Satellite Telemetry of Blue-Throated Macaws in Barba Azul Nature Reserve (Beni, Bolivia) Reveals Likely Breeding Areas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Accessibility
2.2. Study Site: Barba Azul Nature Reserve
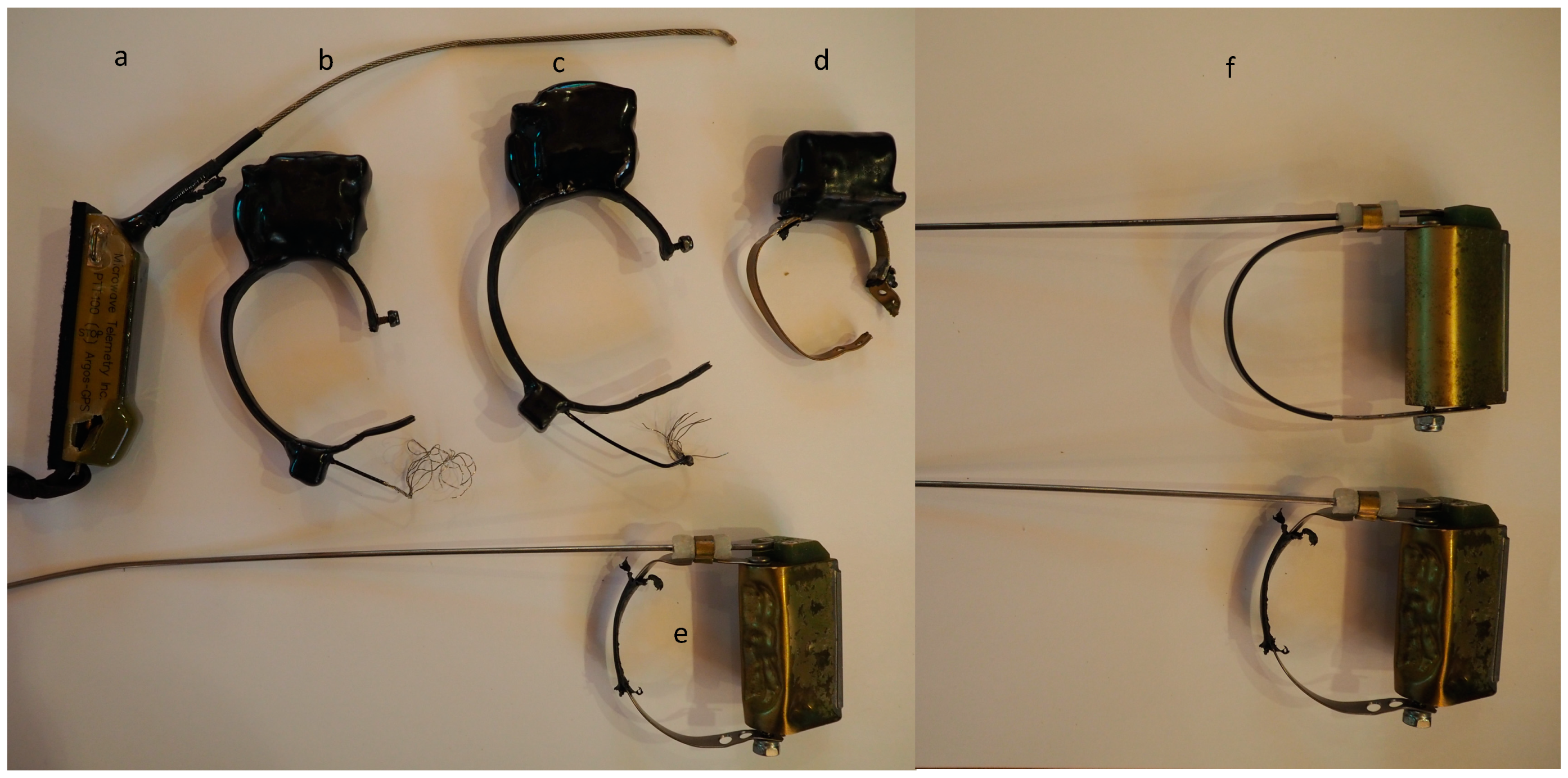
2.3. Attachment Tests
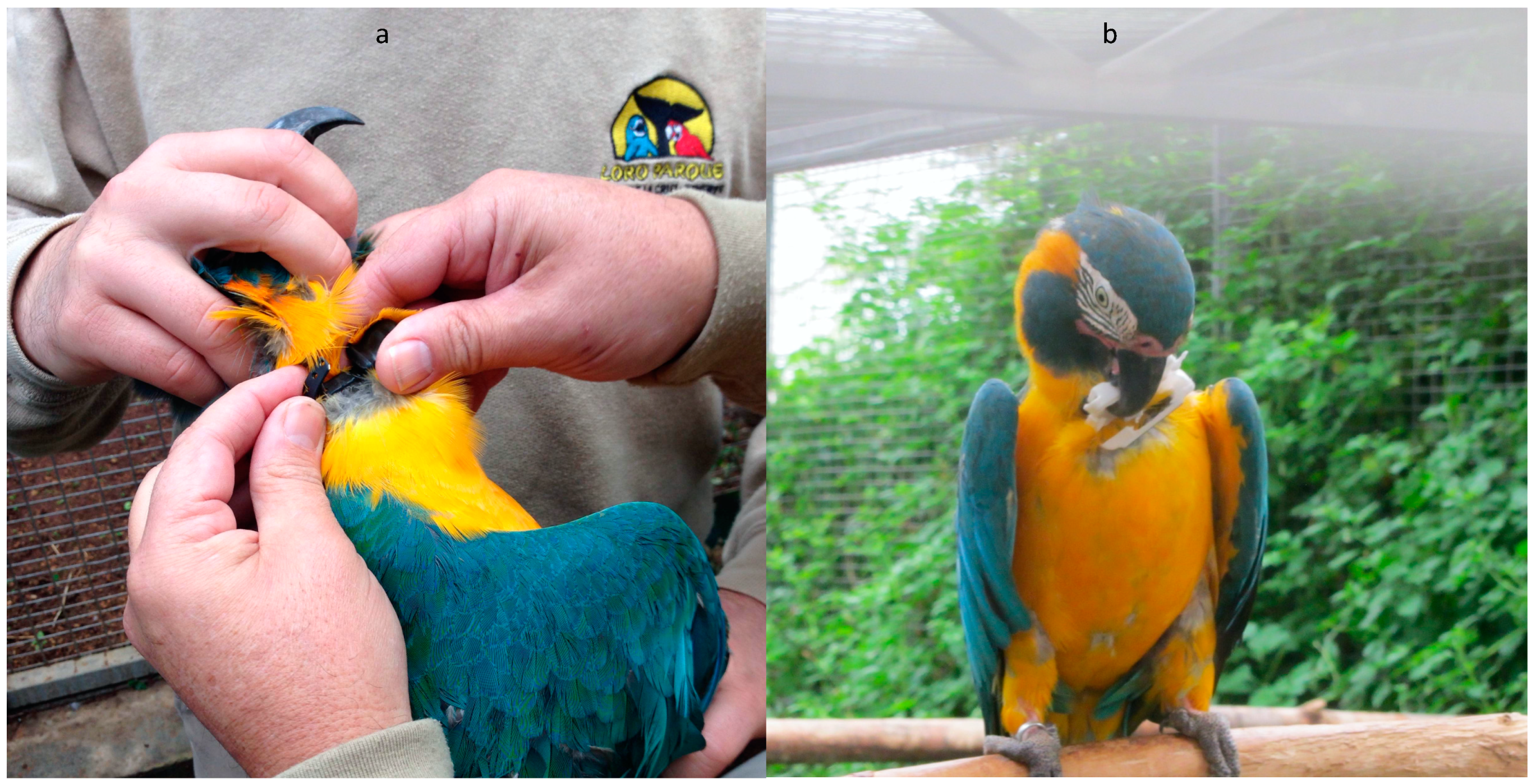
2.4. Capture of Wild BTMs
2.5. Tagging
2.6. Programming and Location Accuracy
2.7. Analyses
2.8. Wet Season Nest Surveys
3. Results
3.1. PTT Dummy Testing
3.2. Satellite PTT Performance
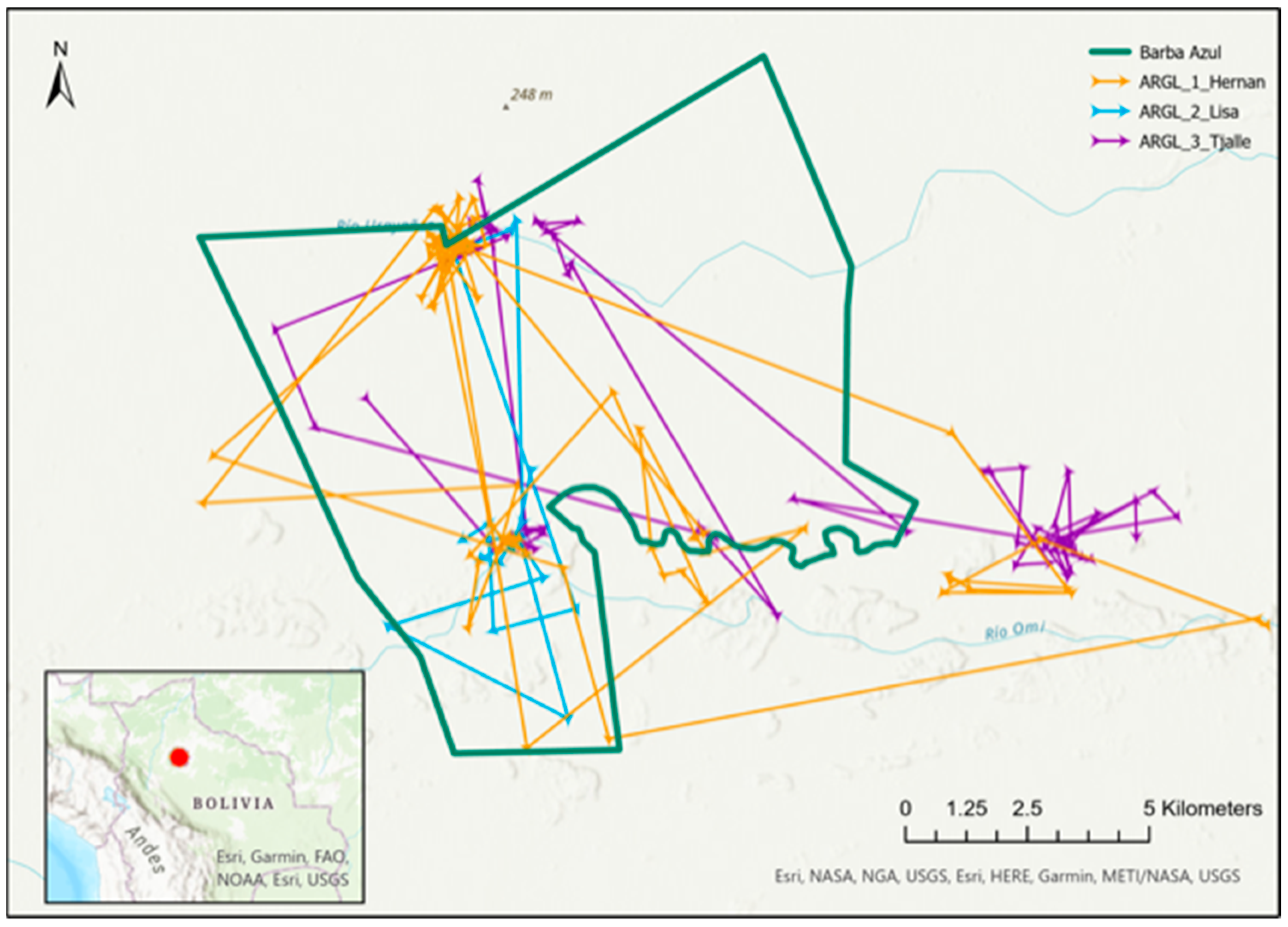
3.3. Range Size, Movements, and Observations around Barba Azul
3.4. Migration, and Breeding Season Ranges and Movements
3.5. Wet Season Ground–Truthing Surveys
4. Discussion
4.1. Tracking Results
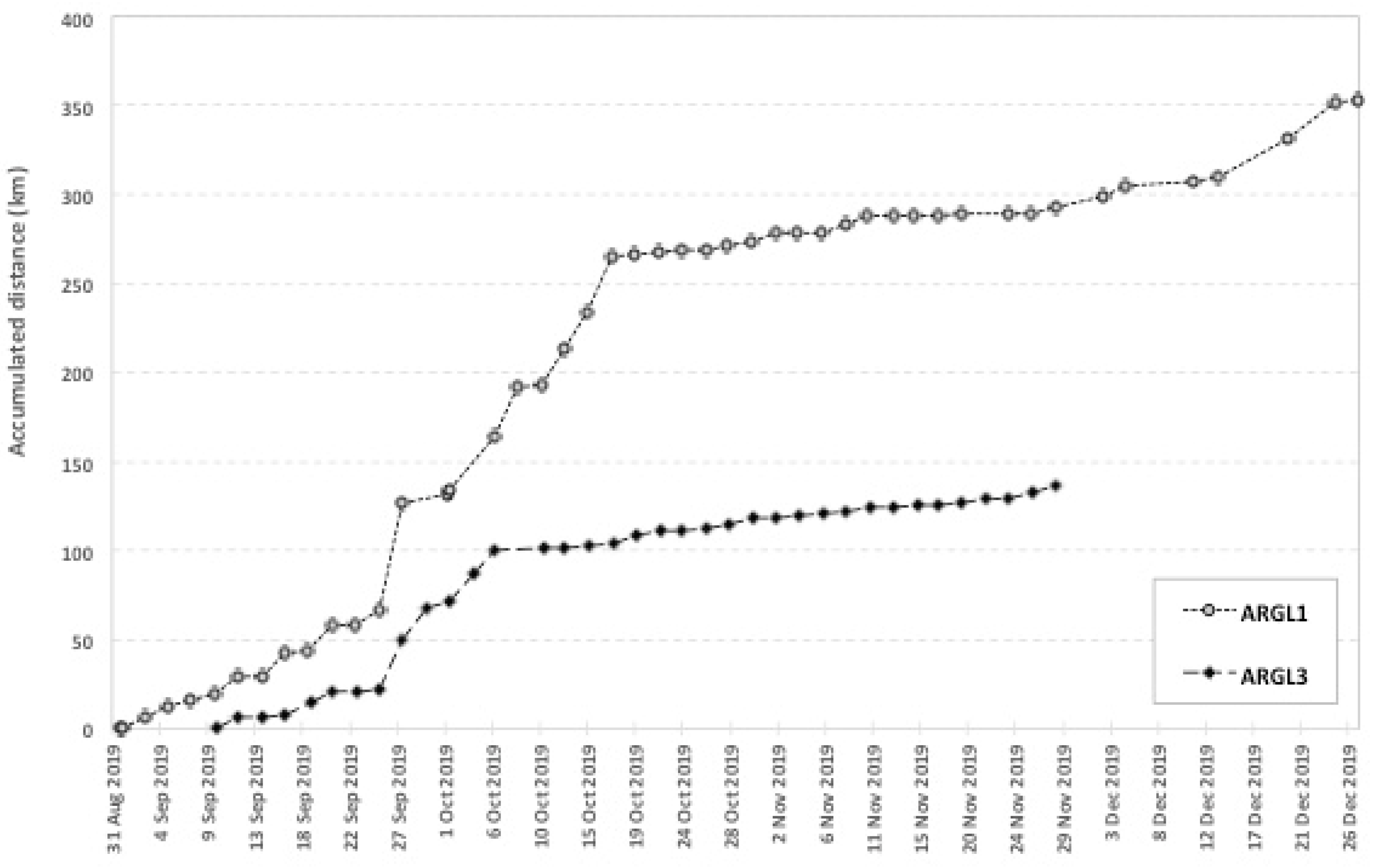
4.2. Distances Traveled
4.3. Challenges Working with the Psittacidae
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bridge, E.S.; Thorup, K.; Bowlin, M.S.; Chilson, P.B.; Diehl, R.H.; Fléron, R.W.; Hartl, P.; Kays, R.; Kelly, J.F.; Robinson, W.D.; et al. Technology on the move: Recent and forthcoming innovations for tracking migratory birds. BioScience 2011, 61, 689–698. [Google Scholar] [CrossRef] [Green Version]
- Crossin, G.T.; Cooke, S.J.; Goldbogen, J.A.; Phillips, R.A. Tracking fitness in marine vertebrates: Current knowledge and opportunities for future research. Mar. Ecol. Prog. Ser. 2014, 496, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Guilford, T.; Åkesson, S.; Gagliardo, A.; Holland, R.A.; Mouritsen, H.; Muheim, R.; Wiltschko, R.; Wiltschko, W.; Bingman, V.P. Migratory navigation in birds: New opportunities in an era of fast-developing tracking technology. J. Exp. Biol. 2011, 214, 3705–3712. [Google Scholar] [CrossRef] [Green Version]
- Robinson, W.D.; Bowlin, M.S.; Bisson, I.; Shamoun-Baranes, J.; Thorup, K.; Diehl, R.H.; Kunz, T.H.; Mabey, S.; Winkler, D.W. Integrating concepts and technologies to advance the study of bird migration. Front. Ecol. Environ. 2010, 8, 354–361. [Google Scholar] [CrossRef] [Green Version]
- Wikelski, M.; Kays, R.W.; Kasdin, N.J.; Thorup, K.; Smith, J.A.; Swenson, G.W., Jr. Going wild: What a global small-animal tracking system could do for experimental biologists. J. Exp. Biol. 2007, 210, 181–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skewgar, E.; Boersma, P.D.; Simeone, A. Winter migration of Magellanic Penguins (Spheniscus magellanicus) along the southeastern Pacific. Waterbirds 2014, 37, 203–209. [Google Scholar] [CrossRef]
- Martell, M.S.; Henny, C.J.; Nye, P.E.; Solensky, M.J. Fall migration routes, timing, and wintering sites of North American ospreys as determined by satellite telemetry. Condor 2001, 103, 715–724. [Google Scholar] [CrossRef]
- McIntyre, C.L. Quantifying Sources of Mortality and Wintering Ranges of Golden Eagles from Interior Alaska Using Banding and Satellite Tracking. J. Raptor Res. 2012, 46, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Davenport, L.C.; Goodenough, K.; Haugaasen, T. Birds of two oceans? Trans-Andean and divergent migration of Black Skimmers (Rynchops niger) from the Peruvian Amazon. PLoS ONE 2016, 11, e0144994. [Google Scholar] [CrossRef] [PubMed]
- Cappelle, J.; Iverson, S.A.; Takekawa, J.Y.; Newman, S.H.; Dodman, T.; Gaidet, N. Implementing telemetry on new species in remote areas: Recommendations from a large-scale satellite tracking study of African waterfowl. Ostrich 2011, 82, 17–26. [Google Scholar] [CrossRef]
- Hill, J.M.; Sandercock, B.K.; Renfrew, R.B. Migration Patterns of Upland Sandpipers in the Western Hemisphere. Front. Ecol. Evol. 2019, 7, 426. [Google Scholar] [CrossRef] [Green Version]
- Pedler, R.D.; Ribot, R.F.H.; Bennett, A.T.D. Extreme nomadism in desert waterbirds: Flights of the banded stilt. Biol. Lett. 2014, 10, 20140547. [Google Scholar] [CrossRef]
- Lutcavage, M.E.; Brill, R.W.; Skomal, G.B.; Chase, B.C.; Howey, P.W. Results of pop-up satellite tagging of spawning size class fish in the Gulf of Maine: Do North Atlantic Bluefin tuna spawn in the mid-Atlantic? Can. J. Fish. Aquat. Sci. 1999, 56, 173–177. [Google Scholar] [CrossRef]
- Pilcher, N.E.; Rodriguez-Zarate, C.J.; Antonopoulou, M.A.; Mateos-Molina, D.; Sekhar-Das, H.; Abdullah-Bugla, I. Combining laparoscopy and satellite tracking: Successful round-trip tracking of female green turtles from feeding areas to nesting grounds and back. Glob. Ecol. Cons. 2020, 23, e01169. [Google Scholar] [CrossRef]
- Takekawa, J.Y.; Newman, S.H.; Xiao, X.; Prosser, D.J.; Spragens, K.A.; Palm, E.C.; Yan, B.; Li, T.; Lei, F.; Zhao, D.; et al. Migration of waterfowl in the East Asian flyway and spatial relationship to HPAI H5N1 outbreaks. Avian Dis. 2010, 54 (Suppl. 1), 466–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, Y.C.; Tibbitts, T.L.; Lok, T.; Hassell, C.J.; Peng, H.B.; Ma, Z.; Zhang, Z.; Piersma, T. Filling knowledge gaps in a threatened shorebird flyway through satellite tracking. J. Appl. Ecol. 2019, 56, 2305–2315. [Google Scholar] [CrossRef] [Green Version]
- Sick, H.; Teixeira, D.M.; Gonzaga, L.P. A nossa descoberta da pátria da arara Anodorhynchus leari. Anais da Acadamia Bras Ciências 1979, 51, 575–576. [Google Scholar]
- Styles, F.G.; Skutch, A.F. A Guide to the Birds of Costa Rica; Comstock Publishing Associates: Ithaca, NY, USA, 1989. [Google Scholar]
- Forshaw, J.M. Parrots of the World, 3rd ed.; Landsdowne Editions: Melbourne, Australia, 1989. [Google Scholar]
- Jordan, O.C.; Munn, C.A. First observations of the Blue-throated Macaw in Bolivia. Wilson Bull. Wilson Ornithol. Soc. 1993, 105, 694–695. [Google Scholar]
- Ortiz-von Halle, B. Bird’s-Eye View: Lessons from 50 Years of Bird Trade Regulation & Conservation in Amazon Countries; TRAFFIC: Cambridge, UK, 2018. [Google Scholar]
- Herzog, S.K.; Maillard, O.Z.; Boorsma, T.; Sanchez-Avila, G.; Garcia-Soliz, V.H.; Paca-Condori, A.; Vailez de Abajo, M.; Soria-Auza, W. First systematic sampling approach to estimate the global population size of the Critically Endangered Blue-throated Macaw (Ara glaucogularis). Bird Conserv. Int. 2021, 31, 293–311. [Google Scholar] [CrossRef]
- Hordijk, I.; Meijer, F.; Nissen, E.; Boorsma, T.; Poorter, L. Cattle affect regeneration of the palm species Attalea princeps in a Bolivian forest-savannah mosaic. Biotropica 2019, 51, 28–38. [Google Scholar] [CrossRef] [Green Version]
- Lombardo, U.; Veit, H. The origin of oriented lakes: Evidence from the Bolivian Amazon. Geomorphology 2014, 204, 502–509. [Google Scholar] [CrossRef]
- Lombardo, U.; Iriarte, J.; Hilbert, L.; Ruiz-Pérez, J.; Capriles, J.M.; Veit, H. Early Holocene crop cultivation and landscape modification in Amazonia. Nature 2020, 581, 190–193. [Google Scholar] [CrossRef]
- Berkunsky, I.; Daniele, G.; Kacoliris, F.P.; Díaz-Luque, J.A.; Silva Frias, C.P.; Aramburu, R.M.; Gilardi, J.D. Reproductive parameters in the critically endangered Blue-throated Macaw: Limits to the recovery of a parrot under intensive management. PLoS ONE 2014, 9, e99941. [Google Scholar] [CrossRef] [PubMed]
- Boorsma, T.; Asociación Armonia, Santa Cruz, Bolivia. 2020; Unpublished data.
- Kennedy, E.M.; Kemp, J.R.; Mosen, C.C.; Perry, G.L.W.; Dennis, T.E. GPS telemetry for parrots: A case study with the Kea (Nestor notabilis). Auk Ornithol. Adv. 2015, 132, 389–396. [Google Scholar] [CrossRef]
- Antas, P.T.Z.; Carrara, L.A.; Yabe, R.S.; Ubaid, F.K.; Oliveira-Junior., S.B.; Vasques, E.R.; Ferreira, L.P. A Arara-Azul na Reserva Particular do Patrimônio Natural SESC Pantanal; The Hyacinth Macaw in the SESC Pantanal Natural Heritage Private Reserve; Conhecendo o Pantanal 6; SESC Serviço Social do Comércio, Departamento Nacional: Rio de Janeiro, Brazil, 2010; Available online: http://www.sescpantanal.com.br/arquivos/cadastro-itens/layout-6/arquivos/file-635877033528123910.pdf (accessed on 4 November 2021). (In Portuguese)
- Brightsmith, D.J.; Boyd, J.; Hobson, E.A.; Randal, C.J. Satellite telemetry reveals complex migratory movement patterns of two large macaw species in the western Amazon basin. Avian Conserv. Ecol. 2021, 16, 14. [Google Scholar] [CrossRef]
- Rycken, S.; Warren, K.S.; Yeap, L.; Jackson, B.; Riley, K.; Page, M.; Dawson, R.; Smith, K.; Mawson, P.R.; Shephard, J.M. Assessing Integration of Black Cockatoos Using Behavioral Change Point Analysis. J. Wildl. Manag. 2019, 83, 334–342. [Google Scholar] [CrossRef]
- Davenport, L.C.; Boorsma, T.; Carrara, L.A.; Antas, P.; Faria, L.; Brightsmith, D.J.; Herzog, S.K.; Soria-Auza, R.W.; Hennessey, A.B. Data from: Satellite telemetry of Blue-throated macaws in Barba Azul Nature Reserve (Beni, Bolivia) reveals likely breeding areas. Movebank Data Repos. 2021. [Google Scholar] [CrossRef]
- Haase, R.; Beck, G. Structure and composition of savanna vegetation in northern Bolivia: A preliminary report. Brittonia 1989, 41, 80–100. [Google Scholar] [CrossRef]
- Wittmann, F.; Householder, E.; de Oliveira, A.; Lopes, A.; Junk, W.J.; Piedade, M.T. Implementation of the Ramsar Convention on South American wetlands: An update. Res. Rep. Biodivers. Stud. 2015, 4, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Capriles, J.M.; Lombardo, U.; Maley, B.; Zuna, C.; Veit, H.; Kennett, D.J. Persistent Early to Middle Holocene tropical foraging in southwestern Amazonia. Sci. Adv. 2019, 5, eaav5449. [Google Scholar] [CrossRef] [Green Version]
- Mayle, F.E.; Langstroth, R.P.; Fisher, R.A.; Meir, P. Long-term forest-savannah dynamics in the Bolivian Amazon: Implications for conservation. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 291–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callaú, L.N.M.; Boorsma, T. Guía Práctica Para Ganadería de Armonización: La Ganadería Sostenible Para el Beni; Asociación Civil Armonía: Santa Cruz de la Sierra, Bolivia, 2019; Available online: http://armoniabolivia.org/wp-content/uploads/2020/01/Gu%C3%ADa-Ganader%C3%ADa-Sostenible-baja.pdf (accessed on 8 August 2020).
- Davenport, L.C.; Nole, I.; Carlos, N. East with the Night: Longitudinal migration of the Orinoco Goose (Neochen jubata) between Manú National Park, Peru and the Llanos de Moxos, Bolivia. PLoS ONE 2012, 7, e46886. [Google Scholar] [CrossRef]
- Barron, D.G.; Brawn, J.D.; Weatherhead, P.J. Meta analysis of transmitter effects on avian behaviour and ecology. Methods Ecol. Evol. 2010, 1, 180–187. [Google Scholar] [CrossRef]
- CLS (Collecte Localisation Satellites). Argos User’s Manual; Collecte Localisation Satellites: Ramonville Saint-Agne, France, 2011; 69p, Available online: http://www.argos-system.org/manual/ (accessed on 14 February 2021).
- Boyd, J.D.; Brightsmith, D.J. Error properties of Argos satellite telemetry locations using Least Squares and Kalman Filtering. PLoS ONE 2013, 8, e63051. [Google Scholar] [CrossRef] [Green Version]
- Douglas, D.C.; Weinzierl, R.C.; Davidson, S.; Kays, R.; Wikelski, M.; Bohrer, G. Moderating Argos location errors in animal tracking data. Methods Ecol. Evol. 2012, 3, 999–1007. [Google Scholar] [CrossRef]
- Fleming, C.H.; Calabrese, J.M.; Mueller, T.; Olson, K.A.; Leimgruber, P.; Fagan, W.F. Non-Markovian maximum likelihood estimation of autocorrelated movement processes. Methods Ecol. Evol. 2014, 5, 462–472. [Google Scholar] [CrossRef]
- Calabrese, J.M.; Fleming, C.H.; Gurarie, E. ctmm: An r package for analyzing animal relocation data as a continuous-time stochastic process. Methods Ecol. Evol. 2016, 7, 1124–1132. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 9 February 2021).
- Uhlenbeck, G.E.; Ornstein, L.S. On the theory of the Brownian motion. Phys. Rev. 1930, 36, 823. [Google Scholar] [CrossRef]
- Silva, I.; Fleming, C.H.; Noonan, M.J.; Alston, J.; Folta, C.; Fagan, W.F.; Calabrese, J.M. Autocorrelation-informed home range estimation: A review and practical guide. EcoEvoRxiv 2021. preprint. Available online: https://ecoevorxiv.org/23wq7/ (accessed on 9 February 2021).
- Asociación Civil Armonía. Discoveries from 2020 Nest Search Expedition Guide Next Steps for Blue-Throated Macaw Research and Conservation 2020; Asociación Civil Armonía: Santa Cruz de la Sierra, Bolivia, 2020; Available online: http://armoniabolivia.org/2020/04/28/discoveries-guide-next-steps-for-research-and-conservation (accessed on 9 February 2021).
- Weinzetll, M.; (Loro Parque Foundation, Tenerife, Spain). Personal communication, 2018.
- Asociación Civil Armonía. Barba Azul Nature Reserve; May 2020 Update Report; Asociación Civil Armonía: Santa Cruz de la Sierra, Bolivia, 2020; Available online: http://armoniabolivia.org/wp-content/uploads/2020/09/Barba-Azul-Nature-Reserve-May-2020-Update-Report.pdf (accessed on 9 February 2021).
- Da-Ré, M.A.; (Centro de Economia Verde, Fundação Certi Florionopolis, Florianópolis, Brazil). Personal communication, 2020.
- Meyer, C. Spatial Ecology and Conservation of the Endemic and Endangered Red-Fronted MACAW (Ara Rubrogenys) in the Bolivian Andes. Diploma Thesis, Georg-August University Göttingen, Göttingen, Germany, 2010. Available online: http://armoniabolivia.org/wp-content/uploads/2016/07/01_2010-Meyer-DiplomArbeit-GoettingenUniversity.pdf (accessed on 9 February 2021).
- Guedes, N.M.R.; Seixas, G.H.F. Métodos para estudos de reprodução de Psitacídeos. In Ecologia e Conservação de Psitacídeos no Brasil; Melopsittacus Publicações Científicas: Belo Horizonte, Brasil, 2020; pp. 123–140. [Google Scholar]
- Antas, P.; Fundação Pró-Natureza (Funatura), Brasilia, Brazil. 2020; Unpublished data.
- Powell, G.; Wright, P.; Aleman, U.; Guindon, C.; Palminteri, S.; Bjork, R. Research Findings and Conservation Recommendations for the Great Green Macaw (Ara Ambigua) in Costa Rica; Centro Cientifico Tropica: San Jose, Costa Rica, 1999. [Google Scholar]
- Brightsmith, D.J.; Hobson, E.A.; Martinez, G. Food availability and breeding season as predictors of geophagy in Amazonian parrots. IBIS 2018, 160, 112–129. [Google Scholar] [CrossRef] [Green Version]
- Le Sage, K.; (Geotrak, Raleigh, NC, USA). Personal communication, 2018.

| Total 95% AKDE (ha) | Wet 95% AKDE (ha) | Dry (Barba Azul) 95% AKDE (ha) | Core Wet 70% AKDE (ha) | Core Dry 70% AKDE (ha) | |
|---|---|---|---|---|---|
| ARGL1 | 325,851 (221,430–450,124) | 4587 (3551–5773) | 22,593 (14,723–32,122) | 1292 (1000–1620) | 8415 (5484–11,963) |
| ARGL3 | 118,970 (79,393–166,441) | 2068 (1729–2436) | 27,066 (5484–11,963) | 506 (423–597) | 12,551 (6078–21,332) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davenport, L.C.; Boorsma, T.; Carrara, L.; Antas, P.d.T.Z.; Faria, L.; Brightsmith, D.J.; Herzog, S.K.; Soria-Auza, R.W.; Hennessey, A.B. Satellite Telemetry of Blue-Throated Macaws in Barba Azul Nature Reserve (Beni, Bolivia) Reveals Likely Breeding Areas. Diversity 2021, 13, 564. https://doi.org/10.3390/d13110564
Davenport LC, Boorsma T, Carrara L, Antas PdTZ, Faria L, Brightsmith DJ, Herzog SK, Soria-Auza RW, Hennessey AB. Satellite Telemetry of Blue-Throated Macaws in Barba Azul Nature Reserve (Beni, Bolivia) Reveals Likely Breeding Areas. Diversity. 2021; 13(11):564. https://doi.org/10.3390/d13110564
Chicago/Turabian StyleDavenport, Lisa C., Tjalle Boorsma, Lucas Carrara, Paulo de Tarso Zuquim Antas, Luciene Faria, Donald J. Brightsmith, Sebastian K. Herzog, Rodrigo W. Soria-Auza, and A. Bennett Hennessey. 2021. "Satellite Telemetry of Blue-Throated Macaws in Barba Azul Nature Reserve (Beni, Bolivia) Reveals Likely Breeding Areas" Diversity 13, no. 11: 564. https://doi.org/10.3390/d13110564
APA StyleDavenport, L. C., Boorsma, T., Carrara, L., Antas, P. d. T. Z., Faria, L., Brightsmith, D. J., Herzog, S. K., Soria-Auza, R. W., & Hennessey, A. B. (2021). Satellite Telemetry of Blue-Throated Macaws in Barba Azul Nature Reserve (Beni, Bolivia) Reveals Likely Breeding Areas. Diversity, 13(11), 564. https://doi.org/10.3390/d13110564






