Conservation Biogeography of Tenebrionid Beetles: Insights from Italian Reserves
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Médail, F.; Quézel, P. Biodiversity Hotspots in the Mediterranean Basin: Setting Global Conservation Priorities. Conserv. Biol. 1999, 13, 1510–1513. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.L.; Underwood, E.C. The importance of conserving biodiversity outside of protected areas in mediterranean ecosystems. PLoS ONE 2011, 6, e14508. [Google Scholar] [CrossRef]
- Ruffo, S.; Stoch, F. (Eds.) Checklist and Distribution of the Italian Fauna. 10,000 Terrestrial and Freshwater Species. 2 Serie, Sez. Scienze della Vita, 2nd revised ed.; Memorie del Museo Civico di Storia Naturale di Verona: Verona, Italy, 2007; Volume 17. [Google Scholar]
- Blasi, C.; Biondi, E. La flora in Italia; Ministero dell’Ambiente e della Tutela del Territorio e del Mare, Sapienza Università Editrice: Rome, Italy, 2017; pp. 1–704. [Google Scholar]
- Blasi, C.; Boitani, L.; La Posta, S.; Manes, F.; Marchetti, M. Stato della Biodiversità in Italia; Palombi Editori: Roma, Italy, 2005; pp. 1–468. [Google Scholar]
- Dennis, R.L.H.; Williams, W.R.; Shreeve, T.G. Faunal structures among European butterflies: Evolutionary implications of bias for geography, endemism and taxonomic affiliation. Ecography 1998, 21, 181–203. [Google Scholar] [CrossRef]
- Fattorini, S.; Baselga, A. Species richness and turnover patterns in European tenebrionid beetles. Insect Conserv. Diver. 2012, 5, 331–345. [Google Scholar] [CrossRef]
- Fattorini, S.; Ulrich, W. Spatial distributions of European Tenebrionidae point to multiple postglacial colonization trajectories. Biol. J. Linn. Soc. 2012, 105, 318–329. [Google Scholar] [CrossRef]
- Fattorini, S.; Ulrich, W. Drivers of species richness in European Tenebrionidae (Coleoptera). Acta Oecol. 2012, 43, 22–28. [Google Scholar] [CrossRef]
- Sergio, C. Elenco Ufficiale delle Aree Protette (EUAP): Supplemento Ordinario alla “Gazzetta Ufficiale”; Ministero dell’ambiente e della tutela del territorio e del mare: Roma, Italy, 2010. [Google Scholar]
- Dajoz, R. Les Coléoptères Carabides et Ténébrionidés; Lavoisier: Paris, France, 2002; pp. 1–522. [Google Scholar]
- Fattorini, S.; Mantoni, C.; Audisio, P.; Biondi, M. Taxonomic variation in levels of endemism: A case study of Italian tenebrionid beetles. Insect Conserv. Divers. 2019, 12, 351–361. [Google Scholar] [CrossRef]
- Matthews, T.; Triantis, K.; Whittaker, R. (Eds.) The Species-Area Relationship: Theory and Application (Ecology, Biodiversity and Conservation); Cambridge University Press: Cambridge, UK, 2020; pp. 1–481. [Google Scholar]
- Desmet, P.; Cowling, R. Using the species–area relationship to set baseline targets for conservation. Ecol. Soc. 2004, 9, 11. [Google Scholar] [CrossRef]
- Fattorini, S. The use of cumulative area curves in biological conservation: A cautionary note. Acta Oecol. 2010, 36, 255–258. [Google Scholar] [CrossRef]
- Fattorini, S. Urban biodiversity hotspots are not related to the structure of green spaces: A case study of tenebrionid beetles from Rome, Italy. Urban Ecosyst. 2014, 17, 1033–1045. [Google Scholar] [CrossRef]
- Fattorini, S. Detecting biodiversity hotspots by species-area relationships: A case study of Mediterranean beetles. Conserv. Biol. 2006, 20, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Mazel, F.; Guilhaumon, F.; Mouquet, N.; Devictor, V.; Gravel, D.; Renaud, J.; Cianciaruso, M.V.; Loyola, R.; Diniz-Filho, J.A.F.; Mouillot, D.; et al. Global hotspots of multifaceted mammal diversity. Global Ecol. Biogeogr. 2014, 23, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, S. The Identification of Biodiversity Hotspots Using the Species–Area Relationship. In The Species-Area Relationship: Theory and Application (Ecology, Biodiversity and Conservation); Matthews, T., Triantis, K., Whittaker, R., Eds.; Cambridge University Press: Cambridge, UK, 2020; pp. 321–344. [Google Scholar]
- Fattorini, S.; Borges, P.V.A. Species–area relationships underestimate extinction rates. Acta Oecol. 2012, 40, 27–30. [Google Scholar] [CrossRef]
- Fattorini, S.; Matthews, T.; Ulrich, W. Using the Species–Area Relationship to Predict Extinctions Resulting from Habitat Loss. In The Species-Area Relationship: Theory and Application (Ecology, Biodiversity and Conservation); Matthews, T., Triantis, K., Whittaker, R., Eds.; Cambridge University Press: Cambridge, UK, 2020; pp. 345–367. [Google Scholar]
- Lomolino, M.V.; Riddle, B.R.; Whittaker, R.J.; Brown, J.H. Biogeography, 4th ed.; Sinauer Associates: Sunderland, MA, USA, 2010; pp. 1–878. [Google Scholar]
- Ashton, K.G. Are ecological and evolutionary rules being dismissed prematurely? Divers. Distrib. 2001, 7, 289–295. [Google Scholar] [CrossRef]
- Fattorini, S. Testing the latitudinal gradient: A narrow-scale analysis of tenebrionid richness (Coleoptera, Tenebrionidae) in the Aegean archipelago (Greece). Ital. J. Zool. 2006, 73, 203–211. [Google Scholar] [CrossRef]
- Whittaker, R.J.; Nogues-Bravo, D.; Araujo, M.B. Geographical gradients of species richness: A test of the water energy conjecture of Hawkins et al. (2003) using European data for five taxa. Global Ecol. Biogeogr. 2007, 16, 76–89. [Google Scholar] [CrossRef]
- Baselga, A. Determinants of species richness, endemism and turnover in European longhorn beetles. Ecography 2008, 31, 263–271. [Google Scholar] [CrossRef]
- Schuldt, A.; Assmann, T. Environmental and historical effects on richness and endemism patterns of carabid beetles in the western Palaearctic. Ecography 2009, 32, 705–714. [Google Scholar] [CrossRef]
- Battisti, C. ‘Peninsula effect’ and Italian peninsula: Matherials for a review and implications in applied biogeography. Biogeographia 2006, 27, 153–188. [Google Scholar] [CrossRef][Green Version]
- Battisti, C. Peninsular patterns in biological diversity: Historical arrangement, methodological approaches and causal processes. J. Nat. Hist. 2014, 48, 43–44. [Google Scholar] [CrossRef]
- Fattorini, S. Disentangling the effects of available area, mid-domain constraints, and species environmental tolerance on the altitudinal distribution of tenebrionid beetles in a Mediterranean area. Biodivers. Conserv. 2014, 23, 2545–2560. [Google Scholar] [CrossRef]
- Fattorini, S.; Mantoni, C.; Di Biase, L.; Pace, L. Mountain Biodiversity and Sustainable Development. In Encyclopedia of the UN Sustainable Development Goals. Life on Land; Leal Filho, W., Azul, A., Brandli, L., Özuyar, P., Wall, T., Eds.; Springer International Publishing: New York, NY, USA, 2020; pp. 1–31. [Google Scholar]
- Fattorini, S.; Mantoni, C.; Di Biase, L.; Strona, G.; Pace, L.; Biondi, M. Elevational Patterns of Generic Diversity in the Tenebrionid Beetles (Coleoptera Tenebrionidae) of Latium (Central Italy). Diversity 2020, 12, 47. [Google Scholar] [CrossRef]
- Fattorini, S.; Dapporto, L.; Strona, G.; Borges, P.V.A. Calling for a new strategy to measure environmental (habitat) diversity in Island Biogeography: A case study of Mediterranean tenebrionids (Coleoptera: Tenebrionidae). Fragm. Entomol. 2015, 47, 1–14. [Google Scholar] [CrossRef]
- Fattorini, S. Ecology and conservation of tenebrionid beetles in Mediterranean coastal areas. In Insect Ecology and Conservation; Fattorini, S., Ed.; Research Signpost: Trivandrum, Kerala, India, 2008; pp. 165–297. [Google Scholar]
- Fattorini, S. Endemism in historical biogeography and conservation biology: Concepts and implications. Biogeographia 2017, 32, 47–75. [Google Scholar] [CrossRef]
- Aliquò, V.; Leo, P. I Coleotteri Tenebrionidi delle Madonie (Sicilia). Naturalista Sicil. 1996, 4, 281–304. [Google Scholar]
- Andreetti, A.; Di Gaetano, B.; Di Marco, C.; Osella, G.; Riti, M. Coleoptera Tenebrionidae (Insecta). In Ricerche sulla Valle Peligna (Italia Centrale, Abruzzo). Quaderni di Provincia oggi, 23/II; Osella, B.G., Biondi, M., Di Marco, C., Riri, M., Eds.; Amministrazione provinciale de L’Aquila: L’Aquila, Italy, 1997; Volume 2, pp. 425–443. [Google Scholar]
- Angelini, F. Contribution to the knowledge of beetles (Insecta Coleoptera) of some protected areas of Apulia, Basilicata and Calabria (Italy). Biodivers. J. 2020, 11, 85–254. [Google Scholar] [CrossRef]
- Cavanna, C. Parte, II. Catalogo dogli animali raccolti al Vulture, al Pollino ed in altri luoghi dell’Italia meridionale e centrale. Boll. Soc. Ent. Ital. 1882, 14, 31–87. [Google Scholar]
- Chelazzi, L.; DeMatthaeis, E.; Colombini, I.; Fallaci, M.; Bandini, V.; Tozzi, C. Abundance, zonation and ecological indices of a coleopteran community from a sandy beach-dune ecosystem of the southern Adriatic coast, Italy. Vie Milieu 2005, 55, 127–141. [Google Scholar]
- Crucitti, P.; Brocchieri, D.; Bubbico, F.; Castelluccio, P.; Cervoni, F.; Di Russo, E.; Emiliani, F.; Giardini, M.; Pulvirenti, E. Checklist di alcuni gruppi selezionati dell’entomofauna del Parco Naturale Archeologico dell’Inviolata (Guidonia Montecelio, Roma): XlI contributo allo studio della biodiversità della Campagna Romana a nord-est di Roma. Boll. Soc. Ent. Ital. 2019, 151, 65–92. [Google Scholar] [CrossRef]
- Fattorini, S. The Tenebrionidae (Coleoptera) of a Tyrrhenian coastal area: Diversity and zoogeographical composition. Biogeographia 2002, 23, 103–126. [Google Scholar] [CrossRef]
- Fattorini, S. I Coleotteri Tenebrionidi del Parco Nazionale del Circeo (Italia Centrale) (Coleoptera, Tenebrionidae). Boll. Assoc. Rom. Entomol. 2005, 60, 47–104. [Google Scholar]
- Fattorini, S. The tenebrionid beetles of Mt Vesuvius: Species assemblages and biogeographic kinetics on an active volcano (Coleoptera Tenebrionidae). In Artropodi del Parco Nazionale del Vesuvio: Ricerche preliminari. Conservazione Habitat Invertebrati; Nardi, G., Vomero, V., Eds.; Cierre edizioni: Verona, Italy, 2007; Volume 4, pp. 221–243. [Google Scholar]
- Fattorini, S.; Maltzeff, P.; Salvati, L. Use of insect distribution across landscape-soil units to assess conservation priorities in a Mediterranean coastal reserve: The tenebrionid beetles of Castelporziano (Central Italy). Rend. Lincei-Sci. Fis. 2015, 26, 353–366. [Google Scholar] [CrossRef]
- Fattorini, S.; Vigna Taglianti, A. Use of taxonomic and chorological diversity to highlight the conservation value of insect communities in a Mediterranean coastal area: The carabid beetles (Coleoptera, Carabidae) of Castelporziano (Central Italy). Rend. Lincei-Sci. Fis. 2015, 26, 625–641. [Google Scholar] [CrossRef]
- Fattorini, S.; Romiti, F.; Carpaneto, G.M.; Poeta, G.; Bergamaschi, D. I Coleotteri Tenebrionidi del Sito d’Importanza Comunitaria “Foce Saccione—Bonifica Ramitelli” (Molise) (Coleoptera Tenebrionidae). Boll. Soc. Ent. Ital. 2016, 148, 57–62. [Google Scholar] [CrossRef]
- Furlanetto, D. Atlante della Biodiversità nel Parco Ticino—Edizione 2002. Elenchi Sistematici (Monografie); Consorzio Parco Lombardo della Valle del Ticino: Pontevecchio di Magenta, Milan, Italy, 2002; 406p. [Google Scholar]
- Gatti, E. Ricerche sull’entomofauna della Riserva Naturale Vincheto di Celarda—(BL) Collana Verde, 86; Ministero dell’Agricoltura e delle Foreste: Rome, Italy, 1991; p. 200. [Google Scholar]
- Hardersen, S.; Toni, I.; Cornacchia, P.; Curletti, G.; Leo, P.; Nardi, G.; Penati, F.; Piattella, E.; Platia, G. Survey of selected beetle families in a floodplain remnant in northern Italy. Bull. Insectology 2012, 65, 199–207. [Google Scholar]
- Lucarelli, E.; Chelazzi, L.; Colombini, I.; Fallaci, M.; Mascagni, A. La coleotterofauna del tombolo antistante la laguna di Burano (GR): Lista e zonazioni delle specie raccolte durante un intero anno di campionamenti. Boll. Ass. Romana Entomol. 1993, 47, 7–34. [Google Scholar]
- Scupola, A. Tenebrionidae. In Invertebrati di una foresta della Pianura Padana. Bosco della Fontana. Centro Nazionale per lo Studio e la Conservazione della Biodiversià Forestale Bosco della Fontana; Mason, F., Cerretti, P., Tagliapietra, A., Speight, M.C.D., Zapparoli, M., Eds.; Gianluigi Arcari Editore: Mantova, Italy, 2002; p. 93. [Google Scholar]
- Scupola, A. Coleoptera, Tenebrionidae (excluding Alleculinae, Lagriinae). In Invertebrati di una Foresta della Pianura Padana, Bosco della Fontana, Secondo Contributo, Conservazione Habitat Invertebrati; Cerretti, P., Hardersen, S., Mason, F., Nardi, G., Tisato, M., Zapparoli, M., Eds.; Cierre Grafica Editore: Verona, Italy, 2004; Volume 3, p. 285. [Google Scholar]
- Aliquò, V.; Rastelli, M.; Rastelli, S.; Soldati, F. Coleotteri Tenebrionidi d’Italia; CD-ROM Museo Civico di Storia Naturale di Carmagnola: Carmagnola, Italy, 2006. [Google Scholar]
- Löbl, I.; Smetana, A. Catalogue of Palaearctic Coleoptera. Vol. 5. Tenebrionoidea; Apollo Books: Stenstrup, UK, 2008; pp. 1–670. [Google Scholar]
- Angelini, F. Coleotterofauna del Promontorio del Gargano (Coleoptera). Atti Mus. Civ. Stor. Nat. Grosseto 1987, 11/12, 5–84. [Google Scholar]
- Nardi, G.; Vomero, V. Introduzione. In Artropodi del Parco Nazionale del Vesuvio: Ricerche preliminari. Conservazione Habitat Invertebrati; Nardi, G., Vomero, V., Eds.; Cierre edizioni: Verona, Italy, 2007; pp. 15–28. [Google Scholar]
- Angelini, F. Coleotterofauna del Massiccio del Pollino (Basilicata-Calabria) (Coleoptera). Entomologica 1986, 21, 37–125. [Google Scholar]
- Angelini, F. Coleotterofauna dell’Altipiano della Sila (Calabria, Italia) (Coleoptera). Mem. Soc. Entomol. Ital. 1991, 70, 171–254. [Google Scholar]
- Connor, E.F.; McCoy, E.D. The statistics and biology of the species–area relationship. Am. Nat. 1979, 113, 791–833. [Google Scholar] [CrossRef]
- Triantis, K.A.; Guilhaumon, F.; Whittaker, R.J. The island species–area relationship: Biology and statistics. J. Biogeogr. 2012, 39, 215–231. [Google Scholar] [CrossRef]
- Matthews, T.J.; Guilhaumon, F.; Triantis, K.A.; Borregaard, M.K.; Whittaker, R.J. On the form of species–area relationships in habitat islands and true islands. Global Ecol. Biogeogr. 2016, 25, 847–858. [Google Scholar] [CrossRef]
- Fattorini, S.; Borges, P.V.A.; Dapporto, L.; Strona, G. What can the parameters of the species–area relationship (SAR) tell us? Insights from Mediterranean islands. J. Biogeogr. 2017, 44, 1018–1028. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002; p. 488. [Google Scholar]
- Zurlini, G.; Grossi, L.; Rossi, O. Spatial-accumulation pattern and extinction rates of Mediterranean flora as related to species confinement to habitats in preserves and larger areas. Conserv. Biol. 2002, 16, 948–963. [Google Scholar] [CrossRef]
- Báldi, A.; Vörös, J. Extinction debt of Hungarian reserves: A historical perspective. Basic Appl. Ecol. 2006, 7, 289–295. [Google Scholar] [CrossRef]
- Benedick, S.; Hill, J.K.; Mustaffa, N.; Chey, V.K.; Maryati, M.; Searle, J.B.; Schilthuizen, M.; Hamer, K.C. Impacts of rain forest fragmentation on butterflies in northern Borneo: Species richness, turnover and the value of small fragments. J. Appl. Ecol. 2006, 43, 967–977. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing. 2018. Available online: http://www.r-project.org/ (accessed on 10 September 2018).
- Bartoń, K. MuMIn: Multi-Model Inference. R Package Version 1.43.17. 2020. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 10 June 2020).
- Imdadullah, M.; Aslam, M.; Altaf, S. mctest: An R Package for Detection of Collinearity among Regressors. R Package Version 1. 3.1. 2020. Available online: https://CRAN.R-project.org/package=mctest (accessed on 29 June 2020).
- Carpaneto, G.; Fattorini, S. Flightlessness in psammophilous beetles inhabiting a Mediterranean coastal area: Ecological and biogeographical implications. Biogeographia 2002, 23, 71–80. [Google Scholar] [CrossRef]
- Fattorini, S. Tenebrionid beetle distributional patterns in Italy: Multiple colonisation trajectories in a biogeographical crossroad. Insect Conserv. Divers. 2014, 7, 144–160. [Google Scholar] [CrossRef]
- Fattorini, S.; Di Biase, L.; Chiarucci, A. Recognizing and interpreting vegetational belts: New wine in the old bottles of a von Humboldt’s legacy. J. Biogeogr. 2019, 46, 1643–1651. [Google Scholar] [CrossRef]

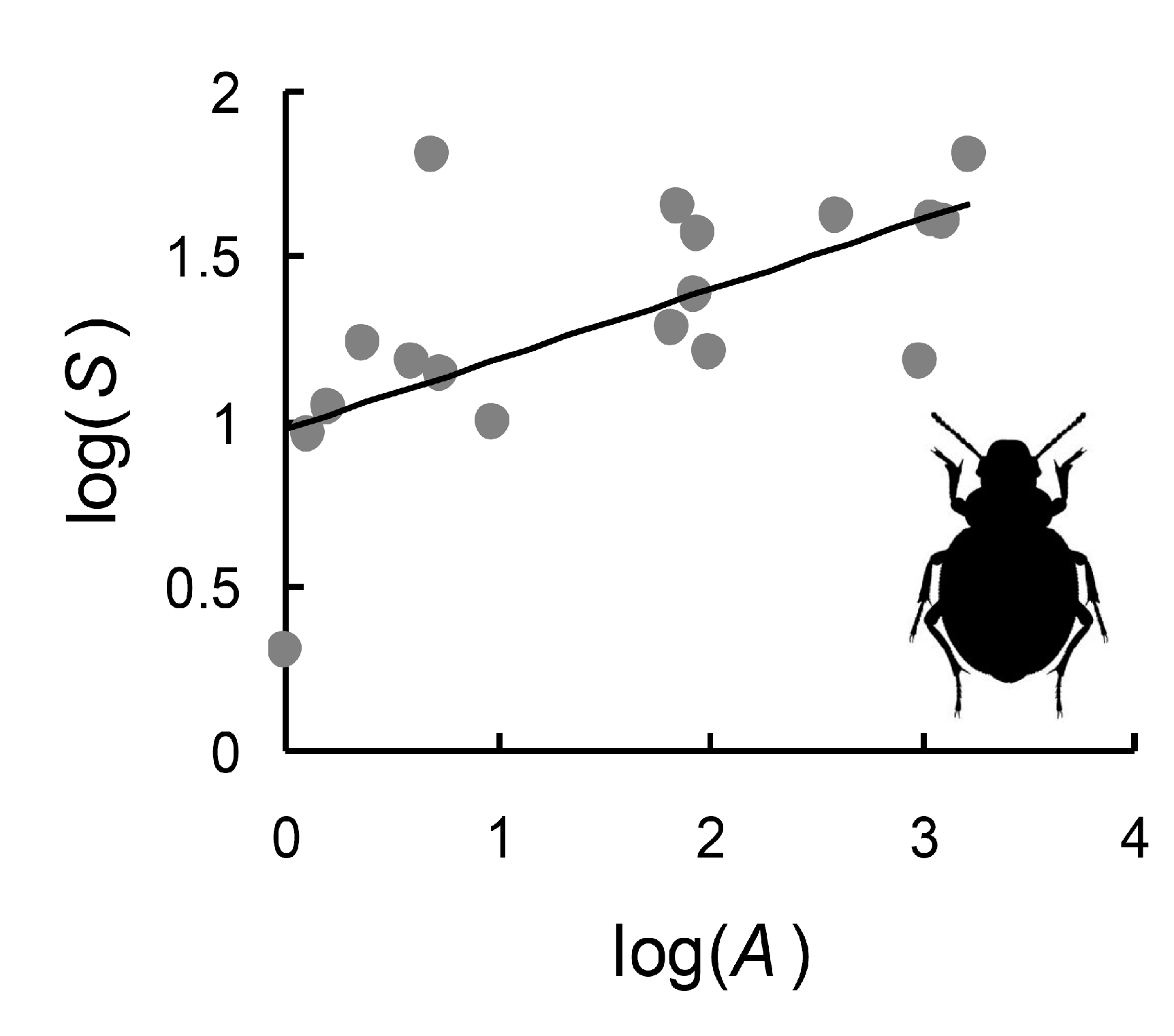
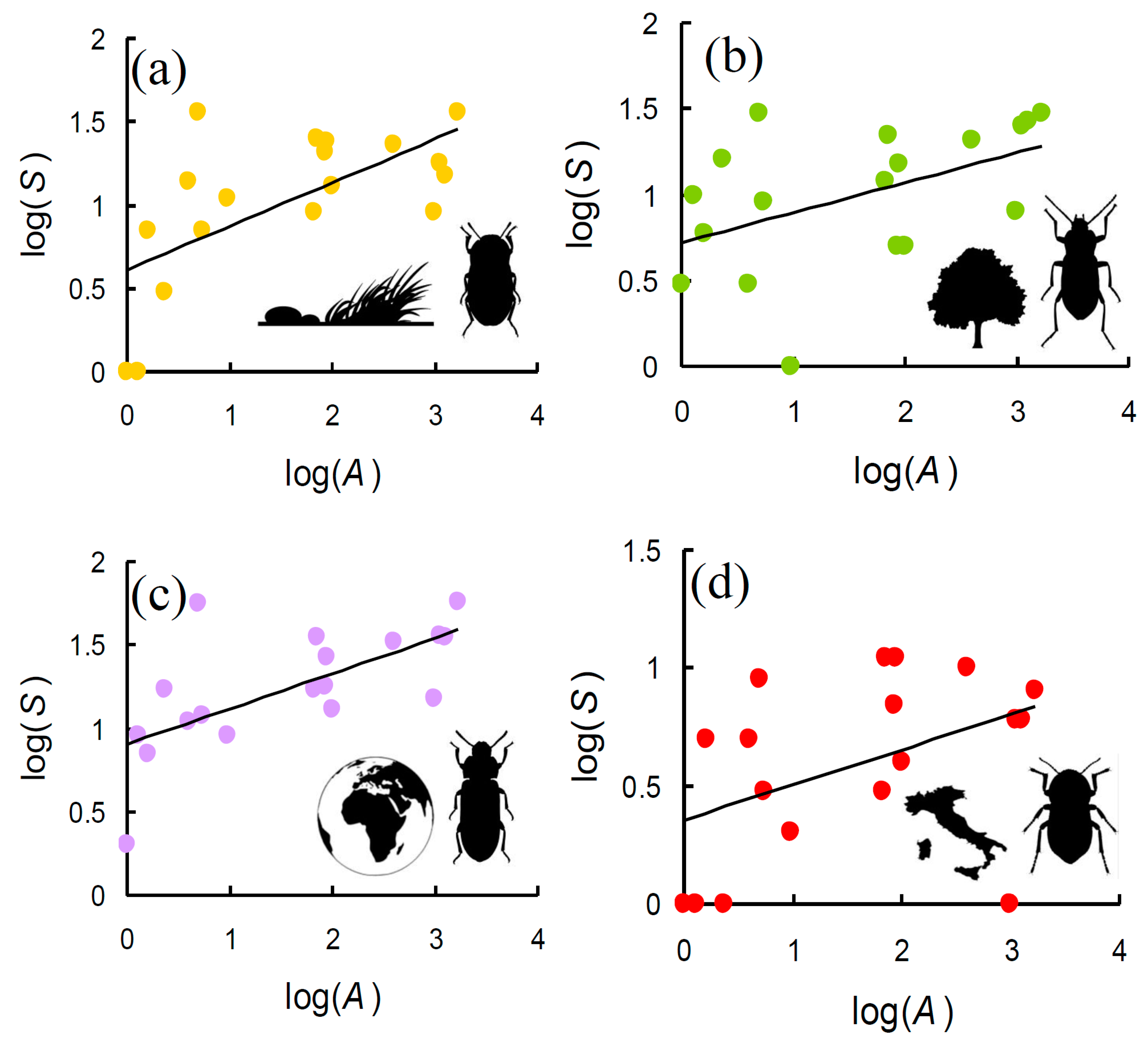
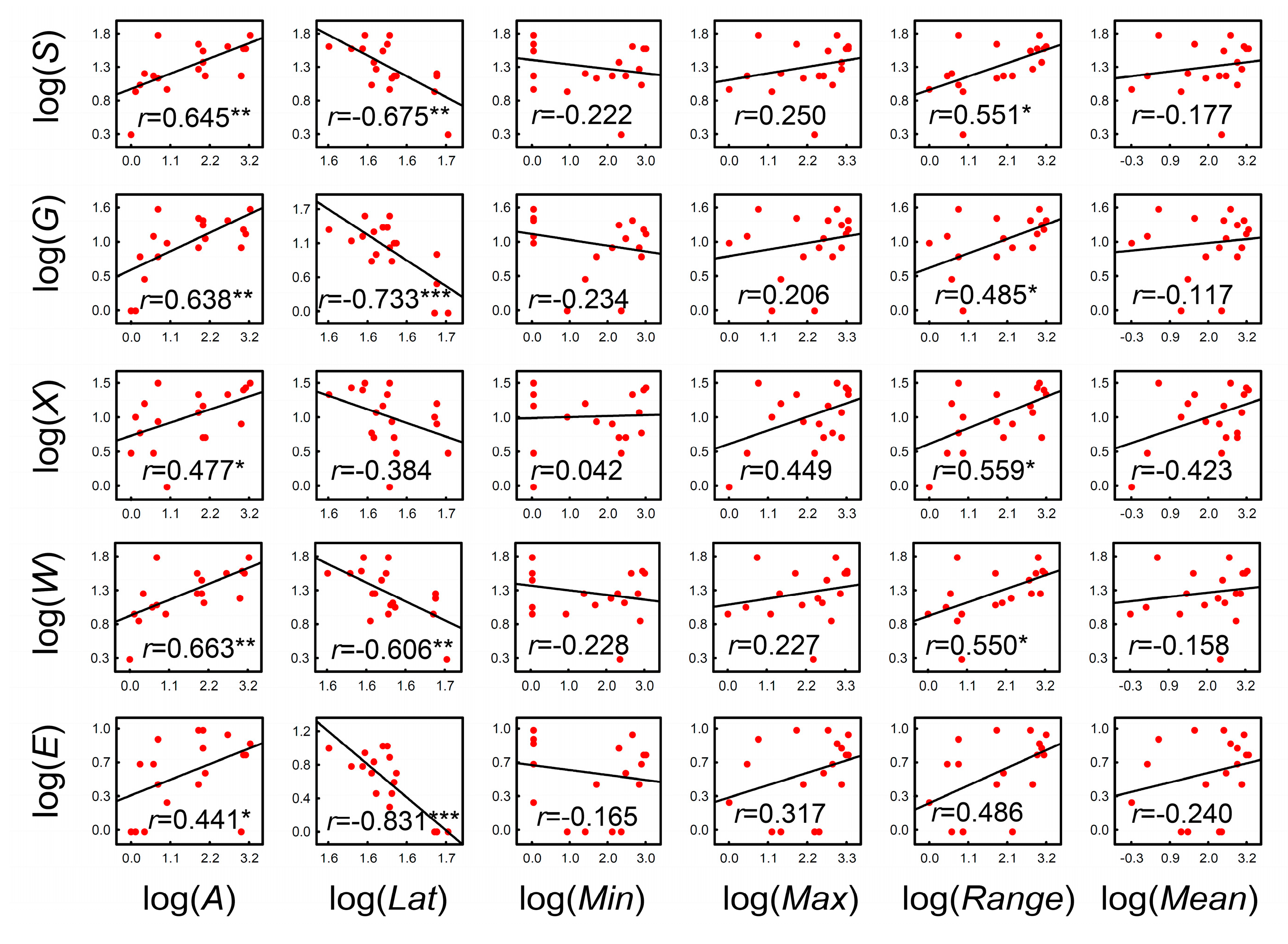
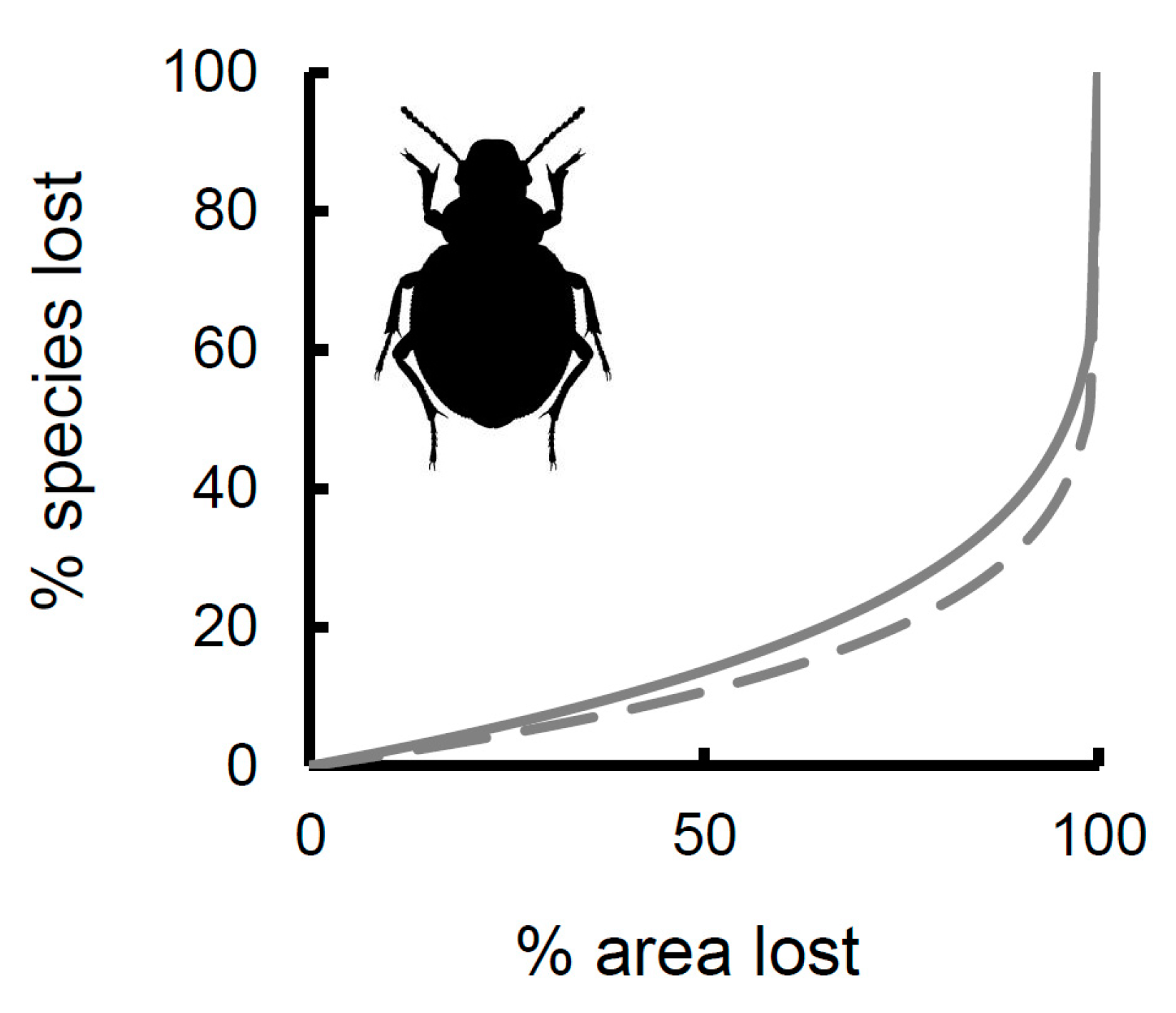
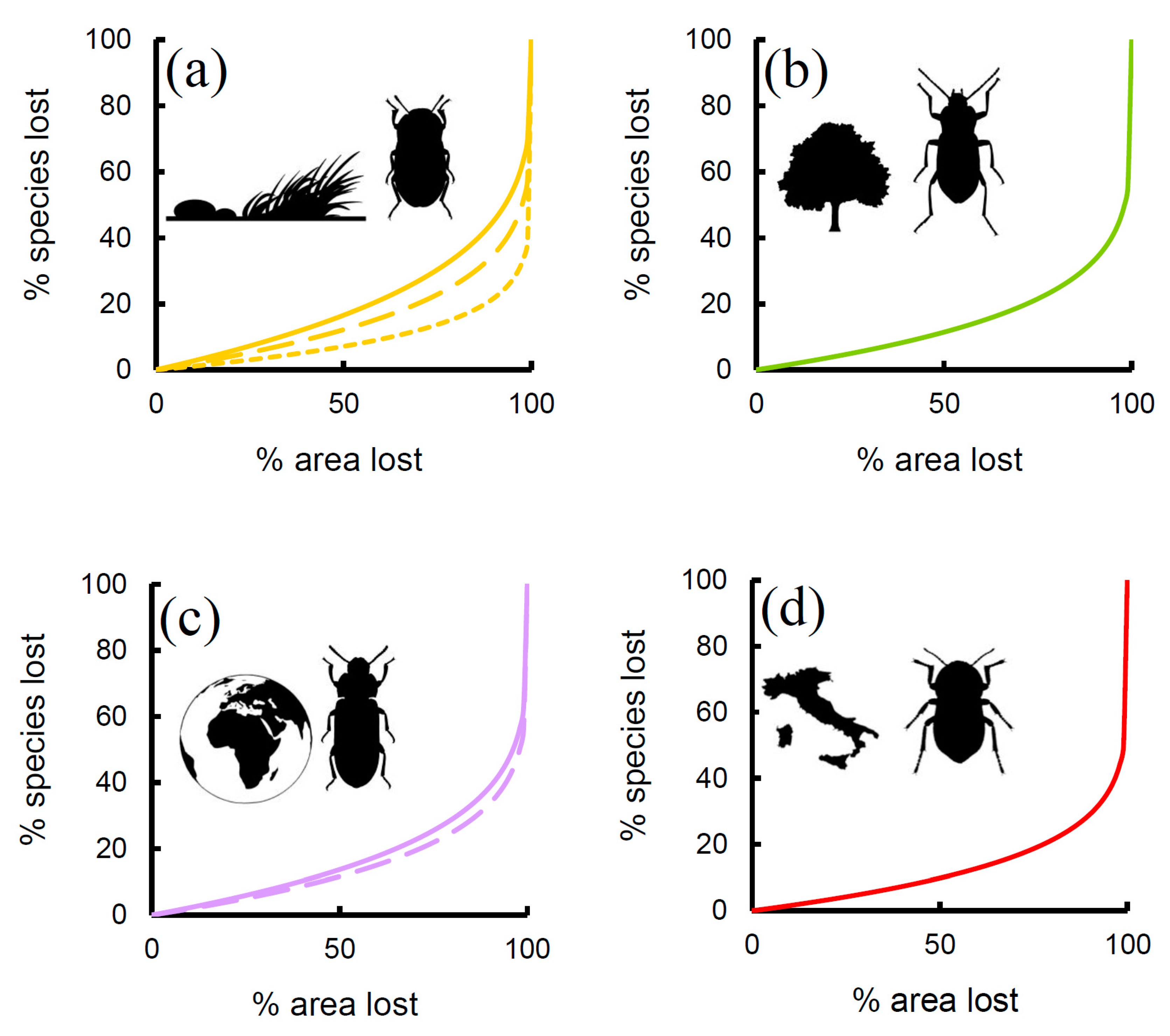
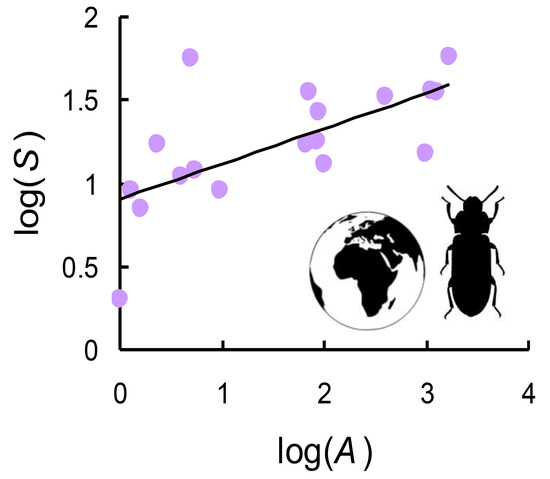
| Res | Lat | Lon | Area | Min | Max | Range | Mean | S | G | X | W | E |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 46.0208 | 11.9794 | 1 | 225 | 233 | 8 | 229 | 2 | 0 | 2 | 2 | 0 |
| 2 | 45.3117 | 8.9264 | 983 | 116 | 300 | 184 | 208 | 15 | 8 | 7 | 15 | 0 |
| 3 | 45.2011 | 10.7428 | 2.3 | 22 | 26 | 4 | 24 | 17 | 2 | 15 | 17 | 0 |
| 4 | 45.0403 | 11.2403 | 1.3 | 7 | 15 | 8 | 14 | 9 | 0 | 9 | 9 | 0 |
| 5 | 42.4006 | 11.2117 | 4 | 0 | 3 | 3 | 1.5 | 15 | 13 | 2 | 11 | 4 |
| 6 | 42.1614 | 13.8230 | 100 | 300 | 400 | 100 | 350 | 16 | 12 | 4 | 13 | 3 |
| 7 | 41.9769 | 12.6675 | 5.4 | 50 | 120 | 70 | 85 | 14 | 6 | 8 | 12 | 2 |
| 8 | 41.9325 | 15.1053 | 9.6 | 0 | 1 | 1 | 0.5 | 10 | 10 | 0 | 9 | 1 |
| 9 | 41.8050 | 15.8506 | 1700 | 0 | 950 | 950 | 456 | 64 | 35 | 29 | 57 | 7 |
| 10 | 41.7036 | 12.3754 | 70 | 0 | 70 | 70 | 35 | 45 | 24 | 21 | 35 | 10 |
| 11 | 41.3383 | 13.0383 | 88 | 0 | 541 | 541 | 270.5 | 37 | 23 | 14 | 27 | 10 |
| 12 | 40.9453 | 15.6339 | 66 | 650 | 1326 | 676 | 988 | 19 | 8 | 11 | 17 | 2 |
| 13 | 40.8211 | 14.4275 | 84.8 | 200 | 1281 | 1081 | 740.5 | 24 | 20 | 4 | 18 | 6 |
| 14 | 40.5881 | 15.7483 | 1.6 | 764 | 770 | 6 | 767 | 11 | 6 | 5 | 7 | 4 |
| 15 | 40.1753 | 16.6975 | 5 | 0 | 6 | 6 | 3 | 64 | 35 | 29 | 56 | 8 |
| 16 | 39.9650 | 16.2147 | 1100 | 800 | 2000 | 1200 | 1482 | 41 | 17 | 24 | 36 | 5 |
| 17 | 39.3197 | 16.5783 | 1250 | 1000 | 1900 | 900 | 1425 | 40 | 14 | 26 | 35 | 5 |
| 18 | 37.8858 | 13.9992 | 399.4 | 400 | 1979 | 1579 | 1189.5 | 42 | 22 | 20 | 33 | 9 |
| Beetle group, R2 Values, and Estimated Parameters | Estimate ± SE | t | p |
|---|---|---|---|
| All tenebrionids (R2 = 0.416) | |||
| log(c) | 0.976 ± 0.121 | 8.094 | <<0.001 |
| z | 0.213 ± 0.063 | 3.374 | 0.004 |
| Geophilous species (R2 = 0.407) | |||
| log(c) | 0.613 ± 0150 | 4.075 | <0.001 |
| z | 0.261 ± 0.079 | 3.317 | 0.004 |
| Xylophilous species (R2 = 0.228) | |||
| log(c) | 0.721 ± 0.154 | 4.693 | <0.001 |
| z | 0.175 ± 0.080 | 2.172 | 0.045 |
| Widespred species (R2 = 0.440) | |||
| log(c) | 0.905 ± 0.114 | 7.911 | <<0.001 |
| z | 0.212 ± 0.060 | 3.544 | 0.003 |
| Endemic species (R2 = 0.195) | |||
| log(c) | 0.355 ± 0.145 | 2.449 | 0.026 |
| z | 0.149 ± 0.076 | 1.967 | 0.067 |
| Intercept | Area | Latitude | Minimum Elevation | Maximum Elevation | Mean Elevation | Elevational Range | DF | R2adj | AICc | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total | ||||||||||
| 14.417 ± 3.823 (**) | 0.164 ± 0.047 (***) | −8.733 ± 2.333 (**) | −0.1312 ± 0.039 | 5 | 0.730 | 2.4 | ||||
| 15.143 ± 4.133 (**) | −8.575 ± 2.517 (**) | −0.162 ± 0.044 (**) | 0.173 ± 0.057 (**) | 5 | 0.699 | 4.3 | ||||
| Geophilous | 29.551 ± 10.335 (*) | −7.711 ± 2.695 (*) | 2.821 ± 0.535 (***) | −2.719 ± 0.506 (***) | 5 | 0.819 | 2.9 | |||
| 48.590 ± 9.024 (***) | 0.187 ± 0.049 (**) | −12.402 ± 2.391 (***) | −0.171 ± 0.040 (***) | 5 | 0.817 | 3.3 | ||||
| 31.222 ± 9.956 (**) | 0.104 ± 0.069 (ns) | −8.075 ± 2.592 (**) | 2.151 ± 0.678 (**) | −2.139 ± 0.619 (**) | 6 | 0.8341 | 4.6 | |||
| Xylophilous | 0.603 ± 0.167 (**) | 0.212 ± 0.079 (*) | 3 | 0.270 | 19.1 | |||||
| Widespread | 12.356 ± 4.049 (**) | 0.179 ± 0.050 (**) | −6.908 ± 2.470 (*) | −0.128 ± 0.042 (**) | 5 | 0.677 | 4.5 | |||
| Endemic | 22.353 ± 2.496 (***) | −13.436 ± 1.524 (***) | −0.214 ± 0.040 (***) | 0.173 ± 0.048 (**) | 5 | 0.876 | −10.7 | |||
| 21.607 ± 2.566 (***) | −13.004 ± 1.563 (***) | −0.199 ± 0.0373 (***) | 0.169 ± 0.169 (**) | 5 | 0.876 | −10.7 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fattorini, S. Conservation Biogeography of Tenebrionid Beetles: Insights from Italian Reserves. Diversity 2020, 12, 348. https://doi.org/10.3390/d12090348
Fattorini S. Conservation Biogeography of Tenebrionid Beetles: Insights from Italian Reserves. Diversity. 2020; 12(9):348. https://doi.org/10.3390/d12090348
Chicago/Turabian StyleFattorini, Simone. 2020. "Conservation Biogeography of Tenebrionid Beetles: Insights from Italian Reserves" Diversity 12, no. 9: 348. https://doi.org/10.3390/d12090348
APA StyleFattorini, S. (2020). Conservation Biogeography of Tenebrionid Beetles: Insights from Italian Reserves. Diversity, 12(9), 348. https://doi.org/10.3390/d12090348