Taxonomy, Distribution and Life Cycle of the Maghrebian Endemic Rhithrogena sartorii (Ephemeroptera: Heptageniidae) in Algeria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Molecular Analyses
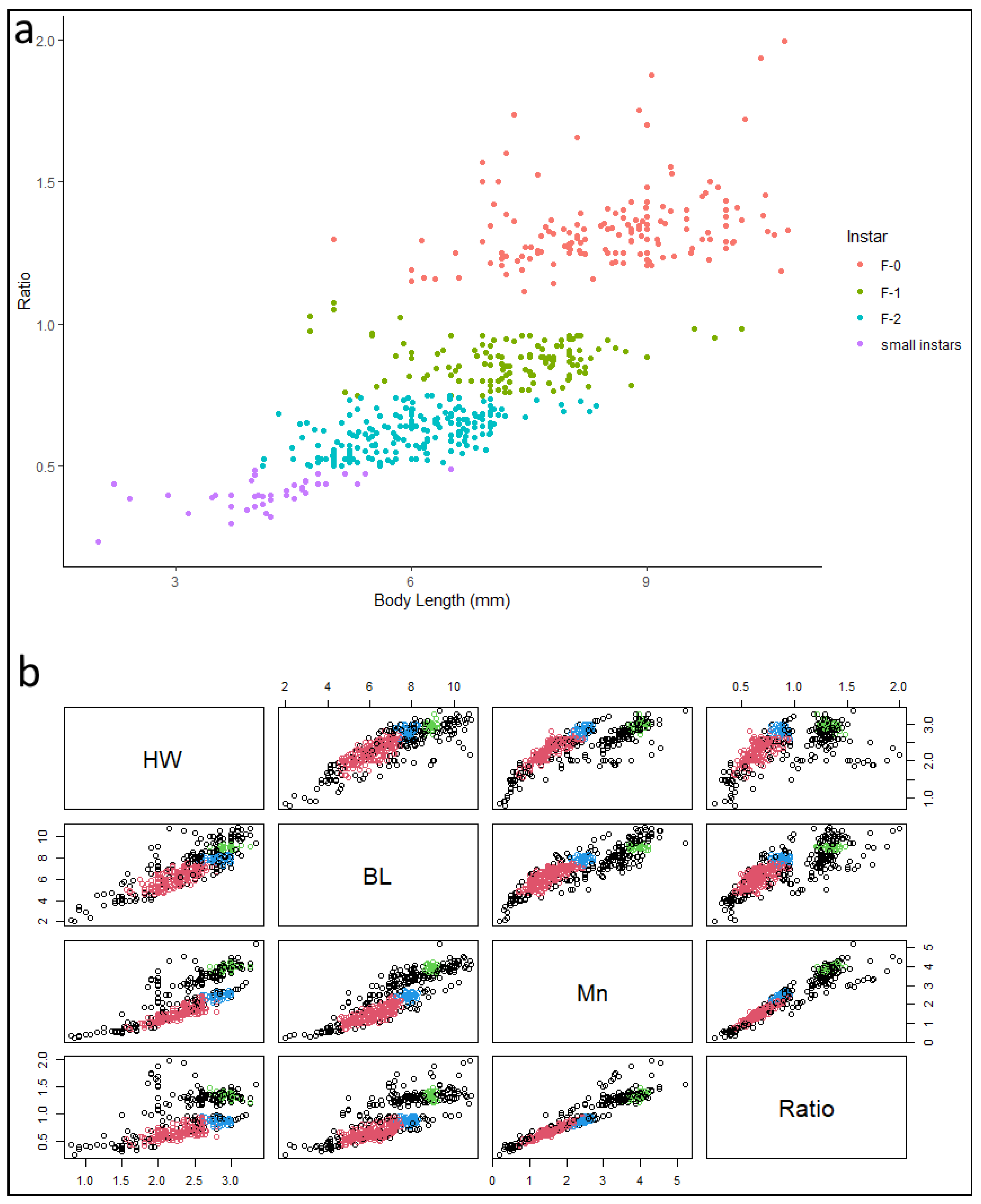
2.4. Morphometry
2.5. Statistical Analysis

3. Results
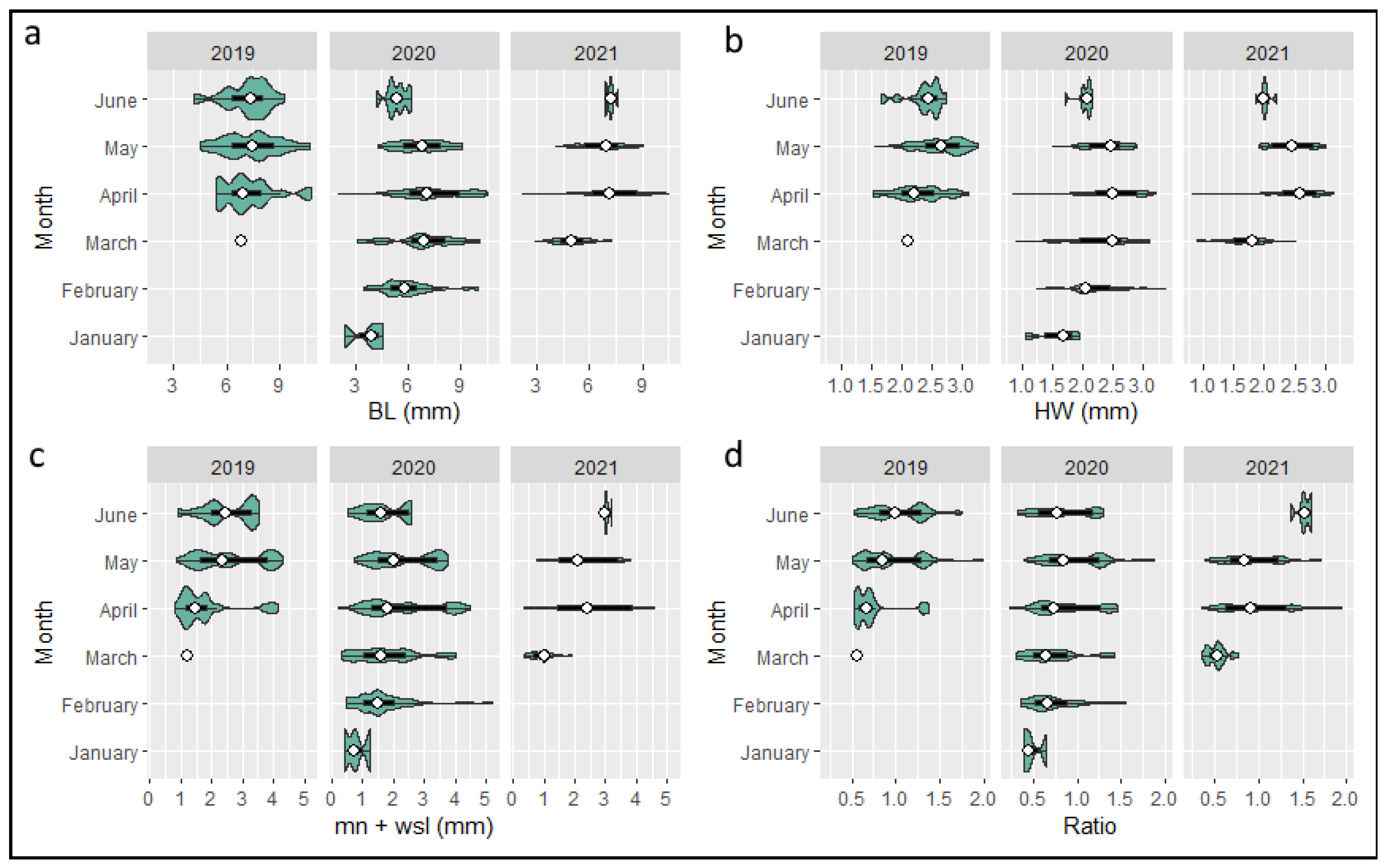
3.1. Distribution and Phenology
3.2. Taxonomy
3.3. Molecular Analyses

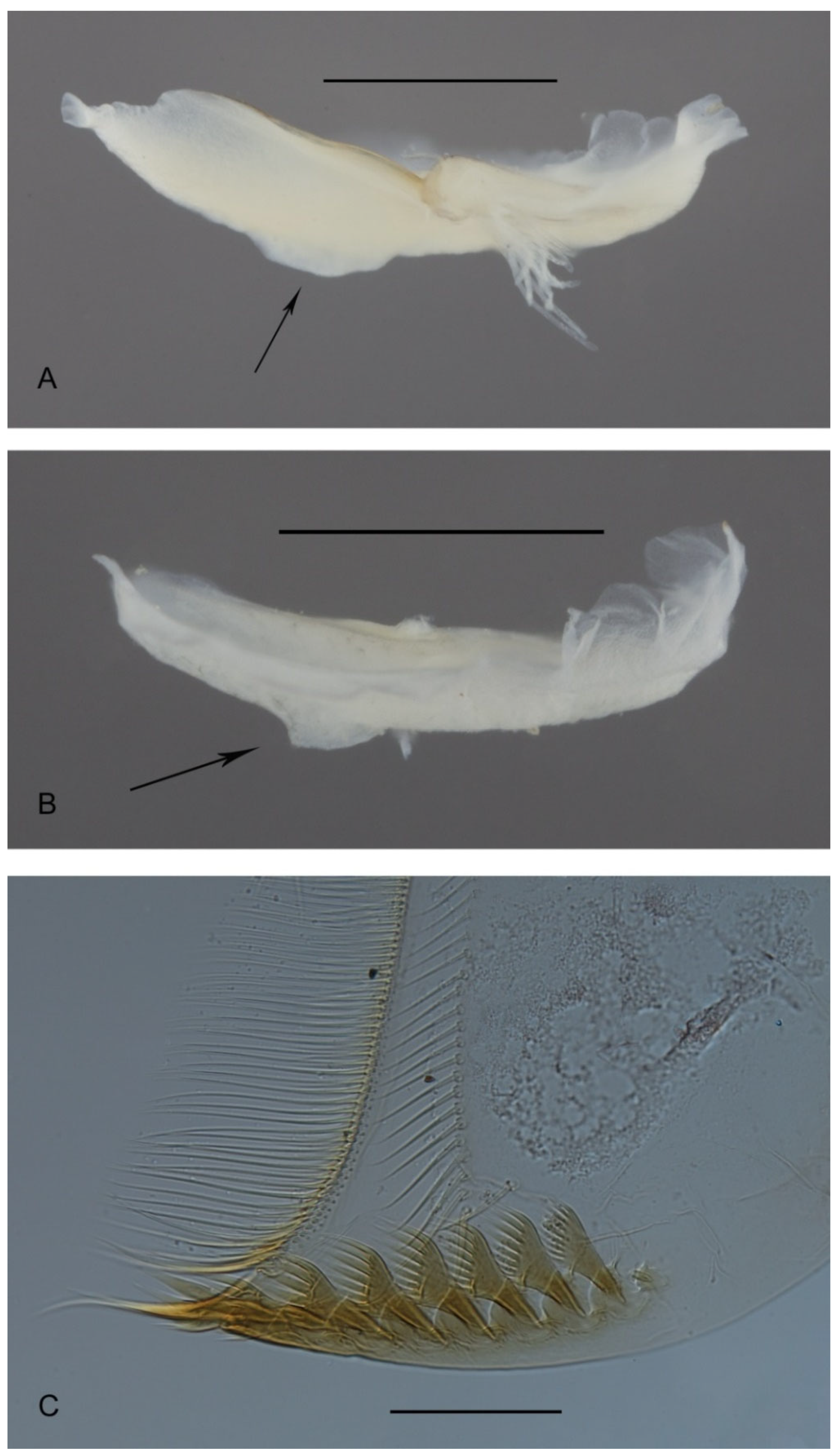
3.4. Morphometry
3.5. Life Cycle
4. Discussion
4.1. Distribution
4.2. Taxonomy
4.3. Eaton’s Rhithrogena
4.4. Life Cycle
4.5. Conservation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barber-James, H.M.; Gattolliat, J.-L.; Sartori, M.; Hubbard, M.D. Global diversity of mayflies (Ephemeroptera, Insecta) in freshwater. Hydrobiologia 2007, 595, 339–350. [Google Scholar] [CrossRef]
- Webb, J.M.; McCafferty, W.P. Heptageniidae of the world. Part II. Key to the genera. Can. J. Arthropod Identif. 2008, 7, 1–55. [Google Scholar]
- Jacobus, L.M.; Macadam, C.R.; Sartori, M. Mayflies (Ephemeroptera) and their contributions to ecosystem services. Insects 2019, 10, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuataz, L.; Sartori, M.; Wagner, A.; Monaghan, M.T. Toward a DNA taxonomy of Alpine Rhithrogena (Ephemeroptera: Heptageniidae) using a mixed yule-coalescent analysis of mitochondrial and nuclear DNA. PLoS ONE 2011, 6, e19728. [Google Scholar] [CrossRef] [Green Version]
- Vuataz, L.; Rutschmann, S.; Monaghan, M.T.; Sartori, M. Molecular phylogeny and timing of diversification in Alpine Rhithrogena (Ephemeroptera: Heptageniidae). BMC Evol. Biol. 2016, 16, 194. [Google Scholar] [CrossRef] [Green Version]
- Sowa, R. Contribution à la connaissance des espèces européennes de Rhithrogena Eaton (Ephemeroptera, Heptageniidae) avec le rapport particulier aux espèces des Alpes et des Carpates. In Proceedings of the Fourth International Conference on Ephemeroptera, Bechyne, Czechia, 4–10 September 1983; Landa, V., Soldán, T., Tonner, M., Eds.; CSAV: Bechyne, Czechia, 1984; pp. 37–52. [Google Scholar]
- Zurwerra, A.; Metzler, M.; Tomka, I. Biochemical systematic and evolution of the European Heptageniidae (Ephemeroptera). Arch. Hydrobiol. 1987, 109, 481–510. [Google Scholar]
- Eaton, A.E. List of Ephemeridae hitherto observed in Algeria with localities. Ent. Mon. Mag. 1899, 35, 4–5. [Google Scholar]
- Thomas, A.G.; Mohati, A. Rhithrogena ourika n. sp., Ephéméroptère nouveau du Haut Atlas marocain (Heptageniidae). Ann. Limnol.-Int. J. Limnol. 1985, 21, 145–148. [Google Scholar] [CrossRef] [Green Version]
- Dakki, M.; Thomas, A.G. Rhithrogena ayadi n. sp., Ephéméroptère nouveau du Moyen Atlas marocain (Heptageniidae). Ann. Limnol.-Int. J. Limnol. 1986, 22, 27–29. [Google Scholar] [CrossRef]
- Thomas, A.G.B.; Bouzidi, A. Trois Ephéméroptères nouveaux du Haut Atlas marocain (Heptageniidae, Baetidae, Leptophlebidae). Bull. Soc. Hist. Nat. Toulouse 1986, 122, 7–11. [Google Scholar]
- Thomas, A.G.; Vitte, B.; Soldán, T. Rhithrogena ryszardi n. sp., Ephéméroptère nouveau du Moyen Atlas (Maroc) et redescription de Rh. soteria Navás, 1917 (Heptageniidae). Ann. Limnol.-Int. J. Limnol. 1987, 23, 169–177. [Google Scholar] [CrossRef]
- Vitte, B. Rhithrogena mariae n. sp. Ephéméroptère nouveau du Rif marocain (Ephemeroptera, Heptageniidae). Nouv. Rev. D’Entomologie 1991, 8, 89–96. [Google Scholar]
- Zrelli, S.; Sartori, M.; Bejaoui, M.; Boumaiza, M. Rhithrogena sartorii, a new mayfly species (Ephemeroptera: Heptageniidae) from North Africa. Zootaxa 2011, 3139, 63–68. [Google Scholar] [CrossRef]
- Samraoui, B.; Márquez-Rodríguez, J.; Ferreras-Romero, M.; El-Serehy, H.A.; Samraoui, F.; Sartori, M.; Gattolliat, J. Biogeography, ecology, and conservation of mayfly communities of relict mountain streams, north-eastern Algeria. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021. [Google Scholar] [CrossRef]
- Samways, M.J. Insect Conservation Biology; Chapman & Hall: London, UK, 1984. [Google Scholar]
- Samraoui, B.; Márquez-Rodríguez, J.; Ferreras-Romero, M.; Sartori, M.; Gattolliat, J.-L.; Samraoui, F. Life history and ecology of the Maghrebian endemic Choroterpes atlas Soldán & Thomas, 1983 (Ephemeroptera: Leptophlebiidae). Limnologica 2021, 89, 125887. [Google Scholar] [CrossRef]
- Bouhala, Z.; Márquez-Rodríguez, J.; Chakri, K.; Samraoui, F.; El-Serehy, H.A.; Ferreras-Romero, M.; Samraoui, B. The life history of the Ibero-Maghrebian endemic Oligoneuriopsis skhounate Dakki and Guidicelli (Ephemeroptera: Oligoneuriidae). Limnologica 2020, 81, 125761. [Google Scholar] [CrossRef]
- Bouhala, Z.; Márquez-Rodríguez, J.; Chakri, K.; Samraoui, F.; El-Serehy, H.A.; Ferreras-Romero, M.; Samraoui, B. The life cycle of the Maghrebian endemic Ecdyonurus rothschildi Navás, 1929 (Ephemeroptera: Heptageniidae) and its potential importance for environmental monitoring. Limnology 2021, 22, 17–26. [Google Scholar] [CrossRef]
- Samraoui, B.; Bouhala, Z.; Chakri, K.; Márquez-Rodríguez, J.; Ferreras-Romero, M.; El-Serehy, H.A.; Samraoui, F.; Sartori, M.; Gattolliat, J.-L. Environmental determinants of mayfly assemblages in the Seybouse River, north-eastern Algeria (Insecta: Ephemeroptera). Biologia 2021, 76, 2277–2289. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2—A multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Stecher, G.; Tamura, K.; Kumar, S. Molecular Evolutionary Genetics Analysis (MEGA) for macOS. Mol. Biol. Evol. 2020, 37, 1237–1239. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Tennessen, K. A method for determining stadium number of late stage Dragonfly Nymphs (Odonata: Anisoptera). Èntomol. News 2017, 126, 299–306. [Google Scholar] [CrossRef]
- Ester, M.; Kriegel, H.P.; Sander, J.; Xu, X. A density-based algorithm for discovering clusters in large spatial databases with noise. In Proceedings of the 2nd International Conference on Knowledge Discovery and Data Mining KDD-96, Portland, OR, USA, 2–4 August 1996; pp. 226–231. [Google Scholar]
- Hahsler, M.; Piekenbrock, M.; Doran, D. dbscan: Fast Density-Based Clustering with R. J. Stat. Softw. 2019, 91, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Team, R.C. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Bauernfeind, E.; Soldan, T. The Mayflies of Europe (Ephemeroptera); Apollo Books: Ollerup, Denmark, 2012. [Google Scholar]
- Sartori, M.; Hughes, S.J. Description of a peculiar Rhithrogena nymph from the Iberian Peninsula (Ephemeroptera, Heptageniidae). Limnetica 2007, 26, 415–434. [Google Scholar]
- Ball, S.L.; Hebert, P.D.N.; Burian, S.K.; Webb, J.M. Biological identifications of mayflies (Ephemeroptera) using DNA barcodes. J. North Am. Benthol. Soc. 2005, 24, 508–524. [Google Scholar] [CrossRef]
- Webb, J.M.; Jacobus, L.M.; Funk, D.H.; Zhou, X.; Kondratieff, B.; Geraci, C.J.; DeWalt, R.E.; Baird, D.J.; Richard, B.; Phillips, I.; et al. A DNA barcode library for North American Ephemeroptera: Progress and prospects. PLoS ONE 2012, 7, e38063. [Google Scholar] [CrossRef] [PubMed]
- Cardoni, S.; Tenchini, R.; Ficulle, I.; Piredda, R.; Simeone, M.C.; Belfiore, C. DNA barcode assessment of Mediterranean mayflies (Ephemeroptera), benchmark data for a regional reference library for rapid biomonitoring of freshwaters. Biochem. Syst. Ecol. 2015, 62, 36–50. [Google Scholar] [CrossRef]
- Morinière, J.; Hendrich, L.; Balke, M.; Beermann, A.J.; König, T.; Hess, M.; Koch, S.; Müller, R.; Leese, F.; Hebert, P.D.N.; et al. A DNA barcode library for Germany′s mayflies, stoneflies and caddisflies (Ephemeroptera, Plecoptera and Trichoptera). Mol. Ecol. Resour. 2017, 17, 1293–1307. [Google Scholar] [CrossRef]
- Lubini, V.; Sartori, M. Current status, distribution, life cycle and ecology of Rhithrogena germanica Eaton, 1885 in Switzerland: Preliminary results (Ephemeroptera, Heptageniidae). Aquat. Sci. 1994, 56, 388–397. [Google Scholar] [CrossRef]
- Demarteau, B. Rhithrogena germanica (Eaton, 1885) nouvelle espèce pour la faune belge (Ephemeroptera: Heptageniidae). Bull. Soc. R. Belg. d’Entomol. 2015, 151, 243–249. [Google Scholar]
- Sweeney, B.W. Factors influencing life-history patterns of aquatic insects. In The Ecology of Aquatic Insects; Resh, V.H., Rosenberg, D.M., Eds.; Praeger: New York, NY, USA, 1984; pp. 56–100. [Google Scholar]
- Brittain, J.E.; Campbell, I.C. The effect of temperature on egg development in the Australian mayfly genus Coloburiscoides (Ephemeroptera: Coloburiscidae) and its relationship to distribution and life history. J. Biogeogr. 1991, 18, 231. [Google Scholar] [CrossRef]
- Humpesch, U.H.; Elliott, J.M. Effect of temperature on the hatching time of eggs of three Rhithrogena spp. (Ephemeroptera) from Austrian Streams and an English Stream and River. J. Anim. Ecol. 1980, 49, 643. [Google Scholar] [CrossRef]
- Knispel, S.; Sartori, M.; Brittain, J.E. Egg development in the mayflies of a Swiss glacial floodplain. J. N. Am. Benthol. Soc. 2006, 25, 430–443. [Google Scholar] [CrossRef]
- Clifford, H.F. Life cycles of mayflies (Ephemeroptera), with special reference to voltinism. Quaest. Entomol. 1982, 18, 15–90. [Google Scholar]
- Sowa, R. Ecology and biogeography of mayflies (Ephemeroptera) of running waters in the Polish part of the Carpathians, 1. Distribution and quantitative analysis. Acta Hydrobiol. 1975, 17, 223–297. [Google Scholar]
- Thibault, M.; Valdes, H.; Bosviel, A.; Vignes, J.-C. Le développement des éphéméroptères d’un ruisseau à truites des Pyrénées-Atlantiques, le lissuraga. Ann. Limnol.-Int. J. Limnol. 1971, 7, 53–120. [Google Scholar] [CrossRef]
- Neveu, A.; Lapchin, L.; Vignes, J.C. Le macrobenthos de la basse Nivelle, petit fleuve côtier des Pyrennées-Atlantiques. Ann. Zool. Ecol. Anim. 1979, 11, 1293–1370. [Google Scholar]
- Bauernfeind, E.; Moog, O. Mayflies (Insecta: Ephemeroptera) and the assessment of ecological integrity: A methodological approach. Assess. Ecol. Integr. Run. Waters 2000, 422, 71–83. [Google Scholar] [CrossRef]
- Brittain, J.E.; Sartori, M. Ephemeroptera. In Encyclopedia of Insects; Elsevier: Amsterdam, The Netherlands, 2009; pp. 328–334. [Google Scholar]
- Benslimane, N.; Chakri, K.; Haiahem, D.; Guelmami, A.; Samraoui, F.; Samraoui, B. Anthropogenic stressors are driving a steep decline of hemipteran diversity in dune ponds in north-eastern Algeria. J. Insect Conserv. 2019, 23, 475–488. [Google Scholar] [CrossRef]
- Morghad, F.; Samraoui, F.; Touati, L.; Samraoui, B. The times they are a changin’: Impact of land-use shift and climate warming on the odonate community of a Mediterranean stream over a 25-year period. Vie Milieu 2019, 69, 25–33. [Google Scholar]
- Zrelli, S.; Boumaïza, M.; Béjaoui, M.; Gattolliat, J.-L.; Sartori, M. New data and revision of the Ephemeroptera of Tunisia. Inland Water Biol. Suppl. 2016, 3, 99–106. [Google Scholar]
- Korbaa, M.; Ferreras-Romero, M.; Bejaoui, M.; Boumaiza, M. Two species of Odonata newly recorded from Tunisia. Afr. Èntomol. 2014, 22, 291–296. [Google Scholar] [CrossRef]


| GBIF Code | Country | Locality | Latitude | Longitude | Date | GenBank ID | Source |
|---|---|---|---|---|---|---|---|
| GBIFCH00671210 | Tunisia | Ennour | 36.8018 | 8.6568 | 28.IV.2010 | LN868554 | Vuataz et al. (2016) |
| GBIFCH00671211 | Tunisia | Ennour | 36.8018 | 8.6568 | 28.IV.2010 | MZ433256 | This study |
| GBIFCH00671212 | Tunisia | Ennour | 36.8018 | 8.6568 | 28.IV.2010 | MZ433257 | This study |
| GBIFCH00671213 | Tunisia | Ennour | 36.8018 | 8.6568 | 28.IV.2010 | MZ433258 | This study |
| GBIFCH00673108 | Algeria | Guitna inf | 36.6379 | 8.3652 | 06.VI.2019 | MZ433260 | This study |
| GBIFCH00673114 | Algeria | Guitna inf | 36.6181 | 8.3462 | 06.VI.2019 | MZ433259 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samraoui, B.; Vuataz, L.; Sartori, M.; Gattolliat, J.-L.; Al-Misned, F.A.; El-Serehy, H.A.; Samraoui, F. Taxonomy, Distribution and Life Cycle of the Maghrebian Endemic Rhithrogena sartorii (Ephemeroptera: Heptageniidae) in Algeria. Diversity 2021, 13, 547. https://doi.org/10.3390/d13110547
Samraoui B, Vuataz L, Sartori M, Gattolliat J-L, Al-Misned FA, El-Serehy HA, Samraoui F. Taxonomy, Distribution and Life Cycle of the Maghrebian Endemic Rhithrogena sartorii (Ephemeroptera: Heptageniidae) in Algeria. Diversity. 2021; 13(11):547. https://doi.org/10.3390/d13110547
Chicago/Turabian StyleSamraoui, Boudjéma, Laurent Vuataz, Michel Sartori, Jean-Luc Gattolliat, Fahad A. Al-Misned, Hamed A. El-Serehy, and Farrah Samraoui. 2021. "Taxonomy, Distribution and Life Cycle of the Maghrebian Endemic Rhithrogena sartorii (Ephemeroptera: Heptageniidae) in Algeria" Diversity 13, no. 11: 547. https://doi.org/10.3390/d13110547
APA StyleSamraoui, B., Vuataz, L., Sartori, M., Gattolliat, J.-L., Al-Misned, F. A., El-Serehy, H. A., & Samraoui, F. (2021). Taxonomy, Distribution and Life Cycle of the Maghrebian Endemic Rhithrogena sartorii (Ephemeroptera: Heptageniidae) in Algeria. Diversity, 13(11), 547. https://doi.org/10.3390/d13110547







