The Early Branching Group of Orbiniida Sensu Struck et al., 2015: Parergodrilidae and Orbiniidae
Abstract
1. Introduction
2. Parergodrilidae Reisinger, 1925
2.1. Parergodrilus Reisinger, 1925
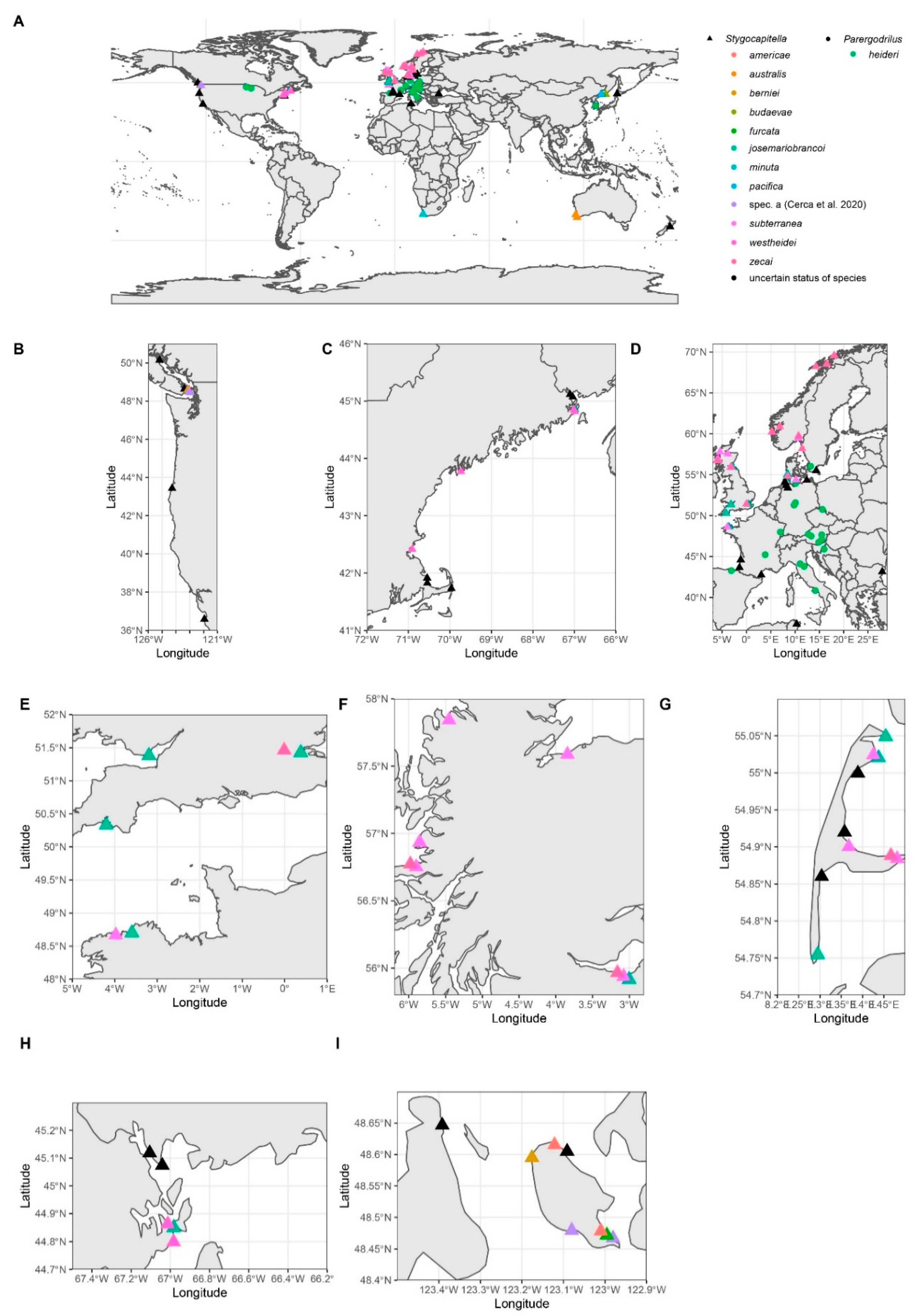
Distribution
2.2. Stygocapitella Knöllner, 1934
2.2.1. Distribution
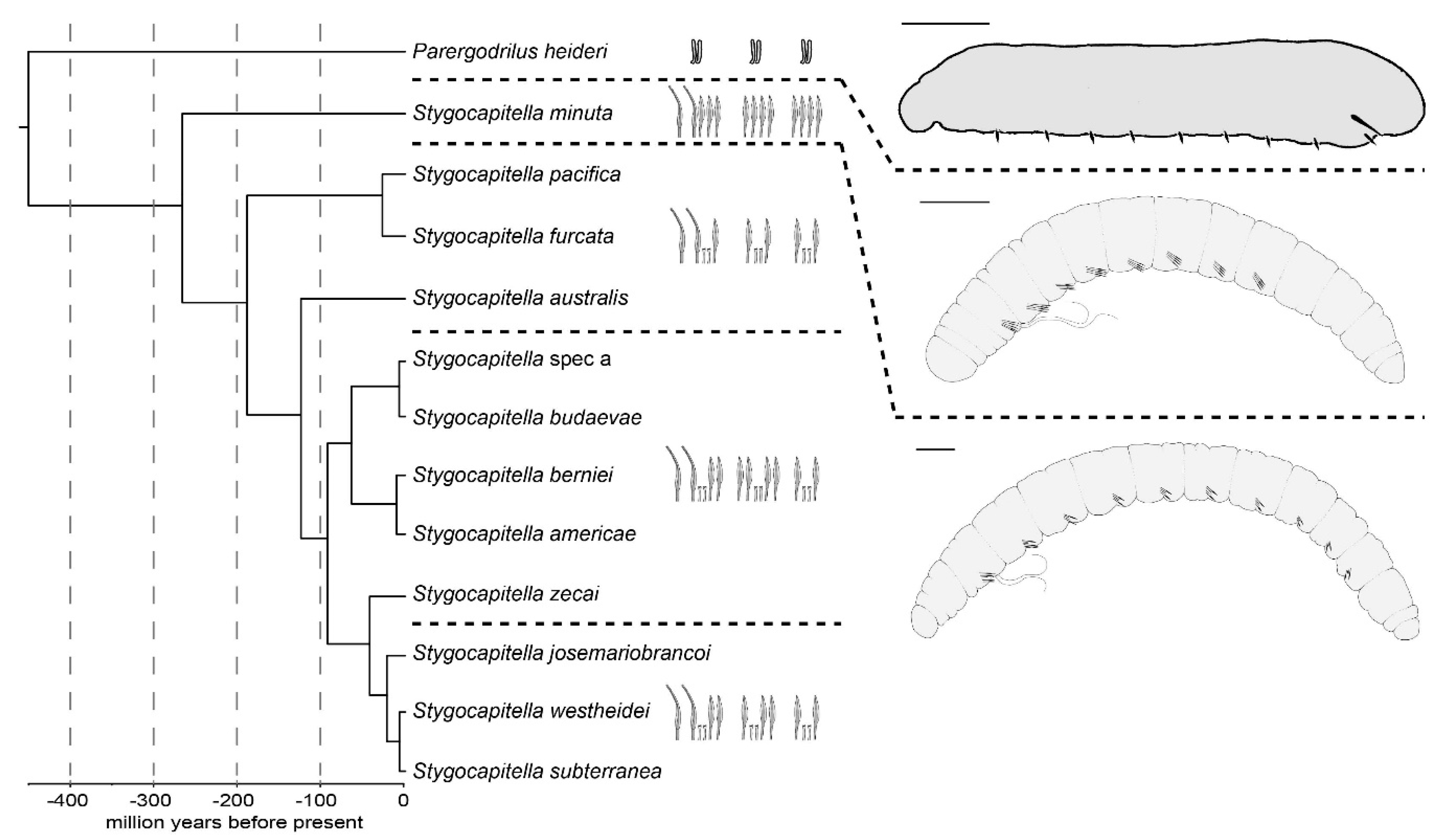
2.2.2. Taxonomy
2.2.3. Conclusions
3. Orbiniidae Hartman, 1942
3.1. Systematics
3.1.1. Morphological Era
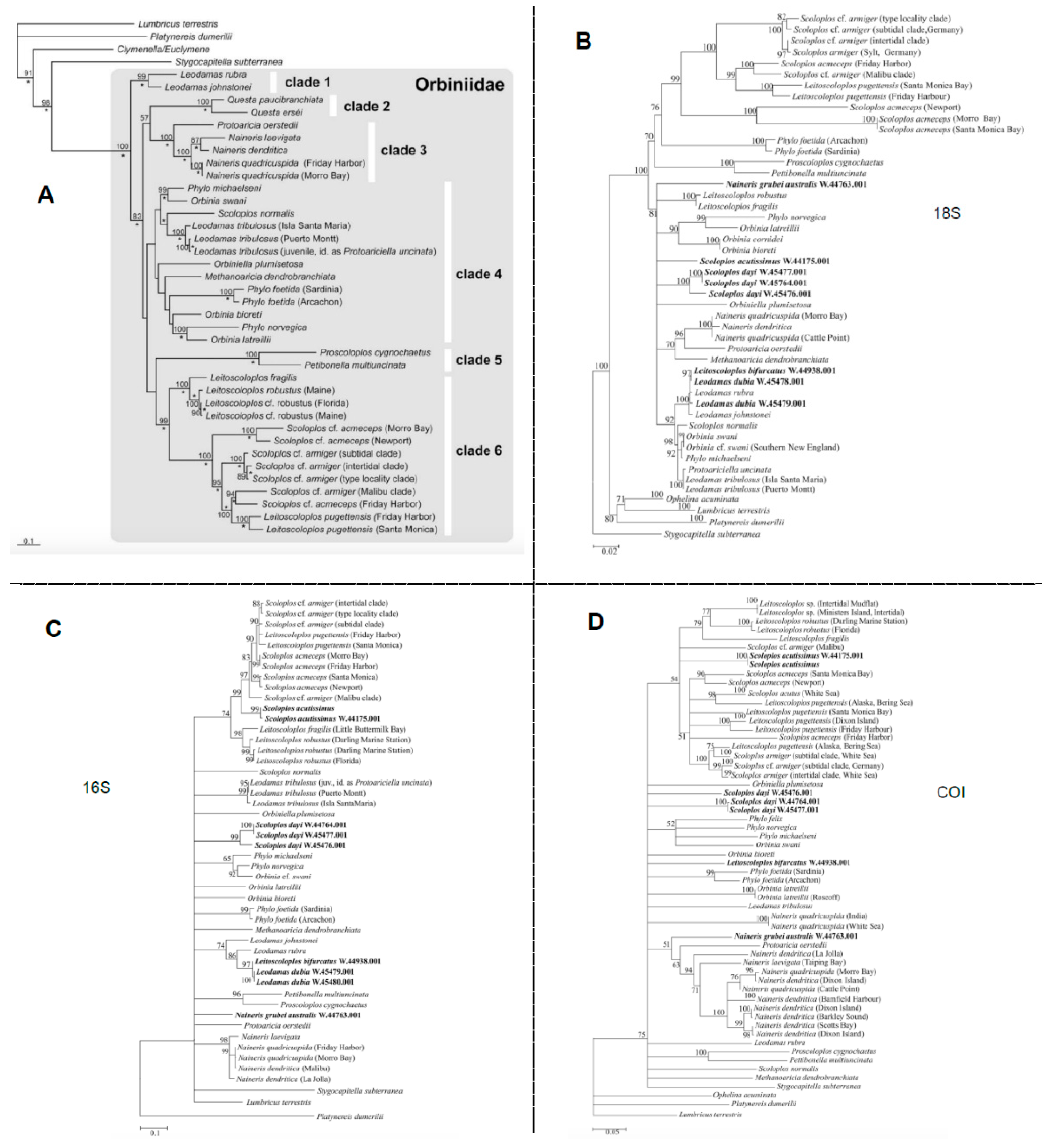
3.1.2. Genetics Era
3.1.3. Current State: Traditional Taxonomy vs. Genetics
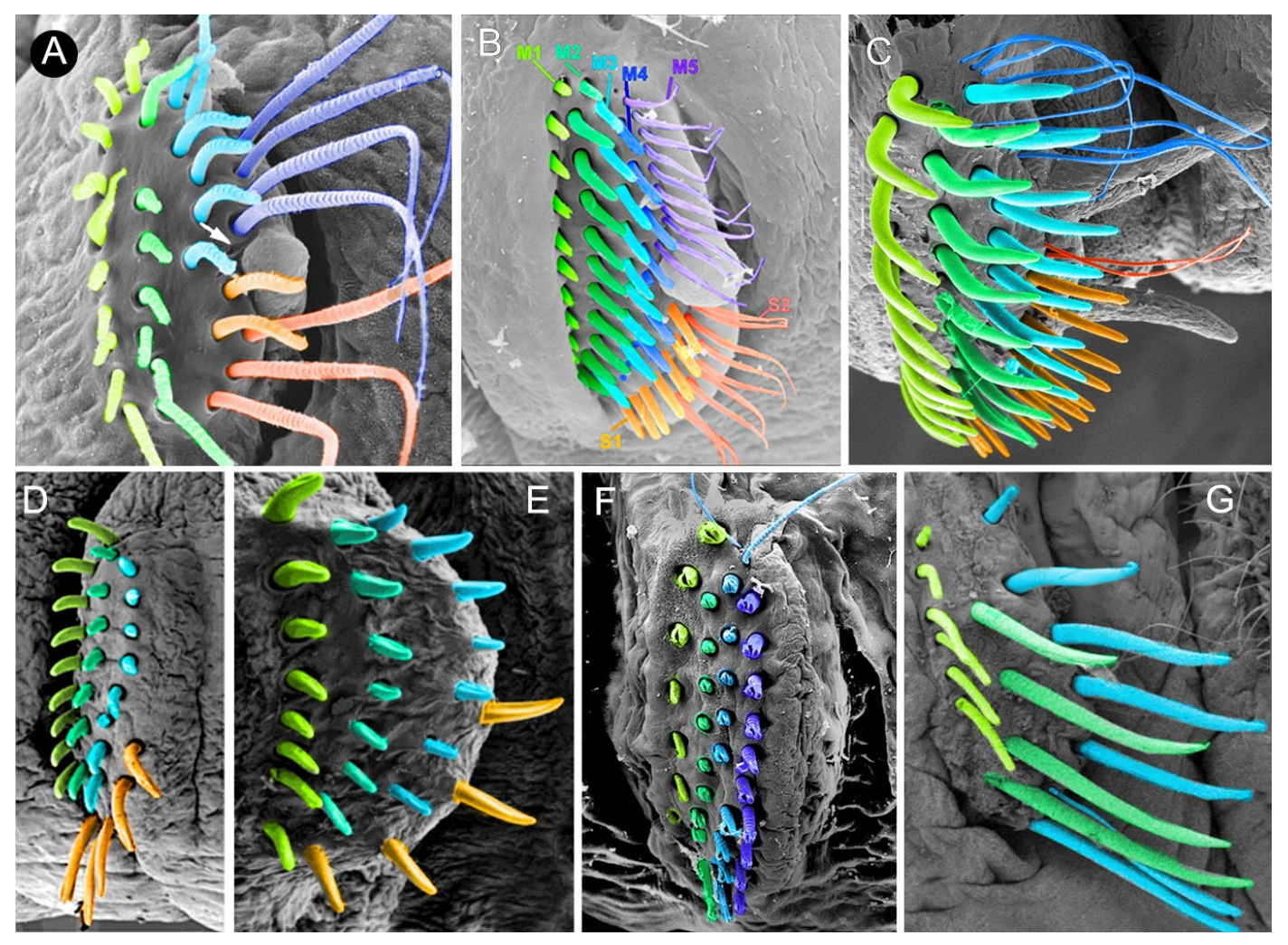
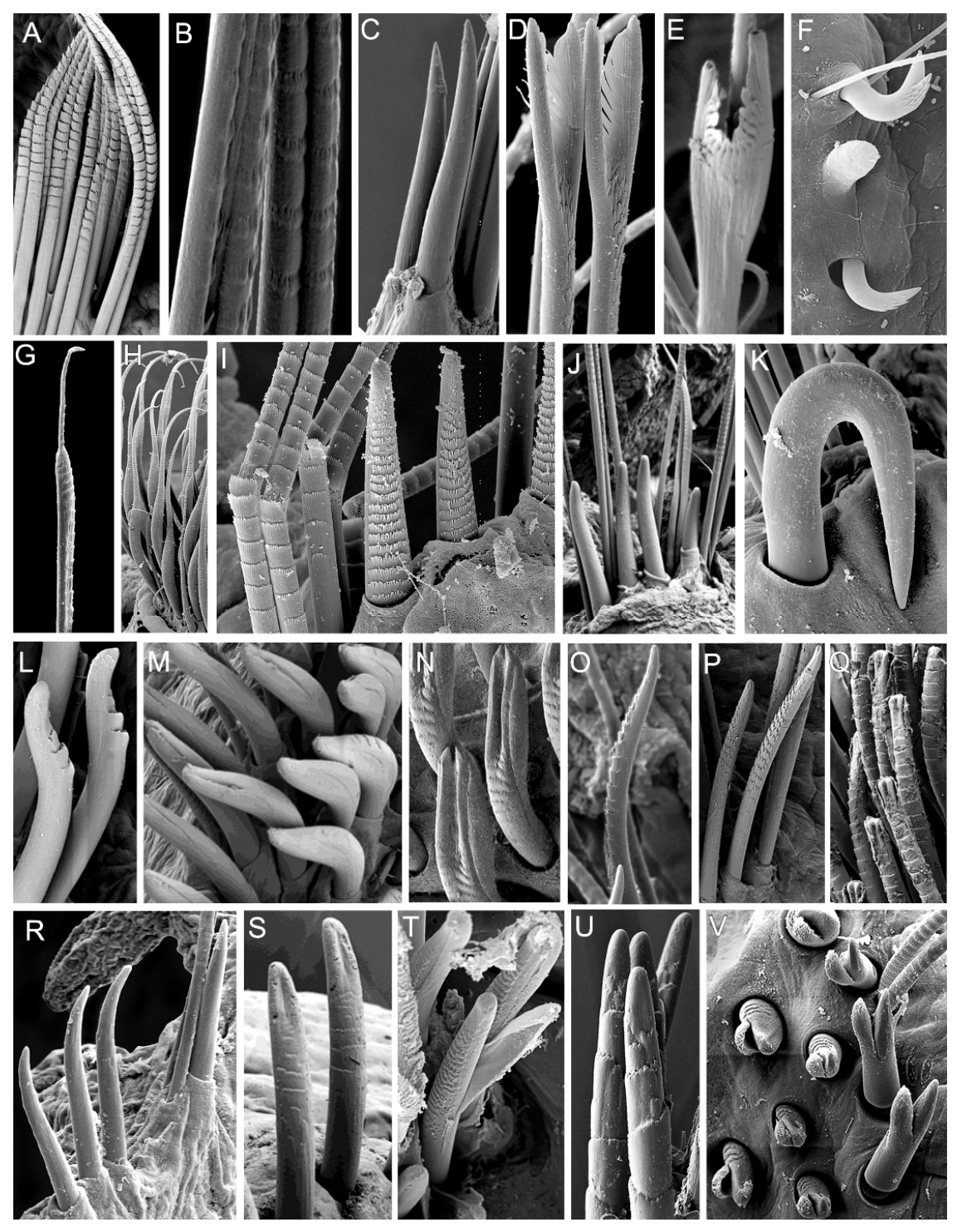
3.2. Discussion on Taxonomical Characters
3.3. Diversity
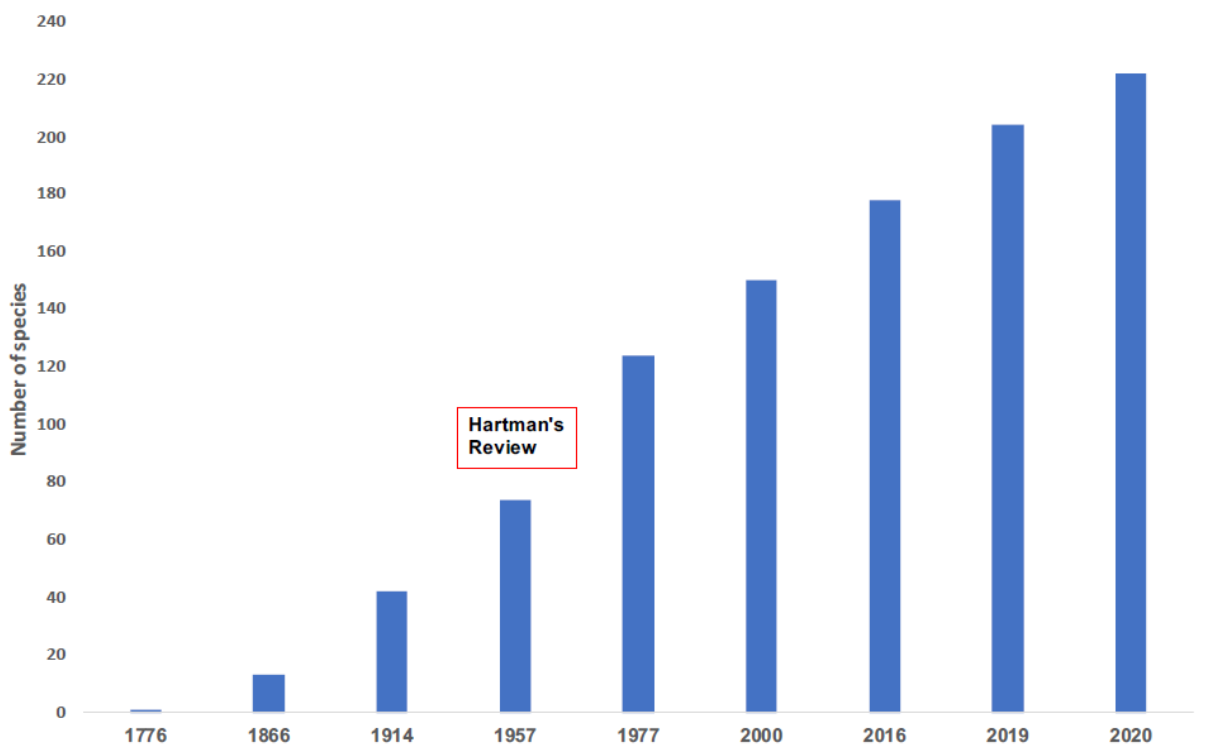
3.3.1. Species Numbers
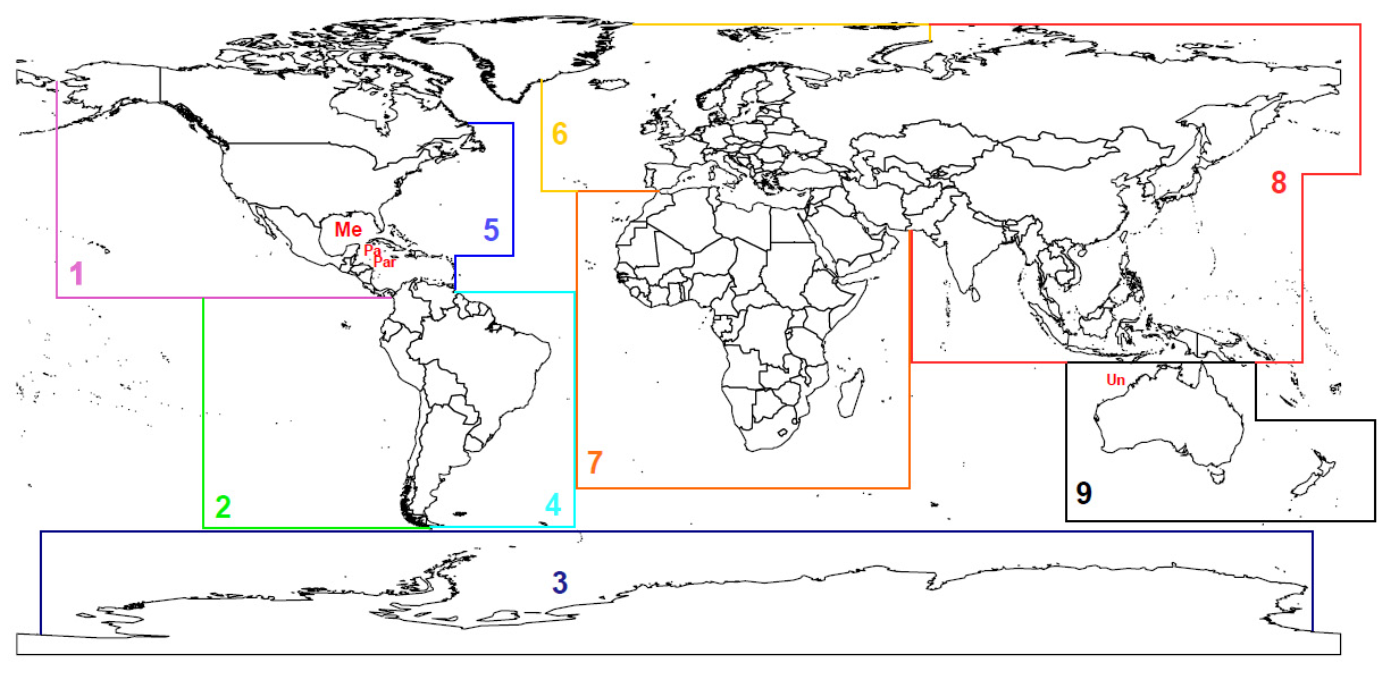
3.3.2. Species Distribution
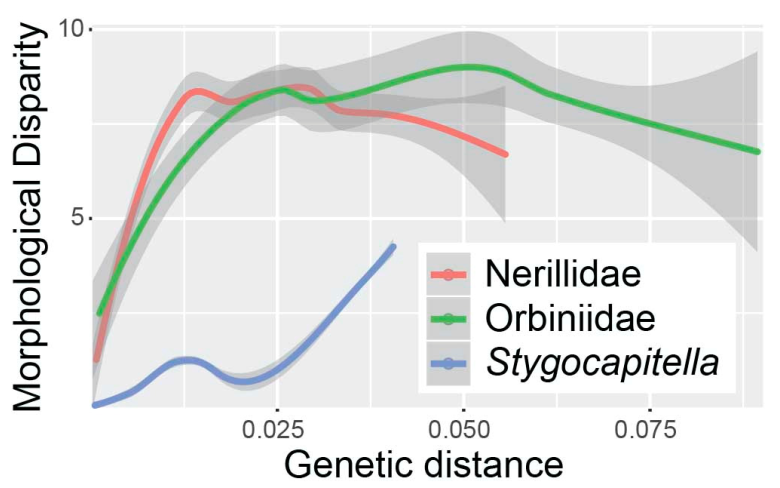
3.3.3. Cryptic Diversity and Subspecies
3.4. Ecology
3.4.1. Habitat
3.4.2. Relation to Pollution
3.5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fauchald, K. The polychaete worms. Definitions and keys to the orders, families and genera. Nat. Hist. Mus. Los Angeles Cty. 1977, 28, 188. [Google Scholar]
- Fauvel, P. Polychètes Sédentaires. Addenda aux Errantes, Archiannélides, Myzostomaires. Faune Fr. 1927, 16, 1–494. [Google Scholar]
- Day, J.H. A Monograph on the Polychaeta of South Africa. Part 2. Sedentaria.; British Museum (Natural History): London, UK, 1967. [Google Scholar]
- Hartmann-Schröder, G. Teil 58. Annelida, Borstenwürmer, Polychaeta; VEB Gustav Fischer Verlag: Jena, Germany, 1971. [Google Scholar]
- Rouse, G.W.; Fauchald, K. Cladistics and polychaetes. Zoöl. Scr. 1997, 26, 139–204. [Google Scholar] [CrossRef]
- Bleidorn, C.; Helm, C. 7.1.4 Orbiniidae Hartman, 1942. In Annelida Basal Groups and Pleistoannelida; Sedentaria, I., Purschke, G., Böggemann, M., Westheide, W., Eds.; De Gruyter: Berlin/Boston, Germany, 2019; pp. 237–250. [Google Scholar] [CrossRef]
- Struck, T.H. 7.2 Phylogeny. In Annelida Basal Groups and Pleistoannelida; Sedentaria, I., Purschke, G., Böggemann, M., Westheide, W., Eds.; De Gruyter: Berlin/Boston, Germany, 2019; pp. 37–68. [Google Scholar] [CrossRef]
- Struck, T.H.; Paul, C.; Hill, N.; Hartmann, S.; Hösel, C.; Kube, M.; Lieb, B.; Meyer, A.; Tiedemann, R.; Purschke, G.; et al. Phylogenomic analyses unravel annelid evolution. Nat. Cell Biol. 2011, 471, 95–98. [Google Scholar] [CrossRef]
- Struck, T.H. The Impact of Paralogy on Phylogenomic Studies—A Case Study on Annelid Relationships. PLoS ONE 2013, 8, e62892. [Google Scholar] [CrossRef]
- Weigert, A.; Bleidorn, C. Current status of annelid phylogeny. Org. Divers. Evol. 2016, 16, 345–362. [Google Scholar] [CrossRef]
- Martín-Durán, J.M.; Vellutini, B.C.; Marlétaz, F.; Cetrangolo, V.; Cvetesic, N.; Thiel, D.; Henriet, S.; Grau-Bové, X.; Carrillo-Baltodano, A.M.; Gu, W.; et al. Conservative route to genome compaction in a miniature annelid. Nat. Ecol. Evol. 2020, 1–12. [Google Scholar] [CrossRef]
- Struck, T.H.; Golombek, A.; Weigert, A.; Franke, F.A.; Westheide, W.; Purschke, G.; Bleidorn, C.; Halanych, K.M. The Evolution of Annelids Reveals Two Adaptive Routes to the Interstitial Realm. Curr. Biol. 2015, 25, 1993–1999. [Google Scholar] [CrossRef]
- Worsaae, K.; Kristensen, R.M. Evolution of interstitial Polychaeta (Annelida). Hydrobiologia 2005, 535, 319–340. [Google Scholar] [CrossRef]
- Struck, T.H.; Purschke, G.; Dordel, J.; Hösel, C.; Nesnidal, M.P.; Diersing, F.; Bleidorn, C.; Paul, C.; Hill, N.; Tiedemann, R.; et al. Phylogeny and evolution of Annelida based on molecular data. In Deep Metazoan Phylogeny: The Backbone of the Tree of Life—New Insights from Analyses of Molecules, Morphology, and Theory of Data Analysis; Wägele, J.W., Bartolomaeus, T., Eds.; De Gruyter: Berlin, Germany, 2014; pp. 143–160. [Google Scholar]
- Struck, T.H.; Nesnidal, M.; Purschke, G.; Halanych, K.M. Detecting possibly saturated positions in 18S and 28S sequences and their influence on phylogenetic reconstruction of Annelida (Lophotrochozoa). Mol. Phylogenet. Evol. 2008, 48, 628–645. [Google Scholar] [CrossRef]
- Jordens, J.; Struck, T.; Purschke, G. Phylogenetic inference regarding Parergodrilidae and Hrabeiella periglandulata (’Polychaeta’, Annelida) based on 18S rDNA, 28S rDNA and COI sequences. J. Zoöl. Syst. Evol. Res. 2004, 42, 270–280. [Google Scholar] [CrossRef]
- Struck, T.H.; Hessling, R.; Purschke, G. The phylogenetic position of the Aeolosomatidae and Parergodrilidae, two enigmatic oligochaete-like taxa of the ’Polychaeta’, based on molecular data from 18S rDNA sequences. J. Zoöl. Syst. Evol. Res. 2002, 40, 155–163. [Google Scholar] [CrossRef]
- Bleidorn, C. The role of character loss in phylogenetic reconstruction as exemplified for the Annelida. J. Zoöl. Syst. Evol. Res. 2007, 45, 299–307. [Google Scholar] [CrossRef]
- Bleidorn, C. Phylogenetic relationships and evolution of Orbiniidae (Annelida, Polychaeta) based on molecular data. Zoöl. J. Linn. Soc. 2005, 144, 59–73. [Google Scholar] [CrossRef]
- Bleidorn, C.; Vogt, L.; Bartolomaeus, T. New insights into polychaete phylogeny (Annelida) inferred from 18S rDNA sequences. Mol. Phylogenet. Evol. 2003, 29, 279–288. [Google Scholar] [CrossRef]
- Zrzavý, J.; Riha, P.; Piálek, L.; Janouškovec, J. Phylogeny of Annelida (Lophotrochozoa): Total-evidence analysis of morphology and six genes. BMC Evol. Biol. 2009, 9, 189. [Google Scholar] [CrossRef]
- Reisinger, E. Ein landbewohnender archiannelide. Zoomorphology 1925, 3, 197–254. [Google Scholar] [CrossRef]
- Meyer, A. Ist Parergodrilus heideri (Reisinger) ein Archiannelide? Zool. Anz. 1927, 72, 19–35. [Google Scholar]
- Karling, T.G. Zur Kenntnis von Stygocapitella subterranea Knöllner und Parergodrilus heideri Reisinger. Ark. Zool. 1958, 1, 307–324. [Google Scholar]
- Knöllner, F. Stygocapitella subterranea nov.gen., nov.spec. Schriften Naturwissenschaftlichen Ver. Schlesw. Holst. 1934, 20, 468–472. [Google Scholar]
- Hartmann-Schröder, G. Teil 58. Annelida, Borstenwürmer, Polychaeta, 2nd ed.; Gustav Fischer: Stuttgart, Germany, 1996. [Google Scholar]
- Pizl, V.; Chalupsky, J. Hrabeiella periglandulata gen. et sp. n.(Annelida): A curious worm from Czechoslovakia. Veštn. Českoslov. Spol. Zool. 1984, 48, 291–295. [Google Scholar]
- Rota, E. Morphology and adaptations of Parergodrilus Reisinger and Hrabeiella Pizl & Chalupsky, two enigmatic soil-dwelling annelids. Ital. J. Zoöl. 1998, 65, 75–84. [Google Scholar] [CrossRef]
- Purschke, G. Male genital organs, spermatogenesis and spermatozoa in the enigmatic terrestrial polychaete Parergodrilus heideri (Annelida, Parergodrilidae). Zoomorphology 2002, 121, 125–138. [Google Scholar] [CrossRef]
- Purschke, G. Terrestrial polychaetes—Models for the evolution of the Clitellata (Annelida)? Hydrobiologia 1999, 406, 87–99. [Google Scholar] [CrossRef]
- Rota, E.; De Nicola, F.; Bargagli, R. Parergodrilus heideri Reisinger, 1925 (Annelida: Polychaeta) from a holm oak wood in an extinct volcano of southern Italy. Zootaxa 2010, 2687, 65–68. [Google Scholar] [CrossRef]
- Rota, E. First Italian record of the terrestrial polychaeteparergodrilus heiderireisinger, with anatomical and ecological notes. Ital. J. Zoöl. 1997, 64, 91–96. [Google Scholar] [CrossRef]
- Reisinger, E. Die Lösung des Parergodrilus-Problems. Z. Morphol. Ökol. Tiere 1960, 48, 517–544. [Google Scholar] [CrossRef]
- Graefe, U. Die Gliederung der Zersetzergesellschaften für die standortsökologische Ansprache. Mitteilungen Dtsch. Bodenkd. Ges. 1993, 69, 95–98. [Google Scholar]
- Graefe, U.; Schmelz, R.M. Indicator values, strategy types and life forms of terrestrial Enchytraeidae and other microannelids. In Newsletter on Enchytraeidae 6, Proceedings of the 3rd International Symposium on Enchytraeidae, Osnabrück, Germany, 3–4 July 1998; Schmelz, R.M., Sühlo, K., Eds.; Universitätsverlag Rasch: Osnabrück, Germany, 1999; pp. 59–67. [Google Scholar]
- Martinez-Ansemil, E.; Parapar, J. Primera cita en la península Ibérica del poliqueto terrestre Parergodrilus heideri Reisinger, 1925 (Polychaeta, Parergodrilidae). Graellsia 2009, 65, 235–240. [Google Scholar] [CrossRef]
- Schlaghamerský, J.; Frelich, L.E. First records of Parergodrilus heideri (Annelida: “Polychaeta”) from North America. Zootaxa 2012, 3498, 81–86. [Google Scholar] [CrossRef]
- Rota, E.; De Jong, Y. Fauna Europaea: Annelida—Terrestrial Oligochaeta (Enchytraeidae and Megadrili), Aphanoneura and Polychaeta. Biodivers. Data J. 2015, 3, e5737. [Google Scholar] [CrossRef] [PubMed]
- Römbke, J.; Jans, W. On terrestrial Polychaeta (Annelida) from forest soils of southern Germany. Verhandlungen Dtsch. Zool. Ges. 1991, 84, 447–448. [Google Scholar]
- Beylich, A.; Graefe, U. Investigations on the enchytraeid fauna in floodplain soils of the Lower Middle Elbe. Folia Fac. Sci. Nat. Univ. Masaryk. Brun. Brno Biol. 2007, 110, 107–122. [Google Scholar]
- Chalupský, J. Terrestrial Enchytraeidae (Oligochaeta) and Parergodrilidae (Polychaeta) from Sweden, with description of a new enchytraeid species. Zool. Scr. 1992, 21, 133–150. [Google Scholar] [CrossRef]
- Rouse, G.W.; Pleijel, F. Polychaetes; Oxford University Press: New York, NY, USA, 2001; p. 354. [Google Scholar]
- Purschke, G. Problematic Annelid Groups. In Reproductive Biology and Phylogeny of Annelida; Rouse, G.W., Pleijel, F., Eds.; Science Publisher: Plymouth, UK, 2006; Volume 4, pp. 639–667. [Google Scholar]
- Westheide, W. Polychaetes: Interstitial Families, 2nd ed.; Field Studies Council: Shrewsbury, UK, 2008. [Google Scholar]
- Purschke, G. 7.1.3 Parergoldrilidae Reisinger, 1925. In Annelida Basal Groups and Pleistoannelida; Sedentaria, I., Purschke, G., Böggemann, M., Westheide, W., Eds.; De Gruyter: Berlin/Boston, Germany, 2019; pp. 237–250. [Google Scholar] [CrossRef]
- Cerca, J.; Meyer, C.; Purschke, G.; Struck, T.H. Delimitation of cryptic species drastically reduces the geographical ranges of marine interstitial ghost-worms (Stygocapitella; Annelida, Sedentaria). Mol. Phylogenet. Evol. 2020, 143, 106663. [Google Scholar] [CrossRef] [PubMed]
- Cerca, J.; Meyer, C.; Stateczny, D.; Siemon, D.; Wegbrod, J.; Purschke, G.; Dimitrov, D.; Struck, T.H. Deceleration of morphological evolution in a cryptic species complex and its link to paleontological stasis. Evolution 2019, 74, 116–131. [Google Scholar] [CrossRef]
- Struck, T.H.; Koczula, J.; Stateczny, D.; Meyer, C.; Purschke, G. Two new species in the annelid genus Stygocapitella (Orbiniida, Parergodrilidae) with comments on their biogeography. Zootaxa 2017, 4286, 301. [Google Scholar] [CrossRef]
- Jans, W.; Römbke, J. Funde eines terrestrischen Polychaeten (Annelida) in Wäldern Baden-Württembergs. Carolinea 1989, 47, 158–162. [Google Scholar]
- Rota, E.; Martin, P.; Erséus, C. Soil-dwelling polychaetes: Enigmatic as ever? Some hints on their phylogenetic relationships as suggested by a maximum parsimony analysis of 18S rRNA gene sequences. Contrib. Zool. 2001, 70, 127–138. [Google Scholar] [CrossRef]
- Dózsa-Farkas, K.; Hong, Y. Three new Hemienchytraeus species (Enchytraeidae, Oligochaeta, Annelida) from Korea, with first records of other enchytraeids and terrestrial polychaetes (Annelida). Zootaxa 2010, 2406, 29–56. [Google Scholar] [CrossRef]
- Riser, N.W. The aberrant polychaete Stygocapitella from some American beaches. Wasmann J. Biol. 1980, 38, 10–17. [Google Scholar]
- Westheide, W. Zur Polychaetenfauna des Eulitorals der Nordseeinsel Sylt. Helgol. Mar. Res. 1966, 13, 203–209. [Google Scholar] [CrossRef]
- Riser, N.W. General observations on the intertidal interstitial fauna of New Zealand. Tane 1984, 30, 239–250. [Google Scholar]
- Worsfold, T.M.; Dyer, M.F. UK Marine SACs Project. Monitoring Methods Workshop at Plymouth (April 1997) and Millport (May 1997). (Contractor: Unicomarine, Letchworth); Joint Nature Conservation Committee: Peterborough, UK, 1997. [Google Scholar]
- Hartmann-Schröder, G. Teil 9—Die Polychaeten der antiborealen Südwestküste Australiens (zwischen Dunsborough im Norden und Denmark im Süden). Mitteilungen Hambg. Zool. Mus. Inst. 1983, 80, 123–167. [Google Scholar]
- Schmidt, H.; Westheide, W. Are the meiofaunal polychaetes Hesionides arenaria and Stygocapitella subterranea true cosmopolitan species?—Results of RAPD-PCR investigations. Zool. Scr. 2000, 29, 17–27. [Google Scholar] [CrossRef]
- Schmidt, P. Zonation of the interstitial polychaeteStygocapitella subterranea (Stygocapitellidae) in European sandy beaches. Mar. Biol. 1970, 7, 319–323. [Google Scholar] [CrossRef]
- Schmidt, P. Die quantitative Verteilung und Populationsdynamik des Mesopsammons am Gezeiten-Sandstrand der Nordsee-Insel Sylt1). II. Quantitative Verteilung und Populationsdynamik einzelner Arten. Int. Rev. Hydrobiol. 1969, 54, 95–174. [Google Scholar] [CrossRef]
- Westheide, W. The geographical distribution of interstitial polychaetes. Mikrofauna Meeresbod. 1977, 61, 287–302. [Google Scholar]
- Schmidt, P. Zonierung und jahreszeitliche Fluktuationen des Mesopsammons im Sandstrand von Schilksee (Kieler Bucht). Mikrofauna Meeresbod. 1972, 10, 1–60. [Google Scholar]
- Schmidt, P. Zonierung und jahreszeitliche Fluktuationen der interstitiellen Fauna in Sandstränden des Gebiets von Tromsø (Norwegen). Mikrofauna Meeresbod. 1972, 10, 81–164. [Google Scholar]
- Renaud-Debysier, J. Recherches écologiques sur la faune interstitielle des sables. Bassin d’Arcachon, Île de Bimini, Bahamas. Vie Milieu Suppl. 1963, 15, 1–157. [Google Scholar]
- Valkanov, A. Zwei neue Polychaeten für das Schwarze Meer [Bulgar]. Trud. Chernomorsk. Biol. Sta. Varna 1954, 18, 55–58. [Google Scholar]
- Westheide, W. La faune des Polychètes et des Archiannélides dans les plages à Ressac de la côte méditerranéenne de la Tunisie. Bull. Insti. Natl. Sci. Technol. Mer 1972, 2, 449–468. [Google Scholar]
- Worsfold, T.M. British records of the interstitial polychaete Stygocapitella subterranea (Annelida: Parergodrilidae). Mar. Biodivers. Rec. 2008, 1, 40. [Google Scholar] [CrossRef]
- Brinck, P.; Dahl, E.; Wieser, W. On the littoral subsoil fauna of the Simrishamn Beach in Eastern Scania. K. Fysiogr. Sällsk. Lund Förh. 1955, 25, 109–129. [Google Scholar]
- Itô, T. Studies on the interstitial animals in the Ishikari beach, Hokkaido, northern Japan-A preliminary report. BENTHOS Res. 1984, 1–14. [Google Scholar] [CrossRef]
- Westheide, W. Meiofauna geographic distribution: Vicariance and dispersal. Meiofauna Mar. 2005, 14, 201–207. [Google Scholar]
- Cerca, J.; Purschke, G.; Struck, T.H. Marine connectivity dynamics: Clarifying cosmopolitan distributions of marine interstitial invertebrates and the meiofauna paradox. Mar. Biol. 2018, 165, 123. [Google Scholar] [CrossRef]
- Struck, T.H.; Feder, J.L.; Bendiksby, M.; Birkeland, S.; Cerca, J.; Gusarov, V.I.; Kistenich, S.; Larsson, K.-H.; Liow, L.H.; Nowak, M.D.; et al. Finding Evolutionary Processes Hidden in Cryptic Species. Trends Ecol. Evol. 2018, 33, 153–163. [Google Scholar] [CrossRef]
- Gerlach, S.A. Means of meiofauna dispersal. Mikrofauna Meeresbod. 1977, 61, 89–103. [Google Scholar]
- Hedgpeth, J.W. Polychaeta Errantia of Antarctica. Volume 3, Antarctic Research Series.Olga HartmanPolychaeta Myzostomidae and Sedentaria of the Antarctica. Volume 7, Antarctic Research Series.Olga HartmanPolychaetous Annelids Collected by the USNS Eltanin and Staten Island Cruises Chiefly from Antarctic Seas. Allan Hancock Monographs in Biology, No. 2.Olga Hartman. Q. Rev. Biol. 1968, 43, 206. [Google Scholar] [CrossRef]
- Blake, J.A. Polychaeta Orbiniidae from Antarctica, the Southern Ocean, the Abyssal Pacific Ocean, and off South America. Zootaxa 2017, 4218, 1–145. [Google Scholar] [CrossRef] [PubMed]
- Day, J.H. New polychaeta from Beaufort: With a key to all species recorded from North Carolina. Biodivers. Herit. Libr. 1973, 375, 1–140. [Google Scholar] [CrossRef]
- Day, J. A review of the Australian and New Zealand Orbiniidae (Annelida: Polychaeta). In Essays on Polychaetous Annelids in Memory of Dr. Olga Hartman; Reish, D.J., Fauchald, K., Eds.; Allan Hancock Foundation: Los Angeles, CA, USA, 1977; pp. 217–243. [Google Scholar]
- Zhadan, A. Review of Orbiniidae (Annelida, Sedentaria) from Australia. Zootaxa 2020, 4860, 451–502. [Google Scholar] [CrossRef] [PubMed]
- Kirkegaard, J. Havborsteorme II. Sedentaria; Dansk Naturhistorisk Forening: Copenhagen, Denmark, 1996; Volume 86, p. 451. [Google Scholar]
- Blake, J.A. Family Orbiniidae Hartman, 1942. In Taxonomic Atlas of the Benthic Fauna of Santa Maria Basin and Western Santa Barbara Channel. The Annelida Part 3—Polychaeta: Orbiniidae to Cossuridae; Blake, J.A., Hilbig, B., Scott, P.H., Eds.; Santa Barbara Museum of Natural History: Santa Barbara, CA, USA, 1996; Volume 6, pp. 1–26. [Google Scholar]
- López, E. Familia Orbiniidae Hartman, 1942. In Fauna Iberica. Annelida Polychaeta III; Parapar, J., Alós, C., Núñez, J., Moreira, J., López, E., Aguirrezabalaga, F., Besteiro, C., Martinez, A., Eds.; Museo Nacional de Ciencias Naturales. CSIC: Madrid, Spain, 2012; Volume 36, pp. 96–160. [Google Scholar]
- Díaz-Díaz, O.; Vanegas-Espinosa, V.; Cárdenas-Oliva, A.; Liñero-Arana, I. Orbiniidae Hartman, 1942 (Annelida: Polychaeta) de las costas de Venezuela. Biota Colomb. 2012, 13, 18–34. [Google Scholar]
- Gillet, P. A new species of Orbiniella (Orbiniidae: Polychaeta) from Marion Island, Indian Ocean. Proc. Biol. Soc. Wash. 1999, 112, 592–597. [Google Scholar]
- Parapar, J.; Moreira, J.; Helgason, G.V. First record of genus Orbiniella Day, 1954 (Polychaeta: Orbiniidae) in North Atlantic Ocean with the description of a new species. Zootaxa 2015, 4006, 330–346. [Google Scholar] [CrossRef]
- Solis-Weiss, V.; Fauchald, K. Orbiniidae (Annelida: Polychaeta) from mangrove root-mats in Belize, with a revision of protoariciin genera. Proc. Biol. Soc. Wash. 1989, 102, 772–792. [Google Scholar]
- Sun, Y.; Li, X. Orbinia wui, a new species from China, with redescription of O. dicrochaeta Wu, 1962 (Annelida, Orbiniidae). Zootaxa 2018, 4403, 351–364. [Google Scholar] [CrossRef]
- Sun, Y.; Sui, J.; Li, X. A new species of Leodamas Kinberg, 1866 (Polychaeta: Orbiniidae) from the Yellow Sea and the East China Sea. Acta Oceanol. Sin. 2018, 37, 130–135. [Google Scholar] [CrossRef]
- Blake, J.A. New species and records of deep-water Orbiniidae (Annelida, Polychaeta) from the Eastern Pacific continental slope, abyssal Pacific Ocean, and the South China Sea. Zootaxa 2020, 4730, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Malmgren, A.J. Annulata Polychaeta Spetsbergiae, Groenlandiae, Islandiae et Scandinaviae hactenus cognita. Ofvers. K. Vetenskapsakademiens Forh. 1867, 24, 127–235. [Google Scholar]
- Hartman, O. A review of the types of polychaetous annelids at the Peabody Museum of Natural History, Yale University. Bull. Bingham Oceanogr. Collect. Yale Univ. 1942, 8, 1–98. [Google Scholar]
- Savigny, J.-C.; Benedict, J.E. Systèmes de Diverses Clases D’animaux Sans Vertèbres. Principalement de Celles des Côtes de l’Égypte et de la Syrie, Offrant les Caractères Tant Distinctifs que Naturels des Ordres, Familles et Genres, avec la Description des espèces. Available online: https://www.biodiversitylibrary.org/bibliography/66284#/summary (accessed on 18 December 2020).
- Quatrefages, A. Note sur la Classification des Annelides. Ann. Sci. Nat. Paris 1865, 5, 253–296. [Google Scholar]
- Hartman, O. Nomenclatural changes involving California polychaete worms. J. Wash. Acad. Sci. 1936, 26, 31–32. [Google Scholar]
- Eisig, H. Zur Systematik, Anatomie und Morphologie der ariciiden nebst beiträgen zur generellen Systematik. Mitteilungen Zool. Station Neapel 1914, 21, 153–500. [Google Scholar]
- Hartman, O. Orbiniidae, Apistobranchidae, Paraonidae and Longosomidae. Allan Hancock Pac. Exped. 1957, 15, 211–393. [Google Scholar]
- Pettibone, M.H. North American genera of the family Orbiniidae (Annelida: Polychaeta), with descriptions of new species. J. Wash. Acad. Sci. 1957, 47, 159–167. [Google Scholar]
- Mackie, A.S. A review of species currently assigned to the genus Leitoscoloplos Day, 1977 (Polychaeta: Orbiniidae), with descriptions of species newly referred to Scoloplos Blainville, 1828. Sarsia 1987, 72, 1–28. [Google Scholar] [CrossRef]
- Badalamenti, F.; Castelli, A. Schroederella laubieri, a new species of the subfamily protoariicinae (polychaeta, orbiniidae), with some notes on the genusschroederellalaubier, 1962. Boll. Zool. 1991, 58, 95–98. [Google Scholar] [CrossRef]
- Blake, J.A. A new genus of polychaete worm (Family Orbiniidae) from methane seeps in the Gulf of Mexico, with a review of the systematics and phylogenetic interrelationships of the genera of Orbiniidae. Cah. Biol. Mar. 2000, 4, 435–449. [Google Scholar]
- Bleidorn, C.; Vogt, L.; Bartolomaeus, T. A contribution to sedentary polychaete phylogeny using 18S rRNA sequence data. J. Zool. Syst. Evol. Res. 2003, 41, 186–195. [Google Scholar] [CrossRef]
- Hartman, O. Quantitative survey of the benthos of SanPedro Basin, Southern California. Part 11. Final results and conclusions. Allan Hancock Pac. Exped. 1966, 19, 187–456. [Google Scholar]
- Bleidorn, C.; Hill, N.; Erséus, C.; Tiedemann, R. On the role of character loss in orbiniid phylogeny (Annelida): Molecules vs. morphology. Mol. Phylogenet. Evol. 2009, 52, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Zhadan, A.; Stupnikova, A.N.; Neretina, T. Orbiniidae (Annelida: Errantia) from Lizard Island, Great Barrier Reef, Australia with notes on orbiniid phylogeny. Zootaxa 2015, 4019, 773–801. [Google Scholar] [CrossRef] [PubMed]
- Hartmann-Schroeder, G. Zweiter Beitrag zur Polychaetenfauna von Peru. Kiel. Meeresforsch. 1962, 18, 109–147. [Google Scholar]
- Ehlers, E. Ergebnisse der Hamburger Magalhaensischen Sammelreise 1892/93; L. Friederichsen & Co.: Hamburg, Germany, 1897; p. 148. [Google Scholar]
- Hernández-Alcántara, P.; Solís-Weiss, V. Anatomical and morphometric analysis of a new species of Leitoscoloplos (Annelida: Orbiniidae) with numerous stomach papillae, from the Gulf of California, Eastern Pacific. Contrib. Zool. 2014, 83, 133–150. [Google Scholar] [CrossRef]
- Verrill, A.E. Additions to the Turbellaria, Nemertina, and Annelida of the Bermudas, with revisions of some New England genera and species. Trans. Connect. Acad. Arts Sci. 1900, 10, 595–671. [Google Scholar] [CrossRef]
- Leão, L.S.D.; Santos, C.S.G. Orbinia (Polychaeta: Orbiniidae) from the Brazilian coast: Two new species and two new records. Zootaxa 2016, 4105, 145–158. [Google Scholar] [CrossRef]
- Kruse, I.; Reusch, T.B.; Schneider, M.V. Sibling species or poecilogony in the polychaete Scoloplos armiger? Mar. Biol. 2003, 142, 937–947. [Google Scholar] [CrossRef]
- Meyer, A.; Bleidorn, C.; Rouse, G.W.; Hausen, H. Morphological and molecular data suggest a cosmopolitan distribution of the polychaete Proscoloplos cygnochaetus Day, 1954 (Annelida, Orbiniidae). Mar. Biol. 2007, 153, 879–889. [Google Scholar] [CrossRef]
- Carr, C.M.; Hardy, S.M.; Brown, T.M.; Macdonald, T.A.; Hebert, P.D.N. A Tri-Oceanic Perspective: DNA Barcoding Reveals Geographic Structure and Cryptic Diversity in Canadian Polychaetes. PLoS ONE 2011, 6, e22232. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, S.; Hausen, H. Chaetal arrangement in Orbiniidae (Annelida, Polychaeta) and its significance for systematics. Zoomorphology 2007, 126, 215–227. [Google Scholar] [CrossRef]
- Hartmann-Schröder, G. Zur Kenntnis des eulitorals der australischen Kuesten unter besounderer Beruecksichtigung des Polychaeten und Ostracoden. Teil 16. Die Polychaeten der subtropisch-tropischen bis tropischen Ostkueste Australiens zwischen Maclean (New South Wales) und Gladstone (Queensland) sowie von Heron Island (Grosses Barrier Riff). Mitteilungen Hamburgischen Zool. Mus. Inst. 1991, 88, 17–71. [Google Scholar]
- Kinberg, J. Annulata nova. Ofversight K. Vetensk.–Adakemiens Forh. Stockh. 1866, 22, 239–258. [Google Scholar]
- Tebble, N. The polychaete fauna of the Gold Coast [=Ghana]. Bulletin of the British Museum (Natural History), Ser. Zoology 1955, 3, 59–148. [Google Scholar]
- De Oliveira, V.M.; Cutrim, A.S.T.; Vieira, A.K.M.; Ferreira, C.N.; Almeida, Z.S.; Júnior, M.N. Description of Scoloplos maranhensis sp. nov. (Orbiniidae, Annelida) from tropical Brazilian mangrove. Iheringia. Série Zool. 2019, 109. [Google Scholar] [CrossRef]
- Merz, R.A. Polychaete chaetae: Function, fossils, and phylogeny. Integr. Comp. Biol. 2006, 46, 481–496. [Google Scholar] [CrossRef]
- Martin, D.; Meca, M.A.; Gil, J.; Drake, P.; Nygren, A. Another brick in the wall: Population dynamics of a symbiotic species of Oxydromus (Annelida, Hesionidae), described as new based on morphometry. Contrib. Zool. 2017, 86, 181–211. [Google Scholar] [CrossRef]
- Meca, M.A.; Drake, P.; Martin, D. Does polyxenous symbiosis promote sympatric divergence? A morphometric and phylogeographic approach based on Oxydromus okupa (Annelida, Polychaeta, Hesionidae). Contrib. Zool. 2019, 88, 1–28. [Google Scholar] [CrossRef]
- Teixeira, M.A.L.; Vieira, P.E.; Pleijel, F.; Sampieri, B.R.; Ravara, A.; Costa, F.O.; Nygren, A. Molecular and morphometric analyses identify new lineages within a large Eumida (Annelida) species complex. Zool. Scr. 2019, 49, 222–235. [Google Scholar] [CrossRef]
- Lattig, P.; Martín, G.S.; Martin, D. Análisis toxinómico y morfométrico del completo Haplosyllis spongicola (Polychaeta: Syllidae: Syllinae) de los mares de España, con la re-descripción de la especie tipo y la descripción de dos nuevas especies. Sci. Mar. 2007, 71, 551–570. [Google Scholar] [CrossRef]
- Coutinho, M.C.L.; Paiva, P.C.; Santos, C.S.G. Morphometric analysis of two sympatric species ofPerinereis (Annelida: Nereididae) from the Brazilian coast. J. Mar. Biol. Assoc. U. K. 2015, 95, 953–959. [Google Scholar] [CrossRef]
- Monro, C.C.A. The Polychaeta Sedentaria collected by Dr. C. Crossland at Colón, in the Panama Region, and the Galapagos Islands during the Expedition of the S.Y. ‘St. George’. Proc. Zool. Soc. Lond. 2009, 103, 1039–1092. [Google Scholar] [CrossRef]
- Müller, O.F.; Dall, W.H. Zoologiae Danicae Prodromus, seu, Animalium Daniae et Norvegiae Indigenarum Characteres, Nomina, et Synonyma Imprimis Popularium. Available online: https://www.biodiversitylibrary.org/bibliography/13268#/summary (accessed on 18 December 2020).
- Wu, B. New species of polychaete worms of the family Orbiniidae and Paraonidae from the Yellow Sea. Acta Zool. Sin. 1962, 14, 421–426. [Google Scholar]
- Hartmann-Schröder, G. Die Polychaeten des Sublitorals. zur Kentnnis des Sublitorals der chilenischen Küste unter besonderer Berücksichtigung der Polychaeten und Ostracoden (Mit Bemerkungen über den Einfluss sauerstoffarmer strömungen auf die Besiedlung von marinen Sedimenten). Mitteilungen Hamburg. Zool. Mus. Inst. 1965, 62, 59–305. [Google Scholar]
- Gallardo, V.A. Polychaeta from the bay of Nha Trang, South Viet Nam. NAGA Rep. 1968, 4, 91–96. [Google Scholar]
- Fauchald, K. Benthic polychaetous annelids from deep water off western Mexico and adjacent areas in the eastern Pacific Ocean. Allan Hancock Monogr. Mar. Biol. 1972, 7, 162–171. [Google Scholar]
- Maciolek, N.J.; Holland, J. Scoloplos texana: A new orbiniid polychaete from south Texas, with notes on the related species Scoloplos treadwelli Eisig. Contrib. Mar. Sci. 1978, 21, 163–169. [Google Scholar]
- Hartmann-Schröder, G. Die Polychaeten der tropischen Nordwestküste Australiens (zwischen Derby im Norden und Port Hedland im Süden). Mitteilungen Hamburgisch. Zool. Mus. Inst. 1979, 76, 77–218. [Google Scholar]
- Hartmann-Schröder, G. des Eulitorals der australischen Küsten unter besonder Berücksichtigung der Polychaeten und Ostracoden. Mitteilungen Hamburgisch. Zool. Mus. Inst. 1980, 77, 67–68. [Google Scholar]
- Hartmann-Schröder, G. Zur Kenntnis des Eulitorals der australischen Kusten unter besonderer Berucksichtigung der Polychaeten und Ostracoden. Teil 6. Die Polychaeten der tropisch-subtropischen Westkuste Australiens (zwischen Exmouth im Norden und Cervantes im Suden). Mitteilungen Hamburgisch. Zool. Mus. Inst. 1981, 78, 48–49. [Google Scholar]
- Hartmann-Schröder, G. Zur Polychaetenfauna der Polynesischen Inseln Huahiné (Gesellschafts inseln) und Rangiroa (Tuamotu-Inseln). Mitteilungen Hamburgisch. Zool. Mus. Inst. 1992, 89, 68. [Google Scholar]
- Mohammad, M. Polychaete annelids from Kuwaitian islands, Arabian Gulf, with descriptions of four new species. Zool. J. Linn. Soc. 1980, 69, 31–42. [Google Scholar] [CrossRef]
- Westheide, W. Interstitielle Fauna von Galapagos. XXVI. Questidae, Cirratulidae, Acrocirridae, Ctenodrilidae (Polychaeta). Mikrofauna Meeresbod. 1981, 82, 6–8. [Google Scholar]
- Jamieson, B.; Webb, R. The morphology, spermatozoal ultrastructure and phylogenetic affinities of a new species of questid (Polychaeta, Annelida). In Proceedings of the 1st International Polychaete Conference, Sydney, Australia, 1–5 August 2016; Linnean Society of New South Wales: Sydney, Australia, 2016; pp. 21–34. [Google Scholar]
- Blake, J.A. Polychaeta from the vicinity of deep-sea geothermal vents in the eastern Pacific. I. Euphrosinidae, Phyllodocidae, Hesionidae, Nereididae, Glyceridae, Dorvilleidae, Orbiniidae, and Maldanidae. Bull. Biol. Soc. Wash. 1985, 6, 67–101. [Google Scholar]
- Mackie, A.S. A new species of Scoloplos (Polychaeta: Orbiniidae) from Hong Kong and a comparison with closely related Scoloplos marsupialis Southern, 1921 from India. Asian Mar. Biol. 1991, 8, 35–44. [Google Scholar]
- Blake, J.A.; Hilbig, B. Polychaeta from the vicinity of deep-sea hydrothermal vents in the Eastern Pacific Ocean. II. New species and records from the Juan de Fuca and Explorer Ridge systems. Pac. Sci. 1990, 44, 219–253. [Google Scholar]
- Buzhinskaya, G.N. Occurrence of Orbiniella plumisetosa sp. n. (Polychaeta: Orbiniidae) in the north-western Pacific Ocean and a key to species of the genus Orbiniella. Explor. Fauna Seas 1993, 43, 76–81. [Google Scholar]
- Branch, M.L. Four new species of Polychaeta from subantarctic Marion Island. Ann. S. Afr. Mus. 1998, 105, 249–265. [Google Scholar]
- Giere, O.; Erséus, C. A systematic account of the Questidae (Annelida, Polychaeta), with description of new taxa. Zool. Scr. 1998, 27, 345–360. [Google Scholar] [CrossRef]
- Mirsa, A. Polychaete [Fauna of West Bengal]. In Zoological Survey of India, Calcutta, Series 3; State Fauna Series, Fauna of West Bengal, Part 10, Invertebrates; Gosh, A.K., Ed.; Zoological survey of India: Calcuttam, India, 1999; pp. 125–225. [Google Scholar]
- Rouse, G.W. Orbiniidae Hartman, 1942. In Polychaetes; Rouse, G., Pleijel, F., Eds.; University Press: Oxford, UK, 2001; pp. 57–60. [Google Scholar]
- Eibye-Jacobsen, D. The Orbiniidae (Annelida: Polychaeta) of the BIOSHELF Project, Andaman Sea, Thailand. Phuket Mar. Biol. Cent. Spec. Publ. 2002, 24, 77–99. [Google Scholar]
- López, E.; Cladera, P.; Martín, G.S.; Lpez, E.; Martn, G.S. Two new species of the genus Leodamas (Orbiniidae: Scolecida: Polychaeta) from the Pacific coast of Panama. J. Mar. Biol. Assoc. U. K. 2003, 83, 367–374. [Google Scholar] [CrossRef]
- López, E.; Cladera, P.; San Martín, G. Orbiniidae polychaetes (Polychaeta: Scolecida) from Coiba Island, eastern Pacific of Panama, with description of a new species. Rev. Biol. Trop. 2006, 54, 1307–1318. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Narayanaswamy, B.E.; Blake, J.A. A new species of Orbiniella (Polychaeta: Orbiniidae) from deep basins of Antarctica. J. Mar. Biol. Assoc. U. K. 2005, 85, 843–846. [Google Scholar] [CrossRef]
- Giere, O.; Ebbe, B.; Erseus, C. Questa (Annelida, Polychaeta, Orbiniidae) from Pacific regions—New species and reassessment of the genus Periquesta. Org. Divers. Evol. 2008, 7, 304–319. [Google Scholar] [CrossRef][Green Version]
- Imajima, M. Deep-sea benthic polychaetes off Pacific coast of the northern Honshu, Japan. In Deep-Sea Fauna and Pollutants off Pacific Coast of Northern Japan; Fujita, T., Ed.; National Museum of Nature and Science Monographs: Tokyo, Japan, 2009; Volume 39, pp. 39–192. [Google Scholar]
- Gopal, A.; Useph, A.J.K.; Varghese, S.A.; Narayana, S.V. A new species of polychaete, Pettibonella shompenssp. nov. (Orbiniidae), from the Nicobar Islands, North Indian Ocean. Mar. Biol. Res. 2014, 10, 1033–1037. [Google Scholar] [CrossRef]
- Dean, H.K.; Blake, J.A. The Orbiniidae (Annelida: Polychaeta) of Pacific Costa Rica. Zootaxa 2015, 3956, 183–198. [Google Scholar] [CrossRef][Green Version]
- Blake, J.A. Life history analysis of five dominant infaunal polychaete species from the continental slope off North Carolina. J. Mar. Biol. Assoc. U. K. 1993, 73, 123–141. [Google Scholar] [CrossRef]
- Fabricius, O. Fauna Groenlandica, systematice sistens, Animalia Groenlandiae occidentalis hactenus indagata, quoad nomen specificum, triviale, vernaculumque; synonyma auctorum plurium, descriptionem, locum, victum, generationem, mores, usum, capturamque singuli; prout detegendi occasion fuit, maximaque parti secundum proprias observationes. Hafniae Lipsiae 1780, i-xvi, 1–452. [Google Scholar]
- Sars, G.O. Diagnoser af nye Annelider fra Christianiaforden, efter Professor M. Sar’s efterladte Manuskripter. Forh. Vidensk. Christiania 1872, 1871, 406–417. [Google Scholar]
- Claparède, É. Glanures zootomiques parmi les annélides de Port-Vendres (Pyrénées Orientales). Mém. Société Phys. d’Histoire Nat. Genève 1864, 17, 463–600. [Google Scholar]
- Day, J.H. The Polychaeta of Tristan da Cunha. Results Nor. Sci. Exped. Tristan Cunha 1954, 29, 1–35. [Google Scholar]
- Blake, J.A.; Giangrande, A. Naineris setosa (Verrill) (Polychaeta, Orbiniidae), an American subtropical–tropical polychaete collected from an aquaculture facility in Brindisi (Adriatic Sea, Italy): A possible alien species. Ital. J. Zool. 2011, 78, 20–26. [Google Scholar] [CrossRef]
- Khedhri, I. First record of Naineris setosa (Verrill, 1900) (Annelida: Polychaeta: Orbiniidae) in the Western Mediterranean Sea. BioInvasions Rec. 2014, 3, 83–88. [Google Scholar] [CrossRef]
- Raman, A.; Ganapati, P. Occurrence of Scolaricia capensis Day, 1961 (Polychaeta: Orbiniidae) off the east coast of India, Bay of Bengal. Indian J. Mar. Sci. 1977, 6, 185–186. [Google Scholar]
- Zenetos, A.; Gofas, S.; Verlaque, M.; Cinar, M.; Raso, J.G.; Bianchi, C.; Morri, C.; Azzurro, E.; Bilecenoglu, M.; Froglia, C.; et al. Alien species in the Mediterranean Sea by 2010. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part I. Spatial distribution. Mediterr. Mar. Sci. 2010, 11, 381. [Google Scholar] [CrossRef]
- Day, J.H. Israel South Red Sea Expedition, 1962, Reports no. 7. Some Polychaeta from the Israel South Red Sea Expedition, 1962. Bull. Sea Fish. Res. Stn. Haifa 1965, 38, 15–27. [Google Scholar]
- Harmelin, J.G. Contribution à l’étude de l’endofaune des prairies d’Halophila stipulacea de Méditerranée Orientale. Recl. Trav. Stn. Mar. d’Endoume 1969, 45, 305–316. [Google Scholar]
- McIntosh, W.C. Report on the Annelida Polychaeta collected by HMS “Challenger” during the years 1873–1876. Reports on the Scientific Results of the Voyage of HMS “Challenger”. Zoology 1885, 12, 1–554. [Google Scholar]
- Ramos, J.M. Haploscoloplos kerguelensis McIntosh, 1885. Nouvel Orbiniidae en Méditerranée Occidentale. Vie Milieu 1976, 26, 1–9. [Google Scholar]
- Claparède, R.-É. Royal College of Physicians of Edinburgh. In Les Annelides Chétopodes du Golfe de Naples; Smithsonian Institution: Washington, DC, USA, 1870; Volume 19, pp. 313–584. [Google Scholar]
- Chamberlin, R.V. New polychaetous annelids from Laguna Beach, California. Pomona Coll. J. Entomol. Zool. 1919, 11, 1–23. [Google Scholar]
- Kinberg, J. Annulata Nova. Öfversigt Königlich Vetensk. Förhandlingar 1866, 22, 337. [Google Scholar]
- Bleidorn, C.; Kruse, I.; Albrecht, S.; Bartolomaeus, T. Mitochondrial sequence data expose the putative cosmopolitan polychaete Scoloplos armiger (Annelida, Orbiniidae) as a species complex. BMC Evol. Biol. 2006, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Verrill, A.E. XVIII. Report upon the Invertebrate Animals of Vineyard Sound and the Adjacent Waters, with an Account of the Physical Characters of the Region. Available online: https://www.biodiversitylibrary.org/bibliography/11688#/summary (accessed on 18 December 2020).
- Holte, B.; Gulliksen, B. Common macrofaunal dominant species in the sediments of some north Norwegian and Svalbard glacial fjords. Polar Biol. 1998, 19, 375–382. [Google Scholar] [CrossRef]
- Carvalho, S.; Ravara, A.; Quintino, V.; Rodrigues, A. Macrobenthic community characterisation of an estuary from the western coast of Portugal (Sado estuary) prior to dredging operations. Boletín Inst. Español Oceanogr. 2001, 17, 179–190. [Google Scholar]
- Belan, T.A. Marine environmental quality assessment using polychaete taxocene characteristics in Vancouver Harbour. Mar. Environ. Res. 2004, 57, 89–101. [Google Scholar] [CrossRef]
- Kraan, C.; Dekinga, A.; Piersma, T. Now an empty mudflat: Past and present benthic abundances in the western Dutch Wadden Sea. Helgol. Mar. Res. 2010, 65, 51–58. [Google Scholar] [CrossRef]
- Luttikhuizen, P.C.; Bol, A.; Cardoso, J.F.; Dekker, R. Overlapping distributions of cryptic Scoloplos cf. armiger species in the western Wadden Sea. J. Sea Res. 2011, 66, 231–237. [Google Scholar] [CrossRef]
- Faulwetter, S.; Simboura, N.; Katsiaras, N.; Chatzigeorgiou, G.; Arvanitidis, C. Polychaetes of Greece: An updated and annotated checklist. Biodivers. Data J. 2017, 5, e20997. [Google Scholar] [CrossRef]
- Kruse, I.; Reise, K. Reproductive isolation between intertidal and subtidal Scoloplos armiger (Polychaeta, Orbiniidae) indicates sibling species in the North Sea. Mar. Biol. 2003, 143, 511–517. [Google Scholar] [CrossRef]
- Kruse, I.; Strasser, M.; Thiermann, F. The role of ecological divergence in speciation between intertidal and subtidal Scoloplos armiger (Polychaeta, Orbiniidae). J. Sea Res. 2004, 51, 53–62. [Google Scholar] [CrossRef]
- Francoeur, A.A.; Dorgan, K.M. Burrowing Behavior in Mud and Sand of Morphologically Divergent Polychaete Species (Annelida: Orbiniidae). Biol. Bull. 2014, 226, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Blake, J.A.; Hilbig, B. Dense infaunal assemblages on the continental slope off Cape Hatteras, North Carolina. Deep. Sea Res. Part II Top. Stud. Oceanogr. 1994, 41, 875–899. [Google Scholar] [CrossRef]
- Zhadan, A. Taxonomy of the genus Scoloplos (orbiniidae) in the white, barents and north seas. Зooлoгический журнал 1998, 77, 177–190. [Google Scholar]
- Fauvel, P. Sur l’Aricia foetida, Claparède et ses variétés. Bull. Société Zool. Fr. 1924, 49, 518–528. [Google Scholar]
- Hartman, O. Systematic account of some marine invertebrate animals from the deep basins off southern California. Allan Hancock Pac. Exped. 1960, 22, 69–216. [Google Scholar]
- Grube, A.E. Beschreibungen neuer oder wenig bekannter Anneliden. Archiv für Naturgeschichte 1855, 21, 81–136. [Google Scholar] [CrossRef]
- Day, F.J.H. The polychaet fauna of south africa. part 6. sedentary species dredged off cape coasts with a few new records from the shore. J. Linn. Soc. Lond. Zool. 1961, 44, 463–560. [Google Scholar] [CrossRef]
- Southern, R. Polychaeta of the Chilka Lake and also of fresh and brackish waters in other parts of India. Mem. Indian Mus. 1921, 5, 563–659. [Google Scholar]
- Giangrande, A.; Fraschetti, S. A population study of Naineris laevigata (Polychaeta, Orbiniidae) in a fluctuating environment (Mediterranean Sea). Sci. Mar. 1995, 59, 39–48. [Google Scholar]
- Khan, S.A.; Murugesan, P. Polychaete diversity in Indian estuaries. Indian J. Mar. Sci. 2005, 34, 114–119. [Google Scholar]
- Grieshaber, M. Adaptation of the polychaete worm Scoloplos armiger to hypoxic conditions. Mar. Biol. 1988, 99, 215–222. [Google Scholar] [CrossRef]
- Linnaeus, C. Systema Naturae per regna tria naturae secundum classes, ordines, genera, species cum characteribus, differentiis, synonymis, locis. Editio decima, reformata. Laurentius Salvius Holmiae 1758, 2, 824. [Google Scholar]
- Gustafson, R.G.; Turner, R.D.; Lutz, R.; Vrijenhoek, R.C. A new genus and five new species of mussels (Bivalvia, Mytilidae) from deep-sea sulfide/hydrocarbon seeps in the Gulf of Mexico. Malacologia 1998, 40, 63–112. [Google Scholar]
- Hourdez, S.; Schernecke, A.; Fisher, C.R.; Frederick, L.-A. Functional respiratory anatomy of a deep-sea orbiniid polychaete from the Brine Pool NR-1 in the Gulf of Mexico. Invertebr. Biol. 2005, 120, 29–40. [Google Scholar] [CrossRef]
- Hourdez, S.; Weber, R.E.; Green, B.N.; Kenney, J.M.; Fisher, C.R. Respiratory adaptations in a deep-sea orbiniid polychaete from Gulf of Mexico brine pool NR-1: Metabolic rates and hemoglobin structure/function relationships. J. Exp. Biol. 2002, 205, 1669–1681. [Google Scholar]
- Van Gaest, A.L.; Young, C.M.; Young, J.J.; Helms, A.R.; Arellano, S.M. Physiological and behavioral responses of Bathynerita naticoidea (Gastropoda: Neritidae) and Methanoaricia dendrobranchiata (Polychaeta: Orbiniidae) to hypersaline conditions at a brine pool cold seep. Mar. Ecol. 2007, 28, 199–207. [Google Scholar] [CrossRef]
- Will, K.W.; Mishler, B.D.; Wheeler, Q.D. The Perils of DNA Barcoding and the Need for Integrative Taxonomy. Syst. Biol. 2005, 54, 844–851. [Google Scholar] [CrossRef]
- Padial, J.M.; Miralles, A.; De La Riva, I.; Vences, M. The integrative future of taxonomy. Front. Zool. 2010, 7, 16. [Google Scholar] [CrossRef]
- Giribet, G. Morphology should not be forgotten in the era of genomics—A phylogenetic perspective. Zool. Anz.-A J. Comp. Zool. 2015, 256, 96–103. [Google Scholar] [CrossRef]
- Fauvel, P. Annélides Polychètes nouvelles de l’Afrique orientale: 2ième note. Bull. Mus. D’histoire Nat. 1919, 1, 33–39. [Google Scholar]
- Borja, A.; Franco, J.; Pérez, V. A Marine Biotic Index to Establish the Ecological Quality of Soft-Bottom Benthos Within European Estuarine and Coastal Environments. Mar. Pollut. Bull. 2000, 40, 1100–1114. [Google Scholar] [CrossRef]
- Bigot, L.; Grémare, A.; Amouroux, J.-M.; Frouin, P.; Maire, O.; Gaertner, J.C. Assessment of the ecological quality status of soft-bottoms in Reunion Island (tropical Southwest Indian Ocean) using AZTI marine biotic indices. Mar. Pollut. Bull. 2008, 56, 704–722. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, H.; Weisberg, S.B.; Borja, A.; Ranasinghe, J.A.; Cadien, D.B.; Velarde, R.G.; Lovell, L.L.; Pasko, D.; Phillips, C.A.; Montagne, D.E.; et al. Calibration and validation of the AZTI’s Marine Biotic Index (AMBI) for Southern California marine bays. Ecol. Indic. 2012, 12, 84–95. [Google Scholar] [CrossRef]
- Driscoll, S.K.; McElroy, A.E. Bioaccumulation and metabolism of benzo[A]pyrene in three species of polychaete worms. Environ. Toxicol. Chem. 1996, 15, 1401–1410. [Google Scholar] [CrossRef]
- Hartman, O. The littoral marine annelids of the Gulf of Mexico. Publ. Inst. Mar. Sci. Port Aransas Texas 1951, 2, 7–124. [Google Scholar]
- Rakocinski, C.F.; Brown, S.S.; Gaston, G.R.; Heard, R.W.; Walker, W.W.; Summers, J.K. Macrobenthic Responses to Natural and Contaminant-Related Gradients in Northern Gulf of Mexico Estuaries. Ecol. Appl. 1997, 7, 1278. [Google Scholar] [CrossRef]
- Elías, R.; Rivero, M.S.; Vallarino, E.A. Sewage impact on the composition and distribution of Polychaeta associated to intertidal mussel beds of the Mar del Plata rocky shore, Argentina. Iheringia. Série Zool. 2003, 93, 309–318. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meca, M.A.; Zhadan, A.; Struck, T.H. The Early Branching Group of Orbiniida Sensu Struck et al., 2015: Parergodrilidae and Orbiniidae. Diversity 2021, 13, 29. https://doi.org/10.3390/d13010029
Meca MA, Zhadan A, Struck TH. The Early Branching Group of Orbiniida Sensu Struck et al., 2015: Parergodrilidae and Orbiniidae. Diversity. 2021; 13(1):29. https://doi.org/10.3390/d13010029
Chicago/Turabian StyleMeca, Miguel A., Anna Zhadan, and Torsten H. Struck. 2021. "The Early Branching Group of Orbiniida Sensu Struck et al., 2015: Parergodrilidae and Orbiniidae" Diversity 13, no. 1: 29. https://doi.org/10.3390/d13010029
APA StyleMeca, M. A., Zhadan, A., & Struck, T. H. (2021). The Early Branching Group of Orbiniida Sensu Struck et al., 2015: Parergodrilidae and Orbiniidae. Diversity, 13(1), 29. https://doi.org/10.3390/d13010029




