Insect Communities Associated with Siam Weed: Evaluation after Three Decades of Cecidochares connexa Release as Biocontrol Agent
Abstract
1. Introduction
2. Materials and Methods
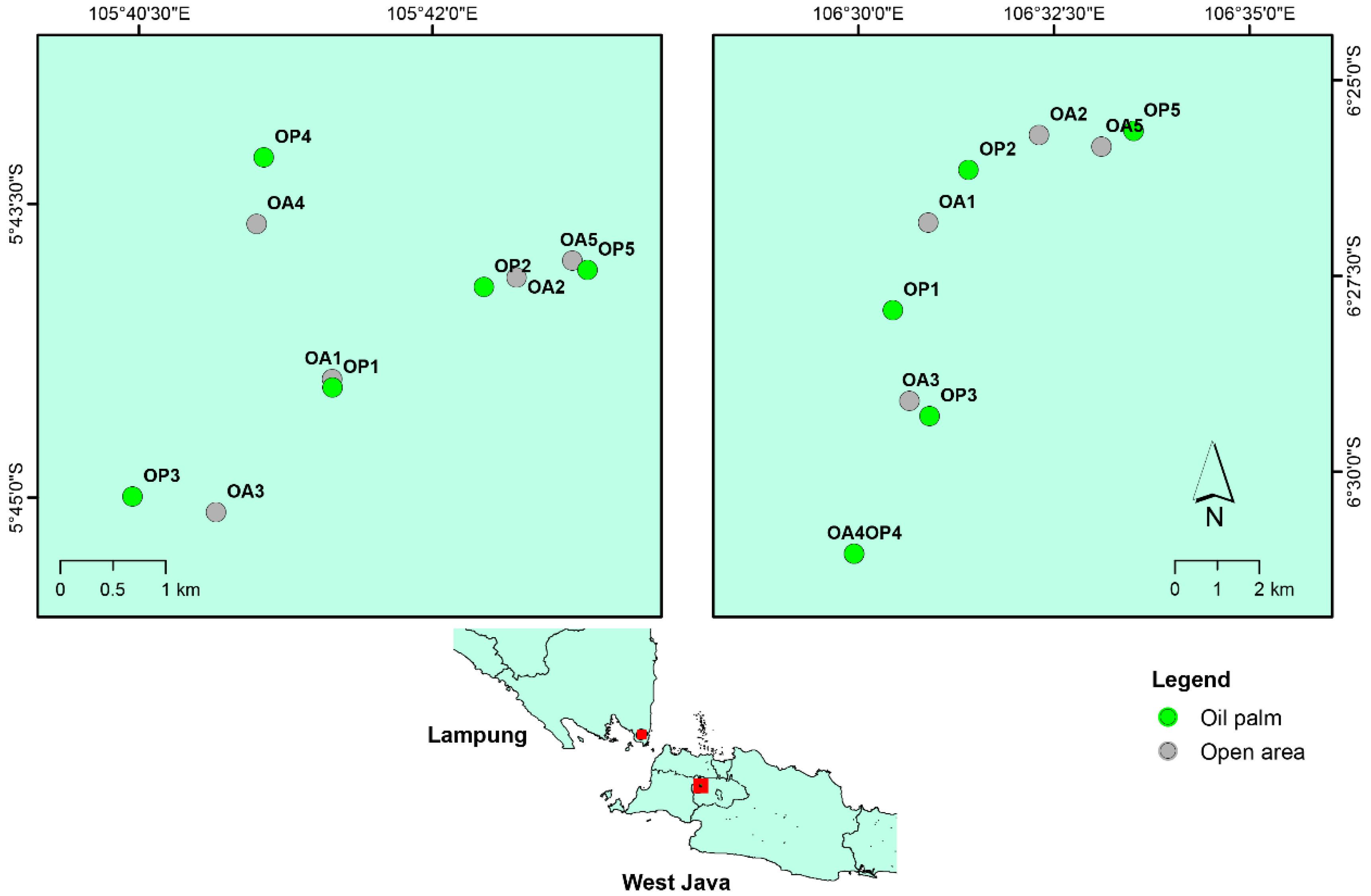
2.1. Research Site and Plot Selection
2.2. Sampling and Identification of Insects
2.3. Data Analysis
3. Results
3.1. Insect Diversity Associated with C. odorata
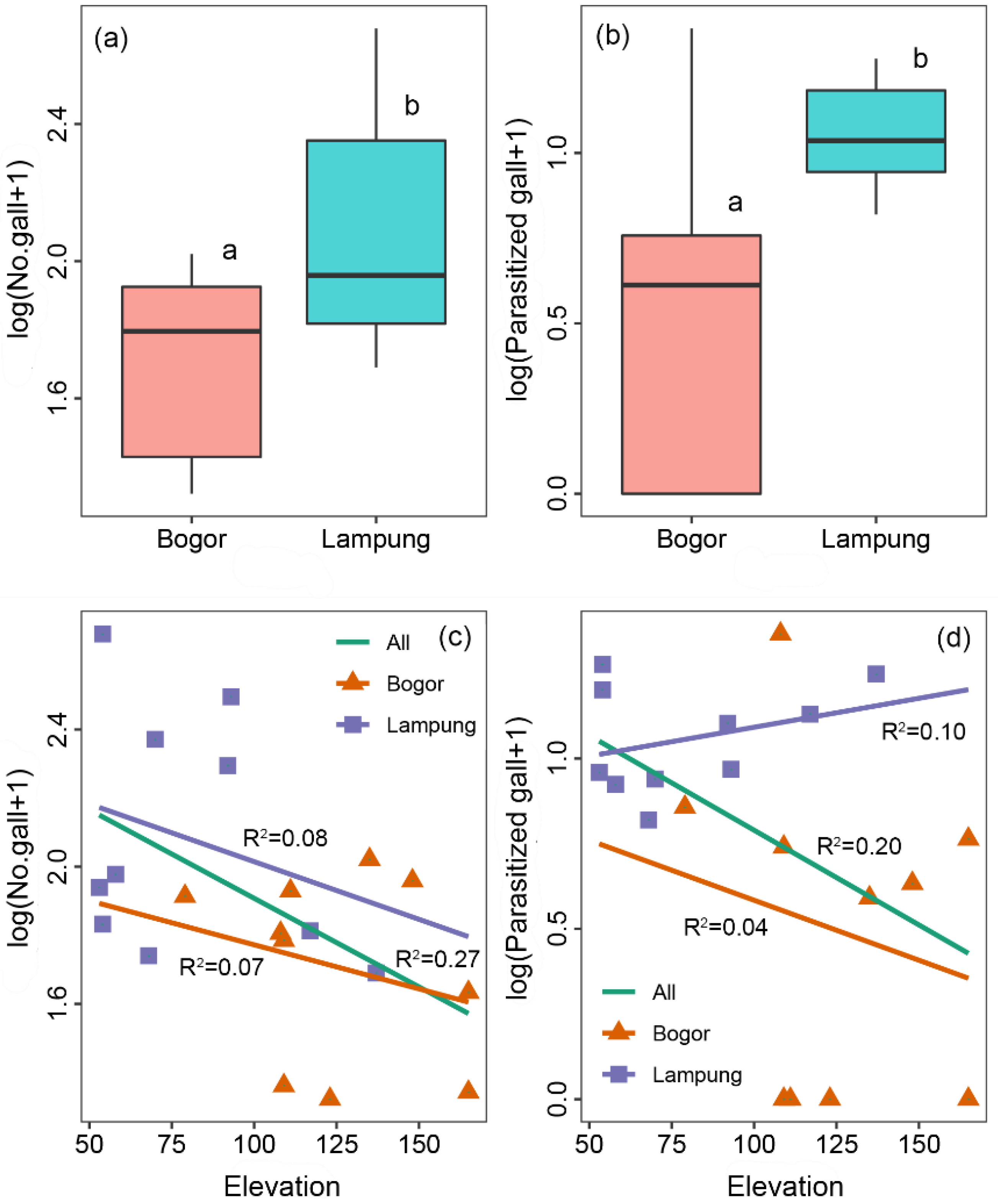
3.2. Evaluation of the Release of C. connexa as the Natural Enemy of C. odorata
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zachariades, C.; Day, M.; Muniappan, R.; Reddy, G.V.P. Chromolaena odorata (L.) King and Robinson (Asteraceae). In Biological Control of Tropical Weeds Using Arthropods; Muniappan, R., Reddy, G.V.P., Raman, A., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 130–162. [Google Scholar]
- Gautier, L. Taxonomy and distribution of a tropical weed, Chromolaena odorata (L.) R. King and H. Robinson. Candollea 1992, 47, 645–662. [Google Scholar]
- Tjitrosemito, S. The establishment of Procecidocares connexa in West Java, Indonesia: A biological control agent of Chromolaena odorata. Biotropia 1999, 12, 19–24. [Google Scholar]
- Paini, D.R.; Sheppard, A.W.; Cook, D.C.; Barro, P.J.D.; Worner, S.P.; Thomas, M.B. Global threat to agriculture from invasive species. Proc. Natl. Acad. Sci. USA 2016, 113, 7575–7579. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Zhang, Z. Allelopathic effects of Chromolaena odorata on native and non-native invasive herbs. J. Food Agric. Environ. 2013, 11, 878–882. [Google Scholar]
- Mandal, G.; Joshi, S.P. Invasion establishment and habitat suitability of Chromolaena odorata (L.) King and Robinson over time and space in the western Himalayan forests of India. J. Asia-Pac. Biodivers. 2014, 7, 391–400. [Google Scholar] [CrossRef]
- Mangla, S.; Callaway, R.M. Exotic invasive plant accumulates native soil pathogens which inhibit native plants. J. Ecol. 2008, 96, 58–67. [Google Scholar] [CrossRef]
- Schoonhoven, L.M.; Jermy, T.; van Loon, J.J.A. Insect-Plant Biology: From Physiology to Evolution; Chapman & Hall: London, UK, 1996. [Google Scholar]
- Van Driesche, R.G.; Carruthers, R.I.; Center, T.; Hoddle, M.S.; Hough-Goldstein, J.; Morin, L.; Smith, L.; Wagner, D.L.; Blossey, B.; Brancatini, V.; et al. Classical biological control for the protection of natural ecosystems. Biol. Control 2010, 54, S2–S33. [Google Scholar] [CrossRef]
- Day, M.; Witt, A.; Winston, R. Weed biological control in low- and middle-income countries. Curr. Opin. Insect Sci. 2020, 38, 92–98. [Google Scholar] [CrossRef]
- Desmier de Chenon, R.; Sipayung, A.; Sudharto, P. A decade of biological control against Chromolaena odorata at the Indonesian Oil Palm Research Institute in Marihat. In Proceedings of the Fifth International Workshop on Biological Control and Management of Chromolaena odorata, Durban, South Africa, 23–25 October 2000; Zachariades, C., Muniappan, R., Strathie, L.W., Eds.; ARC-PPRI: Durban, South Africa, 2002; pp. 46–52. [Google Scholar]
- Tjitrosemito, S. Integrated management of Chromolaena odorata: Emphasizing the classical biological control. Biotropia 1998, 11, 9–21. [Google Scholar]
- McFadyen, R.E.C.; Desmier de Chenon, R.D.; Sipayung, A. Biology and host specificity of the chromolaena stem gall fly, Cecidochares connexa (Macquart) (Diptera: Tephritidae). Aust. J. Entomol. 2003, 42, 294–297. [Google Scholar] [CrossRef]
- Rizali, A.; Hadi, M.S.; Pudjianto, P.; Buchori, D. A new trophic interaction between invasive weed, its biological control agent, and local insects: A case study of Chromolaena odorata. Biodiversitas 2019, 20, 1006–1011. [Google Scholar] [CrossRef]
- Pearson, D.E.; Callaway, R.M. Indirect effects of host-specific biological control agents. Trends Ecol. Evol. 2003, 18, 456–461. [Google Scholar] [CrossRef]
- Bellows, T.S. Restoring population balance through natural enemy introductions. Biol. Control 2001, 21, 199–205. [Google Scholar] [CrossRef]
- Lonsdale, W.M.; Briese, D.T.; Cullen, J.M. Risk analysis and weed biological control. In Evaluating Indirect Ecological Effects of Biological Control; Wajnberg, E., Scott, J.K., Quimby, P.C., Eds.; CABI Publishing: Wallingford, CT, USA, 2001; pp. 185–210. [Google Scholar]
- Simberloff, D.; Stiling, P. How risky is biological control? Ecology 1996, 77, 1965–1974. [Google Scholar] [CrossRef]
- Aigbedion-Atalor, P.O.; Adom, M.; Day, M.D.; Uyi, O.; Egbon, I.N.; Idemudia, I.; Igbinosa, I.B.; Paterson, I.D.; Braimah, H.; Wilson, D.D.; et al. Eight decades of invasion by Chromolaena odorata (Asteraceae) and its biological control in West Africa: The story so far. Biocontrol Sci. Technol. 2019, 29, 1215–1233. [Google Scholar] [CrossRef]
- Carino, F.O.; Kenmore, P.E.; Dyck, V.A. The FARMCOP suction sampler for hoppers and predators in flooded rice fields. IRRN 1979, 4, 21–22. [Google Scholar]
- Borror, D.; Triplehorn, C.H.; Johnson, N.F. An Introduction to the Study of Insects, 6th ed.; Saunders College Publishing: Philadelphia, PA, USA, 1996. [Google Scholar]
- CSIRO. The Insects of Australia, 2nd ed.; Melbourne University Press: Melbourne, Australia, 1996. [Google Scholar]
- McAlpine, J.F. Manual of Nearctic Diptera Volume 2; Research Branch Agriculture Canada: Ottawa, ON, Canada, 1987. [Google Scholar]
- Goulet, H.; Huber, J.T. Hymenoptera of the World: An. Identification Guide to Families; Canada Communication Group Publishing: Ottawa, ON, Canada, 1993. [Google Scholar]
- Bolton, B. Identification Guide to the Ant Genera of the World; Harvard University Press: Cambridge, UK, 1994; p. 222. [Google Scholar]
- Colwell, R.K.; Coddington, J.A. Estimating terrestrial biodiversity through extrapolation. Philos. Trans. R. Soc. B Biol. Sci. 1994, 345, 101–118. [Google Scholar]
- Clarke, K.R. Non-parametric multivariate analysis of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed.; Elsevier: Dordrecht, The Netherlands, 1998. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Kindt, R.; Coe, R. Tree Diversity Analysis: A Manual and Software for Common Statistical Methods for Ecological and Biodiversity Studies; World Agroforestry Centre (ICRAF): Nairobi, Kenya, 2005. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-3. 2018. [Google Scholar]
- Cruttwell, R.E. Insects and mites attacking Eupatorium odoratum in the neotropics: An annotated list of the insects and mites recorded from Eupatorium odoratum L., with a key to the types of damage found in Trinidad. Tech. Bull. Commonw. Inst. Biol. Control 1974, 17, 87–125. [Google Scholar]
- Hokkanen, H.M.T.; Pimentel, D. New associations in biological control: Theory and practice. Can. Entomol. 1989, 121, 829–840. [Google Scholar] [CrossRef]
- La Salle, J.; Ramadan, M.; Kumashiro, B.R. A new parasitoid of the Erythrina Gall Wasp, Quadrastichus erythrinae Kim (Hymenoptera: Eulophidae). Zootaxa 2009, 2083, 19–26. [Google Scholar] [CrossRef]
- Kim, I.-K.; Mendel, Z.; Protasov, A.; Blumberg, D.; La Salle, J. Taxonomy, biology, and efficacy of two Australian parasitoids of the eucalyptus gall wasp, Leptocybe invasa Fisher & La Salle (Hymenoptera: Eulophidae: Tetrastichinae). Zootaxa 2008, 1910, 1–20. [Google Scholar]
- MacGowan, I.; Merz, B.; Wermelinger, B. Six species of Lonchaea Fallén (Diptera, Lonchaeidae) new to Switzerland. Bull. De La Société Entomol. Suisse 2007, 80, 31–35. [Google Scholar]
- Watanabe, S.; Murakami, Y.; Hasegawa, E. Effects of aphid parasitism on host plant fitness in an aphid-host relationship. PLoS ONE 2018, 13, e0202411. [Google Scholar] [CrossRef]
- Aigbedion-Atalor, P.O.; Day, M.D.; Idemudia, I.; Wilson, D.D.; Paterson, I.D. With or without you: Stem-galling of a tephritid fly reduces the vegetative and reproductive performance of the invasive plant Chromolaena odorata (Asteraceae) both alone and in combination with another agent. BioControl 2018, 64, 103–114. [Google Scholar] [CrossRef]
- Aigbedion-Atalor, P.O.; Idemudia, I.; Adom, M.; Day, M. First record of a specialist folivore of Chromolaena odorata (Asteraceae) in Togo, and indices of its range expansion in Nigeria: Implications for biological control. Biocontrol Sci. Technol. 2018, 28, 805–810. [Google Scholar] [CrossRef]
- McFadyen, R.E.C. The ecology of Chromolaena odorata in the neotropics. In Proceedings of the Second International Workshop on Biological Control of Chromolaena Odorata, Bogor, Indonesia, 4–8 February 1991; Muniappan, R., Ferrar, P., Eds.; BIOTROP Special Publication: Bogor, Indonesia, 1991; Volume 44, pp. 1–9. [Google Scholar]
- McFadyen, R.E.C. Siam weed: A new threat to Australia’s north. Plant. Prot. Q. 1989, 4, 3–7. [Google Scholar]
- Wolda, H. Altitude, habitat and tropical insect diversity. Biol. J. Linn. Soc. 1987, 39, 313–323. [Google Scholar] [CrossRef]
- McCoy, E.D. The distribution of insects along elevational gradients. Oikos 1990, 58, 313–322. [Google Scholar] [CrossRef]
- Day, M.D.; Riding, N.; Senaratne, K.A.D.W. The host specificity and climatic suitability of the gall flyCecidochares connexa(Diptera: Tephritidae), a potential biological control agent forChromolaena odorata(Asteraceae) in Australia. Biocontrol Sci. Technol. 2016, 26, 691–706. [Google Scholar] [CrossRef]
- Aigbedion-Atalor, P.O.; Idemudia, I.; Witt, A.B.R.; Day, M.D. First record of the impact of the parasitism of Cecidochares connexa (Diptera: Tephritidae) by a solitary larval ectoparasitoid in West Africa: Cause for concern? J. Plant. Dis. Prot. 2018, 126, 93–95. [Google Scholar] [CrossRef]
- Van Driesche, R.; Hoddle, M. Non-target effects of insect biocontrol agents and trends in host specificity since 1985. CAB Rev. 2016, 11, 1–66. [Google Scholar] [CrossRef]
- Hopper, K.R. Research needs concerning non-target impacts of biological control introductions. In Evaluating Indirect Ecological Effects of Biological Control; Wajnberg, E., Scott, J.K., Quimby, P.C., Eds.; CABI Publishing: Wallingford, CT, USA, 2001; pp. 39–56. [Google Scholar]



| No | Order | Bogor | Lampung | Entire Area | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Open Area | Oil Palm | Open Area | Oil Palm | ||||||||
| S | N | S | N | S | N | S | N | S | N | ||
| 1. | Araneae | 13 | 31 | 15 | 41 | 11 | 15 | 9 | 13 | 25 | 100 |
| 2. | Blattodea | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 2 | 2 |
| 3. | Coleoptera | 14 | 29 | 9 | 17 | 6 | 16 | 5 | 6 | 25 | 68 |
| 4. | Diptera | 16 | 40 | 24 | 118 | 13 | 69 | 9 | 48 | 30 | 275 |
| 5. | Hemiptera | 14 | 2352 | 13 | 6755 | 13 | 162 | 12 | 1193 | 32 | 10,462 |
| 6. | Hymenoptera | 45 | 1159 | 61 | 299 | 65 | 357 | 50 | 450 | 124 | 2265 |
| 7. | Lepidoptera | 2 | 8 | 0 | 0 | 2 | 2 | 1 | 1 | 3 | 11 |
| 8. | Mantodea | 2 | 7 | 3 | 33 | 3 | 10 | 1 | 2 | 3 | 52 |
| 9. | Odonata | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 |
| 10. | Orthoptera | 8 | 22 | 7 | 28 | 5 | 12 | 5 | 19 | 9 | 81 |
| 11. | Thysanoptera | 0 | 0 | 1 | 4 | 1 | 3 | 1 | 1 | 1 | 8 |
| Total | 255 | 13,325 | |||||||||
| No. | Family | Species | Bogor | Lampung | Entire Area | Role | ||
|---|---|---|---|---|---|---|---|---|
| OA | OP | OA | OP | |||||
| DIPTERA | ||||||||
| 1. | Tephritidae | Cecidochares connexa | 13 | 36 | 194 | 227 | 470 | Gall fly |
| 2. | Lonchaeidae | Lonchaea sp | 4 | 1 | 5 | Gall fly | ||
| HYMENOPTERA | ||||||||
| 1. | Bethylidae | Bethylidae sp | 1 | 1 | Parasitoid | |||
| 2. | Braconidae | Braconidae sp1 | 3 | 7 | 10 | Parasitoid | ||
| 3. | Braconidae sp2 | 1 | 2 | 3 | Parasitoid | |||
| 4. | Encyrtidae | Encyrtidae sp | 1 | 1 | Parasitoid | |||
| 5. | Figitidae | Eucoilinae sp1 | 1 | 1 | 2 | Parasitoid | ||
| 6. | Eucoilinae sp2 | 1 | 1 | Parasitoid | ||||
| 7. | Eucoilinae sp3 | 1 | 1 | Parasitoid | ||||
| 8. | Eulophidae | Quadrastichus sp | 14 | 8 | 52 | 102 | 146 | Parasitoid |
| 9. | Eupelmidae | Eupelmidae sp | 1 | 1 | Parasitoid | |||
| 10. | Eurytomidae | Eurytomidae sp1 | 1 | 3 | 4 | Parasitoid | ||
| 11. | Eurytomidae sp2 | 1 | 1 | Parasitoid | ||||
| 12. | Pteromalidae | Pteromalidae sp1 | 2 | 1 | 1 | 4 | 8 | Parasitoid |
| 13. | Pteromalidae sp2 | 1 | 1 | Parasitoid | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchori, D.; Rizali, A.; Lukvitasari, L.; Triwidodo, H. Insect Communities Associated with Siam Weed: Evaluation after Three Decades of Cecidochares connexa Release as Biocontrol Agent. Diversity 2020, 12, 344. https://doi.org/10.3390/d12090344
Buchori D, Rizali A, Lukvitasari L, Triwidodo H. Insect Communities Associated with Siam Weed: Evaluation after Three Decades of Cecidochares connexa Release as Biocontrol Agent. Diversity. 2020; 12(9):344. https://doi.org/10.3390/d12090344
Chicago/Turabian StyleBuchori, Damayanti, Akhmad Rizali, Luna Lukvitasari, and Hermanu Triwidodo. 2020. "Insect Communities Associated with Siam Weed: Evaluation after Three Decades of Cecidochares connexa Release as Biocontrol Agent" Diversity 12, no. 9: 344. https://doi.org/10.3390/d12090344
APA StyleBuchori, D., Rizali, A., Lukvitasari, L., & Triwidodo, H. (2020). Insect Communities Associated with Siam Weed: Evaluation after Three Decades of Cecidochares connexa Release as Biocontrol Agent. Diversity, 12(9), 344. https://doi.org/10.3390/d12090344






