 2020, 367, eaay1838. [Google Scholar] [CrossRef] [PubMed]
- Lips, K.R.; Diffendorfer, J.; Mendelson, J.R.; Sears, M.W. Riding the Wave: Reconciling the Roles of Disease and Climate Change in Amphibian Declines. PLoS Biol. 2008, 6, e72. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.L.; Rovito, S.M.; Wake, D.B.; Vredenburg, V.T. Coincident Mass Extirpation of Neotropical Amphibians with the Emergence of the Infectious Fungal Pathogen Batrachochytrium dendrobatidis. Proc. Natl. Acad. Sci. USA 2011, 108, 9502–9507. [Google Scholar] [CrossRef] [PubMed]
- Pounds, J.A.; Crump, M.L. Amphibian Declines and Climate Disturbance: The Case of the Golden Toad and the Harlequin Frog. Conserv. Biol. 1994, 8, 72–85. [Google Scholar] [CrossRef]
- Crump, M.L.; Hensley, F.R.; Clark, K.L. Apparent Decline of the Golden Toad: Underground or Extinct? Copeia 1992, 1992, 413–420. [Google Scholar] [CrossRef]
- Lips, K.R. Decline of a Tropical Montane Amphibian Fauna. Conserv. Biol. 1998, 12, 106–117. [Google Scholar] [CrossRef]
- Puschendorf, R.; Bolaños, F.; Chaves, G. The Amphibian Chytrid Fungus along an Altitudinal Transect before the First Reported Declines in Costa Rica. Biol. Conserv. 2006, 132, 136–142. [Google Scholar] [CrossRef]
- Puschendorf, R.; Carnaval, A.C.; VanDerWal, J.; Zumbado-Ulate, H.; Chaves, G.; Bolaños, F.; Alford, R.A. Distribution Models for the Amphibian Chytrid Batrachochytrium dendrobatidis in Costa Rica: Proposing Climatic Refuges as a Conservation Tool. Divers. Distrib. 2009, 15, 401–408. [Google Scholar] [CrossRef]
- Bolaños, F. Situación de Los Anfibios de Costa Rica. Biocenosis 2009, 22, 95–108. [Google Scholar]
- Zumbado-Ulate, H.; García-Rodríguez, A.; Searle, C.L. Species Distribution Models Predict the Geographic Expansion of an Enzootic Amphibian Pathogen. Biotropica 2021, 53, 221–321. [Google Scholar] [CrossRef]
- Bolom-Huet, R.; Pineda, E.; Díaz-Fleischer, F.; Muñoz-Alonso, A.L.; Galindo-González, J. Known and Estimated Distribution in Mexico of Batrachochytrium dendrobatidis, a Pathogenic Fungus of Amphibians. Biotropica 2019, 51, 731–746. [Google Scholar] [CrossRef]
- Brem, F.; Lips, K. Batrachochytrium dendrobatidis Infection Patterns among Panamanian Amphibian Species, Habitats and Elevations during Epizootic and Enzootic Stages. Dis. Aquat. Organ. 2008, 81, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Zumbado-Ulate, H.; García-Rodríguez, A.; Vredenburg, V.T.; Searle, C.L. Infection with Batrachochytrium dendrobatidis Is Common in Tropical Lowland Habitats: Implications for Amphibian Conservation. Ecol. Evol. 2019, 9, 4917–4930. [Google Scholar] [CrossRef]
- Woodhams, D.C.; Kilburn, V.L.; Reinert, L.K.; Voyles, J.; Medina, D.; Ibáñez, R.; Hyatt, A.D.; Boyle, D.G.; Pask, J.D.; Green, D.M.; et al. Chytridiomycosis and Amphibian Population Declines Continue to Spread Eastward in Panama. EcoHealth 2008, 5, 268–274. [Google Scholar] [CrossRef]
- Knapp, R.A.; Fellers, G.M.; Kleeman, P.M.; Miller, D.A.W.; Vredenburg, V.T.; Rosenblum, E.B.; Briggs, C.J. Large-Scale Recovery of an Endangered Amphibian despite Ongoing Exposure to Multiple Stressors. Proc. Natl. Acad. Sci. USA 2016, 113, 11889. [Google Scholar] [CrossRef]
- Reeder, N.M.M.; Pessier, A.P.; Vredenburg, V.T. A Reservoir Species for the Emerging Amphibian Pathogen Batrachochytrium dendrobatidis Thrives in a Landscape Decimated by Disease. PLoS ONE 2012, 7, e33567. [Google Scholar] [CrossRef]
- Taylor, E.H. A Review of the Frogs and Toads of Costa Rica. Univ. Kans. Sci. Bull. 1952, 35, 577–941. [Google Scholar] [CrossRef]
- Robinson, D.C. A New Dwarf Salamander of the Genus Bolitoglossa (Plethodontidae) from Costa Rica. Proc. Biol. Soc. Wash. 1976, 89, 289–294. [Google Scholar]
- García-París, M.; Good, D.A.; Parra-Olea, G.; Wake, D.B. Biodiversity of Costa Rican Salamanders: Implications of High Levels of Genetic Differentiation and Phylogeographic Structure for Species Formation. Proc. Natl. Acad. Sci. USA 2000, 97, 1640. [Google Scholar] [CrossRef]
- Lips, K.R.; Green, D.E.; Papendick, R. Chytridiomycosis in Wild Frogs from Southern Costa Rica. J. Herpetol. 2003, 37, 215–218. [Google Scholar] [CrossRef]
- Abarca, J.; Chaves, G.; García-Rodríguez, A.; Vargas, R. Reconsidering Extinction: Rediscovery of Incilius holdridgei (Anura: Bufonidae) in Costa Rica after 25 Years. Herpetol. Rev. 2010, 41, 150. [Google Scholar]
- Chaves, G.; Zumbado-Ulate, H.; García-Rodríguez, A.; Gómez, E.; Vredenburg, V.T.; Ryan, M.J. Rediscovery of the Critically Endangered Streamside Frog, Craugastor taurus (Craugastoridae), in Costa Rica. Trop. Conserv. Sci. 2014, 7, 628–638. [Google Scholar] [CrossRef]
- Zumbado-Ulate, H.; Willink, B. Craugastor ranoides. Geographic Distribution. Herpetol. Rev. 2011, 42, 236. [Google Scholar]
- García-Rodríguez, A.; Arias, E.; Chaves, G. Multiple Lines of Evidence Support the Species Status of the Poorly Known Diasporus tigrillo and the Recently Described Diasporus citrinobapheus (Anura: Eleutherodactylidae). Neotropical Biodivers. 2016, 2, 59–68. [Google Scholar] [CrossRef]
- Boza-Oviedo, E.; Rovito, S.M.; Chaves, G.; Garcia-Rodriguez, A.; Artavia, L.G.; Bolaños, F.; Wake, D.B. Salamanders from the Eastern Cordillera de Talamanca, Costa Rica, with Descriptions of Five New Species (Plethodontidae: Bolitoglossa, Nototriton, and Oedipina) and Natural History Notes from Recent Expeditions. Zootaxa 2012, 3309, 36–61. [Google Scholar] [CrossRef]
- Zumbado-Ulate, H.; Nelson, K.N.; García-Rodríguez, A.; Chaves, G.; Arias, E.; Bolaños, F.; Whitfield, S.M.; Searle, C.L. Endemic Infection of Batrachochytrium dendrobatidis in Costa Rica: Implications for Amphibian Conservation at Regional and Species Level. Diversity 2019, 11, 129. [Google Scholar] [CrossRef]
- García-Rodríguez, A.; Chaves, G.; Benavides-Varela, C.; Puschendorf, R. Where Are the Survivors? Tracking Relictual Populations of Endangered Frogs in Costa Rica. Divers. Distrib. 2012, 18, 204–212. [Google Scholar] [CrossRef]
- Acosta-Chaves, V.J.; Madrigal-Elizondo, V.; Chaves, G.; Morera-Chacón, B.; Garcia-Rodriguez, A.; Bolaños, F. View of Shifts in the Diversity of an Amphibian Community from a Premontane Forest of San Ramón, Costa Rica. Rev. Biol. Trop. 2019, 67, 259–273. [Google Scholar] [CrossRef]
- Acosta-Chaves, V.; Chaves, G.; Abarca, J.G.; García-Rodríguez, A.; Bolaños, F. A Checklist of the Amphibians and Reptiles of Río Macho Biological Station, Provincia de Cartago, Costa Rica. Check List 2015, 11, 1784. [Google Scholar] [CrossRef][Green Version]
- Rodríguez, J.; Chaves, G.; Neam, K.; Luedtke, J.; Carrillo, J.; Bolaños, F.; Matamoros, Y. Taller de Evaluación de Las Necesidades de Conservación de Anfibios—Arca de Los Anfibios y de La Lista Roja de La UICN: Un Esfuerzo Para La Segunda Evaluación Global de Anfibios; UICN SSC and CPSG Mesoamérica: San José, Costa Rica, 2019. [Google Scholar]
- Wilson, L.D.; McCranie, J.R. The Conservation Status of the Herpetofauna of Honduras. Amphib. Reptile Conserv. 2004, 3, 6–33. [Google Scholar] [CrossRef][Green Version]
- Chaves, G.; Bolaños, F.; Rodríguez, J.E.; Matamoros, Y. Actualización de Las Listas Rojas Nacionales de Costa Rica. Anfibios y Reptiles.; IUCN SSC and CBSG Mesoamerica: San José, Costa Rica, 2014. [Google Scholar]
- International Union for Conservation of Nature (IUCN) The IUCN Red List of Threatened Species. Version 2019-1. Available online: https://www.iucnredlist.org/en (accessed on 1 September 2021).
- Sasa, M.; Chaves, G.; Porras, L.W. The Costa Rican herpetofauna: Conservation status and future perspectives. In Conservation of Mesoamerican Amphibians and Reptiles; Townsend, J.H., Johnson, J.D., Eds.; Eagle Mountain Press: Salt Lake City, UT, USA, 2010; pp. 510–603. [Google Scholar]
- Hoffmann, H. Some Ecological Notes on Agalychnis annae (Anura: Hylidae). Brenesia 2006, 73–77. [Google Scholar]
- IUCN SSC Amphibian Specialist Group. Agalychnis annae. Available online: https://www.iucnredlist.org/species/55288/158518518 (accessed on 8 September 2021).
- Arguedas-Porras, V. Parque Zoológico y Jardín Botánico Simón Bolívar El Hogar de La Rana Cafetalera Agalychnis Annae; Editorial Sobrevuelo: San José, Costa Rica, 2018. [Google Scholar]
- Hertz, A.; Lotzkat, S.; Stadler, L.; Hamad, N.; Carrizo, A.; Köhler, G. Noteworthy Records of Amphibians from Western Panama. Herpetol. Rev. 2011, 42, 245–250. [Google Scholar]
- Kubicki, B. Leaf-Frogs of Costa Rica; INBio: Heredia, Costa Rica, 2004. [Google Scholar]
- De León, M.E.; Zumbado-Ulate, H.; García-Rodríguez, A.; Alvarado, G.; Sulaeman, H.; Bolaños, F.; Vredenburg, V.T. Batrachochytrium Dendrobatidis Infection in Amphibians Predates First Known Epizootic in Costa Rica. PLoS ONE 2019, 14, e0208969. [Google Scholar] [CrossRef]
- Salazar Zuñiga, J.A.; Chaves Acuña, W.J.; Chaves, G.; Acuña, A.; Abarca Odio, J.I.; Lobon, J.; Gómez Méndez, E.; Gutiérrez Vannucchi, A.C.; Bolaños, F. The Most Frog-Diverse Place in Middle America, with Notes on Conservation Status of Six Threatened Species of Amphibians. Amphib. Reptile Conserv. 2019, 13, 304–322. [Google Scholar]
- IUCN SSC Amphibian Specialist Group. Agalychnis Lemur. Available online: https://www.iucnredlist.org/species/55855/3033153 (accessed on 8 September 2021).
- Leenders, T. Amphibians of Costa Rica; Cornell University Press: New York, NY, USA, 2016. [Google Scholar]
- IUCN SSC Amphibian Specialist Group. Duellmanohyla uranochroa. Available online: https://www.iucnredlist.org/species/55314/54345228 (accessed on 8 September 2021).
- Savage, J.M.; Kubicki, B. A New Species of Fringe-Limb Frog, Genus Ecnomiohyla (Anura: Hylidae), from the Atlantic Slope of Costa Rica, Central America. Zootaxa 2010, 2719, 21–34. [Google Scholar] [CrossRef]
- IUCN SSC Amphibian Specialist Group. Isthmohyla angustilineata. Available online: https://www.iucnredlist.org/species/55390/54345829 (accessed on 8 September 2021).
- Chaves-Acuña, W.; Chaves, G.; Klank, J.; Arias, E.; Bolaños, F.; Shepack, A.; Leenders, T.; Cossel, J.; Faivovich, J. Recent Findings of Isthmohyla pictipes (Anura: Hylidae) in Costa Rica: Variation and Implications for Conservation. Zootaxa 2020, 4881, 499–514. [Google Scholar] [CrossRef]
- IUCN SSC Amphibian Specialist Group. Isthmohyla pictipes. Available online: https://www.iucnredlist.org/species/55603/54362143 (accessed on 8 September 2021).
- IUCN SSC Amphibian Specialist Group. Isthmohyla pseudopuma. Available online: https://www.iucnredlist.org/species/55616/54347154 (accessed on 8 September 2021).
- IUCN SSC Amphibian Specialist Group. Isthmohyla rivularis. Available online: https://www.iucnredlist.org/species/55627/3031396 (accessed on 8 September 2021).
- Flemons, P.; Guralnick, R.; Krieger, J.; Ranipeta, A.; Neufeld, D. A Web-Based GIS Tool for Exploring the World’s Biodiversity: The Global Biodiversity Information Facility Mapping and Analysis Portal Application (GBIF-MAPA). Ecol. Inform. 2007, 2, 49–60. [Google Scholar] [CrossRef]
- Salazar, S. Redescubrimiento de La Rana Marsupial Gastrotheca Cornuta (Anura: Hemiphractidae) En Costa Rica. Brenesia 2015, 83–84, 81–82. [Google Scholar]
- Hidalgo-Mora, E.; Valverde-Castillo, A.; Abarca, J.G. Range Extension of the Blue-Sided Leaf Frog, Agalychnis annae (Anura: Hylidae): Using Citizen Science across Suburban Areas in Costa Rica. Reptil. Amphib. 2021, 28, 264–267. [Google Scholar] [CrossRef]
- Olsen, A.C.; Cossel JR, J.O. Observations of a Remnant Population of the Critically Endangered Hylid Frog Isthmohyla rivularis on the Monteverde Cloud Forest Preserve, Costa Rica. Herpetol. Rev. 2014, 45, 205–208. [Google Scholar]
- Whitfield, S.M.; Alvarado, G.; Abarca, J.; Zumbado-Ulate, H.; Zuñiga, I.; Wainwright, M.; Kerby, J. Differential Patterns of Batrachochytrium dendrobatidis Infection in Relict Amphibian Populations Following Severe Disease-Associated Declines. Dis. Aquat. Organ. 2017, 126, 33–41. [Google Scholar] [CrossRef]
- Barrio-Amorós, C.; Torres, H. Ecnomiohyla miliaria. Distribution in Pacific Costa Rica. Mesoamerican Herpetol. 2017, 4, 660–661. [Google Scholar]
- Zizka, A.; Silvestro, D.; Andermann, T.; Azevedo, J.; Ritter, C.D.; Edler, D.; Farooq, H.; Herdean, A.; Ariza, M.; Scharn, R.; et al. CoordinateCleaner: Automated Cleaning of Occurrence Records from Biological Collections. Methods Ecol. Evol. 2020, 10, 744–751. [Google Scholar]
- Brown, J.L.; Carnaval, A.C. A Tale of Two Niches: Methods, Concepts, and Evolution. Front. Biogeogr. 2019, 11. [Google Scholar] [CrossRef]
- Booth, T.H.; Nix, H.A.; Busby, J.R.; Hutchinson, M.F. BIOCLIM: The First Species Distribution Modelling Package, Its Early Applications and Relevance to Most Current MaxEnt Studies. Divers. Distrib. 2014, 20, 1–9. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Title, P.O.; Bemmels, J.B. ENVIREM: An Expanded Set of Bioclimatic and Topographic Variables Increases Flexibility and Improves Performance of Ecological Niche Modeling. Ecography 2018, 41, 291–307. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Van Etten, J.; Cheng, J.; Mattiuzzi, M.; Sumner, M.; Greenberg, J.A.; Lamigueiro, O.P.; Bevan, A.; Racine, E.B.; Shortridge, A. Package ‘Raster’. R Package. 2015. Available online: https://cran.r-project.org/web/packages/raster/index.html (accessed on 1 September 2021).
- Naimi, B. Usdm: Uncertainty Analysis for Species Distribution Models. R Package Version 1.1-15. 2015. Available online: https://cran.r-project.org/web/packages/usdm/index.html (accessed on 1 September 2021).
- Ortiz-Malavasi, E. Atlas Digital Costa Rica. 2014. Available online: https://repositoriotec.tec.ac.cr/handle/2238/6749?show=full (accessed on 1 September 2021).
- Bland, L.M.; Keith, D.A.; Miller, R.M.; Murray, N.J.; Rodríguez, J.P. (Eds.) Guidelines for the Application of IUCN Red List of Ecosystems Categories and Criteria, Version 1.1; IUCN International Union for Conservation of Nature: Gland, Switzerland, 2017; ISBN 978-2-8317-1769-2. [Google Scholar]
- Brooks, T.M.; Pimm, S.L.; Akçakaya, H.R.; Buchanan, G.M.; Butchart, S.H.M.; Foden, W.; Hilton-Taylor, C.; Hoffmann, M.; Jenkins, C.N.; Joppa, L.; et al. Measuring Terrestrial Area of Habitat (AOH) and Its Utility for the IUCN Red List. Trends Ecol. Evol. 2019, 34, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Dudík, M. Modeling of Species Distributions with Maxent: New Extensions and a Comprehensive Evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R Package for Conducting Spatially Independent Evaluations and Estimating Optimal Model Complexity for Maxent Ecological Niche Models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the Black Box: An Open-Source Release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Shcheglovitova, M.; Anderson, R.P. Estimating Optimal Complexity for Ecological Niche Models: A Jackknife Approach for Species with Small Sample Sizes. Ecol. Model. 2013, 269, 9–17. [Google Scholar] [CrossRef]
- Radosavljevic, A.; Anderson, R.P. Making Better MAXENT Models of Species Distributions: Complexity, Overfitting and Evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. Dismo: Species Distribution Modeling. R Package Version 1.3-5. 2021. Available online: https://cran.r-project.org/web/packages/dismo/index.html (accessed on 1 September 2021).
- Liu, C.; Newell, G.; White, M. On the Selection of Thresholds for Predicting Species Occurrence with Presence-Only Data. Ecol. Evol. 2016, 6, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, E.B.; Pedersen, S.; Linnell, J.D.C. Can Minimum Convex Polygon Home Ranges Be Used to Draw Biologically Meaningful Conclusions? Ecol. Res. 2008, 23, 635–639. [Google Scholar] [CrossRef]
- Nishida, K. Encounter with Hyla angustilineata Taylor, 1952 (Anura: Hylidae) in a Cloud Forest of Costa Rica. Brenesia 2006, 79–81. [Google Scholar]
- Scheele, B.C.; Foster, C.N.; Hunter, D.A.; Lindenmayer, D.B.; Schmidt, B.R.; Heard, G.W. Living with the Enemy: Facilitating Amphibian Coexistence with Disease. Biol. Conserv. 2019, 236, 52–59. [Google Scholar] [CrossRef]
- Puschendorf, R.; Hoskin, C.J.; Cashins, S.D.; McDonald, K.; Skerratt, L.F.; Vanderwal, J.; Alford, R.A. Environmental Refuge from Disease-Driven Amphibian Extinction. Conserv. Biol. 2011, 25, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Granados-Martínez, S.; Zumbado-Ulate, H.; Searle, C.L.; Oliveira, B.F.; García-Rodríguez, A. Niche Contraction of an Endangered Frog Species Driven by the Amphibian Chytrid Fungus. EcoHealth 2021, 18, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Christie, M.R.; Searle, C.L. Evolutionary Rescue in a Host–Pathogen System Results in Coexistence Not Clearance. Evol. Appl. 2018, 11, 681–693. [Google Scholar] [CrossRef] [PubMed]
- DiRenzo, G.V.; Zipkin, E.F.; Grant, E.H.C.; Royle, J.A.; Longo, A.V.; Zamudio, K.R.; Lips, K.R. Eco-Evolutionary Rescue Promotes Host–Pathogen Coexistence. Ecol. Appl. 2018, 28, 1948–1962. [Google Scholar] [CrossRef]
- Price, P.W.; Westoby, M.; Rice, B.; Atsatt, P.R.; Fritz, R.S.; Thompson, J.N.; Mobley, K. Parasite Mediation in Ecological Interactions. Annu. Rev. Ecol. Syst. 1986, 17, 487–505. [Google Scholar] [CrossRef]
- Scheele, B.C.; Hunter, D.A.; Brannelly, L.A.; Skerratt, L.F.; Driscoll, D.A. Reservoir-Host Amplification of Disease Impact in an Endangered Amphibian. Conserv. Biol. 2017, 31, 592–600. [Google Scholar] [CrossRef]
- Acosta-Chaves, V.J.; Cossel, J., Jr. Smilisca phaeota. Colonization. Mesoamerican Herpetol. 2016, 3, 713–715. [Google Scholar]
- Morera-Chacón, B.H.; Chaves-Acosta, V.J. Amphibians from the Cloud Forest of El Silencio de Los Ángeles, San Ramón de Alajuela, Costa Rica. Pensam. Actual 2019, 19, 190–204. [Google Scholar] [CrossRef]
- Abarca, J.G. Cambios En La Estructura de La Comunidad de Anuros (Amphibia: Anura) En El Cerro Chompipe, Costa Rica. Cuad. Investig. UNED 2012, 4, 9–15. [Google Scholar]
- Valencia-Aguilar, A.; Cortés-Gómez, A.M.; Ruiz-Agudelo, C.A. Ecosystem Services Provided by Amphibians and Reptiles in Neotropical Ecosystems. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2013, 9, 257–272. [Google Scholar] [CrossRef]



| Amphibian Species | EVS | IUCN Status | Distribution in Costa Rica | Elevation (m) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2004 | 2019 | CL | CT | MSCC | PN | PS | |||
| Hemiphractidae [1] (1) | |||||||||
| Gastrotheca cornuta | 15 | EN | U | X | X | 300–1000 | |||
| Hylidae [12] (40) | |||||||||
| Boana rosenbergi | 10 | LC | LC | X | X | 0–900 | |||
| Boana rufitela | 10 | LC | LC | X | X | 0–750 | |||
| Dendropsophus ebraccatus | 8 | LC | LC | X | X | X | X | X | 0–1600 |
| Dendropsophus microcephalus | 7 | LC | LC | X | X | X | X | X | 0–1200 |
| Dendropsophus phlebodes | 8 | LC | LC | X | X | 0–750 | |||
| Duellmanohyla legleri | 13 | EN | LC | X | X | 600–1500 | |||
| Duellmanohyla lythrodes | 14 | EN | EN | X | 150–450 | ||||
| Duellmanohyla rufioculis | 12 | LC | LC | X | X | X | X | X | 650–1600 |
| Duellmanohyla uranochroa | 12 | EN | VU | X | X | X | 300–1750 | ||
| Ecnomiohyla bailarina | 15 | NA | NT | X | 300–750 | ||||
| Ecnomiohyla fimbrimembra | 14 | EN | VU | X | 750–1900 | ||||
| Ecnomiohyla miliaria | 12 | VU | VU | X | X | X | 0–1350 | ||
| Ecnomiohyla sukia | 14 | NA | LC | X | X | 400–1000 | |||
| Ecnomiohyla veraguensis | 14 | NA | VU | X | NE | ||||
| Hyloscirtus colymba | 9 | CR | EN | X | 600–1200 | ||||
| Hyloscirtus palmeri | 10 | LC | LC | X | X | 400–1000 | |||
| Isthmohyla angustilineata | 10 | CR | CR | X | X | X | 1500–2350 | ||
| Isthmohyla calypsa | 13 | CR | CR (PE) | X | 1700–2300 | ||||
| Isthmohyla debilis | 11 | CR | CR | X | X | 900–1450 | |||
| Isthmohyla lancasteri | 10 | LC | LC | X | X | 350–1400 | |||
| Isthmohyla picadoi | 14 | LC | LC | X | X | 1700–2900 | |||
| Isthmohyla pictipes | 12 | EN | CR | X | X | 1900–2800 | |||
| Isthmohyla pseudopuma | 8 | LC | LC | X | X | 1100–2350 | |||
| Isthmohyla rivularis | 12 | CR | EN | X | X | X | 1200–2450 | ||
| Isthmohyla tica | 10 | CR | CR | X | X | X | 720–1750 | ||
| Isthmohyla xanthosticta | 14 | DD | DD | X | 2150 | ||||
| Isthmohyla zeteki | 14 | NT | VU | X | X | 1200–1800 | |||
| Osteopilus septentrionalis | 9 | LC | LC | X | 0–10 | ||||
| Scinax boulengeri | 6 | LC | LC | X | X | X | 1–700 | ||
| Scinax elaeochroa | 8 | LC | LC | X | X | X | X | X | 0–1200 |
| Scinax staufferi | 9 | LC | LC | X | X | 0–700 | |||
| Smilisca baudinii | 6 | LC | LC | X | X | X | 0–1600 | ||
| Smilisca manisorum | 8 | NA | LC | X | 0–750 | ||||
| Smilisca phaeota | 6 | LC | LC | X | X | X | X | 0–1100 | |
| Smilisca puma | 9 | LC | LC | X | 0–550 | ||||
| Smilisca sila | 10 | LC | LC | X | X | X | X | 0–1000 | |
| Smilisca sordida | 7 | LC | LC | X | X | X | X | X | 0–1550 |
| Tlalocohyla loquax | 9 | LC | LC | X | X | 50–1100 | |||
| Trachycephalus typhonius | 7 | LC | LC | X | X | X | X | 0–1100 | |
| Triprion spinosus | 12 | LC | NT | X | X | X | 100–1400 | ||
| Phyllomedusidae [2] (7) | |||||||||
| Agalychnis annae | 10 | LC | VU | X | X | X | 780–1650 | ||
| Agalychnis callidryas | 8 | LC | LC | X | X | X | X | 0–1250 | |
| Agalychnis lemur | 11 | CR | CR | X | X | 450–1600 | |||
| Agalychnis saltator | 8 | LC | LC | X | X | 0–1000 | |||
| Agalychnis spurrelli | 11 | LC | LC | X | X | X | 0–900 | ||
| Cruziohyla calcarifer | 14 | LC | LC | X | 0–800 | ||||
| Cruziohyla sylviae | 12 | NA | LC | X | 0-800 | ||||
| Species | ESH (km2) | AMCP (km2) | Difference (%) |
|---|---|---|---|
| Agalychnis annae | 6373.9 | 2778.3 | +129.4 |
| Agalychnis lemur | 4352.7 | 7489.4 | −41.9 |
| Duellmanohyla uranochroa | 2853.6 | 5574.3 | −48.9 |
| Ecnomiohyla sukia | 6515.2 | 2905.8 | +124.2 |
| Isthmohyla angustilineata | 238.0 | 400.0 | −40.5 |
| Isthmohyla pictipes | 1891.6 | 2797.0 | −32.4 |
| Isthmohyla pseudopuma | 3212.5 | 4781.9 | −32.8 |
| Isthmohyla rivularis | 2237.6 | 2359.0 | −5.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zumbado-Ulate, H.; Searle, C.L.; Chaves, G.; Acosta-Chaves, V.; Shepack, A.; Salazar, S.; García-Rodríguez, A. Assessing Suitable Habitats for Treefrog Species after Previous Declines in Costa Rica. Diversity 2021, 13, 577. https://doi.org/10.3390/d13110577
Zumbado-Ulate H, Searle CL, Chaves G, Acosta-Chaves V, Shepack A, Salazar S, García-Rodríguez A. Assessing Suitable Habitats for Treefrog Species after Previous Declines in Costa Rica. Diversity. 2021; 13(11):577. https://doi.org/10.3390/d13110577
Chicago/Turabian StyleZumbado-Ulate, Héctor, Catherine L. Searle, Gerardo Chaves, Víctor Acosta-Chaves, Alex Shepack, Stanley Salazar, and Adrián García-Rodríguez. 2021. "Assessing Suitable Habitats for Treefrog Species after Previous Declines in Costa Rica" Diversity 13, no. 11: 577. https://doi.org/10.3390/d13110577
APA StyleZumbado-Ulate, H., Searle, C. L., Chaves, G., Acosta-Chaves, V., Shepack, A., Salazar, S., & García-Rodríguez, A. (2021). Assessing Suitable Habitats for Treefrog Species after Previous Declines in Costa Rica. Diversity, 13(11), 577. https://doi.org/10.3390/d13110577