Abstract
Hinoki cypress (Chamaecyparis obtusa) shows durability against termites and wood decay-causing fungi and is used as a construction material in Japan. However, the effects of the material are still not fully understood. The aim of this study was to evaluate whether Hinoki cypress has antimicrobial effects against airborne microorganisms. We examined the influence of Hinoki cypress on the growth of airborne bacteria and fungi using culture-based methods. The growth of bacterial colonies was observed after day 3 in the control group without Hinoki material. In contrast, the growth of bacterial colonies was observed after day 13 in the experimental group containing Hinoki material. In the experimental group, the number of fungal colonies was smaller than that in the control group, suggesting the antifungal effect of Hinoki cypress to some extent. In addition, we characterized the community structure of airborne bacteria in two rooms with and without cypress wood by the culture-independent method of PCR-denaturing gradient gel electrophoresis. This also suggested differences in the community structure of airborne bacteria depending on the presence or absence of Hinoki cypress wood. These results indicate that Hinoki cypress might be a useful functional material in building environments.
1. Introduction
Hinoki cypress (Chamaecyparis obtusa) is a conifer tree native to central Japan [1,2]. This species shows durability against termites and wood decay causing-fungi; thus, it has been used since ancient times in Japan as a construction material to build temples and shrines [3,4]. Hinoki is currently used for walls, flooring, bathtubs, furniture, and chopping boards. In addition, the Hinoki cypress is a familiar tree to the public because of its popularity for forest bathing in East Asian countries, including Korea, Japan, and China. Forest bathing trips involve visiting a forest for relaxation and recreation while breathing in volatile substances called phytoncides—volatile organic compounds emitted from trees [5,6]. Chemically, the composition of phytoncides is closely related to essential oils produced by plants. Essential oil from Japanese cypress leaves and woods contain terpenes (α-cadinol, hinokitiol) and phenols, which are known to have antibacterial activity [1,7,8,9,10] and antifungal activity [10,11]. However, it is unknown whether cypress has antimicrobial activity on airborne microorganisms.
Owase cypress produced in the southern part of Mie Prefecture, Japan, is highly valued for its dense annual rings, abundant oil, gloss, and excellent durability. The tree was used to build a table for a roundtable discussion by the seven advanced countries (G7) leaders at the Major Leaders’ Summit (Ise Shima Summit) held in Shima City, Mie Prefecture 26–27 May 2016. The news got much attention. To further develop Owase cypress as a valuable construction material, evaluating the aesthetic appeal and performance of houses built using Owase cypress wood is necessary.
Using denaturing gradient gel electrophoresis (DGGE) to acquire genetic fingerprints without the cultivation of microorganisms was introduced by Muyzer et al. [12]. One fingerprinting technique, DGGE of PCR-amplified 16S rRNA gene segments, allows simultaneous analysis of multiple samples and comparisons of microbial communities based on temporal and spatial variations [13]. Furthermore, PCR-DGGE is cheaper than next-generation sequencing methods, and the data are ready to be observed immediately after electrophoresis [14]. Therefore, DGGE has been used to investigate bacterial community structures in air [15,16,17,18].
The aim of the present study was to evaluate whether Hinoki cypress has antimicrobial activities against airborne microorganisms. To achieve this, we examined the influence of Hinoki cypress on the growth of airborne bacteria and fungi using culture-based methods. Moreover, we characterized the community structure of airborne bacteria in two rooms with and without Hinoki cypress wood by the culture-independent method of PCR-DGGE. Our results provide a foundation for detailed future studies of the antimicrobial effect of Hinoki cypress against airborne microorganisms.
2. Materials and Methods
2.1. Influence of Hinoki Cypress on the Growth of Airborne Microorganisms (Culture Method)
Two adjacent rooms, one containing timbers from Hinoki cypress on the floor and walls (experimental group) and one without cypress material (control group), were prepared on the first floor of Owase City Hall (Figure 1). The cypress wood was cut after drying, planing, and unvarnishing. The two rooms were the same size and floor plan and were not ventilated to prevent the loss of volatiles from the cypress. On the third day after preparing these rooms, airborne bacteria and fungi were collected on agar plates using the BioStage Impactor (SKC Inc., Eighty Four, PA, USA) on the roof of Owase City Hall for 5 min at a flow rate of 14 L min−1 [19]. Bacteria were grown on nutrient agar medium (Becton Dickinson, Sparks, MD, USA) with the antifungal agent cycloheximide (Fujifilm Wako Pure Chemical Corporation, Osaka, Japan) at a final concentration of 50 µg mL−1. Fungi were grown on potato dextrose agar (PDA) medium (Becton Dickinson) with the antibacterial agent chloramphenicol (Fujifilm Wako Pure Chemical Corporation) at a final concentration of 50 µg mL−1. The plates were incubated aerobically at room temperature (19 to 23 °C). For each of these two media, five agar plates were used in each room (ten agar plates in total). The colony-forming unit (CFU) counts in media were performed in the two rooms on days 1, 3, 5, 7, 9, 11, 13, 15, and 17, respectively. The antimicrobial effects of cypress were evaluated by comparing the CFUs on each medium type between the two rooms.

Figure 1.
The appearance of the two rooms where the experiments were conducted. These two rooms were adjacent on the first floor of Owase City Hall, Japan. (A) a room containing timbers from Hinoki cypress on the floor and walls (experimental group); (B) a room without Hinoki cypress material (control group).
2.2. Influence of Hinoki Cypress on Airborne Bacterial Community Structure (PCR-DGGE Method)
This experiment used the same rooms as described above. Indoor air samples of each room were collected at six time points: one day prior to starting the experiment, immediately after delivery of Hinoki cypress, and at days 7, 14, 21, and 28. A vacuum pump collected indoor air samples with 0.2-μm pore size and 47-mm-diameter polycarbonate filters (Advantec, Tokyo, Japan) at a flow rate of 10 L min−1 over 6 h. A total volume of 3600 L of air was sampled. After sampling, the filters were transferred to 15 mL sterile centrifuge tubes and stored at −20 °C until DNA extraction.
The filtered samples were processed using a Power Soil DNA Isolation Kit (MO BIO Laboratories, Carlsbad, CA, USA), following the manufacturer’s instructions. Using this extracted DNA as the template for PCR, the V3 region of the bacterial 16S rRNA gene was amplified by a two-step nested PCR method, and DGGE was carried out using the D-code system (Bio-Rad, Hercules, CA, USA) [15,20,21]. The PCR products (approximately 1 μg) were loaded onto an 8% (w/v) polyacrylamide gel in 0.5× Tris-acetate-EDTA (TAE) buffer. The polyacrylamide gel was made with a denaturing gradient ranging from 25% to 65% (where 100% denaturant contained 7 M urea and 40% formamide). Electrophoresis was performed at a constant voltage of 70 V for 12 h at 60 °C, following which the gel was stained with ethidium bromide and photographed under UV illumination.
The DGGE banding patterns were analyzed by non-metric multidimensional scaling (nMDS) analysis using the metaMDS within vegan package v. 2.5-7 [22] in R package 4.1.0 software [23]. The DGGE gel was digitized with ImageJ 1.53e (U.S. National Institutes of Health, Bethesda, MD, USA).
Each DGGE gel band selected was carefully excised using a sterile Pasteur pipette and placed in 50 µL of sterile water. Each gel was kept overnight at 4 °C to elute the DNA, then the eluate was used as a DNA template and reamplified as described above. The sequences were automatically analyzed with an ABI 3130xl Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) using the BigDye terminator v3.1 cycle sequencing kit (Applied Biosystems). Each DNA sequence was compared with published sequences using NCBI BLAST (http://www.ncbi.nlm.nih.gov, accessed on 30 July 2021). A phylogenetic tree was constructed with the maximum-likelihood method in MEGAX software. The dataset was bootstrapped 1000 times.
3. Results
3.1. Influence of Hinoki Cypress on the Growth of Airborne Microorganisms (Culture Method)
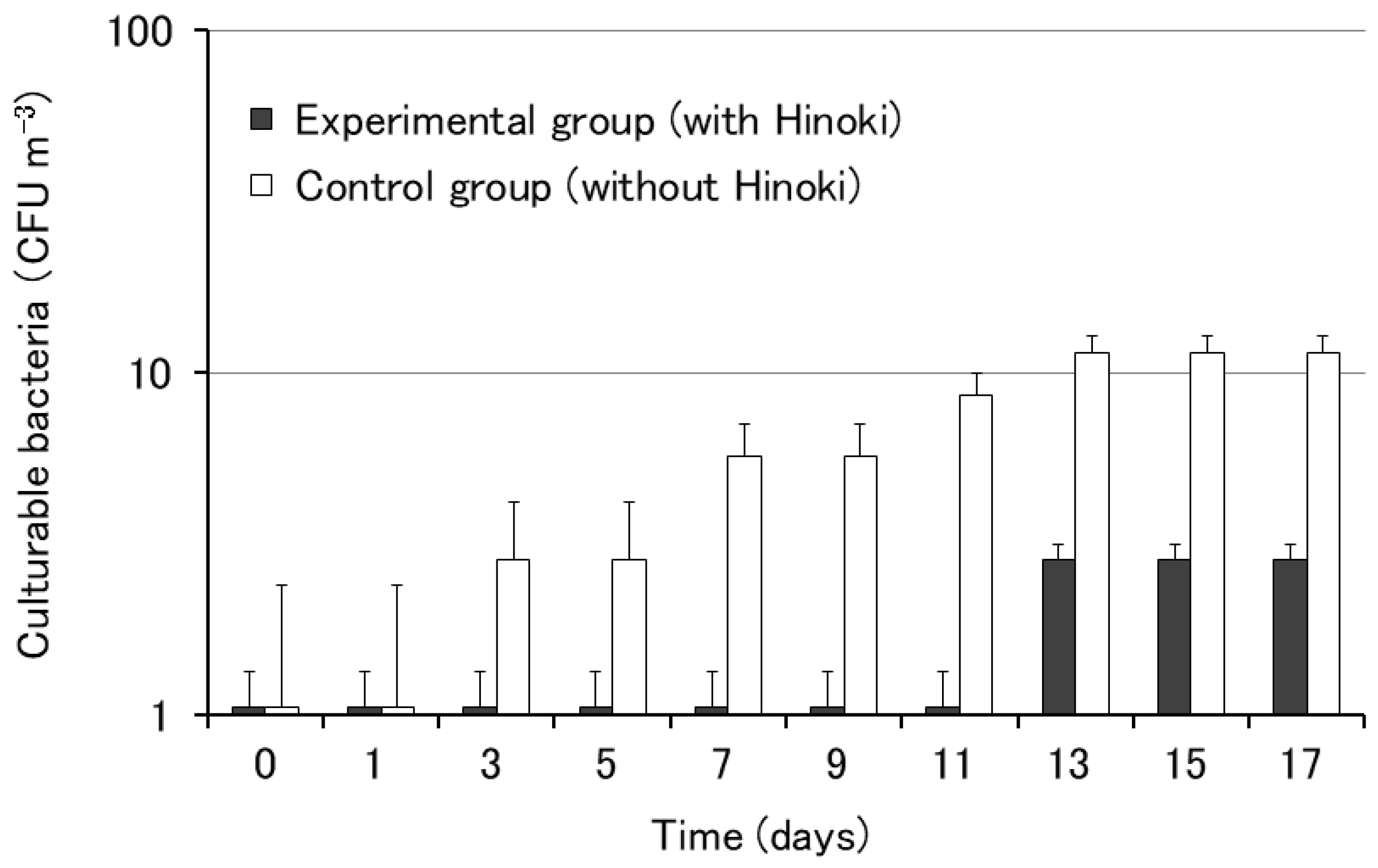
The influence of Hinoki cypress on the growth of airborne bacteria was examined using the rooms of the City Hall. Differences in the growth of airborne bacteria were observed between the rooms with (experimental group) or without (control group) Hinoki cypress material. The growth of bacterial colonies was observed after day 3 in the control group without Hinoki material, whereas the growth of bacterial colonies was observed after day 13 in the experimental group containing Hinoki material (Figure 2). The results of this experiment using the rooms of the City Hall suggested the antibacterial effect of Hinoki cypress against airborne bacteria.
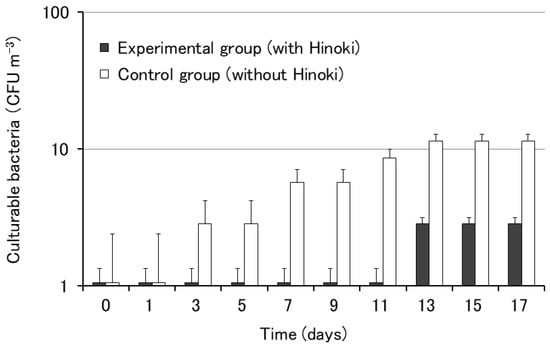
Figure 2.
Growth of airborne bacteria on nutrient agar with and without Hinoki. Each bar represents the mean of five agar plates. The error bars indicate standard errors.
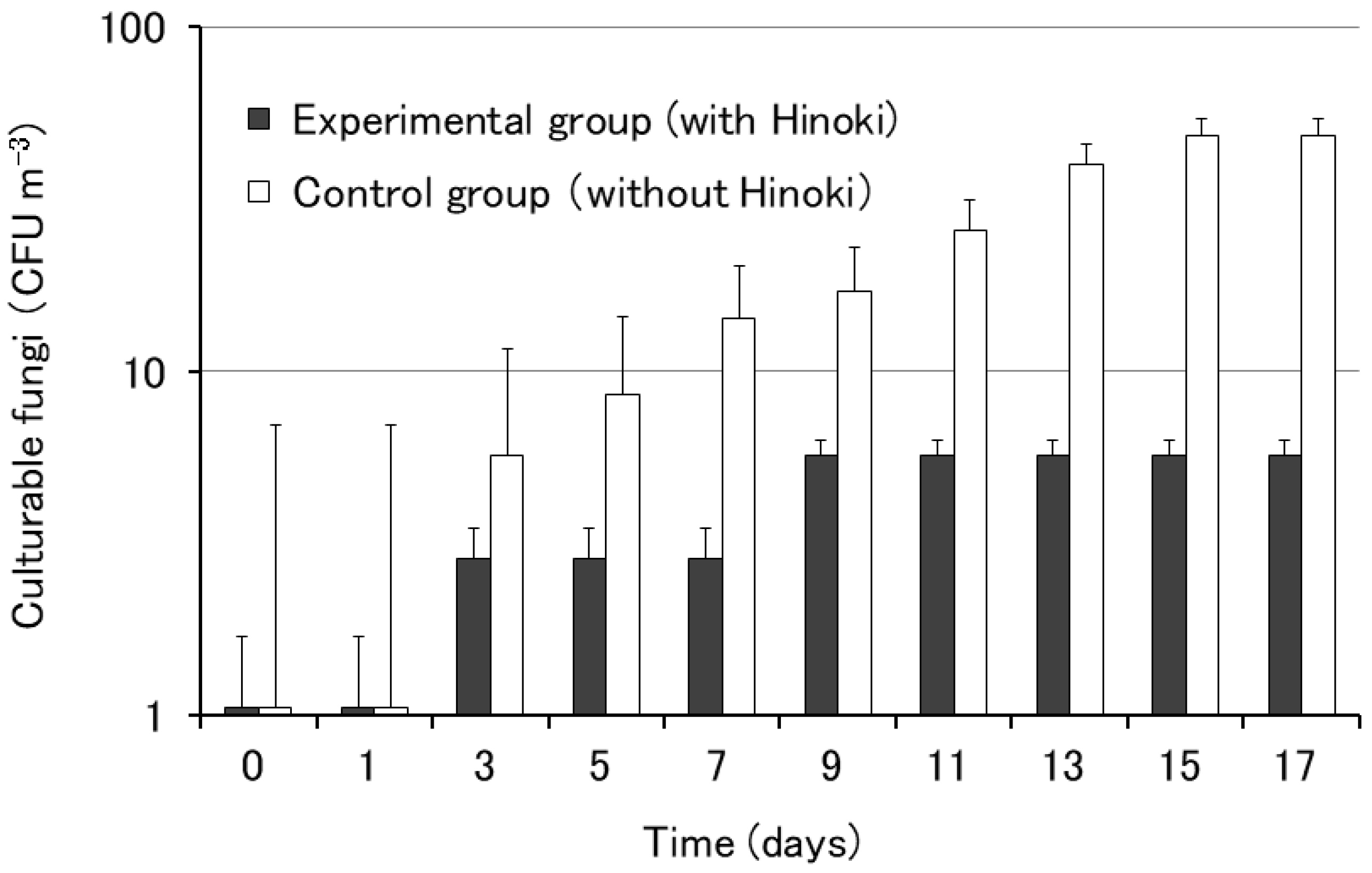
We also examined the influence of Hinoki cypress on the growth of airborne fungi using the rooms of the City Hall. The growth of airborne fungi was observed after three days of culture in both rooms (Figure 3). In the experimental group, the number of colonies was smaller than that in the control group, suggesting some antimicrobial effect of Hinoki cypress against airborne fungi.
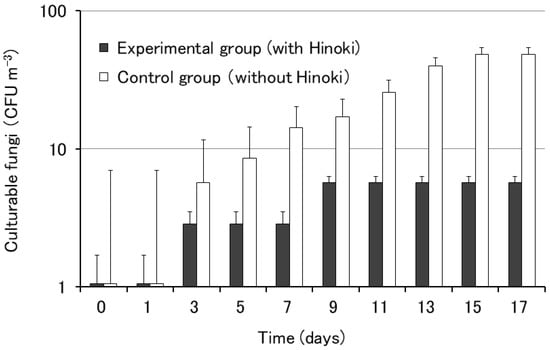
Figure 3.
Growth of airborne fungi on potato dextrose agar with and without Hinoki. Each bar represents the mean of five agar plates. The error bars indicate standard errors.
3.2. Influence of Hinoki Cypress on the Community Structure of Airborne Bacteria (PCR-DGGE Method)
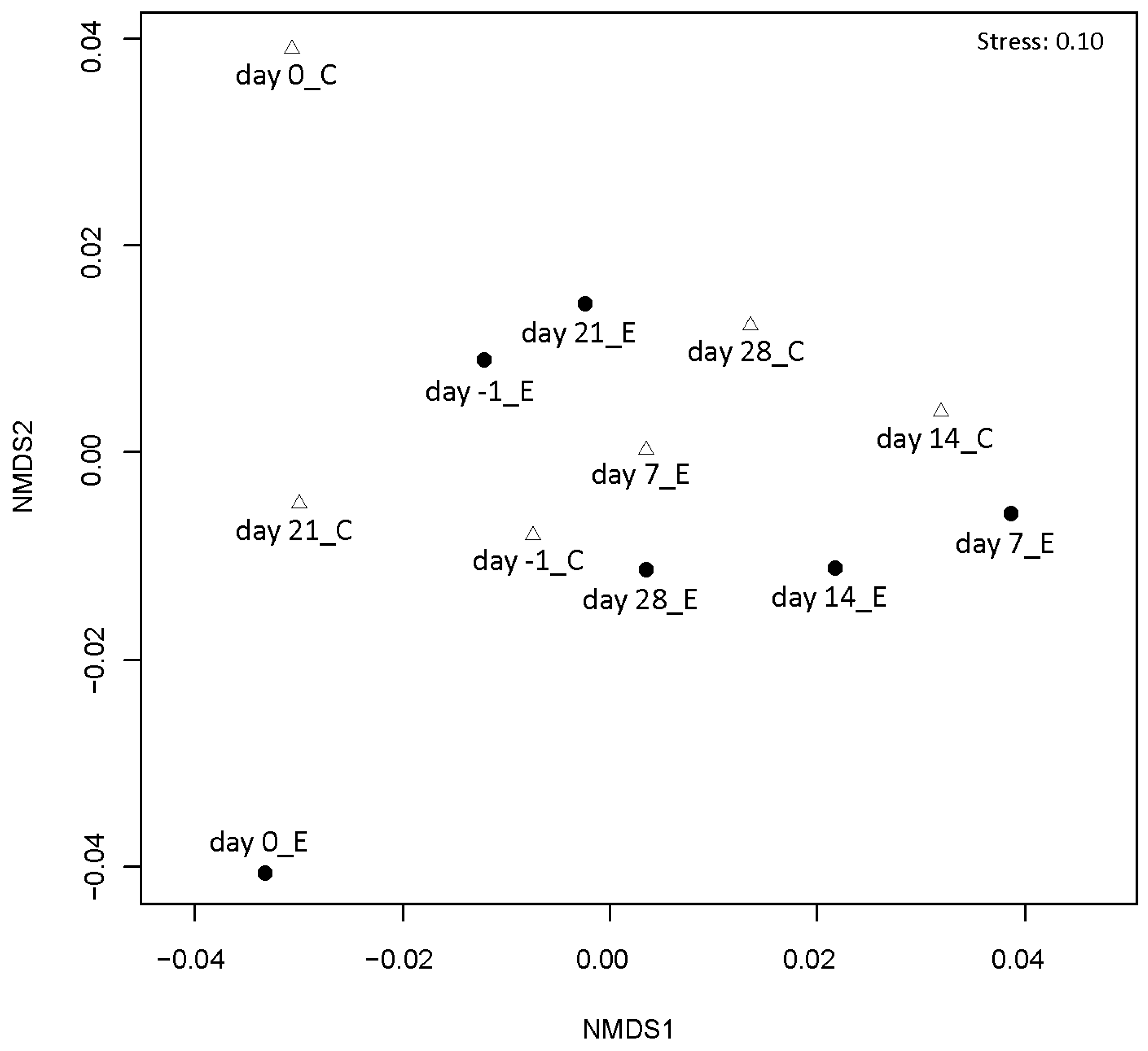
Temporal dynamics of the airborne bacterial community structure in the experimental and control rooms were analyzed by PCR-DGGE. The differences in the DGGE patterns between the experimental and control groups increased over time (Figure 4, Supplementary Figure S1). This suggested differences in the community structure of airborne bacteria depending on the presence or absence of Hinoki cypress wood in the City Hall rooms.
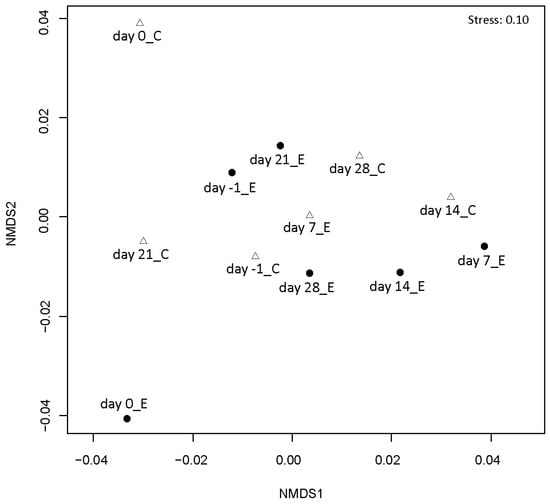
Figure 4.
Non-metric multidimensional scaling (nMDS) analysis of DGGE patterns of air samples. Closed circles and open triangles indicate the air samples collected in the room containing cypress wood (experimental group) and the room not containing cypress wood (control group), respectively.
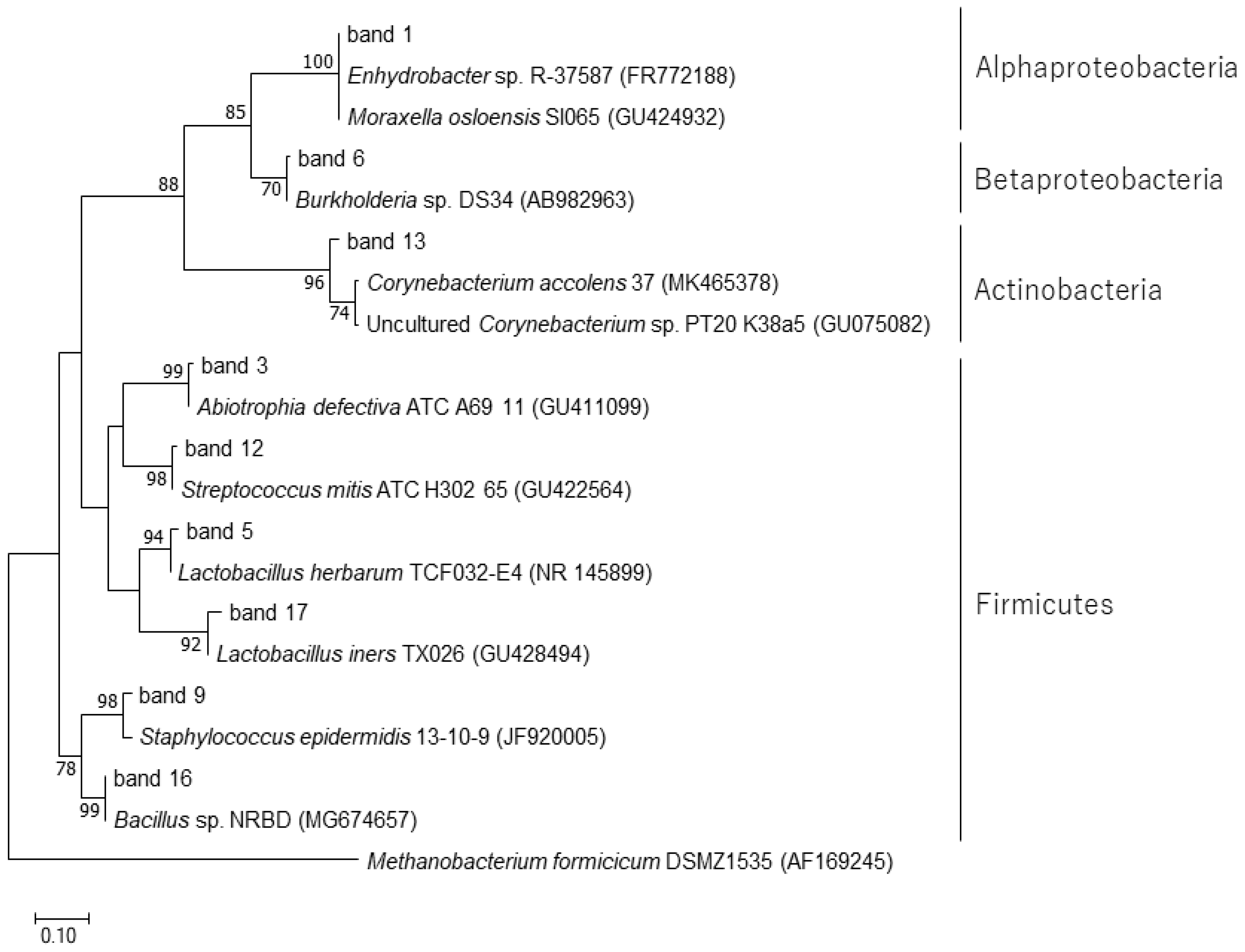
A total of 18 bands were excised from the DGGE gel (Supplementary Figure S1) and successfully sequenced. The results of the BLAST search are summarized in Table 1. The bands commonly detected in many samples (DGGE bands 1, 2, 7, 8, 10, 11, 14, and 18) were closely related to Enhydrobacter sp. R-37587 (accession no. FR772188), which showed the highest homology (100%). The bands 3, 4, 12, 15, and 17 showed the highest homology with three species of bacteria (Abiotrophia defectiva, Streptococcus mitis, and Lactobacillus iners) derived from the human oral cavity. Phylogenetic relationships of the isolated band sequences and their closest related species are shown in Figure 5.

Table 1.
Sequencing analysis of major denaturing gradient gel electrophoresis (DGGE) bands.
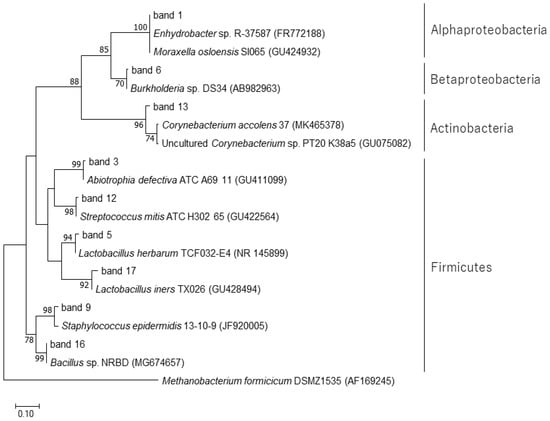
Figure 5.
Phylogenetic analysis of the 16S rRNA gene sequences recovered after excision of DGGE bands. The tree was constructed by the maximum-likelihood method with Methanobacterium formicicum DSMZ1535 (AF169245) as an outgroup. Reference sequences were retrieved from the GenBank (NCBI) database. Bootstrap values were calculated from 1000 replicates, and bootstrap values of 50% or greater are shown on branches. The scale bar represents 0.10 changes per sequence position.
4. Discussion
Hinoki cypress has been used as a material for construction and furniture in Japan for a long time. In addition, essential oil from Hinoki cypress has antibacterial and antifungal activities, as described previously. However, to the best of our knowledge, there has been no study on the influence of Hinoki materials on the growth of airborne microorganisms such as bacteria and fungi. This study aimed to clarify the influence of Hinoki materials against airborne microorganisms using culture-dependent and culture-independent molecular methods.
Hinoki cypress wood exhibited antimicrobial activity against airborne microorganisms (Figure 2 and Figure 3). The culture-based method showed that Hinoki cypress wood effectively inhibited the growth of airborne bacteria and fungi. Phytoncides, which are volatile substances emitted from plants, have insecticidal, antimicrobial, and antifungal activities. Our investigation revealed that volatile substances from Hinoki cypress wood could inhibit the growth of airborne bacteria and fungi.
In addition, we studied the time-dependent changes of airborne bacterial communities in two rooms with (experimental group) or without (control group) Hinoki material using PCR-DGGE. As a culture-independent method, PCR-DGGE effectively monitors microbial dynamics in various environmental samples [15,18,20,24,25]. Our data indicated differences in the structures of airborne bacterial communities depending on the presence or absence of Hinoki material. Environmental factors, such as temperature and humidity, were almost the same between the two rooms (data not shown). The differences between the results of culture-dependent and culture-independent molecular methods may arise from the difference between culturable and unculturable bacterial communities.
Several studies have shown that the primary sources of bacterial communities in indoor air were human occupants, pets, plants, water, plumbing systems, heating, ventilation, air-conditioning systems, and the outdoor environment [26,27]. We obtained similar results in this study (Table 1), where several DGGE bands were considered to be derived from the human oral cavity. The human oral cavity has been reported as one of the significant sources of airborne bacteria in indoor environments [28]. Fujiyoshi et al. showed the effects of temperature, relative humidity, air exchange rate, and occupants on airborne bacteria in built environments [27].
Many of the sequenced DGGE bands in this study were affiliated with Enhydrobacter sp. R-37587 (accession no. FR772188). The genus Enhydrobacter, a rare genus with its single species Enhydrobacter aerosaccus, was proposed by Staley et al. [29] as a gas-vacuolated facultatively anaerobic, heterotrophic rod. Later, the genus was recognized as a member of the family Rhodospirillaceae within the class Alphaproteobacteria [30]. Organisms of this species have been isolated from lake water, seawater, air, sand, and leaf litter compost [30,31]. Metagenomic studies also revealed that members of Enhydrobacter occur in diverse environments [32,33]. Some studies of indoor airborne bacterial communities revealed the dominant bacterial members. For example, members of genera Paracoccus, Acinetobacter, Pseudomonas, Enhydrobacter, Sphingomonas, Staphylococcus, and Streptococcus were dominant in indoor air (Museum) [34]. Moreover, Micrococcus, Paracoccus, Streptococcus, Corynebacterium, Staphylococcus, Bacillus, and Enhydrobacter were dominant in indoor airborne bacterial communities (childcare facilities) [35,36]. Enhydrobacter, Streptococcus, and Micrococcus were the top three most abundant genera in indoor air (elementary school classrooms). At the same time, Methylobacterium, Pseudomonas, and Deinococcus were the top three most abundant genera in outdoor air [26]. Macrococcus, Micrococcus, and Staphylococcus were dominant in indoor air (office rooms) [37,38].
In this study, we found that Hinoki cypress wood possessed antimicrobial activities that inhibit the growth of airborne bacteria and fungi, suggesting its usefulness as a functional material for walls and floors. Further research, including high-throughput sequencing, is needed to understand the antimicrobial activities of Hinoki cypress wood that inhibit the growth of airborne bacteria and fungi.
5. Conclusions
This was the first study to analyze the antimicrobial effects of Hinoki cypress against airborne microorganisms. Our results showed the influence of Hinoki cypress in the rooms of the City Hall on the growth of airborne bacteria and fungi using traditional culture methods and culture-independent molecular methods. Further research should focus on pathogenic microorganisms that can be harmful to the health of human occupants. Nevertheless, the results of this work will help us understand the impact of Hinoki cypress on airborne bacterial communities in the indoor environment to promote human health.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/d13100473/s1, Supplementary Figure S1: Denaturing gradient gel electrophoresis (DGGE) patterns of 16S rRNA gene fragments from air samples collected in rooms with (experimental group) or without (control group) Hinoki cypress material. Lanes marked M represent a DGGE marker lane consisting of 16S rRNA gene sequences from Staphylococcus sp., Exiguobacterium sp., Escherichia coli, Microbacterium sp., Methylobacterium sp., and Kocuria sp. The bands numbered (arrowheads) were excised for the sequence analysis.
Author Contributions
Conceptualization, D.T., D.U. and J.M.; methodology, D.T., D.U., J.M., M.M. (Masahiro Matsunaga) and M.M. (Masaaki Morimoto); investigation, D.T., D.U., J.M., M.M. (Masahiro Matsunaga) and M.M. (Masaaki Morimoto); data curation, D.T., J.M. and F.M.; writing—original draft preparation, D.T. and F.M.; writing—review and editing, D.T., D.U., J.M. and F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Owase City and the Japan Society for the Promotion of Science KAKENHI (Grant numbers 19KK0263 and 21H03611 awarded to D.T.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or supplementary material.
Acknowledgments
We thank Masugi Uchiyama for sampling and helpful comments and Yumi Sano for DGGE analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Park, M.J.; Choi, W.S.; Kang, H.Y.; Gwak, K.S.; Lee, G.S.; Jeung, E.B.; Choi, I.G. Inhibitory effect of the essential oil from Chamaecyparis obtusa on the growth of food-borne pathogens. J. Microbiol. 2010, 48, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Kiyono, Y. The potential of hinoki (Chamaecyparis obtusa [Sieb. et Zucc.] Endlicher) plantation forests for the restoration of the original plant community in Japan. For. Ecol. Manag. 2008, 255, 183–192. [Google Scholar] [CrossRef]
- Ikei, H.; Song, C.; Miyazaki, Y. Physiological Effects of Touching the Wood of Hinoki Cypress (Chamaecyparis obtusa) with the Soles of the Feet. Int. J. Environ. Res. Public Health 2018, 15, 2135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, K.; Uchiyama, Y.; Kurokami, N.; Sugano, K.; Nakanishi, Y. Soil acidification and decline of trees in forests within the precincts of shrines in Kyoto (Japan). Water Air Soil Pollut. 2011, 214, 197–204. [Google Scholar] [CrossRef]
- Ikei, H.; Song, C.; Miyazaki, Y. Physiological effect of olfactory stimulation by Hinoki cypress (Chamaecyparis obtusa) leaf oil. J. Physiol. Anthropol. 2015, 34, 44. [Google Scholar] [CrossRef] [Green Version]
- Bae, D.; Seol, H.; Yoon, H.G.; Na, J.R.; Oh, K.; Choi, C.Y.; Lee, D.W.; Jun, W.; Youl Lee, K.; Lee, J.; et al. Inhaled essential oil from Chamaecyparis obtuse ameliorates the impairments of cognitive function induced by injection of β-amyloid in rats. Pharm. Biol. 2012, 50, 900–910. [Google Scholar] [CrossRef]
- Arima, Y.; Nakai, Y.; Hayakawa, R.; Nishino, T. Antibacterial effect of beta-thujaplicin on staphylococci isolated from atopic dermatitis: Relationship between changes in the number of viable bacterial cells and clinical improvement in an eczematous lesion of atopic dermatitis. J. Antimicrob. Chemother. 2003, 51, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Ishimatsu, S.; Oga, Y.; Ishida, T.; Hori, H. Antibacterial properties of hinokitiol against Legionella pneumophila. J. UOEH 2003, 25, 435–439. (In Japanese) [Google Scholar] [CrossRef] [Green Version]
- Yang, J.K.; Choi, M.S.; Seo, W.T.; Rinker, D.L.; Han, S.W.; Cheong, G.W. Chemical composition and antimicrobial activity of Chamaecyparis obtusa leaf essential oil. Fitoterapia 2007, 78, 149–152. [Google Scholar] [CrossRef]
- Hong, E.J.; Na, K.J.; Choi, I.G.; Choi, K.C.; Jeung, E.B. Antibacterial and antifungal effects of essential oils from coniferous trees. Biol. Pharm. Bull. 2004, 27, 863–866. [Google Scholar] [CrossRef] [Green Version]
- Morikawa, T.; Ashitani, T.; Sekine, N.; Kusumoto, N.; Takahashi, K. Bioactivities of extracts from Chamaecyparis obtusa branch heartwood. J. Wood Sci. 2012, 58, 544–549. [Google Scholar] [CrossRef]
- Muyzer, G.; de Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muyzer, G.; Smalla, K. Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Appl. Environ. Microbiol. 1998, 73, 127–141. [Google Scholar]
- Hanning, I.B.; Ricke, S.C. Prescreening of microbial populations for the assessment of sequencing potential. In High-Throughput Next Generation Sequencing; Kwon, Y.M., Ricke, S.C., Eds.; Humana Press: New York, NY, USA, 2011; Volume 733, pp. 159–170. [Google Scholar]
- Tanaka, D.; Terada, Y.; Nakashima, T.; Sakatoku, A.; Nakamura, S. Seasonal variations in airborne bacterial community structures at a suburban site of central Japan over a 1-year time period using PCR-DGGE method. Aerobiologia 2015, 31, 143–157. [Google Scholar] [CrossRef]
- Lee, S.; Choi, B.; Yi, S.M.; Ko, G. Characterization of microbial community during Asian dust events in Korea. Sci. Total Environ. 2009, 407, 5308–5314. [Google Scholar] [CrossRef] [PubMed]
- Maki, T.; Susuki, S.; Kobayashi, F.; Kakikawa, M.; Tobo, Y.; Yamada, M.; Higashi, T.; Matsuki, A.; Hong, C.; Hasegawa, H.; et al. Phylogenetic analysis of atmospheric halotolerant bacterial communities at high altitude in an Asian dust (KOSA) arrival region, Suzu City. Sci. Total Environ. 2010, 408, 4556–4562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, X.; Li, G.; Yang, W.; Li, D. Distribution characteristics of microbial community structure in atmospheric particulates of the typical industrial city in Jiangsu province, China. Bioengineered 2021, 12, 615–626. [Google Scholar] [CrossRef]
- Ferguson, R.; Garcia-Alcega, S.; Coulon, F.; Dumbrell, A.J.; Whitby, C.; Colbeck, I. Bioaerosol biomonitoring: Sampling optimization for molecular microbial ecology. Mol. Ecol. Resour. 2019, 19, 672–690. [Google Scholar] [CrossRef]
- Tanaka, D.; Tokuyama, Y.; Terada, Y.; Kunimochi, K.; Mizumaki, C.; Tamura, S.; Wakabayashi, M.; Aoki, K.; Shimada, W.; Tanaka, H.; et al. Bacterial communities in Asian dust-containing snow layers on Mt. Tateyama, Japan. Bull. Glaciol. Res. 2011, 29, 31–39. [Google Scholar] [CrossRef]
- Tiodjio, R.E.; Sakatoku, A.; Nakamura, A.; Tanaka, D.; Fantong, W.Y.; Tchakam, K.B.; Tanyileke, G.; Ohba, T.; Hell, V.J.; Kusakabe, M. Bacterial and archaeal communities in Lake Nyos (Cameroon, Central Africa). Sci. Rep. 2014, 4, 6151. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2021; Available online: https://www.R-project.org (accessed on 30 July 2021).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package, R Package Version 2.5–7. 2020. Available online: http://CRAN.R-project.org/package=vegan (accessed on 30 July 2021).
- Tanaka, D.; Takahashi, T.; Yamashiro, Y.; Tanaka, H.; Kimochi, Y.; Nishio, M.; Sakatoku, A.; Nakamura, S. Seasonal variations in bacterioplankton community structures in two small rivers in the Himi region of central Japan and their relationships with environmental factors. World J. Microbiol. Biotechnol. 2017, 33, 212. [Google Scholar] [CrossRef]
- Jorquera, M.A.; Maruyama, F.; Ogram, A.V.; Navarrete, O.U.; Lagos, L.M.; Inostroza, N.G.; Acuña, J.J.; Rilling, J.I.; de La Luz Mora, M. Rhizobacterial Community Structures Associated with Native Plants Grown in Chilean Extreme Environments. Microb. Ecol. 2016, 72, 633–646. [Google Scholar] [CrossRef]
- Lee, B.G.; Yang, J.I.; Kim, E.; Geum, S.W.; Park, J.H.; Yeo, M.K. Investigation of bacterial and fungal communities in indoor and outdoor air of elementary school classrooms by 16S rRNA gene and ITS region sequencing. Indoor Air 2021. [Google Scholar] [CrossRef]
- Fujiyoshi, S.; Tanaka, D.; Maruyama, F. Transmission of airborne bacteria across built environments and its measurement standards: A review. Front. Microbiol. 2017, 8, 2336. [Google Scholar] [CrossRef] [Green Version]
- Hospodsky, D.; Qian, J.; Nazaroff, W.W.; Yamamoto, N.; Bibby, K.; Rismani-Yazdi, H.; Peccia, J. Human occupancy as a source of indoor airborne bacteria. PLoS ONE 2012, 7, e34867. [Google Scholar] [CrossRef] [Green Version]
- Staley, J.T.; Irgens, R.L.; Brenner, D.J. Enhydrobacter aerosaccus gen. nov., sp. nov., a Gas-Vacuolated, Facultatively Anaerobic, Heterotrophic Rod. Int. J. Syst. Evol. Microbiol. 1987, 37, 289–291. [Google Scholar] [CrossRef]
- Kawamura, Y.; Fujiwara, N.; Naka, T.; Mitani, A.; Kubota, H.; Tomida, J.; Morita, Y.; Hitomi, J. Genus Enhydrobacter Staley et al. 1987 should be recognized as a member of the family Rhodospirillaceae within the class Alphaproteobacteria. Microbiol. Immunol. 2012, 56, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Premalatha, N.; Gopal, N.O.; Jose, P.A.; Anandham, R.; Kwon, S.W. Optimization of cellulase production by Enhydrobacter sp. ACCA2 and its application in biomass saccharification. Front. Microbiol. 2015, 6, 1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Oh, D.H.; Jung, J.Y.; Kim, J.C.; Jeon, C.O. Comparative ocular microbial communities in humans with and without blepharitis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5585–5593. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Sharma, S.; Maurya, R.K.; Das De, T.; Thomas, T.; Lata, S.; Singh, N.; Pandey, K.C.; Valecha, N.; Dixit, R. Salivary glands harbor more diverse microbial communities than gut in Anopheles culicifacies. Parasites Vectors 2014, 7, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaüzère, C.; Moletta-Denat, M.; Blanquart, H.; Ferreira, S.; Moularat, S.; Godon, J.J.; Robine, E. Stability of airborne microbes in the Louvre Museum over time. Indoor Air 2014, 24, 29–40. [Google Scholar] [CrossRef]
- Shin, S.K.; Kim, J.; Ha, S.M.; Oh, H.S.; Chun, J.; Sohn, J.; Yi, H. Metagenomic insights into the bioaerosols in the indoor and outdoor environments of childcare facilities. PLoS ONE 2015, 10, e0126960. [Google Scholar] [CrossRef]
- Aydogdu, H.; Asan, A.; Tatman Otkun, M. Indoor and outdoor airborne bacteria in child day-care centers in Edirne City (Turkey), seasonal distribution and influence of meteorological factors. Environ. Monit. Assess. 2010, 164, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Brągoszewska, E.; Biedroń, I.; Kozielska, B.; Pastuszka, J.S. Microbiological indoor air quality in an office building in Gliwice, Poland: Analysis of the case study. Air Qual. Atmos. Health 2018, 11, 729–740. [Google Scholar] [CrossRef] [Green Version]
- Brągoszewska, E.; Biedroń, I. Indoor Air Quality and Potential Health Risk Impacts of Exposure to Antibiotic Resistant Bacteria in an Office Rooms in Southern Poland. Int. J. Environ. Res. Public Health 2018, 15, 2604. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).