Trait–Abundance Relationships of Annual Ephemerals in Response to Nitrogen Addition in Gurbantunggut Desert
Abstract
:1. Introduction
2. Methods
2.1. Study Site
2.2. Experimental Design
2.3. Species Abundance and Ecological Stoichiometric Measurements
2.4. Data Analysis
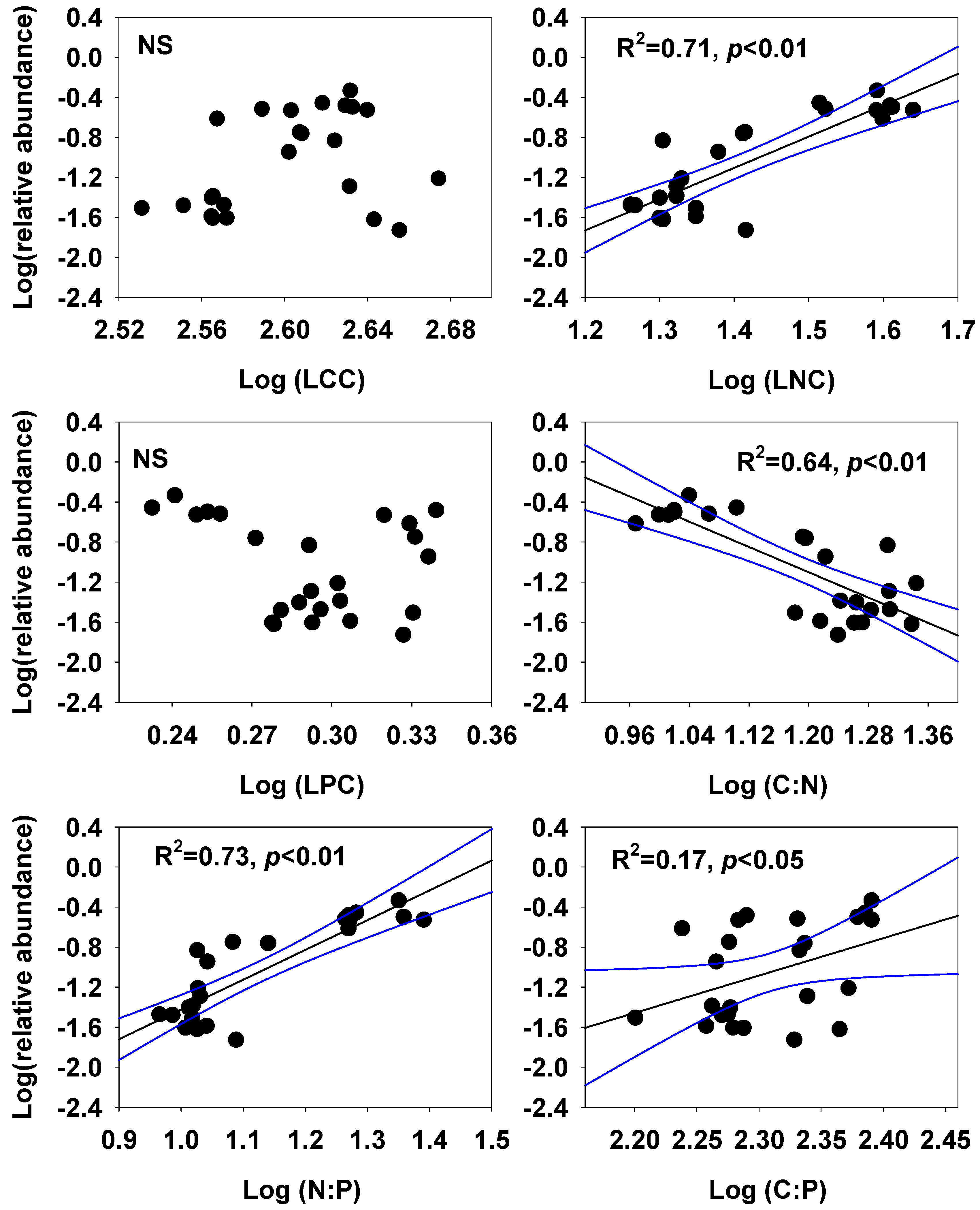
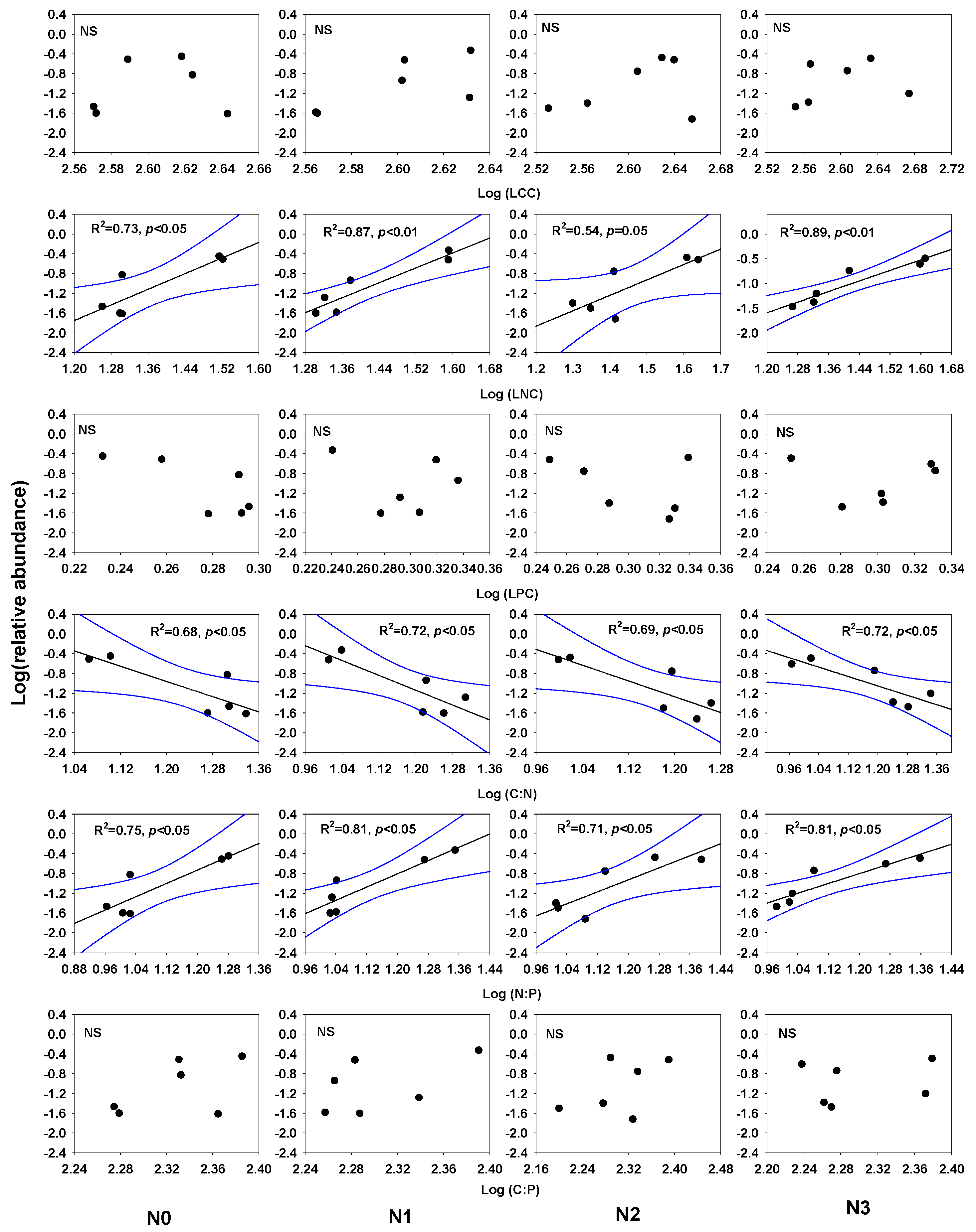
3. Results
4. Discussion
4.1. Which Ecological Stoichiometric Traits Explain Species Abundance?
4.2. Relationships between Species Relative Abundance and Ecological Stoichiometry with Nitrogen Addition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Preston, F.W. The commonness, and rarity, of species. Ecology 1948, 29, 254–283. [Google Scholar] [CrossRef]
- McGill, B.J.; Enquist, B.J.; Weiher, E.; Westoby, M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Umaña, M.N.; Zhang, C.; Cao, M.; Lin, L.; Swenson, N.G. Commonness, rarity, and intraspecific variation in traits and performance in tropical tree seedlings. Ecol. Lett. 2015, 18, 1329–1337. [Google Scholar] [CrossRef]
- Díaz, S.; Cabido, M.; Casanoves, F. Plant functional traits and environmental filters at a regional scale. J. Veg. Sci. 1998, 9, 113–122. [Google Scholar] [CrossRef]
- Weiher, E.; Keddy, P.A. The assembly of experimental wetland plant communities. Oikos 1995, 73, 323–335. [Google Scholar] [CrossRef]
- Wang, M.; Xu, J.; Chai, Y.; Guo, Y.; Liu, X.; Yue, M. Differentiation of environmental conditions promotes variation of two Quercus wutaishanica community assembly patterns. Forests 2020, 11, 43. [Google Scholar] [CrossRef] [Green Version]
- Keddy, P.A. Assembly and response rules: Two goals for predictive community ecology. J. Veg. Sci. 1992, 3, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Kraft, N.J.; Valencia, R.; Ackerly, D.D. Functional traits and niche–based tree community assembly in an Amazonian forest. Science 2008, 322, 580–582. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Wan, P.; Chai, Y.; Guo, Y.; Zhang, X.; Yue, M. Adaptative strategy of leaf traits to drought conditions: Quercus aliena var. acuteserrata forest (The Qinling Mts. China). Pol. J. Ecol. 2015, 63, 77–87. [Google Scholar]
- Zhang, H.; John, R.; Zhu, S.; Liu, H.; Xu, Q.; Qi, W.; Liu, K.; Ye, Q. Shifts in functional trait–species abundance relationships over secondary subalpine meadow succession in the Qinghai–Tibetan Plateau. Oecologia 2018, 188, 547–557. [Google Scholar] [CrossRef]
- Hubbell, S.P. Neutral theory in community ecology and the hypothesis of functional equivalence. Funct. Ecol. 2005, 19, 166–172. [Google Scholar] [CrossRef]
- Suding, K.N.; Goldberg, D.E.; Hartman, K.M. Relationships among species traits: Separating levels of response and identifying linkages to abundance. Ecology 2003, 84, 1–16. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Ackerly, D.D. A link between plant traits and abundance: Evidence from coastal California woody plants. J. Ecol. 2010, 98, 814–821. [Google Scholar] [CrossRef]
- Brenes-Arguedas, T.; Roddy, A.B.; Kursar, T.A. Plant traits in relation to the performance and distribution of woody species in wet and dry tropical forest types in Panama. Funct. Ecol. 2013, 27, 392–402. [Google Scholar] [CrossRef]
- Loranger, J.; Munoz, F.; Shipley, B.; Violle, C. What makes trait-abundance relationships when both environmental filtering and stochastic neutral dynamics are at play? Oikos 2018, 127, 1735–1745. [Google Scholar] [CrossRef]
- Harpole, W.S.; Tilman, D. Non-neutral patterns of species abundance in grassland communities. Ecol. Lett. 2006, 9, 15–23. [Google Scholar]
- Yan, E.; Yang, X.; Chang, S.; Wang, X. Plant trait-species abundance relationships vary with environmental properties in subtropical forests in Eastern China. PLoS ONE 2013, 8, e61113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reader, R.J. Relationship between species relative abundance and plant traits for an infertile habitat. Plant. Ecol. 1998, 134, 43–51. [Google Scholar] [CrossRef]
- Niu, K.; Zhang, S.; Zhao, B.; Du, G. Linking grazing response of species abundance to functional traits in the Tibetan alpine meadow. Plant. Soil. 2010, 330, 215–223. [Google Scholar] [CrossRef]
- Bruelheide, H.; Dengler, J.; Purschke, O.; Lenoir, J.; Jiménez-Alfaro, B.; Hennekens, S.M.; Botta-Dukát, Z.; Chytrý, M.; Field, R.; Jansen, F.; et al. Global trait-environment relationships of plant communities. Nat. Ecol. Evol. 2018, 2, 1906–1917. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Zhang, P.; Guo, Z.; Chu, C.; Du, G. The effects of fertilization on the trait-abundance relationships in a Tibetan alpine meadow community. J. Plant Ecol. 2016, 9, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Li, Y.; Ren, F.; Lin, L.; Zhu, W.; He, J.; Niu, K. Trait-abundance relation in response to nutrient addition in a Tibetan alpine meadow: The importance of species trade-off in resource conservation and acquisition. Ecol. Evol. 2017, 7, 10575–10581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssens, I.A.; Dieleman, W.; Luyssaert, S.; Subke, J.A.; Reichstein, M.; Ceulemans, R.; Ciais, P.; Dolman, A.J.; Grace, J.; Matteucci, G. Reduction of forest soil respiration in response to nitrogen deposition. Nat. Geosci. 2010, 3, 315–322. [Google Scholar] [CrossRef]
- Franklin, J.; Serradiaz, J.M.; Syphard, A.D.; Syphard, A.D.; Regan, H.M. Global change and terrestrial plant community dynamics. Proc. Natl. Acad. Sci. USA 2016, 113, 3725–3734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laliberté, E.; Shipley, B.; Norton, D.A.; Scott, D. Which plant traits determine abundance under long-term shifts in soil resource availability and grazing intensity? J. Ecol. 2012, 100, 662–677. [Google Scholar] [CrossRef]
- Marks, C.O.; Lechowicz, M.J. Alternative designs and the evolution of functional diversity. Am. Nat. 2006, 167, 55–66. [Google Scholar] [CrossRef]
- Reich, P.B. The world-wide ‘fast-slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Mao, Z.; Zhang, D. The conspectus of ephemeral flora in northern Xinjiang. Arid Zone Res. 1994, 3, 1–26, (In Chinese with English Abstract). [Google Scholar]
- Chapin, F.S., III. The mineral nutrition of wild plants. Annu. Rev. Ecol. Syst. 1980, 11, 233–260. [Google Scholar] [CrossRef]
- Han, W.; Fang, J.; Guo, D.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Zechmeister-Boltenstern, S.; Keiblinger, K.M.; Mooshammer, M.; Peñuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The application of ecological stoichiometry to plant-microbial-soil organic matter transformations. Ecol. Monogr. 2015, 85, 133–155. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Yue, P.; Gong, Y.; Li, K.; Tan, D.; Goulding, K.; Liu, X. Impacts of water and nitrogen addition on nitrogen recovery in Haloxylon ammodendron dominated desert ecosystems. Sci. Total. Environ. 2017, 601, 1280–1288. [Google Scholar] [CrossRef]
- Huang, G.; Su, Y.; Zhu, L.; Li, Y. The role of spring ephemerals and soil microbes in soil nutrient retention in a temperate desert. Plant. Soil. 2016, 406, 43–54. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modifed single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta. 1962, 27, 31–36. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Werger, M.J.A.; Castro-Diez, P.; Van Rheenen, J.W.A.; Rowland, A.P. Foliar nutrients in relation to growth, allocation and leaf traits in seedlings of a wide range of woody plant species and types. Oecologia 1997, 111, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Kattge, J.; Knorr, W.; Raddatz, T.; Wirth, C. Quantifying photosynthetic capacity and its relationship to leaf nitrogen content for global-scale terrestrial biosphere models. Glob. Chang. Biol. 2009, 15, 976–991. [Google Scholar] [CrossRef]
- Cui, X.; Yue, P.; Wu, W.; Gong, Y.; Li, K.; Misselbrook, T.; Goulding, K.; Liu, X. The growth and N retention of two annual desert plants varied under different nitrogen deposition rates. Front. Plant Sci. 2019, 10, 356. [Google Scholar] [CrossRef]
- Niu, K.; He, J.; Lechowicz, M.J. Foliar phosphorus content predicts species relative abundance in P-limited Tibetan alpine meadows. Perspect. Plant Ecol. 2016, 22, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural carbon in woody plants. Annu. Rev. Plant. Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; He, F.; Tian, D.; Zou, D.; Yan, Z.; Yang, Y.; Zhou, T.; Huang, K.; Shen, H.; Fang, J. Variations and determinants of carbon content in plants: A global synthesis. Biogeosciences 2018, 15, 693–702. [Google Scholar] [CrossRef] [Green Version]
- Hedin, L.O. Global organization of terrestrial plant-nutrient interactions. Proc. Natl. Acad. Sci. USA 2004, 101, 10849–10850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elser, J.J.; Dobberfuhl, D.R.; MacKay, N.A.; Schampel, J.H. Organism size, life history, and N: P stoichiometry: Toward a unified view of cellular and ecosystem processes. BioScience 1996, 46, 674–684. [Google Scholar] [CrossRef] [Green Version]
- Elser, J.J.; Acharya, K.; Kyle, M.; Cotner, J.; Makino, W.; Markow, T.; Watts, T.; Hobbie, S.; Fagan, W.; Schade, J.; et al. Growth rate–Stoichiometry couplings in diverse biota. Ecol. Lett. 2003, 6, 936–943. [Google Scholar] [CrossRef] [Green Version]
- Wright, I.J.; Reich, P.B.; Westoby, M. Strategy shifts in leaf physiology, structure and nutrient content between species of high-and low-rainfall and high-and low-nutrient habitats. Funct. Ecol. 2001, 15, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Fynn, R.W.S.; Morris, C.D.; Kirkman, K.P. Plant strategies and trait trade-offs influence trends in competitive ability along gradients of soil fertility and disturbance. J. Ecol. 2005, 93, 384–394. [Google Scholar] [CrossRef]
- Stursova, M.; Crenshaw, C.L.; Sinsabaugh, R.L. Microbial responses to long-term N deposition in a semiarid grassland. Microb. Ecol. 2006, 51, 90–98. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.; Downing, A. Non–linear response of microbial activity across a gradient of nitrogen addition to a soil from the Gurbantunggut Desert, northwestern China. Soil. Biol. Biochem. 2012, 47, 67–77. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: San Diego, CA, USA, 2014; pp. 955–956. [Google Scholar]
- Basto, S.; Thompson, K.; Phoenix, G.; Sloan, V.; Leake, J.; Rees, M. Long-term nitrogen deposition depletes grassland seed banks. Nat. Commun. 2015, 6, 6185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Ma, H.; Tan, D. Trait–Abundance Relationships of Annual Ephemerals in Response to Nitrogen Addition in Gurbantunggut Desert. Diversity 2021, 13, 569. https://doi.org/10.3390/d13110569
Wang M, Ma H, Tan D. Trait–Abundance Relationships of Annual Ephemerals in Response to Nitrogen Addition in Gurbantunggut Desert. Diversity. 2021; 13(11):569. https://doi.org/10.3390/d13110569
Chicago/Turabian StyleWang, Mao, Haiyang Ma, and Dunyan Tan. 2021. "Trait–Abundance Relationships of Annual Ephemerals in Response to Nitrogen Addition in Gurbantunggut Desert" Diversity 13, no. 11: 569. https://doi.org/10.3390/d13110569
APA StyleWang, M., Ma, H., & Tan, D. (2021). Trait–Abundance Relationships of Annual Ephemerals in Response to Nitrogen Addition in Gurbantunggut Desert. Diversity, 13(11), 569. https://doi.org/10.3390/d13110569





