Abstract
Floral micromorphology and pollen morphology of two Leonurus (Lamiaceae) species were examined and compared using scanning electron microscopy to evaluate the diagnostic value of these features to facilitate future studies on floral biology and taxonomy. Floral epidermal patterns were similar in both species, with the conical to central-conical epidermal cells on the adaxial side of the lower lip (corolla). Capitate, peltate, scale-like glandular, and non-glandular trichomes were distributed on the surface of the floral organs. Notably, scale-like anther glands and floral stomata were found on the anthers and abaxial side of the calyx, respectively. Pollen grains had bi-reticulate exine with angular primary lumina and rounded secondary lumina. These characteristics provide indirect evidence of a close association between plant-pollinator interactions and effective pollination. In addition, quantitative traits of pollen grains and trichome types on the adaxial side of the lip differed between the two species. These characteristics may have diagnostic and taxonomic value for the genus Leonurus and family Lamiaceae.
1. Introduction
Flower micromorphological characteristics are closely associated with plant–pollinator interactions [1]. For instance, the petals (or corolla) of various angiosperm species may promote pollinator attraction through visual, tactile, and olfactory clues [1,2,3]. Conical or papillose petal epidermal cells may improve light absorption, influencing brightness and color intensity [4,5], increasing the landing grip of pollinating insects to the petal [6,7], and facilitating the diffusion of scent-producing volatile substances [1,8].
Floral trichomes, especially glandular trichomes, are connected with plant-insect interactions. Gland-secreted secondary metabolic compounds such as aromatic compounds, essential oils or floral volatile scents act directly on the attraction of pollinators or defense against herbivory [9,10,11,12,13]. Several functions of anther glands have also been reported, such as containing adherent substances to aggregate pollen grains and attach them to the pollinator body [14,15,16,17,18], being rewarded as food to the pollinator, and containing substances for defense against phytophagous insects [15]. Moreover, floral stomata may act in the exudation of nectar [19,20] or emission of volatile organic compounds [21,22]. Thus, the interactions between plants and insects/pollinators can be inferred based on floral micromorphology.
In Lamiaceae Martinov, floral micromorphology has received less attention than leaves, pollen, fruit, or seeds, which are more commonly used for taxonomic and systematic study. The floral epidermis has been observed using scanning electron microscopy in the following genera or species: Lycopus L. [23], Lycopus maackianus Makino [24], Glechoma L. [25], and Glechoma longituba (Nakai) Kuprian. [26]. Recently, studies on the micromorphology of Lamiaceae focused mainly on the structure and distribution of leaf trichomes [27,28,29,30,31,32,33,34] and on the taxonomic implications of pollen morphology [27,32,35,36,37,38,39].
Leonurus L. (motherwort) is a small genus that belongs to the tribe Leonureae Dumort., subfamily Lamioideae Harley, family Lamiaceae. It comprises 25 species worldwide [40,41], distributed mostly in the temperate, subtropical, and tropical regions of Eurasia. In Eastern Asia, 2–22 species are reported [42,43,44], with two species in Korea, L. japonicus Houtt. and L. macranthus Maxim., which are distributed in open areas and grasslands of all provinces [44]. The Korean Herbal Pharmacopoeia has designated the dried aerial part of L. japonicus (medicinal name: ‘Leonuri Herba’) as an herbal medicine [45,46].
Previous studies on the reproductive biology of Leonurus species reported that their flowers were visited by various insects—L. japonicus: honey bee (Apis mellifera Linnaeus, 1758) and bumblebees [Bombus opifex Smith, 1879, B. morio (Swederus, 1787), and B. bellicosus Smith, 1879] [47]; L. sibiricus L.: bumblebees (Bombus opifex, B. morio, and B. bellicosus) [48]; and L. cardiaca L.: the same as L. japonicus and ladybird beetles, adult blowfly, cabbage white butterfly [Pieris rapae (Linnaeus, 1758)], and European paper wasp [Polistes dominula (Christ, 1791)] [49,50]. However, floral micromorphological and pollen morphological characteristics of Leonurus species have not been approached in this genus.
Therefore, the objectives of the present study were to (1) document and illustrate a detailed description of the micromorphology of various floral organs (calyx, lip, anther, filament, style, and stigma) and pollen (pollen grain and orbicule) of both Leonurus species native to Korea, using field emission scanning electron microscopy (FE-SEM); (2) explore their pollinator-related micromorphological characters as an entomophilous species; and (3) evaluate the taxonomic significance of floral and pollen micromorphology for future studies on the systematics and floral biology of Leonurus.
2. Materials and Methods
2.1. Study Species
Floral samples of two populations of each species (Figure 1) were collected in Korea. The living plants samples were preserved in FAA solution (40% formalin: 40% glacial acetic acid: 70% ethyl alcohol). Voucher specimens were deposited in the Korean Herbarium of Standard Resources, Korean Institute of Oriental Medicine (KIOM). Digital images with collection information are provided in Figure S1 and Table S1.
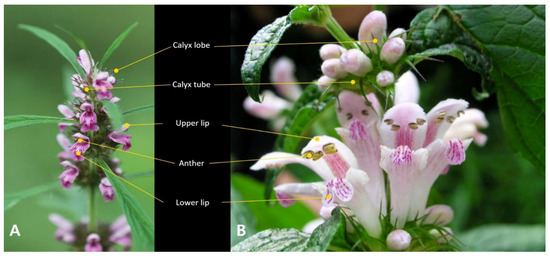
Figure 1.
Inflorescences of the two Korean Leonurus species. (A) L. japonicus. (B) L. macranthus.
2.2. Microscopic Observations and Cell Type Classification
For floral and pollen micromorphology observations, flowers were first examined using a stereomicroscope (SM, Olympus SZX16, Olympus, Tokyo, Japan) to identify fully open mature flowers. For scanning electron microscopic observations, dried materials from herbarium specimens were rehydrated overnight using the wetting agent Agepon® (Agfa Gevaert, Leverkusen, Germany) and distilled water (1:200) at 37 °C, and then dehydrated through an ethanol series (50%, 70%, 90%, 95%, and 100% ethanol; 1 h each) at room temperature. These materials were immersed in liquid carbon dioxide (CO2) for critical point drying (CPD, SPI-13200J-AB, SPI Supplies, West Chester, PA, USA). The dried floral organs and anthers were fixed on aluminum stubs with a double-adhesive carbon disk (05073-BA; SPI Supplies, West Chester, PA, USA). For pollen and orbicule observations, the loculi of the anthers were carefully opened using fine needles prepared from cactus spines, and pollen grains were expelled from the open loculi. All mounted samples were coated with gold using an ion-sputtering device (208HR; Cressington Scientific Instruments Ltd., Watford, UK) and observed using a low-voltage field emission scanning electron microscope (JSM-7600F; JEOL, Tokyo, Japan) at an accelerating voltage of 5–10 kV and working distance of 8–10 mm [51,52]. All scanned images were saved in the original 8-bit tagged image file format (TIFF) of size 1280 × 1024 pixels with 300 DPI resolution. Selected images are herein presented with modifications to image features such as contrast, brightness, exposure, and lightness using the image-editing software Adobe Photoshop® CC 2019 (Adobe Systems® Inc, San Jose, CA, USA).
To investigate the micromorphology of L. japonicus and L. macranthus, and confirm the consistency of characteristics, mature flowers with anthers from all four samples of each species (i.e., two individuals and two collection sites) were selected. The distribution of the epidermal cell types was recorded and compared among the central part of the calyx lobe and tube, upper and lower lips, filament, stigma, and style in both taxa. Moreover, 20 fully developed pollen grains were randomly selected from each sample (total of 80 pollen grains) for measurement and analysis using Digimizer version 5.4.3 (MedCalc Software, Mariakerke, Belgium). An independent two-sample t-test was performed to test the differences in quantitative pollen data between the two species, using R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
Epidermal cell types and pollen terminology followed Barthlott [53,54] and Punt et al. [55], respectively.
3. Results
Floral samples of two natural populations of L. japonicus and L. macranthus were collected in Korea (Figure 1). Details of floral epidermal cell surface ornamentation and pollen morphology are summarized in Table 1 and Table 2, respectively, and Figure 1, Figure 2, Figure 3, Figure 4 and Figure 5. Moreover, details of trichome diversity, including distribution and density, are provided in Table 3 and Figure 6.

Table 1.
Details of the floral epidermal cell surface ornamentation in the two Leonurus species.

Table 2.
Details of pollen morphological characteristics in the two Leonurus species [minimum–(average)–maximum; sample size n = 80; P, significance of difference; N/A, not applicable; * P < 0.05; *** P < 0.001].
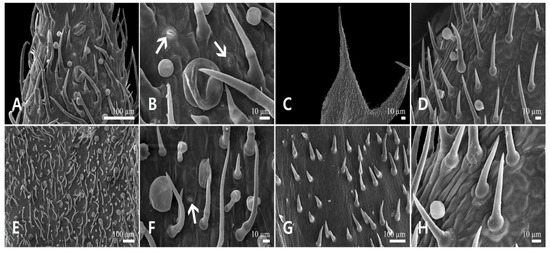
Figure 2.
Scanning electron micrographs of the calyx epidermis in Leonurus japonicus. (A,B) Abaxial side of the calyx lobe. (C,D) Adaxial side of the calyx lobe. (E,F) Abaxial side of the calyx tube. (G,H) Adaxial side of the calyx tube. Arrows indicate the stomata.
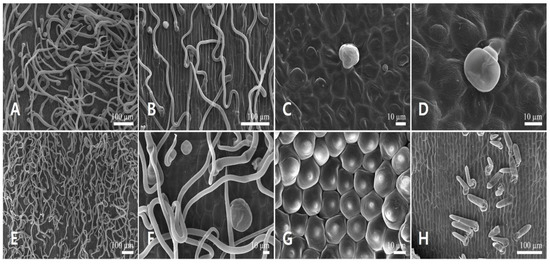
Figure 3.
Scanning electron micrographs of the corolla epidermis in Leonurus japonicus. (A,B) Abaxial side of the upper lip. (C,D) Adaxial side of the upper lip. (E,F) Abaxial side of the lower lip. (G,H) Adaxial side of the lower lip.
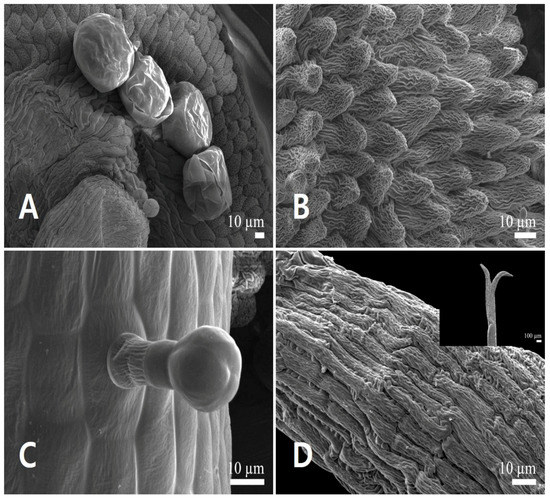
Figure 4.
Scanning electron micrographs of the androecium and gynoecium in Leonurus japonicus. (A,B) Anther. (A) Anther glands. (B) Conical epidermal cells. (C) Capitate stalked glandular trichome on the filament. (D) Stigma surface (bilobate stigmas in detail).
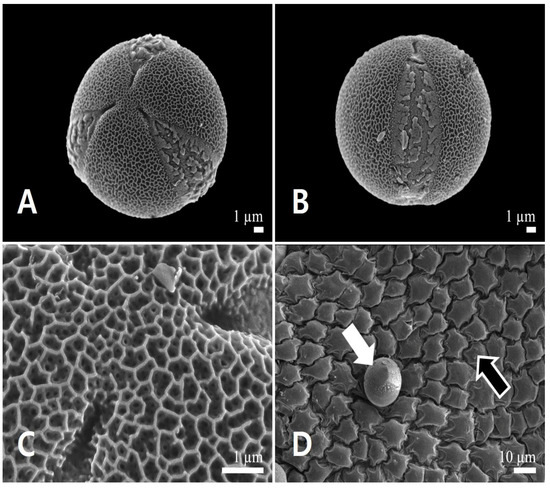
Figure 5.
Scanning electron micrographs of pollen and the inner locule wall in Leonurus japonicus. (A) Polar view of a pollen grain. (B) Equatorial view of a pollen grain. (C) Exine surface ornamentation. (D) Inner locule wall. White arrow indicates pollen grain, and black arrow indicates the inner locule.

Table 3.
Distribution patterns of trichomes and stomata on the floral organs of the two Leonurus species (● = dominance; ◎ = presence; ○ = absence).
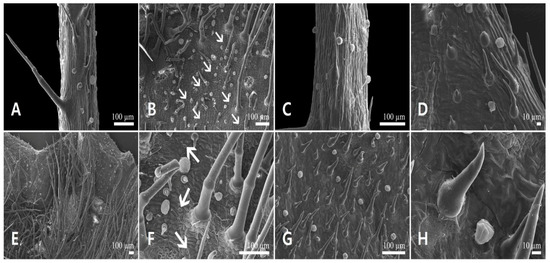
Figure 6.
Scanning electron micrographs of the calyx epidermis in Leonurus macranthus. (A,B) Abaxial side of the calyx lobe. (C,D) Adaxial side of the calyx lobe. (E,F) Abaxial side of the calyx tube. (G,H) Adaxial side of the calyx tube. Arrows indicate floral stomata.
3.1. Leonurus japonicus Houtt
3.1.1. Floral Micromorphology
Calyx lobe epidermal cells were isodiametric, elongated or polygonal in shape, depressed, with straight to curved anticlinal wall (AW), and convex outer periclinal wall (PW) with finely striated relief (FR) in the abaxial (Figure 2A,B) and adaxial side (Figure 2C,D). On both sides of the calyx tube, epidermal cells were similar to those on the calyx lobes; however, the epidermal cells were polygonal rather than elongated (Figure 2E–H). Stomata were found on the abaxial side of the calyx lobe and tube (Figure 2B,F).
Epidermal cells of the abaxial side of the upper lip were isodiametric, elongated to polygonal, with depressed and straight to curved AW, and convex PW with smooth to striated FR (Figure 3A,B). However, the cells of the adaxial side were more rounded and curved with convex to central-conical PW (Figure 3C,D). Epidermal cells of the abaxial side of the lower lip were isodiametric, polygonal, with depressed and straight to curved AW, and convex to central-conical PW with smooth to striated FR (Figure 3E,F). However, the cells of the adaxial side were more rounded, and their PW was more projected-conical to central-conical and rugulate FR (Figure 3G). The smooth-surfaced short simple trichomes were distributed on the basal part of the adaxial side of the lower lip (Figure 3H).
All epidermal cells of the anther were isodiametric, polygonal with depressed, sinuous AW (Figure 4A,B). The outer PW of their adaxial and abaxial sides were convex (Figure 4A), whereas the apical ones were conical with a papillose surface (Figure 4B). The epidermal cells of the filament were isodiametric, elongated to tetragonal with depressed, straight AW, and convex PW with smooth to striated FR (Figure 4C). The bilobate stigmas, consisting of stigmatoid tissues, had irregular, polygonal cells, raised straight AW, and flat to convex PW with striated FR (Figure 4D). The style surface was similar to that of the stigma, but with isodiametric epidermal cells.
3.1.2. Pollen Micromorphology
Pollen grains were monads and small (polar axis 15.98 ± 0.90 μm, equatorial axis 17.06 ± 0.97 μm). Polar outlines were almost circular (Figure 5A) and oblate-spheroidal to prolate-spheroidal (P/E = 0.88–1.03). All pollen grains were radially symmetric, isopolar, and tricolpate (Figure 5A,B). The simple colpi were symmetrically distributed. The colpus length was 15.33 ± 1.63 μm. Exine ornamentation was bi-reticulate (Figure 5C). Lumina of the primary reticulum (0.38 ± 0.07 μm) were angular, and more than four times larger in diameter than those of rounded secondary ones (0.09 ± 0.02 μm). The primary muri (0.07 ± 0.01 μm) were more than twice as thin as the secondary muri (0.12 ± 0.02 μm). The number of secondary lumina was 4.78 ± 1.32 per primary lumen (Table 2). The inner side of the anther (inner locule wall) was smooth; no orbicules were observed (Figure 5D).
3.2. Leonurus macranthus Maxim
3.2.1. Floral Micromorphology
Calyx lobe epidermal cells were isodiametric, elongated or polygonal in shape, depressed, with straight to curved AW, and convex outer PW with striated FR on the abaxial (Figure 6A,B) and adaxial side (Figure 6C,D). On both sides of the calyx tube, epidermal cells were similar to those on the calyx lobes, but their cells were polygonal rather than elongated (Figure 6E–H). Stomata were present on the abaxial side of calyx lobe and tube (Figure 6B,F).
Epidermal cells of the abaxial side of the upper lip were isodiametric, polygonal with depressed and straight to curved AW, and convex PW with smooth FR (Figure 7A,B). However, those on the adaxial side were more rounded and curved with convex PW, and striated FR (Figure 7C,D). Epidermal cells of the abaxial side of the lower lip were isodiametric, polygonal with depressed and straight to curved AW, and convex PW with smooth FR (Figure 7E,F). However, those on the adaxial side were more rounded, and their PW was conical to central-conical with rugulate FR (Figure 7G). The smooth-surfaced short simple trichomes were basally distributed on the adaxial side of the lower lip (Figure 7H).
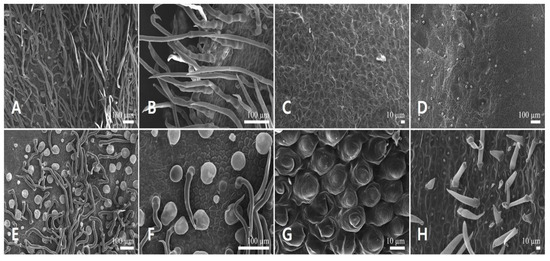
Figure 7.
Scanning electron micrographs of the corolla epidermis in Leonurus macranthus. (A,B) Abaxial side of the upper lip. (C,D) Adaxial side of the upper lip. (E,F) Abaxial side of the lower lip. (G,H) Adaxial side of the lower lip.
All epidermal cells of the anther were isodiametric, polygonal with depressed, sinuous AW (Figure 8A,B). The outer PW of the adaxial and abaxial sides was convex (Figure 8A), whereas that of the apical part was conical (Figure 8B). The epidermal cells of the filament were isodiametric, elongated to tetragonal with depressed, straight AW, and convex PW with smooth to striated FR (Figure 8C). The bilobate stigmas had irregular, polygonal cells and raised, straight AW, flat to convex PW with striated FR (Figure 8D). Style surface was similar to that of the stigma, but with isodiametric epidermal cells.
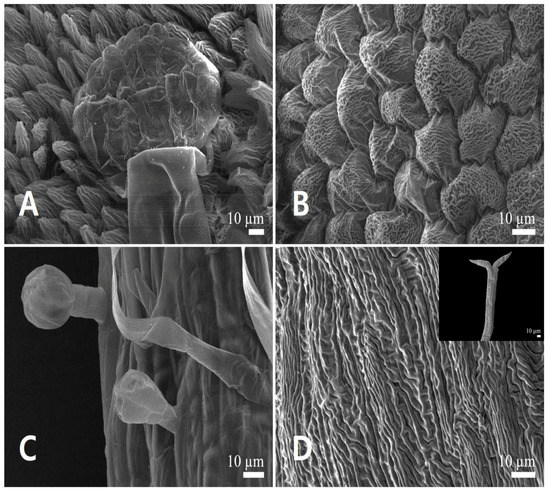
Figure 8.
Scanning electron micrographs of the androecium and gynoecium in Leonurus macranthus. (A,B) Anther. (A) Anther glands. (B) Conical epidermal cells. (C) Capitate stalked glandular trichomes and smooth-surfaced long simple trichome on the filament. (D) Stigma surface (bilobate stigmas in detail).
3.2.2. Pollen Micromorphology
Pollen grains were monads and small-sized (polar axis 23.55 ± 1.31 μm, equatorial axis 20.66 ± 1.22 μm). Polar outlines were almost circular (Figure 9A), spherical to subprolate (P/E = 1.06–1.22). All pollen grains were radially symmetric, isopolar, and tricolpate (Figure 9A,B). The simple colpi were symmetrically distributed. The colpus length was 21.27 ± 1.47 μm. Exine ornamentation was bi-reticulate (Figure 9C). Lumina of the primary reticulum (0.67 ± 0.18 μm) were angular, with diameter more than five times larger than those of rounded secondary ones (0.12 ± 0.02 μm). The primary muri (0.14 ± 0.02 μm) were almost of the same thickness as the secondary muri (0.14 ± 0.04 μm). The number of secondary lumina was 6.35 ± 1.73 per primary lumen (Table 2). The inner side of the anther was smooth; no orbicules were observed (Figure 9D).
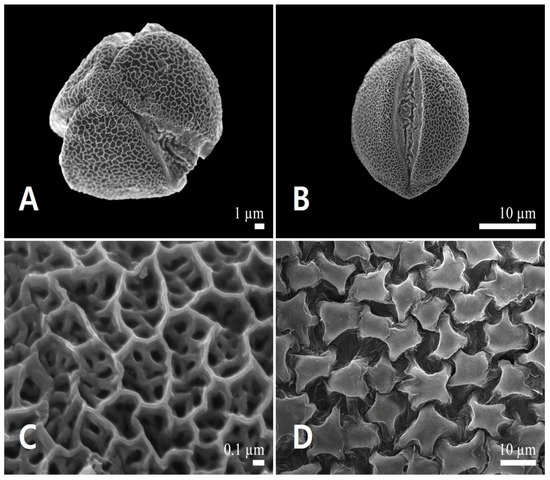
Figure 9.
Scanning electron micrographs of pollen and the inner locule wall in Leonurus macranthus. (A) Polar view of a pollen grain. (B) Equatorial view of a pollen grain. (C) Exine surface ornamentation. (D) Inner locule wall.
3.3. Floral Trichome Diversity
Trichome characteristics, such as distribution, type, length, density, and surface ornamentation, were verified. Three basic types of trichomes were observed on the calyx lobe and tube, upper and lower lips, and filament: capitate stalked glandular trichomes, peltate glandular trichomes, and non-glandular simple trichomes. Scale-like glandular trichomes were found only on the anther.
3.3.1. Glandular Trichomes
Three shapes of glandular trichomes were identified: capitate stalked, peltate, and scale-like glandular trichomes. Capitate stalked glandular trichomes (C, Figure 2A,B,D–F,H and Figure 3C,D) had one (Figure 3C and Figure 10D), three (Figure 10B), four (Figure 3D), or eight cells (Figure 5D), a 10–20 μm diameter head, and a short stalk cell up to 20 μm in height. Their head surface ornamentation could be smooth. They were distributed on the calyces, except on the adaxial side of the tube, abaxial side of the lip, and the filament in L. japonicus and L. macranthus. However, on the adaxial side of the upper lip, this type of trichome was observed only in L. japonicus (Table 3).
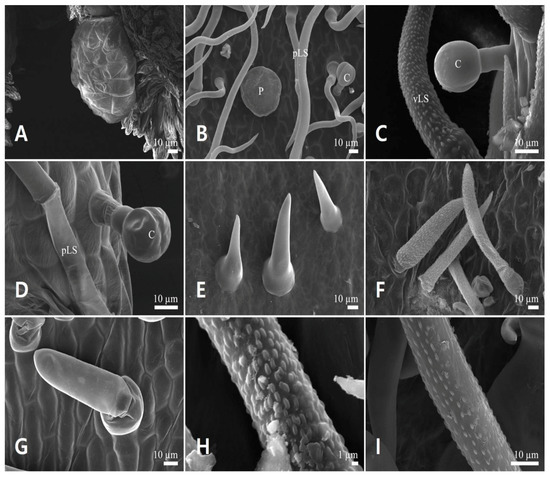
Figure 10.
Floral trichome diversity in the two Leonurus species. (A,B,F,I) Leonurus macranthus. (C–E,G,H) Leonurus japonicus. (A) Scale-like multicellular glandular trichome. (B) Four-celled capitate stalked glandular trichome, peltate glandular trichome, and smooth-surfaced long simple trichomes. (C) Capitate stalked glandular trichome and verrucate-surfaced long simple trichome. (D) Capitate stalked glandular trichome and smooth-surfaced long simple trichome. (E,G) Smooth-surfaced short simple trichomes. (F) Verrucate-surfaced short simple trichomes. (H,I) Verrucate-surfaced trichomes. C, capitate stalked glandular trichome; pLS, smooth-surfaced long simple trichome; P, peltate glandular trichome; vLS, verrucate-surfaced long simple trichome.
Peltate glandular trichomes (P, Figure 10B) had one cell, a 40–80 μm diameter head, and a smooth surface. They were distributed on the abaxial side of the calyx, and on the upper and lower lips in L. japonicus and L. macranthus. However, on the adaxial side of the calyx lobe, this type of trichome was observed in L. japonicus (Table 3).
Scale-like glandular trichomes (S, Figure 10A) had a multi-cellular (up to 24 cells) gland of 68.7 ± 6.35 μm and 100.9 ± 7.17 μm diameter in L. japonicus and L. macranthus, respectively. Moreover, a fragile straight line was observed on the horizontal diametrical region of the head (Figure 4A). These trichomes were distributed on the abaxial sides of anthers in L. japonicus and L. macranthus (Table 3).
3.3.2. Non-Glandular Trichomes
These trichomes were subdivided into four types based on their surface and length: (1) smooth-surfaced short simple trichomes (pSS, up to 100 μm), (2) smooth-surfaced long simple trichomes (pLS, more than 100 μm), (3) verrucate-surfaced short simple trichomes (vSS, up to 100 μm), and (4) verrucate-surfaced long simple trichomes (vLS, more than 100 μm). The distribution patterns of non-glandular trichomes were different between the two species. Leonurus japonicus had smooth-surfaced trichomes (pSS and pLS) on the adaxial side of the calyx and lower lip, whereas L. macranthus had verrucate-surfaced trichomes (vSS and vLS) on the adaxial side of the calyx lobe and lower lip. The distribution pattern of vSS on the calyx was also different.
4. Discussion
Previous studies have shown the existence of specific petal epidermal types related to specific pollinators [1,2,3,4,6,56,57,58]. The petals of melittophilous species, for instance, have conical cells on the adaxial epidermis [3,59], suggesting the existence of a mechanical interaction between the pollinators and the conical cells of melittophilous petals. The non-slippery surface (as a tactile clue) offers stable landing and movement on the petals to pollinators searching for nectar and pollen grains [6,7,60,61]. Our results corroborate these previous studies as conical to central-conical epidermal cells were found on the lower lip’s adaxial side in L. japonicus [47] and L. macranthus (Song, personal observ.), which are well-known melittophilous species. However, the location of the conical to central-conical epidermal cells varies between these species: in L. japonicus, they are on both sides of the lower lip and adaxial side of the upper lip, whereas in L. macranthus they are on the adaxial side of the lower lip. These petal cellular patterns may be related to their different corolla size, color, and pollinators. L. japonicus has 0.9–1.5 cm long petals with a reddish to purplish-red corolla and a 4–8 mm long calyx, whereas L. macranthus has 1.8–2.5 cm long petals with a whitish to reddish-purple corolla and a 1.1–1.5 cm long calyx [44]. Apis mellifera and bumblebees, the most frequent pollinators of L. japonicus, used both surfaces of the lower lip for fixation to the small lip (Figure 1B,C of Galetto & Torres [47]). In our field survey, we found that the flower of L. macranthus was frequently visited by butterflies, which used the adaxial surfaces of the lower lip and long calyx; thus, this species might be melittophilous and psychophilous (Song, personal observ.). These different pollinator and visitor patterns of the two species might be related to their different cellular pattern on petals. Comprehensive monitoring, including of generalist, specialist, and simple visitors is necessary to verify additional details concerning floral biology and their adaptations for pollination.
Six types of floral trichomes were found on the calyx and lips of the two Leonurus species. Trichome diversity and density were higher on the abaxial side of the calyx and lip than on the adaxial side. Non-glandular trichomes are traditionally considered a physical protection against biotic and abiotic stress such as low humidity, high temperatures, sun radiation [62], and damage from herbivores [63,64]. Thus, the high density of non-glandular and glandular trichomes, especially on the abaxial side of the calyx and lip, may have several roles in the two Leonurus species, including physical protection and deterrence of herbivory. Recent investigations have also suggested that edible floral non-glandular trichomes, which contain starch, may act as a food reward for visitors and pollinators [65,66], and that most lamiacean plants produce and store aromatic compounds, essential oils, or floral scents—which may increase the attractiveness of flowers to insects [67,68,69,70,71]—in glandular trichomes, especially the peltate and capitate glands on the calyx and corolla [23,30,67,68,69]. Thus, field surveys and histochemical studies are necessary to explore whether the floral non-glandular trichomes of Leonurus can be provided as a food material to pollinators and to describe secondary metabolic compounds.
The functions of anther glands are related to pollinator attraction or defense against herbivory, which depends on their metabolic compounds [9,10]. However, anther glands rarely occur in angiosperms (Leite et al. [18] and references therein). In Lamiaceae, the anther glands of L. sibiricus were reported to secrete an adhesive substance that facilitates pollen transfer by pollinators [48]. In fact, the acquisition of anther glands has been suggested to have occurred in the very early stages of evolution in Lamiaceae, and this feature may be more frequent in this family than is currently known [48]. We herein confirmed the presence of anther glands (scale-like glandular trichome) in the two Leonurus species, possibly with a function similar to that of L. sibiricus. Ultrastructural and histochemical analysis of capitate, peltate, and scale-like anther glands are required for detailing the composition of their secretion. Moreover, further studies are necessary to evaluate the variation of anther glands in Lamiaceae in a phylogenetic context.
Notably, stomata were present exclusively on the abaxial surface of the calyx in the two Leonurus species. Several studies have proposed an association between the emission of volatile compounds and floral stomata, typically located on the abaxial surface of petals and/or sepals [22,72,73]. Although the present study does not provide evidence of a direct relationship between floral scent and floral stomata, the presence of stomata in calyx epidermis suggests their function in attracting pollinators.
The micromorphological structures of floral parts (anther, filament, style, and stigma) were highly similar in the two Leonurus species investigated herein. However, quantitative characteristics of the pollen grains and the size of anther glands were significantly different (all non-overlapping data) between the two species. Moreover, each species exhibited different trichome types on the lower lip’s adaxial side. Similar to previous studies [74,75,76,77,78], observations of the present study contribute to the knowledge on the diversity of pollen and trichome micromorphology in lamiacean plants. Nevertheless, further studies are necessary to evaluate species-level variation in pollen and floral trichome morphology in Leonurus based on the taxonomic and phylogenetic context, with an expanded and targeted sampling strategy.
5. Conclusions
Our study is the first to observe floral micromorphology using scanning electron microscopy to explore pollinator-related characters in two Korean Leonurus. Our micromorphological analysis provides additional cases of entomophilous species with conical epidermal cells on the adaxial lip. The presence of conical to central-conical epidermal cells, floral trichomes, including peltate and capitate glands, anther glands, and floral stomata, may be essential for interactions with pollinators. Moreover, the taxonomic applicability of pollen grains, floral trichomes, and anther glands was verified. The findings of this study have potential taxonomic value for the genus Leonurus and family Lamiaceae, and will contribute to further studies on floral biology of Lamiaceae.
Supplementary Materials
The following materials are available online at https://www.mdpi.com/article/10.3390/d13110533/s1, Figure S1: Digital image of voucher specimen of Leonurus species that are examined in the present study, Table S1: Voucher information of Leonurus species that are examined in the present study.
Author Contributions
Conceptualization, M.-K.O. and J.-H.S.; methodology, M.-K.O. and J.-H.S.; software, M.-K.O. and J.-H.S.; validation, S.Y. and G.C.; formal analysis, M.-K.O. and J.-H.S.; investigation, M.-K.O. and J.-H.S.; resources, S.Y., G.C. and J.-H.S.; data curation, M.-K.O.; writing—original draft preparation, M.-K.O. and J.-H.S.; writing—review and editing, S.Y., G.C. and J.-H.S.; visualization, M.-K.O. and S.Y.; supervision, J.-H.S.; project administration, J.-H.S.; funding acquisition, J.-H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2020R1A2C1100147) to J.-H. Song and the Korea Institute of Oriental Medicine, Naju, South Korea (grant number KSN2013320).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no competing interest or conflict of interest.
References
- Whitney, H.M.; Bennett, K.V.; Dorling, M.; Sandbach, L.; Prince, D.; Chittka, L.; Glover, B.J. Why do so many petals have conical epidermal cells? Ann. Bot. 2011, 108, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Glover, B.J.; Martin, C. The role of petal cell shape and pigmentation in pollination success in Antirrhinum majus. Heredity 1998, 80, 778–784. [Google Scholar] [CrossRef]
- Costa, V.B.S.; Pimentel, R.M.M.; Chagas, M.G.S.; Alves, G.D.; Castro, C.C. Petal micromorphology and its relationship to pollination. Plant Biol. 2017, 19, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Kay, Q.O.N.; Daoud, H.S.; Stirton, C.H. Pigment distribution, light reflection and cell structure in petals. Bot. J. Linn. Soc. 1981, 83, 57–83. [Google Scholar] [CrossRef]
- Gkikas, D.; Argiropoulos, A.; Rhizopoulou, S. Epidermal focusing of light and modelling of reflectance in floral-petals with conically shaped epidermal cells. Flora 2015, 212, 38–45. [Google Scholar] [CrossRef]
- Keven, P.G.; Lane, M.A. Flower petal microtexture is a tactile cue for bees. Proc. Natl. Acad. Sci. USA. 1985, 82, 4750–4752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitney, H.M.; Chittka, L.; Bruce, T.J.; Glover, B.J. Conical epidermal cells allow bees to grip flowers and increase foraging efficiency. Curr. Biol. 2009, 19, 948–953. [Google Scholar] [CrossRef] [Green Version]
- Kolosova, N.; Sherman, D.; Karlson, D.; Dudareva, N. Cellular and subcellular localization of S-adenosyl-l-methionine: Benzoic acid carboxyl methyltransferase, the enzyme responsible for biosynthesis of the volatile ester methylbenzoate in snapdragon flowers. Plant Physiol. 2001, 126, 956–964. [Google Scholar] [CrossRef] [Green Version]
- Fahn, A. Secretory tissues in plants. New Phytol. 1988, 108, 229–257. [Google Scholar] [CrossRef]
- Palo, R.T.; Robbins, C.T. Plant Defenses against Mammalian Herbivory; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Ferreira, J.F.; Janick, J. Floral morphology of Artemisia annua with special reference to trichomes. Int. J. Plant Sci. 1995, 156, 807–815. [Google Scholar] [CrossRef]
- Mithöfer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mithöfer, A.; Boland, W.; Maffei, M.E. Chemical ecology of plant–insect interactions. Annu. Plant Rev. 2018, 34, 261–291. [Google Scholar] [CrossRef]
- Endress, P.K. Floral structure and evolution of primitive angiosperms: Recent advances. Plant Syst. Evol. 1994, 192, 79–97. [Google Scholar] [CrossRef]
- Luckow, M.; Grimes, J. A survey of anther glands in the mimosoid legume tribes Parkieae and Mimoseae. Am. J. Bot. 1997, 84, 285–297. [Google Scholar] [CrossRef] [PubMed]
- de Barros, T.C.; Teixeira, S.P. Revisited anatomy of anther glands in mimosoids (Leguminosae). Int. J. Plant Sci. 2016, 177, 18–33. [Google Scholar] [CrossRef]
- de Barros, T.C.; Pedersoli, G.D.; Teixeira, S.P. Anther glands in Mimosoideae (Leguminosae) are emergences with a conserved meristematic origin. Flora 2017, 226, 1–9. [Google Scholar] [CrossRef]
- Leite, V.G.; Mansano, V.F.; Pansarin, E.R.; Teixeira, S.P. Presence of the anther gland is a key feature in pollination of the early-branching papilionoids Dipteryx alata and Pterodon pubescens (Leguminosae). Plant Biol. 2019, 21, 1016–1023. [Google Scholar] [CrossRef]
- Davies, K.L.; Stpiczyńska, M.; Gregg, A. Nectar-secreting floral stomata in Maxillaria anceps Ames & C. Schweinf. (Orchidaceae). Ann. Bot. 2005, 96, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Subedi, A.; Chaudhary, R.P.; van Achterberg, C.; Heijerman, T.; Lens, F.; Van Dooren, T.J.; Gravendeel, B. Pollination and protection against herbivory of Nepalese Coelogyninae (Orchidaceae). Am. J. Bot. 2011, 98, 1095–1103. [Google Scholar] [CrossRef] [Green Version]
- Hadacek, F.; Weber, M. Club-shaped organs as additional osmophores within the Sauromatum inflorescence: Odour analysis, ultrastructural changes and pollination aspects. Plant Biol. 2002, 4, 367–383. [Google Scholar] [CrossRef]
- Song, J.H.; Hong, S.P. Identity and localization of floral scent components in an androdioecious species, Chionanthus retusus (Oleaceae). J. Asia-Pac. Biodivers. 2020, 13, 288–294. [Google Scholar] [CrossRef]
- Moon, H.K.; Hong, S.P. The taxonomic consideration of petal and sepal micromorphology in Lycopus L. (Mentheae-Lamiaceae). Korean J. Pl. Taxon. 2004, 34, 273–285. [Google Scholar] [CrossRef]
- Hong, S.P.; Moon, H.K. Gynodioecy in Lycopus maackianus Makino (Lamiaceae) in Korea: Floral dimorphism and nutlet production. Flora 2003, 198, 461–467. [Google Scholar] [CrossRef]
- Jang, T.S.; Lee, J.; Hong, S.P. A systematic study of Glechoma L. (Lamiaceae) based on micromorphological characters and nuclear ribosomal ITS sequences. Korean J. Pl. Taxon. 2014, 44, 22–32. [Google Scholar] [CrossRef]
- Jang, T.S.; Hong, S.P. Floral micromorphology and microsporogenesis of the gynodioecious herb Glechoma longituba (Lamiaceae). Nord. J. Bot. 2015, 33, 708–714. [Google Scholar] [CrossRef]
- Atalay, Z.; Celep, F.; Bara, F.; Doğan, M. Systematic significance of anatomy and trichome morphology in Lamium (Lamioideae; Lamiaceae). Flora 2016, 225, 60–75. [Google Scholar] [CrossRef]
- Santos Tozin, L.R.D.; de Melo Silva, S.C.; Rodrigues, T.M. Non-glandular trichomes in Lamiaceae and Verbenaceae species: Morphological and histochemical features indicate more than physical protection. N. Z. J. Bot. 2016, 54, 446–457. [Google Scholar] [CrossRef]
- Eiji, S.; Salmaki, Y. Evolution of trichomes and its systematic significance in Salvia (Mentheae; Nepetoideae; Lamiaceae). Bot. J. Linn. Soc. 2016, 180, 241–257. [Google Scholar] [CrossRef] [Green Version]
- Haratym, W.; Weryszko-Chmielewska, E. Ultrastructural and histochemical analysis of glandular trichomes of Marrubium vulgare L. (Lamiaceae). Flora 2017, 231, 11–20. [Google Scholar] [CrossRef]
- Mannethody, S.; Purayidathkandy, S. Trichome micromorphology and its systematic significance in Asian Leucas (Lamiaceae). Flora 2018, 242, 70–78. [Google Scholar] [CrossRef]
- Gul, S.; Ahmad, M.; Zafar, M.; Bahadur, S.; Sultana, S.; Begum, N.; Shah, S.N.; Zaman, W.; Ullah, F.; Ayaz, A.; et al. Taxonomic study of subfamily Nepetoideae (Lamiaceae) by palynomorphological approach. Microsc. Res. Tech. 2019, 82, 1021–1031. [Google Scholar] [CrossRef]
- de Almeida, V.P.; Raman, V.; Raeski, P.A.; Urban, A.M.; Swiech, J.N.; Miguel, M.D.; Farago, P.V.; Khan, I.A.; Budel, J.M. Anatomy, micromorphology, and histochemistry of leaves and stems of Cantinoa althaeifolia (Lamiaceae). Microsc. Res. Tech. 2020, 83, 551–557. [Google Scholar] [CrossRef]
- Siadati, S.; Salmaki, Y.; Bräuchler, C. Trichome morphology provides phylogenetically informative signal for generic delimitation in tribe Marrubieae (Lamiaceae). Flora 2020, 273, 151720. [Google Scholar] [CrossRef]
- Ma, Z.; Bramley, G.L.; Zhang, D. Pollen morphology of Callicarpa L. (Lamiaceae) from China and its systematic implications. Plant Syst. Evol. 2016, 302, 67–88. [Google Scholar] [CrossRef]
- Marzouk, R.I.; Salama, M.; Askar, A.B.M. Pollen morphology of Teucrium L. (Lamiaceae, Ajugoideae) in Libya. Bangladesh J. Plant Taxon. 2017, 24, 219–226. [Google Scholar] [CrossRef]
- Doaigey, A.R.; El-Zaidy, M.; Alfarhan, A.; Milagy, A.E.S.; Jacob, T. Pollen morphology of certain species of the family Lamiaceae in Saudi Arabia. Saudi J. Biol. Sci. 2018, 25, 354–360. [Google Scholar] [CrossRef]
- Bedolla-Garcia, B.Y.; Castro-Morales, M.; Cultid-Medina, C.A. Comparative assessment of pollen micromorphology of Salvia assurgens (Lamiaceae), an endemic sage from Mexico. Phytotaxa 2020, 458, 183–194. [Google Scholar] [CrossRef]
- Özler, H.; Kahraman, A.; Pehlivan, S.; Dogan, M.; Baser, B.; Fisne, A.Y.; Bagherpour, S. Contribution to the knowledge of the pollen morphology of the genus Salvia (Lamiaceae). Phytotaxa 2020, 428, 228–240. [Google Scholar] [CrossRef]
- Krestovskaja, T. Systematics and phytogeography of Leonurus L. In Advances in Labiate Science; Harley, R.M., Reynolds, R.M., Eds.; Royal Botanic Gardens, Kew: Richmond, UK, 1992; pp. 139–148. [Google Scholar]
- Harley, R.M.; Atkins, S.; Budantsey, A.L.; Cantino, P.D.; Conn, B.J.; Grayer, R.; Harley, M.M.; de Kok, R.; Krestovskaja, T.; Morales, R.; et al. Labiatae. In Families and Genera of Vascular Plants, Flowering Plants: Dicotyledons; Lamiales (except Acanthaceae including Avicenniaceae) Vol. 7; Kubitzki, K., Kadereit, J.W., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 167–275. [Google Scholar]
- Murata, G.; Yamazaki, T. Lamiaceae. In Flora of Japan Vol. 3a; Iwatsuki, K., Yamazaki, T., Boufford, D.E., Ohba, H., Eds.; Kodansha: Tokyo, Japan, 1993; pp. 273–321. [Google Scholar]
- Li, X.W.; Hedge, I.C. Leonurus. In Flora of China Vol. 17; Wu, C.Y., Raven, P.H., Eds.; Science Press and Missouri Botanical Garden Press: St. Louis, UK; Beijing, China, 1994; pp. 162–165. [Google Scholar]
- Park, S.J. Leonurus. In Flora of Korea Asteridae: Loganiaceae to Oleaceae Vol. 6a; Flora of Korea Editorial Committee: Seoul, Korea, 2018; pp. 105–106. ISBN 978-89-6811-334-5. [Google Scholar]
- Korea Food and Drug Administration. The Korean Pharmacophoeia, 12th ed.; Korea Food and Drug Administration: Seoul, Korea, 2019. [Google Scholar]
- Korea Institute of Oriental Medicine. Defining Dictionary for Medicinal Herbs. 2021. Available online: http://https://oasis.kiom.re.kr/herblib/hminfo/hbmcod/hbmcodList.do (accessed on 21 February 2021).
- Galetto, L.; Torres, C. Nectar sugar composition and pollinators for the naturalized exotic Leonurus japonicus (Lamiaceae) in Central Argentina. Int. J. Plant Reprod. Biol. 2010, 2, 1–4. [Google Scholar]
- Moyano, F.; Cocucci, A.; Sersic, A. Accessory pollen adhesive from glandular trichomes on the anthers of Leonurus sibiricus L. (Lamiaceae). Plant Biol. 2003, 5, 411–418. [Google Scholar] [CrossRef]
- Borna, F.; Ahmad, N.M.; Luo, S.; Trethowan, R. Reproductive biology of a medicinally important plant Leonurus cardiaca (Lamiaceae). Aust. J. Bot. 2016, 64, 342–358. [Google Scholar] [CrossRef]
- Shekari, A.; Mahdipour, M.H.; Nazeri, V.; Shokrpour, M. The reproductive biology of motherwort (Leonurus cardiaca L.). J. Biodivers. Environ. Sci. 2018, 12, 109–116. [Google Scholar]
- Park, I.; Yang, S.; Song, J.-H.; Moon, B.C. Dissection for floral micromorphology and plastid genome of valuable medicinal borages Arnebia and Lithospermum (Boraginaceae). Front. Plant Sci. 2020, 11, 606463. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-H.; Hong, S.P. A taxonomic revision of the genus Sorbaria (Rosaceae) with a new infrageneric classification based on morphology, micromorphology, and palynology. Phytotaxa 2021, 487, 1–25. [Google Scholar] [CrossRef]
- Barthlott, W. Epidermal and seed surface characters of plants: Systematic applicability and some evolutionary aspects. Nord. J. Bot. 1981, 1, 345–355. [Google Scholar] [CrossRef]
- Barthlott, W. Scanning electron microscopy of the epidermal surface in plants. In Scanning Electron Microscopy in Taxonomy and Functional Morphology; Claugher, D., Ed.; Clarendon Press: Oxford, UK, 1990; pp. 69–94. [Google Scholar]
- Punt, W.; Hoen, P.P.; Blackmore, S.; Nilsson, S.; Le Thomas, A. Glossary of pollen and spore terminology. Rev. Palaeobot. Palynol. 2007, 143, 1–81. [Google Scholar] [CrossRef]
- Millner, H.J.; Baldwin, T.C. Floral micromorphology of the genus Restrepia (Orchidaceae) and the potential consequences for pollination. Flora 2016, 225, 10–19. [Google Scholar] [CrossRef]
- Ojeda, D.I.; Valido, A.; Fernández de Castro, A.G.; Ortega-Olivencia, A.; Fuertes-Aguilar, J.; Carvalho, J.A.; Santos-Guerra, A. Pollinator shifts drive petal epidermal evolution on the Macaronesian Islands bird-flowered species. Biol. Lett. 2016, 12, 20160022. [Google Scholar] [CrossRef] [Green Version]
- Piwowarczyk, R.; Kasińska, J. Petal epidermal micromorphology in holoparasitic Orobanchaceae and its significance for systematics and pollination ecology. Aust. Syst. Bot. 2017, 30, 48–63. [Google Scholar] [CrossRef]
- Christensen, K.I.; Hansen, H.V. SEM studies of epidermal patterns of petals in the angiosperms. Opera Bot. 1998, 135, 5–91. [Google Scholar]
- Kevan, P.G.; Baker, H.G. Insects as flower visitors and pollinators. Annu. Rev. Entomol. 1983, 28, 407–453. [Google Scholar] [CrossRef]
- Rands, S.A.; Glover, B.J.; Whitney, H.M. Floral epidermal structure and flower orientation: Getting to grips with awkward flowers. Arthropod Plant Interact. 2011, 5, 279–285. [Google Scholar] [CrossRef] [Green Version]
- Werker, E. Trichome diversity and development. Adv. Bot. Res. 2000, 31, 1–35. [Google Scholar] [CrossRef]
- Levin, D.A. The role of trichomes in plant defense. Q. Rev. Biol. 1973, 48, 3–15. [Google Scholar] [CrossRef]
- Dalin, P.; Ågren, J.; Björkman, C.; Huttunen, P.; Kärkkäinen, K. Leaf trichome formation and plant resistance to herbivory. In Induced Plant Resistance to Herbivory; Schaller, A., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 89–105. [Google Scholar]
- Pansarin, E.R.; Maciel, A.A. Evolution of pollination systems involving edible trichomes in orchids. AoB Plants 2017, 9, plx033. [Google Scholar] [CrossRef] [Green Version]
- Lustofin, K.; Świątek, P.; Stolarczyk, P.; Miranda, V.F.; Płachno, B.J. Do food trichomes occur in Pinguicula (Lentibulariaceae) flowers? Ann. Bot. 2020, 126, 1039–1048. [Google Scholar] [CrossRef]
- Kaya, A.; Demirci, B.; Baser, K.H.C. Micromorphology of glandular trichomes of Nepeta congesta Fisch. & Mey. var. congesta (Lamiaceae) and chemical analysis of the essential oils. S. Afr. J. Bot. 2007, 73, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Schmiderer, C.; Grassi, P.; Novak, J.; Weber, M.; Franz, C. Diversity of essential oil glands of clary sage (Salvia sclarea L., Lamiaceae). Plant Biol. 2008, 10, 433–440. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Tozin, L.R.; Rodrigues, T.M. Glandular trichomes in the tree-basil (Ocimum gratissimum L., Lamiaceae): Morphological features with emphasis on the cytoskeleton. Flora 2019, 259, 151459. [Google Scholar] [CrossRef]
- Konarska, A.; Chmielewski, P. Taxonomic traits in the microstructure of flowers of parasitic Orobanche picridis with particular emphasis on secretory structures. Protoplasma 2020, 257, 299–317. [Google Scholar] [CrossRef] [Green Version]
- Raguso, R.A. Functions of essential oils and natural volatiles in plant-insect interactions. In Handbook of Essential Oils. Science, Technology, and Applications, 3rd ed.; Hüsnü Can Başer, K., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 481–496. ISBN 9781351246460. [Google Scholar]
- de Melo, M.C.; Borba, E.L.; Paiva, E.A.S. Morphological and histological characterization of the osmophores and nectaries of four species of Acianthera (Orchidaceae: Pleurothallidinae). Plant Syst. Evol. 2010, 286, 141–151. [Google Scholar] [CrossRef]
- Maiti, S.; Mitra, A. Morphological, physiological and ultrastructural changes in flowers explain the spatio-temporal emission of scent volatiles in Polianthes tuberosa L. Plant Cell Physiol. 2017, 58, 2095–2111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdtman, G. Pollen morphology and plant taxonomy. IV. Labiatae, Verbanaceae and Avicenniaceae. Sven. Bot. Tidskr. 1945, 39, 279–285. [Google Scholar]
- Metcalfe, C.R.; Chalk, L. Anatomy of the Dicotyledons; The Clarendon Press: Oxford, UK, 1950; Volume 1. [Google Scholar]
- Cantino, P.D. The phylogenetic significance of stomata and trichomes in the Labiatae and Verbenaceae. J. Arnold Arbor. 1990, 71, 323–370. [Google Scholar]
- Harley, M.M.; Paton, A.; Harley, R.M.; Cade, P.G. Pollen morphological studies in tribe Ocimeae (Nepetoideae: Labiatae): I. Ocimum L. Grana 1992, 31, 161–176. [Google Scholar] [CrossRef]
- Abu-Asab, M.S.; Cantino, P.D. Systematic implications of pollen morphology in subfamilies Lamioideae and Pogostemonoideae (Labiatae). Ann. Missouri Bot. Gard. 1994, 81, 653–686. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).