Dark Septate Endophytic Fungi Associated with Sugarcane Plants Cultivated in São Paulo, Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sampling and Isolation of Dark Septate Endophytic Fungi
2.2. Identification of the Isolated Dark Septate Endophytic Fungi
3. Results/Discussion
3.1. Isolates of Dark Septate Endophytes
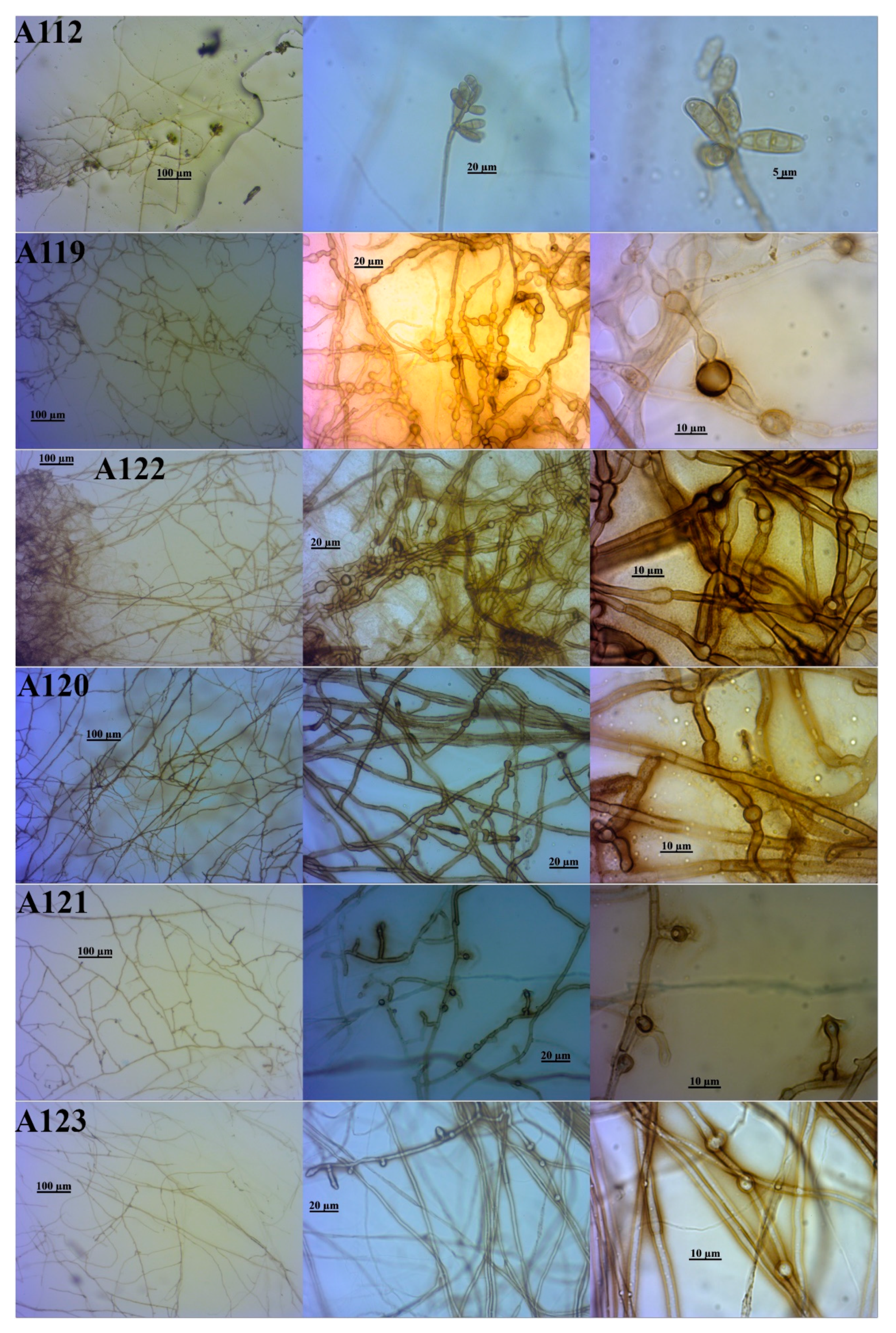
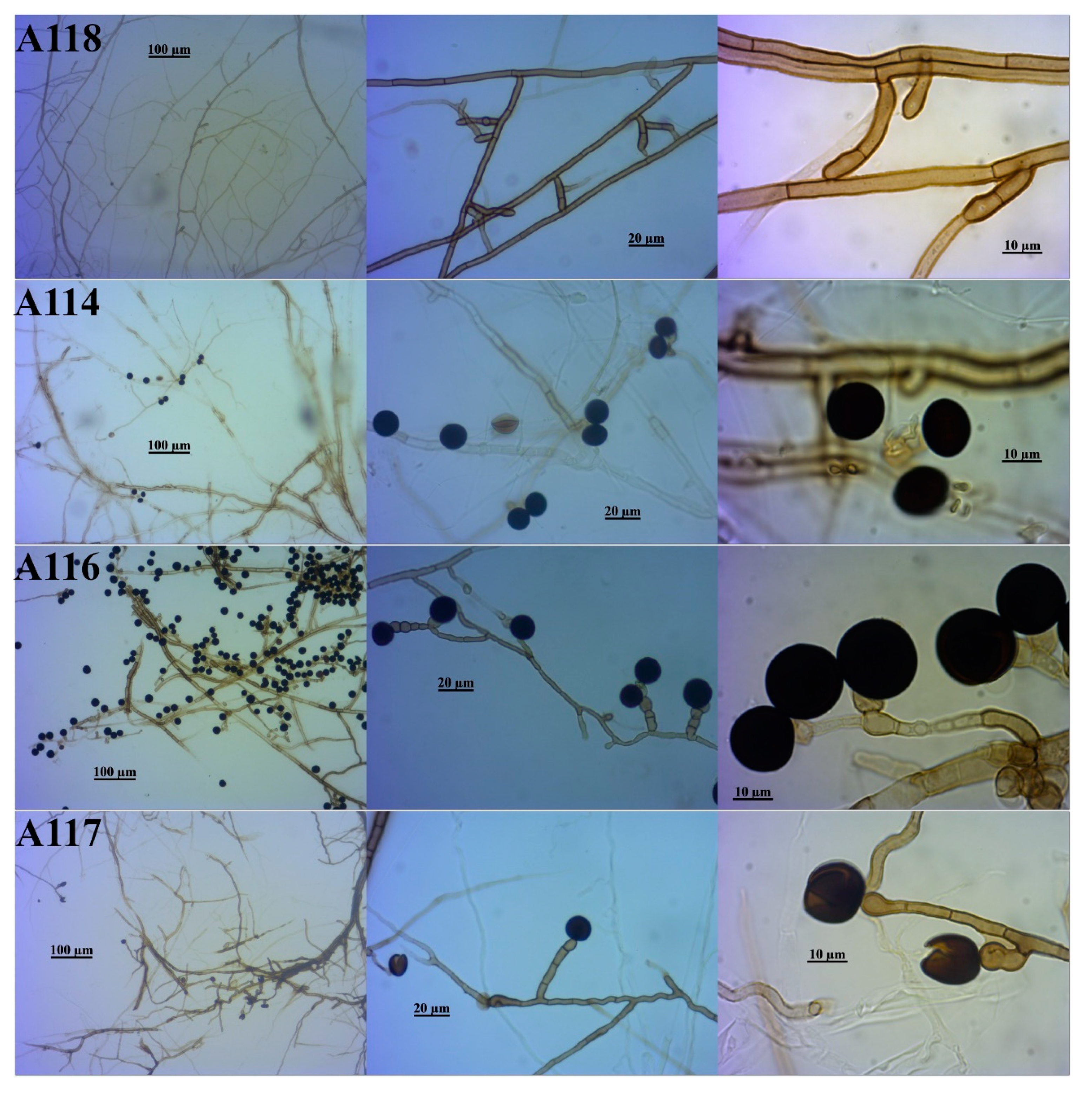
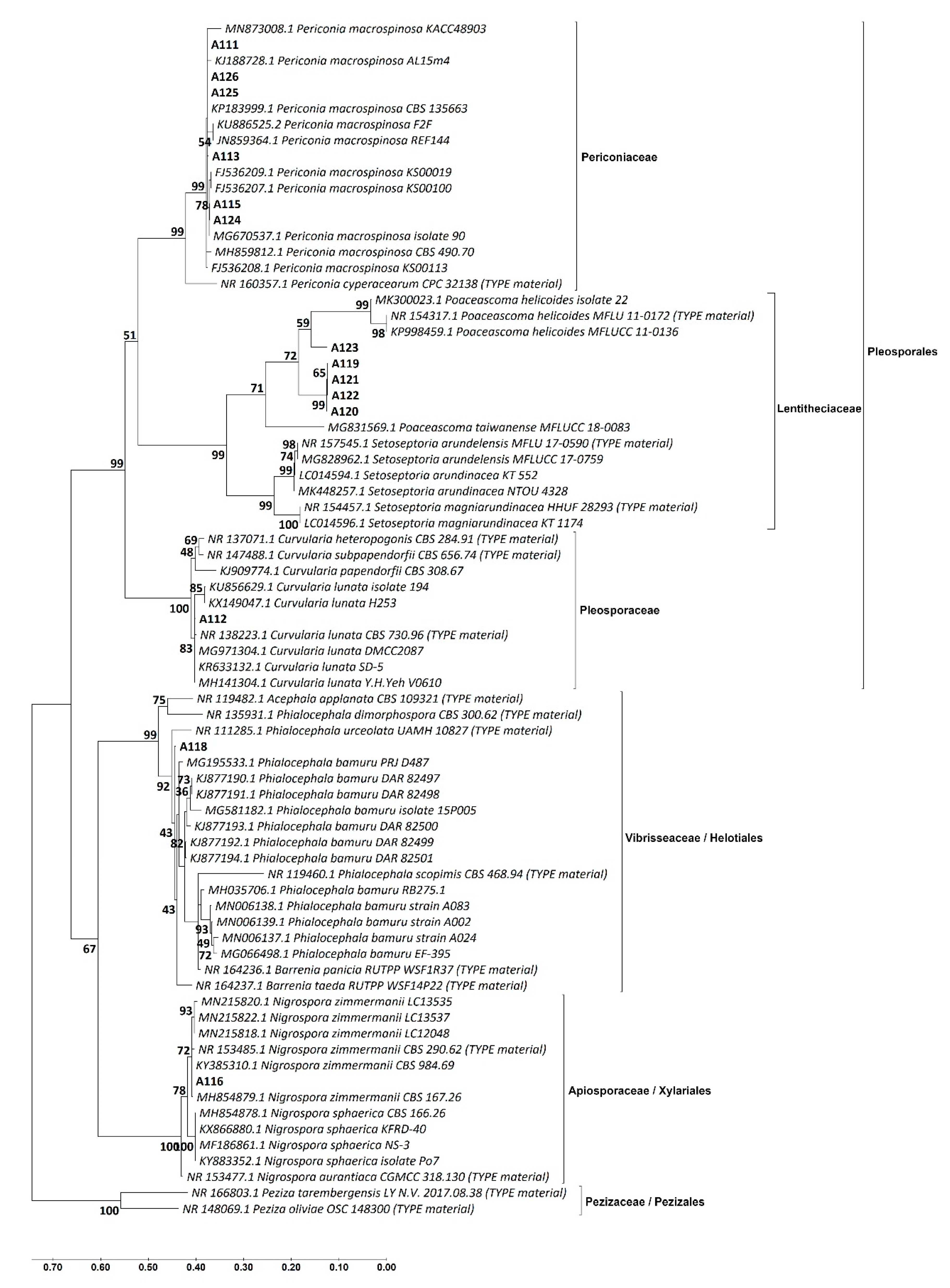
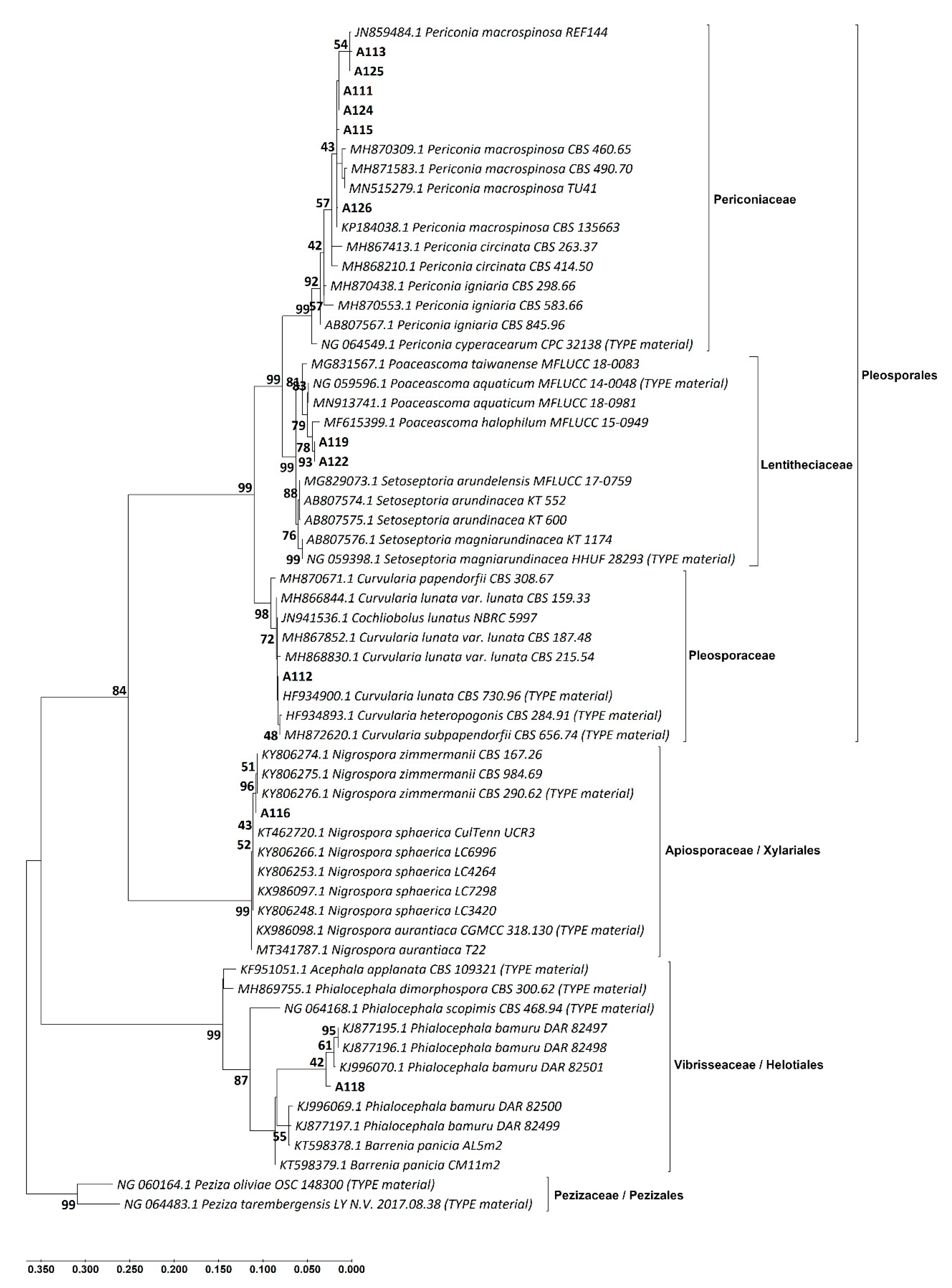
3.2. Identification of the DSE Isolates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grünig, C.R.; Queloz, V.; Sieber, T.N. Structure of Diversity in Dark Septate Endophytes: From Species to Genes. In Endophytes of Forest Trees: Biology and Applications; Pirttilä, A.M., Frank, A.C., Eds.; Forestry Sciences; Springer: Dordrecht, The Netherlands, 2011; pp. 3–30. ISBN 978-94-007-1599-8. [Google Scholar]
- Ruotsalainen, A.L. Dark Septate Endophytes (DSE) in Boreal and Subarctic Forests. In Endophytes of Forest Trees; Pirttilä, A.M., Frank, A.C., Eds.; Springer International Publishing: Cham, Switzerland, 2018; Volume 86, pp. 105–117. ISBN 978-3-319-89832-2. [Google Scholar]
- Rodriguez, R.J.; White, J.F., Jr.; Arnold, A.E.; Redman, R.S. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef]
- Jumpponen, A.; Trappe, J.M. Dark septate endophytes: A review of facultative biotrophic root-colonizing fungi. New Phytol. 1998, 140, 295–310. [Google Scholar] [CrossRef]
- Berthelot, C.; Chalot, M.; Leyval, C.; Blaudez, D. From Darkness to Light: Emergence of the Mysterious Dark Septate Endophytes in Plant Growth Promotion and Stress Alleviation. In Endophytes for a Growing World; Hodkinson, T.R., Doohan, F.M., Saunders, M.J., Murphy, B.R., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 143–164. ISBN 978-1-108-60766-7. [Google Scholar]
- Mandyam, K.G.; Jumpponen, A. Mutualism–parasitism paradigm synthesized from results of root-endophyte models. Front. Microbiol. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Knapp, D.G.; Kovács, G.M.; Zajta, E.; Groenewald, J.Z.; Crous, P.W. Dark septate endophytic pleosporalean genera from semiarid areas. Pers. Mol. Phylogeny Evol. Fungi 2015, 35, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Bonfim, J.A.; Vasconcellos, R.L.F.; Baldesin, L.F.; Sieber, T.N.; Cardoso, E.J.B.N. Dark septate endophytic fungi of native plants along an altitudinal gradient in the Brazilian Atlantic forest. Fungal Ecol. 2016, 20, 202–210. [Google Scholar] [CrossRef]
- Hou, L.; He, X.; Li, X.; Wang, S.; Zhao, L. Species composition and colonization of dark septate endophytes are affected by host plant species and soil depth in the Mu Us sandland, northwest China. Fungal Ecol. 2019, 39, 276–284. [Google Scholar] [CrossRef]
- Knapp, D.G.; Pintye, A.; Kovács, G.M. The Dark Side Is Not Fastidious—Dark Septate Endophytic Fungi of Native and Invasive Plants of Semiarid Sandy Areas. PLoS ONE 2012, 7, e32570. [Google Scholar] [CrossRef]
- Mandyam, K.; Fox, C.; Jumpponen, A. Septate endophyte colonization and host responses of grasses and forbs native to a tallgrass prairie. Mycorrhiza 2012, 22, 109–119. [Google Scholar] [CrossRef]
- Mandyam, K.; Loughin, T.; Jumpponen, A. Isolation and morphological and metabolic characterization of common endophytes in annually burned tallgrass prairie. Mycologia 2010, 102, 813–821. [Google Scholar] [CrossRef]
- Vergara, C.; Araujo, K.E.C.; Urquiaga, S.; Schultz, N.; Balieiro, F.d.C.; Medeiros, P.S.; Santos, L.A.; Xavier, G.R.; Zilli, J.E. Dark Septate Endophytic Fungi Help Tomato to Acquire Nutrients from Ground Plant Material. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Xu, R.; Li, T.; Shen, M.; Yang, Z.L.; Zhao, Z.-W. Evidence for a Dark Septate Endophyte (Exophiala Pisciphila, H93) Enhancing Phosphorus Absorption by Maize Seedlings. Plant Soil 2020, 452, 249–266. [Google Scholar] [CrossRef]
- Santos, S.G.; Silva, P.R.A.; Garcia, A.C.; Zilli, J.É.; Berbara, R.L.L. Dark septate endophyte decreases stress on rice plants. Braz. J. Microbiol. 2017, 48, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, X.-L.; Zhou, Y.; Hou, Y.-T.; Zuo, Y.-L. Effects of Dark Septate Endophytes on the Performance of Hedysarum scoparium Under Water Deficit Stress. Front. Plant. Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, M.J.; Zhang, X.T.; Zhang, H.B.; Sha, T.; Zhao, Z.W. Improved tolerance of maize (Zea mays L.) to heavy metals by colonization of a dark septate endophyte (DSE) Exophiala pisciphila. Sci. Total Environ. 2011, 409, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Likar, M.; Regvar, M. Isolates of dark septate endophytes reduce metal uptake and improve physiology of Salix caprea L. Plant Soil 2013, 370, 593–604. [Google Scholar] [CrossRef]
- Gonzalez Mateu, M.; Baldwin, A.H.; Maul, J.E.; Yarwood, S.A. Dark septate endophyte improves salt tolerance of native and invasive lineages of Phragmites australis. ISME J. 2020, 14, 1943–1954. [Google Scholar] [CrossRef]
- Narisawa, K.; Usuki, F.; Hashiba, T. Control of Verticillium Yellows in Chinese Cabbage by the Dark Septate Endophytic Fungus LtVB3. Phytopathology 2004, 94, 412–418. [Google Scholar] [CrossRef]
- Deng, X.; Song, X.; Halifu, S.; Yu, W.; Song, R. Effects of Dark Septate Endophytes Strain A024 on Damping-off Biocontrol, Plant Growth and the Rhizosphere Soil Enviroment of Pinus sylvestris var. mongolica Annual Seedlings. Plants 2020, 9, 913. [Google Scholar] [CrossRef]
- Harsonowati, W.; Marian, M.; Surono; Narisawa, K. The Effectiveness of a Dark Septate Endophytic Fungus, Cladophialophora chaetospira SK51, to Mitigate Strawberry Fusarium Wilt Disease and With Growth Promotion Activities. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Mandyam, K.G.; Roe, J.; Jumpponen, A. Arabidopsis thaliana model system reveals a continuum of responses to root endophyte colonization. Fungal Biol. 2013, 117, 250–260. [Google Scholar] [CrossRef]
- Rothen, C.; Miranda, V.; Aranda-Rickert, A.; Fracchia, S.; Rodríguez, M.A. Characterization of dark septate endophyte fungi associated with cultivated soybean at two growth stages. Appl. Soil Ecol. 2017, 120, 62–69. [Google Scholar] [CrossRef]
- Mayerhofer, M.S.; Kernaghan, G.; Harper, K.A. The effects of fungal root endophytes on plant growth: A meta-analysis. Mycorrhiza 2013, 23, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Berthelot, C.; Blaudez, D.; Leyval, C. Differential growth promotion of poplar and birch inoculated with three dark septate endophytes in two trace element-contaminated soils. Int. J. Phytoremediat. 2017, 19, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Hulse, J.D. First Report of Dark Septate Endophytes imaged in Cucurbita maxima grown in the Eastern United States. Acta Sci. Agric. 2018, 2, 61–64. [Google Scholar]
- Vergara, C.; Araujo, K.E.C.; Urquiaga, S.; Santa-Catarina, C.; Schultz, N.; Silva Araújo, E.; Carvalho Balieiro, F.; Xavier, G.R.; Zilli, J.É. Dark Septate Endophytic Fungi Increase Green Manure-15N Recovery Efficiency, N Contents, and Micronutrients in Rice Grains. Front. Plant. Sci. 2018, 9. [Google Scholar] [CrossRef]
- Rudorff, B.F.T.; Aguiar, D.A.; Silva, W.F.; Sugawara, L.M.; Adami, M.; Moreira, M.A. Studies on the Rapid Expansion of Sugarcane for Ethanol Production in São Paulo State (Brazil) Using Landsat Data. Remote Sens. 2010, 2, 1057–1076. [Google Scholar] [CrossRef]
- Romão-Dumaresq, A.S.; Dourado, M.N.; de Lima Fávaro, L.C.; Mendes, R.; Ferreira, A.; Araújo, W.L. Diversity of Cultivated Fungi Associated with Conventional and Transgenic Sugarcane and the Interaction between Endophytic Trichoderma virens and the Host Plant. PLoS ONE 2016, 11, e0158974. [Google Scholar] [CrossRef]
- Souza, R.S.C.; Okura, V.K.; Armanhi, J.S.L.; Jorrín, B.; Lozano, N.; Silva, M.J.; González-Guerrero, M.; Araújo, L.M.; Verza, N.C.; Bagheri, H.C.; et al. Unlocking the bacterial and fungal communities assemblages of sugarcane microbiome. Sci. Rep. 2016, 6, 28774. [Google Scholar] [CrossRef]
- Claassens, A.; Nock, C.J.; Rose, M.T.; Zwieten, L.V.; Rose, T.J. Colonisation dynamics of arbuscular mycorrhizal fungi and dark septate endophytes in the sugarcane crop cycle. Rhizosphere 2018, 7, 18–26. [Google Scholar] [CrossRef]
- Fors, R.O.; Júnior, O.J.S.; Carneiro, M.A.C.; Berbara, R.L.L. Selection of arbuscular mycorrhizal fungi for sugarcane in four soils with the presence of dark septate endophytes. Acta Sci. Agron. 2020, 42, e42477. [Google Scholar] [CrossRef]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araujo Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos; Embrapa: Brasília, Brazil, 2018; ISBN 978-85-7035-817-2. [Google Scholar]
- Ribeiro, K.G.; Pereira, G.M.D.; Mosqueira, C.A.; Baraúna, A.C.; Vital, M.J.S.; Silva, K.; Zilli, J.É. Isolamento, armazenamento e determinação da colonização por fungos “dark septate” a partir de plantas de arroz. Rev. Agrombiente Online 2011, 5, 97–105. [Google Scholar] [CrossRef]
- Silvani, V.A.; Fracchia, S.; Fernández, L.; Pérgola, M.; Godeas, A. A simple method to obtain endophytic microorganisms from field-collected roots. Soil Biol. Biochem. 2008, 40, 1259–1263. [Google Scholar] [CrossRef]
- Novais, C.B.; Borges, W.L.; Silva, G.A.; Saggin-Júnior, O.J. Técnicas Básicas em Micorrizas Arbusculares; UFLA: Lavras, Brazil, 2017; ISBN 978-85-8127-062-3. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal lRNA genes for phylogenetics. In PCR Protocols; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. ISBN 978-0-12-372180-8. [Google Scholar]
- Rehner, S.A.; Samuels, G.J. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycol. Res. 1994, 98, 625–634. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- Carbone, I.; Kohn, L.M. A Method for Designing Primer Sets for Speciation Studies in Filamentous Ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Groenewald, J.Z.; Nakashima, C.; Nishikawa, J.; Shin, H.-D.; Park, J.-H.; Jama, A.N.; Groenewald, M.; Braun, U.; Crous, P.W. Species concepts in Cercospora: Spotting the weeds among the roses. Stud. Mycol. 2013, 75, 115–170. [Google Scholar] [CrossRef] [PubMed]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- European and Mediterranean Plant Protection Organization (EPPO) Q-Bank. Available online: https://qbank.eppo.int/fungi/ (accessed on 13 February 2019).
- Morgulis, A.; Coulouris, G.; Raytselis, Y.; Madden, T.L.; Agarwala, R.; Schäffer, A.A. Database indexing for production MegaBLAST searches. Bioinformatics 2008, 24, 1757–1764. [Google Scholar] [CrossRef]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. J. Comput. Mol. Cell Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000; ISBN 9780195135855. [Google Scholar]
- MycoBank Database. Available online: https://www.mycobank.org/ (accessed on 17 June 2020).
- Index Fungorum. Available online: http://www.indexfungorum.org/Names/Names.asp (accessed on 17 June 2020).
- Ali-Shtayeh, M.S.; Jamous, R.M.; Yaghmour, R.M.-R. Mycology Manual; An-Najah National University: Nablus, Palestine, 1998. [Google Scholar]
- Lin, W.; Wu, L.; Lin, S.; Zhang, A.; Zhou, M.; Lin, R.; Wang, H.; Chen, J.; Zhang, Z.; Lin, R. Metaproteomic analysis of ratoon sugarcane rhizospheric soil. BMC Microbiol. 2013, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, C.; He, X.; Su, F.; Hou, L.; Ren, Y.; Hou, Y. Dark septate endophytes improve the growth of host and non-host plants under drought stress through altered root development. Plant Soil 2019, 439, 259–272. [Google Scholar] [CrossRef]
- Yuan, Z.-L.; Zhang, C.-L.; Lin, F.-C.; Kubicek, C.P. Identity, Diversity, and Molecular Phylogeny of the Endophytic Mycobiota in the Roots of Rare Wild Rice (Oryza granulate) from a Nature Reserve in Yunnan, China. Appl. Environ. Microbiol. 2010, 76, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Loro, M.; Valero-Jiménez, C.A.; Nozawa, S.; Márquez, L.M. Diversity and composition of fungal endophytes in semiarid Northwest Venezuela. J. Arid Environ. 2012, 85, 46–55. [Google Scholar] [CrossRef]
- Ellis, M.B. Periconia macrospinosa. Descriptions of Fungi and Bacteria. IMI Descr. Fungi Bact. 1968, 17, 168. [Google Scholar]
- Herrera, J.; Poudel, R.; Bokati, D. Assessment of root-associated fungal communities colonizing two species of tropical grasses reveals incongruence to fungal communities of North American native grasses. Fungal Ecol. 2013, 6, 65–69. [Google Scholar] [CrossRef]
- Lima, A.; Furtado, M. Espécies do género Curvularia (fungos anamórficos: Hyphomycetes) na ilha de Santiago, Cabo Verde. Port. Acta Biol. 2007, 22, 145–156. [Google Scholar]
- Manamgoda, D.S.; Cai, L.; McKenzie, E.H.C.; Crous, P.W.; Madrid, H.; Chukeatirote, E.; Shivas, R.G.; Tan, Y.P.; Hyde, K.D. A phylogenetic and taxonomic re-evaluation of the Bipolaris—Cochliobolus—Curvularia Complex. Fungal Divers. 2012, 56, 131–144. [Google Scholar] [CrossRef]
- Liang, Y.; Ran, S.-F.; Bhat, J.; Hyde, K.D.; Wang, Y.; Zhao, D.-G. Curvularia microspora sp. nov. associated with leaf diseases of Hippeastrum striatum in China. MycoKeys 2018, 49–61. [Google Scholar] [CrossRef]
- Bengyella, L.; Iftikhar, S.; Nawaz, K.; Fonmboh, D.J.; Yekwa, E.L.; Jones, R.C.; Njanu, Y.M.T.; Roy, P. Biotechnological application of endophytic filamentous Bipolaris and Curvularia: A review on bioeconomy impact. World J. Microbiol. Biotechnol. 2019, 35, 69. [Google Scholar] [CrossRef]
- Avinash, K.S.; Ashwini, H.S.; Babu, H.N.R.; Krishnamurthy, Y.L. Antimicrobial Potential of Crude Extract of Curvularia lunata, an Endophytic Fungi Isolated from Cymbopogon caesius. J. Mycol. 2015, 2015, 1–4. [Google Scholar] [CrossRef]
- Ricaud, C.; Egan, B.T. (Eds.) Diseases of Sugarcane: Major Diseases; Elsevier: Amsterdam, The Netherlands, 1989; ISBN 0-444-42797-X. [Google Scholar]
- Mehnaz, S. Microbes—Friends and foes of sugarcane: Microbes and sugarcane. J. Basic Microbiol. 2013, 53, 954–971. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.; Zhang, Z.-F.; Hyde, K.D.; Diao, Y.-Z.; Cai, L. Culturable plant pathogenic fungi associated with sugarcane in southern China. Fungal Divers. 2019, 99, 1–104. [Google Scholar] [CrossRef]
- Tanney, J.B.; Douglas, B.; Seifert, K.A. Sexual and asexual states of some endophytic Phialocephala species of Picea. Mycologia 2016, 108, 255–280. [Google Scholar] [CrossRef]
- Wong, P.T.W.; Dong, C.; Martin, P.M.; Sharp, P.J. Fairway patch—A serious emerging disease of couch (syn. bermudagrass) Cynodon dactylon and kikuyu (Pennisetum clandestinum) turf in Australia caused by Phialocephala bamuru P.T.W. Wong & C. Dong sp. nov. Australas. Plant. Pathol. 2015, 44, 545–555. [Google Scholar] [CrossRef]
- Walsh, E.; Luo, J.; Naik, A.; Preteroti, T.; Zhang, N. Barrenia, a new genus associated with roots of switchgrass and pine in the oligotrophic pine barrens. Fungal Biol. 2015, 119, 1216–1225. [Google Scholar] [CrossRef]
- Tanney, J.B.; Seifert, K.A. Mollisiaceae: An overlooked lineage of diverse endophytes. Stud. Mycol. 2020. [Google Scholar] [CrossRef]
- Wang, M.; Liu, F.; Crous, P.W.; Cai, L. Phylogenetic reassessment of Nigrospora: Ubiquitous endophytes, plant and human pathogens. Persoonia 2017, 39, 118–142. [Google Scholar] [CrossRef]
- Hao, Y.; Aluthmuhandiram, J.V.S.; Chethana, K.W.T.; Manawasinghe, I.S.; Li, X.; Liu, M.; Hyde, K.D.; Phillips, A.J.L.; Zhang, W. Nigrospora Species Associated with Various Hosts from Shandong Peninsula, China. Mycobiology 2020, 48, 169–183. [Google Scholar] [CrossRef]
- Zhao, J.H.; Zhang, Y.L.; Wang, L.W.; Wang, J.Y.; Zhang, C.L. Bioactive secondary metabolites from Nigrospora sp. LLGLM003, an endophytic fungus of the medicinal plant Moringa oleifera Lam. World J. Microbiol. Biotechnol. 2012, 28, 2107–2112. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cai, X.; Pang, Q.; Zhou, M.; Chen, Y.; Zhang, W.; Bian, Q. First Report of Leaf Blight on Mentha canadensis Caused by Nigrospora sphaerica in China. Plant. Dis. 2020. [Google Scholar] [CrossRef]
- Chen, X.; Wang, N.; Yang, M.-F.; Li, H.-X. First Report of Nigrospora Leaf Spot Caused by Nigrospora oryzae on Watermelon in China. Plant. Dis. 2018, 103, 1019. [Google Scholar] [CrossRef]
- Cui, Y.P.; Wu, B.; Peng, A.T.; Li, Z.L.; Lin, J.F.; Song, X.B. First Report of Nigrospora Leaf Blight on Sugarcane Caused by Nigrospora sphaerica in China. Plant. Dis. 2017, 102, 824. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Consortium, F.B. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.A.; Visagie, C.M.; Houbraken, J.; Hong, S.-B.; Hubka, V.; Klaassen, C.H.W.; Perrone, G.; Seifert, K.A.; Susca, A.; Tanney, J.B.; et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 2014, 78, 141–173. [Google Scholar] [CrossRef] [PubMed]
- Raja, H.A.; Miller, A.N.; Pearce, C.J.; Oberlies, N.H. Fungal Identification Using Molecular Tools: A Primer for the Natural Products Research Community. J. Nat. Prod. 2017, 80, 756–770. [Google Scholar] [CrossRef]
- Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Hong, S.-B.; Klaassen, C.H.W.; Perrone, G.; Seifert, K.A.; Varga, J.; Yaguchi, T.; Samson, R.A. Identification and nomenclature of the genus Penicillium. Stud. Mycol. 2014, 78, 343–371. [Google Scholar] [CrossRef] [PubMed]
- Baum, C.; Hrynkiewicz, K.; Szymańska, S.; Vitow, N.; Hoeber, S.; Fransson, P.M.A.; Weih, M. Mixture of Salix Genotypes Promotes Root Colonization with Dark Septate Endophytes and Changes P Cycling in the Mycorrhizosphere. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Shrestha, P.; Szaro, T.M.; Bruns, T.D.; Taylor, J.W. Systematic Search for Cultivatable Fungi That Best Deconstruct Cell Walls of Miscanthus and Sugarcane in the Field. Appl. Environ. Microbiol. 2011, 77, 5490–5504. [Google Scholar] [CrossRef]
- Surono; Narisawa, K. The dark septate endophytic fungus Phialocephala fortinii is a potential decomposer of soil organic compounds and a promoter of Asparagus officinalis growth. Fungal Ecol. 2017, 28, 1–10. [Google Scholar] [CrossRef]
- Martins, T.D.; Menten, J.O.M.; Sanguino, Á. Fungos associados às sementes (Cariopses) de cana-de-açúcar: Métodos para detecção, incidência e relação entre incidência fúngica e ambiente de produção das sementes. Summa Phytopathol. 2009, 35, 173–178. [Google Scholar] [CrossRef]
- Hardoim, P.R.; Hardoim, C.C.P.; van Overbeek, L.S.; van Elsas, J.D. Dynamics of Seed-Borne Rice Endophytes on Early Plant Growth Stages. PLoS ONE 2012, 7, e30438. [Google Scholar] [CrossRef] [PubMed]
- Hardoim, P.R.; Overbeek, L.S.; Elsas, J.D. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 2008, 16, 463–471. [Google Scholar] [CrossRef] [PubMed]



| Municipality | pH | OM | P-resin | K | Ca | Mg | H + Al | Al | CTC | Sampled Point | Sugarcane Variety | Plantation Age |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g dm−3 | mg dm−3 | mmolc dm−3 | ||||||||||
| João Ramalho | 4.66 | 10.4 | 5.2 | 0.98 | 9.60 | 4.80 | 16.8 | 0.6 | 32.2 | 1 | RB867515 | 1st ratoon |
| 2 | RB966928 | 1st ratoon | ||||||||||
| 4 | RB92579 | 1st ratoon | ||||||||||
| Paraguaçu Paulista | 5.43 | 5.0 | 10.5 | 1.30 | 15.17 | 5.17 | 14.0 | 0.5 | 35.5 | 3 | RB867515 | 1st ratoon |
| Paraguaçu Paulista | 5.45 | 10.2 | 16.4 | 2.37 | 17.00 | 6.18 | 14.8 | 0.0 | 40.4 | 11 | RB92579 | 1st ratoon |
| Quatá | 5.58 | 6.1 | 7.0 | 0.96 | 14.31 | 5.92 | 14.5 | 0.9 | 35.8 | 6 | CE2 * | Plant-cane |
| 9 | CE4 * | Plant-cane | ||||||||||
| 12 | CE1 * | Plant-cane | ||||||||||
| 16 | CE3 * | Plant-cane | ||||||||||
| Quatá | 5.70 | 5.0 | 3.0 | 0.53 | 10.67 | 4.67 | 11.0 | 0.0 | 27.3 | 8 | RB867515 | 1st ratoon |
| 10 | RB92579 | 1st ratoon | ||||||||||
| 13 | RB966928 | 1st ratoon | ||||||||||
| Quatá | 6.08 | 12.9 | 15.9 | 1.42 | 17.35 | 5.71 | 12.1 | 0.0 | 36.6 | 15 | RB92579 | 1st ratoon |
| Quatá | 6.20 | 10.5 | 22.2 | 2.63 | 18.50 | 7.33 | 10.8 | 0.0 | 39.3 | 5 | RB867515 | Plant-cane |
| Quatá | 6.20 | 17.0 | 43.5 | 3.55 | 19.25 | 6.75 | 13.2 | 0.0 | 42.7 | 7 | RB966928 | 1st ratoon |
| Quatá | 6.21 | 5.3 | 20.6 | 1.54 | 18.00 | 5.93 | 11.2 | 0.0 | 36.8 | 14 | RB966928 | 1st ratoon |
| Sugarcane Variety | Plantation Age | Sampled Point | Isolated Colonies | ||
|---|---|---|---|---|---|
| Total | Dark | Code Assigned * | |||
| RB867515 | 1st ratoon | 1 | 3 | 2 | A111, A112 |
| 1st ratoon | 3 | 4 | 0 | - | |
| Plant-cane | 5 | 2 | 0 | - | |
| 1st ratoon | 8 | 6 | 3 | A113–A115 | |
| RB92579 | 1st ratoon | 4 | 0 | 0 | - |
| 1st ratoon | 10 | 0 | 0 | - | |
| 1st ratoon | 11 | 3 | 1 | A116 | |
| 1st ratoon | 15 | 2 | 1 | A117 | |
| RB966928 | 1st ratoon | 2 | 12 | 0 | - |
| 1st ratoon | 7 | 8 | 4 | A118–A121 | |
| 1st ratoon | 13 | 2 | 0 | - | |
| 1st ratoon | 14 | 4 | 2 | A122, A123 | |
| CE1 | Plant-cane | 12 | 2 | 0 | - |
| CE2 | Plant-cane | 6 | 7 | 0 | - |
| CE3 | Plant-cane | 16 | 5 | 3 | A124–A126 |
| CE4 | Plant-cane | 9 | 3 | 0 | - |
| Isolate Code * | Identification Based on Morphological Similarity | Closest Identification Based on BLAST and ML Phylogenetic Analysis of | Identification Consensus of This Study | |||
|---|---|---|---|---|---|---|
| ITS Sequences | LSU Sequences | Genus/Species | Family | Order | ||
| Class Dothideomycetes | ||||||
| A111 | Periconia macrospinosa | Periconia macrospinosa | Periconia af. macrospinosa | Periconia macrospinosa | Periconiaceae | Pleosporales |
| A113 | Periconia macrospinosa | Periconia macrospinosa | Periconia af. macrospinosa | Periconia macrospinosa | ||
| A115 | Periconia macrospinosa | Periconia macrospinosa | Periconia af. macrospinosa | Periconia macrospinosa | ||
| A124 | Periconia macrospinosa | Periconia macrospinosa | Periconia af. macrospinosa | Periconia macrospinosa | ||
| A125 | Periconia macrospinosa | Periconia macrospinosa | Periconia af. macrospinosa | Periconia macrospinosa | ||
| A126 | Periconia macrospinosa | Periconia macrospinosa | Periconia af. macrospinosa | Periconia macrospinosa | ||
| A112 | Curvularia sp. | Curvularia lunata | Curvularia af. lunata | Curvularia lunata | Pleosporaceae | |
| A119 | Not identified | Poaceascoma sp. | Poaceascoma af. halophilum | Poaceascoma sp. | Lentitheciaceae | |
| A122 | Not identified | Poaceascoma sp. | Poaceascoma af. halophilum | Poaceascoma sp. | ||
| A120 | Not identified | Poaceascoma sp. | Not identified | Poaceascoma sp. | ||
| A121 | Not identified | Poaceascoma sp. | Not identified | Poaceascoma sp. | ||
| A123 | Not identified | Poaceascoma sp. | Not identified | Poaceascoma sp. | ||
| Class Leotiomycetes | ||||||
| A118 | Phialocephala sp. | Phialocephala sp. | Phialocephala af. bamuru | Phialocephala sp. | Vibrisseaceae | Helotiales |
| Class Sordariomycetes | ||||||
| A114 | Nigrospora sp. | Not identified | Not identified | Nigrospora sp. | Apiosporaceae | Xylariales |
| A116 | Nigrospora sp. | Nigrospora zimmermanii | Nigrospora zimmermanii | Nigrospora zimmermanii | Apiosporaceae | |
| A117 | Nigrospora sp. | Not identified | Not identified | Nigrospora sp. | Apiosporaceae | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fors, R.O.; Patreze, C.M.; Louro Berbara, R.L.; Carbone Carneiro, M.A.; Saggin-Júnior, O.J. Dark Septate Endophytic Fungi Associated with Sugarcane Plants Cultivated in São Paulo, Brazil. Diversity 2020, 12, 351. https://doi.org/10.3390/d12090351
Fors RO, Patreze CM, Louro Berbara RL, Carbone Carneiro MA, Saggin-Júnior OJ. Dark Septate Endophytic Fungi Associated with Sugarcane Plants Cultivated in São Paulo, Brazil. Diversity. 2020; 12(9):351. https://doi.org/10.3390/d12090351
Chicago/Turabian StyleFors, Rosalba Ortega, Camila Maistro Patreze, Ricardo Luis Louro Berbara, Marco Aurélio Carbone Carneiro, and Orivaldo José Saggin-Júnior. 2020. "Dark Septate Endophytic Fungi Associated with Sugarcane Plants Cultivated in São Paulo, Brazil" Diversity 12, no. 9: 351. https://doi.org/10.3390/d12090351
APA StyleFors, R. O., Patreze, C. M., Louro Berbara, R. L., Carbone Carneiro, M. A., & Saggin-Júnior, O. J. (2020). Dark Septate Endophytic Fungi Associated with Sugarcane Plants Cultivated in São Paulo, Brazil. Diversity, 12(9), 351. https://doi.org/10.3390/d12090351





