Diversity of Soil Gamasine Mites (Acari: Mesostigmata: Gamasina) in an Area of Natural Vegetation and Cultivated Areas of the Cerrado Biome in Northern Brazil
Abstract
1. Introduction
2. Material and Methods
2.1. Characterization of the Ecosystems
2.2. Edapho-Climatic Characterization
2.3. Mite Sampling, Extraction, and Identification
2.4. Data Analysis
3. Results
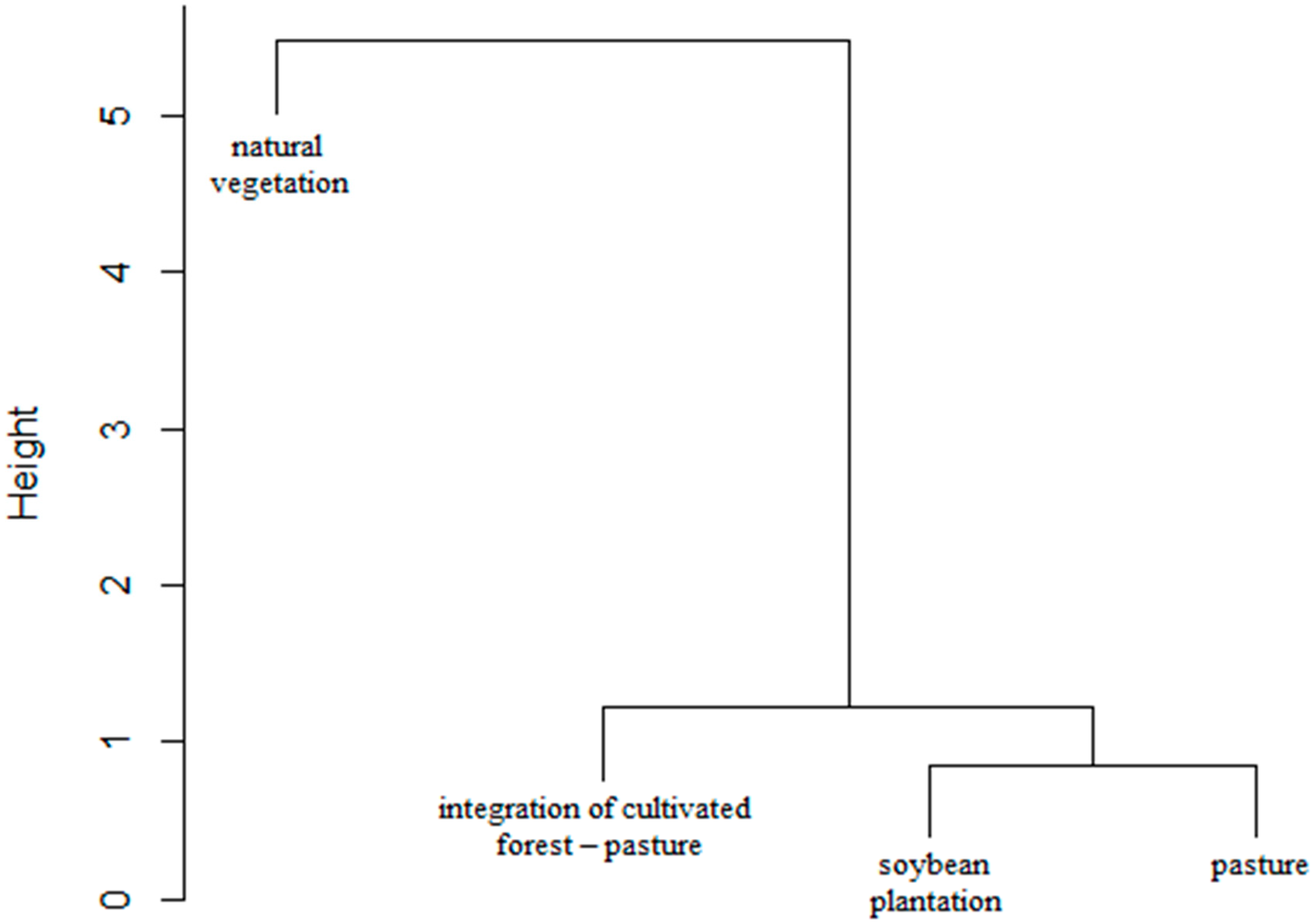
Similarity Analysis
4. Discussion
4.1. Effect of Abiotic Soil Factors
4.2. Interaction of Gamasina with Other Organisms
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- MMA—Ministério do Meio Ambiente. Cerrado Biome. Available online: https://www.mma.gov.br/estruturas/chm/_arquivos/mapas_cobertura_vegetal_ingles.pdf (accessed on 10 May 2020).
- Ribeiro, J.F.; Walter, B.M.T. As principais fitofisionomias do Bioma Cerrado. In Cerrado Ecologia e Flora; Sano, S.M., Almeida, S.P., Ribeiro, J.F., Eds.; Embrapa Informação Tecnológica: Brasília, Brazil, 2008; p. 406. [Google Scholar]
- Companhia Nacional de Abastecimento (2019) Boletim da Safra de Grãos. Available online: https://www.conab.gov.br/info-agro/safras/graos/boletim-da-safra-de-graos (accessed on 18 May 2020).
- Machado, R.B.; Ramos Neto, M.B.; Pereira, P.; Caldas, E.; Gonçalves, D.; Santos, N.; Tabor, K.; Steininger, M. Estimativas de Perda da Área do Cerrado Brasileiro; Conservation International do Brasil: Brasília, Brazil, 2004; relatório técnico não publicado. [Google Scholar]
- El Titi, A. (Ed.) Implications of soil tillage for weed communities. In Soil tillage in Agroecosystems; CRC Press: Boca Raton, FL, USA, 2003; pp. 147–185. [Google Scholar]
- Bedano, J.C.; Domínguez, A.; Arolfo, R.; Wall, L.G. Effect of Good Agricultural Practices under no-till on litter and soil invertebrates in areas with different soil types. Soil Tillage Res. 2016, 158, 100–109. [Google Scholar] [CrossRef]
- Bzuneck, H.L.; Santos, H.R. Efeitos de dois sistemas de preparo do solo e sucessões de cultura, na população de ácaros Galumnidae (Cryptostigmata). Rev. Ciência Agrária 1991, 11, 1–2. [Google Scholar]
- Lopes Assad, M.L. Fauna do solo. In Biologia dos Solos dos Cerrados; Vargas, M.A.T., Hungria, M., Eds.; EMBRAPA, CPAC: Planaltina, Brazil, 1997; pp. 363–444. [Google Scholar]
- Silva, J.; Casalinho, H.; Verona, L.; Schwengber, J. Avaliação da mesofauna (colêmbolos e ácaros) do solo em agroecossistemas de base familiar no Rio Grande do Sul. Rev. Bras. Agroecol. 2007, 2, 539–542. [Google Scholar]
- Kibblewhite, M.G.; Ritz, K.; Swift, M.J. Soil health in agricultural systems. Phil. Trans. R Soc. B Biol. Sci. 2008, 363, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, E.E.; Krantz, G.W.; Walter, D.E. Order Mesostigmata. In A Manual of Acarology, 3rd ed.; Krantz, G.W., Walter, D.E., Eds.; Texas Tech University Press: Lubbock, TX, USA, 2009; p. 807. [Google Scholar]
- Gerson, U.; Smiley, R.L.; Ochoa, R. Mites (Acari) for Pest Control; Blackwell Science: Oxford, MA, USA, 2003; p. 539. [Google Scholar]
- Carrillo, D.; Moraes, G.J.; Peña, J.E. Prospects for Biological Control of Plant Feeding Mites and Other Harmful Organisms; Springer: London, UK, 2015; p. 344. [Google Scholar]
- Roubinet, E.; Straub, C.; Jonsson, T.; Staudacher, K.; Traugott, M.; Ekbom, B.; Jonsson, M. Additive effects of predator diversity on pest control caused by few interactions among predator species. Ecol. Entomol. 2015, 40, 362–371. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- EMBRAPA—Empresa Brasileira de Pesquisa Agropecuária. Sistema Brasileiro de Classificação de Solos; Embrapa Informação Tecnológica: Brasília, Brazil, 2018; p. 195. [Google Scholar]
- Oliveira, A.R.; Moraes, G.J.; Demétrio, C.G.B.; Nardo, E.A.B. Efeito do Vírus da Poliedrose Nuclear de Anticarsia Gemmatalis sobre Oribatida Edáficos (Arachnida: Acari) em um Campo de Soja; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2001; p. 32. [Google Scholar]
- Chant, D.A.; McMurtry, J.A. Illustrated Keys and Diagnoses for the Genera and Subgenera of the Phytoseiidae of the World (Acari: Mesostigmata); Indira Publishing House: West Bloomfield, MI, USA, 2007; p. 219. [Google Scholar]
- Castilho, R.C.; Moraes, G.J.; Halliday, B. Catalogue of the family Rhodacaridae Oudemans, with notes on the classification of Rhodacaroidea (Acari: Mesostigmata). Zootaxa 2012, 3471, 1–69. [Google Scholar] [CrossRef][Green Version]
- Moraes, G.J.; Britto, E.P.J.; Mineiro, J.L.C.; Halliday, B. Catalogue of the mite families Ascidae Voigts & Oudemans, Blattisociidae Garman and Melicharidae Hirschmann (Acari: Mesostigmata). Zootaxa 2016, 4112, 1–299. [Google Scholar] [CrossRef]
- Castilho, R.C.; Silva, E.S.; Moraes, G.J.; Halliday, B. Catalogue of the family Ologamasidae Ryke (Acari: Mesostigmata). Zootaxa 2016, 4197, 1–147. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing, v.3.0.1; R foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Dekkers, T.B.; van der Werff, P.A.; van Amelsvoort, P.A.M. Soil Collembola and Acari related to farming systems and crop rotations in organic farming. Acta Zool. Fennica 1994, 195, 28–31. [Google Scholar]
- Koehler, H. Predatory mites (Gamasina, Mesostigmata). Agric. Ecosys. Environ. 1999, 74, 395–410. [Google Scholar] [CrossRef]
- Bedano, J.C.; Ruf, A. Soil predatory mite communities (Acari: Gamasina) in agroecosystems of Central Argentina. Appl. Soil Ecol. 2007, 36, 22–31. [Google Scholar] [CrossRef]
- Junqueira, B.R. Diversidade de Ácaros Edáficos em um Fragmento de Mata Atlântica e Três Cultivos Agrícolas, em Jaboticabal/SP, com Ênfase nos Gamasina (Mesostigmata). Master’s Thesis, Universidade Estadual Paulista (UNESP), Jaboticabal, Brazil, 2017. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis: Chemical and Microbiological Properties. Part 2; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Soil Science Society of America: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Sisti, C.P.J.; Santos, H.P.; Kohhan, R.; Alves, B.J.R.; Urquiaga, S.; Boddey, R.M. Change in carbon and nitrogen stocks in soil under 13 years of conventional or zero tillage in southern Brazil. Soil Tillage Res. 2003, 76, 39–58. [Google Scholar] [CrossRef]
- Price, D.W. Abundance and vertical distribution of microarthropods in the surface layers of a California pine forest soil. Hilgardia 1973, 42, 121–147. [Google Scholar] [CrossRef]
- Evans, G.O.; Till, W.M. Mesostigmatic mites of Britain and Ireland (Chelicerata: Acari-Parasitiformes): An introduction to their external morphology and classification. Trans. Zoo. Soc. Lond. 1979, 35, 139–262. [Google Scholar] [CrossRef]
- Coleman, D.C.; Crossley, D., Jr. Fundamentals of Soil Ecology; Academic Press, Inc.: San Diego, CA, USA, 1996; p. 205. [Google Scholar]
- Mineiro, J.L.C.; Moraes, G.J. Gamasida (Arachnida: Acari) edáficos de Piracicaba, Estado de São Paulo. Neotrop. Entomol. 2001, 30, 379–385. [Google Scholar] [CrossRef]
- Silva, E.S.; Moraes, G.J.; Krantz, G.W. Diversity of edaphic rhodacaroid mites (Acari: Mesostigmata: Rhodacaroidea) in natural ecosystems in the State of São Paulo, Brazil. Neotrop. Entomol. 2004, 33, 547–555. [Google Scholar] [CrossRef][Green Version]
- Santos, J.C. Ácaros (Arthropoda: Acari) Edáficos do Estado de Alagoas, com Enfase nos Mesostigmata. Master’s Thesis, Universidade Estadual Paulista (UNESP), Jaboticabal, Brazil, 2013. [Google Scholar]
- Karg, W. Zur Systematik der Raubmilbenfamilien Hypoaspididae v. Vitzthum, 1941 und Rhodacaridae Oudemans, 1902 (Acarina, Parasitiformes) mit neuen Arten aus Süd- und Mittelamerika. Mitt. Mus. Naturkunde Berl. 2000, 76, 243–262. [Google Scholar] [CrossRef]
- Castro, M.C. Diversidade de Ácaros Gamasina (Mesostigmata) Edáficos em Sistema de Plantio Direto e Sistema de Integração Lavoura, Pecuária e Floresta (ILPF) no Estado do Mato Grosso. Master’s Thesis, Universidade Estadual Paulista (UNESP), Jaboticabal, Brazil, 2019. [Google Scholar]
- Azevedo, E.B.; Sarmento, R.A.; Castilho, R.C. A new species of Multidentorhodacarus (Mesostigmata: Rhodacaridae) from Brazil, complementary description of Multidentorhodacarus squamosus Karg and a key to the world species of the genus. Syst. Appl. Acarol. 2019, 24, 324–336. [Google Scholar] [CrossRef]
- Yamada, M.; Moraes, G.J. A key to the species of Protogamasellus (Acari: Ascidae), with a new species from the Brazilian Pantanal. Zootaxa 2020, 4801, 343–354. [Google Scholar] [CrossRef]
- Ruf, A.; Beck, L. The use of predatory soil mites in ecological soil classification and assessment concepts, with perspectives for oribatid mites. Ecotox. Environ. Safe 2005, 62, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Mesléard, F.; Garnero, S.; Beck, N.; Rosecchi, E. Uselessness and indirect negative effects of an insecticide on rice field invertebrates. C. R. Biol. 2005, 328, 955–962. [Google Scholar] [CrossRef] [PubMed]
- San Miguel, A.; Raveton, M.; Lempérière, G.; Ravanel, P. Phenylpyrazoles impact on Folsomia candida (Collembola). Soil Biol. Biochem. 2008, 40, 2351–2357. [Google Scholar] [CrossRef]
- Moraes, G.J.; Venancio, R.; Santos, V.L.V.; Paschoal, A.D. Potential of Ascidae, Blattisociidae and Melicharidae (Acari: Mesostigmata) as biological control agents of pest organisms. In Prospects for Biological Control of Plant Feeding Mites and Other Harmful Organisms; Carrillo, D., Moraes, G.J., Peña, J.E., Eds.; Springer: London, UK, 2015; pp. 33–75. [Google Scholar]
- Rueda-Ramírez, D.M. Ácaros Edáficos Mesostigmata de Grandes Altitudes na Colômbia e os Possíveis Efeitos de Mudanças Edafo-Climáticas sobre as Populações destes Ácaros. Master’s Thesis, Escola Superior de Agricultura “Luiz de Queiroz”, Universidade de São Paulo, São Paulo, Brazil, 2012. [Google Scholar]
- Bedano, J.C.; Ruf, A. Sensitivity of different taxonomic levels of soil Gamasina to land use and anthropogenic disturbances. Agric. For. Entomol. 2010, 12, 203–212. [Google Scholar] [CrossRef]
- Torris, A.F. Diversidade e Flutuação Populacional de Ácaros Edáficos em um Fragmento de Caatinga e Três Cultivos Agrícolas, no Vale do São Francisco (Pernambuco), com Ênfase nos Gamasina (Mesostigmata). Master’s Thesis, Universidade Estadual Paulista (UNESP), Jaboticabal, Brazil, 2019. [Google Scholar]
- Birkhofer, K.; Schoning, I.; Alt, F.; Herold, N.; Klarner, B.; Maraun, M.; Marhan, S.; Oelmann, Y.; Wubet, T.; Yurkov, A.; et al. General relationships between abiotic soil properties and soil biota across spatial scales and different land-use types. PLoS ONE 2012, 7, e43292. [Google Scholar] [CrossRef]
- Miller, J.; Battigelli, J.P.; Willms, W.D. Grazing protection influences soil mesofauna in ungrazed and grazed riparian and upland pastures. Rang. Ecol. Manag. 2014, 67, 429–434. [Google Scholar] [CrossRef]
- Bedano, J.C.; Cantú, M.P.; Doucet, M.E. Influence of three different land management practices on soil mite (Arachnida: Acari) densities in relation to a natural soil. Appl. Soil Ecol. 2006, 32, 293–304. [Google Scholar] [CrossRef]
- Edwards, C.A.; Lofty, J.R. The influence of cultivations on soil animal populations. In Progress in Soil Zoology; Vanek, J., Ed.; Academia Publishing House: Prague, Czech Republic, 1975; pp. 399–406. [Google Scholar]
- Fox, C.A.; Fonseca, E.J.A.; Miller, J.J.; Tomlin, A.D. The influence of row position and selected soil attributes on Acarina and Collembola in no-till and conventional continuous corn on a clay loam soil. Appl. Soil Ecol. 1999, 13, 1–8. [Google Scholar] [CrossRef]
- Koukoura, Z.; Mamolos, A.P.; Kalburtji, K.L. Decomposition of dominant plant species litter in a semi-arid grassland. Appl. Soil Ecol. 2003, 23, 13–23. [Google Scholar] [CrossRef]
- Oliveira, L.J.; Roggia, S.; Salvadori, J.R.; Ávila, C.J.; Fernandes, P.M.; Oliveira, C.M. Insetos que atacam raízes e nódulos da soja. In Soja—Manejo Integrado de Insetos e outros Artrópodes-Praga; Hoffmann-Campo, C.B., Corrêa-Ferreira, B.S., Moscardi, F., Eds.; Embrapa: Brasília, Brazil, 2012; pp. 75–144. Available online: http://www.cnpso.embrapa.br/artropodes/Capitulo2.pdf (accessed on 10 June 2020).
- Castilho, R.C.; Moraes, G.J.; Silva, E.S.; Freire, R.A.P.; Eira, F.C. The predatory mite Stratiolaelaps scimitus as a control agent of the fungus gnat Bradysia matogrossensis in commercial production of the mushroom Agaricus bisporus. Int. J. Pest Manag. 2009, 53, 181–185. [Google Scholar] [CrossRef]
- Dias, W.P.; Garcia, A.; Silva, J.F.V.; Carneiro, G.E.S. Nematóides em Soja: Identificação e Controle; Embrapa Soja: Londrina, Brazil, 2010; p. 8. [Google Scholar]
- Mattos, V.S.; Furlanetto, C.; Silva, J.G.P.; Santos, D.F.; Almeida, M.R.A.; Correa, V.R.; Moita, A.W.; Castagnone-Sereno, P.; Carneio, R.M.D.G. Meloidogyne spp. populations from native Cerrado and soybean cultivated areas: Genetic variability and aggressiveness. Nematology 2016, 18, 505–515. [Google Scholar] [CrossRef]

| Parameters | Ecosystems | |||
|---|---|---|---|---|
| Natural Vegetation | Soybean Cultivation | Pasture | Integration of Cultivated Forest–Pasture | |
| Physical properties | ||||
| Clay (g.kg−1) | 250 | 257 | 213 | 244 |
| Sand (g.kg−1) | 703 | 694 | 740 | 710 |
| Silt (g.kg−1) | 50 | 50 | 47 | 47 |
| Chemical Properties | ||||
| pH CaCl2 | ||||
| Dry season | 3.9 | 5.6 | 5.1 | 4.7 |
| Rainy season | 4.0 | 6.5 | 5.4 | 5.0 |
| Al (cmolc·dm−3) | ||||
| Dry season | 0.5 | 0 | 0 | 0 |
| Rainy season | 0.2 | 0 | 0 | 0 |
| OMC (dag·kg−1) | ||||
| Dry season | 1.8 | 1.2 | 1.5 | 1.4 |
| Rainy season | 3.3 | 4.4 | 2.3 | 1.9 |
| TOC (dag·kg−1) | ||||
| Dry season | 1.0 | 0.7 | 0.9 | 0.8 |
| Rainy season | 1.9 | 2.5 | 1.3 | 1.1 |
| P (mg·dm−3) | ||||
| Dry season | 10.8 | 12.4 | 20.3 | 24.5 |
| Rainy season | 14.85 | 60.1 | 25.6 | 34 |
| K (mg·dm−3) | ||||
| Dry season | 36 | 47 | 49 | 41 |
| Rainy season | 50 | 91 | 84 | 57 |
| Ca (cmolc/dm−3) | ||||
| Dry season | 0.2 | 1.2 | 1.5 | 1.9 |
| Rainy season | 0.3 | 6.5 | 5.4 | 4.6 |
| Mg (cmolc/dm−3) | ||||
| Dry season | 0.2 | 0.6 | 0.8 | 1.2 |
| Rainy season | 0.2 | 3.2 | 2.8 | 1.8 |
| S (mg.dm−3) | ||||
| Dry season | 2 | 2 | 2 | 3 |
| Rainy season | 3 | 5 | 4 | 5 |
| B (mg.dm−3) | ||||
| Dry season | 0.1 | 0.1 | 0.1 | 0.1 |
| Rainy season | 0.1 | 0.4 | 0.3 | 0.2 |
| Cu (mg.dm−3) | ||||
| Dry season | 0.2 | 0.3 | 0.3 | 0.4 |
| Rainy season | 0.3 | 0.6 | 0.8 | 1.3 |
| Mn (mg.dm−3) | ||||
| Dry season | 3.8 | 4.7 | 3.4 | 3.4 |
| Rainy season | 5.2 | 5.7 | 11.7 | 5.3 |
| Zn (mg.dm−3) | ||||
| Dry season | 0.5 | 1.1 | 0.6 | 0.8 |
| Rainy season | 0.6 | 1.7 | 0.8 | 0.8 |
| Gamasina Species | Code | Ecosystems/Seasons | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Natural Vegetation | Soybean Cultivation | Pasture | Integration of Cultivated Forest–Pasture | Total | ||||||
| Dry | Rainy | Dry | Rainy | Dry | Rainy | Dry | Rainy | |||
| Rhodacaridae | ||||||||||
| Binodacarus n. sp. | Bino | 15 | 65 | 0 | 2 | 0 | 0 | 0 | 0 | 82 |
| Multidentorhodacarus tocantinensis Azevedo and Castilho | Musp | 24 | 54 | 0 | 1 | 0 | 8 | 1 | 5 | 93 |
| Multidentorhodacarus squamosus Karg | Msqu | 225 | 201 | 0 | 3 | 5 | 30 | 20 | 18 | 502 |
| Protogamasellopsis dioscorus (Manson) | Pdio | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |
| Protogamasellopsis zaheri Abo-Shnaf, Castilho, and Moraes | Pzah | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |
| Rhodacarus n. sp. | Rhod | 2 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 11 |
| Ologamasidae | ||||||||||
| Neogamasellevans sp. | Neog | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Ologamasus sp. | Olog | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Macrochelidae | ||||||||||
| Macrocheles muscaedomesticae (Scopoli) | Macr | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| Podocinidae | ||||||||||
| Podocinium sagax (Berlese) | Podo | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Laelapidae | ||||||||||
| Androlaelaps sp. | Andr | 6 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 8 |
| Cosmolaelaps barbatus Moreira, Klompen and Moraes | Cbar | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 3 | 7 |
| Cosmolaelaps guttulatus (Karg) | Cgut | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 4 |
| Cosmolaelaps pampaensis Duarte, Moreira, Cunha & Moraes | Cpam | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Cosmolaelaps sp. 1 | Cos1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Cosmolaelaps sp. 2 | Cosm | 1 | 14 | 0 | 3 | 1 | 6 | 0 | 0 | 25 |
| Gaeolaelaps sp. 1 | Geo1 | 6 | 11 | 0 | 2 | 1 | 1 | 2 | 4 | 27 |
| Gaeolaelaps sp. 2 | Geo2 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 2 |
| Gaeolaelaps sp. 3 | Geo3 | 0 | 2 | 0 | 0 | 0 | 0 | 2 | 1 | 5 |
| Gaeolaelaps sp. 4 | Geo4 | 2 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 6 |
| Gaeolaelaps sp. 5 | Geo5 | 0 | 0 | 0 | 3 | 0 | 2 | 1 | 0 | 6 |
| Gaeolaelaps sp. 6 | Geo6 | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| Gaeolaelaps sp. 7 | Geo7 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Gaeolaelaps sp. 8 | Geo8 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Gaeolaelaps sp. 9 | Geo9 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Gaeolaelaps sp. 10 | Geo10 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 2 |
| Laelaspisella cavitatis (Karg) | Pogo | 7 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 12 |
| Oloopticus reticulatus Karg | Oloo | 5 | 27 | 0 | 0 | 0 | 1 | 0 | 0 | 33 |
| Pseudoparasitus sp. | Pseu | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Phytoseiidae | ||||||||||
| Euseius citrifolius Denmark and Muma | Econ | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Neoseiulus barkeri Hughes | Nann | 0 | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 4 |
| Neoseiulus gracilis (Muma) | Nide | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Proprioseiopsis mexicanus (Garman) | Pmex | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Typhlodromus transvaalensis (Nesbitt) | Ttra | 2 | 4 | 0 | 3 | 1 | 4 | 3 | 1 | 18 |
| Ascidae | ||||||||||
| Asca garmanni Hurlbutt | Ager | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Asca sp. | Asca | 35 | 9 | 0 | 0 | 2 | 1 | 8 | 3 | 58 |
| New genus | Assp | 4 | 30 | 0 | 0 | 0 | 8 | 1 | 7 | 50 |
| Protogamasellus mica (Athias-Henriot) | Pmic | 18 | 31 | 1 | 18 | 3 | 47 | 4 | 25 | 147 |
| Protogamasellus sigillophorus Mineiro, Lindquist and Moraes | Psig | 8 | 24 | 8 | 7 | 9 | 18 | 38 | 75 | 187 |
| Protogamasellus pantanal Yamada and Moraes | Prot | 7 | 18 | 0 | 1 | 0 | 1 | 1 | 5 | 33 |
| Blattisocidae | ||||||||||
| Cheiroseius pugiunculus Karg | Cher | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Lasioseius n. sp. | Lasi | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 3 |
| Melicharidae | ||||||||||
| Proctolaelaps bickleyi (Bram) | Pbic | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Proctolaelaps paulista Mineiro, Lindquist and Moraes | Ppau | 2 | 5 | 0 | 0 | 0 | 2 | 3 | 6 | 18 |
| Proctolaelaps sp. | Pctl | 2 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 9 |
| Total abundance | 378 | 541 | 13 | 51 | 25 | 135 | 86 | 154 | 1383 | |
| Mean abundance (mites/sample) * | 65.8 ± 9.8 A | 87.3 ±26.7 a | 2.3 ± 0.3 C | 8.5 ± 3.2 c | 4.3 ± 0.7 C | 22.2 ± 5.9 b | 13.7 ± 1.9 B | 26.3 ± 12.9 b | ||
| Species richness | 24 | 31 | 6 | 16 | 8 | 18 | 13 | 13 | ||
| Mean richness (species/sample) * | 12.2 ± 0.5 A | 13.7 ± 2.1 a | 1.8 ± 0.3 C | 4.5 ± 0.9 b | 3.0 ± 0.4 BC | 7.2 ± 0.8 b | 4.8 ± 0.7 B | 6.0 ± 1.6 b | ||
| Shannon-Weaver index | 1.70 | 2.34 | 1.29 | 2.27 | 1.79 | 2.03 | 1.77 | 1.75 | ||
| Simpson index | 0.63 | 0.82 | 0.59 | 0.83 | 0.79 | 0.82 | 0.73 | 0.72 | ||
| Equitability | 0.54 | 0.68 | 0.72 | 0.82 | 0.86 | 0.69 | 0.69 | 0.68 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandão de Azevedo, E.; Henrique Azevedo, L.; Moreira, G.F.; Santos, F.A.d.; Carvalho, M.A.F.d.; Sarmento, R.d.A.; de Campos Castilho, R. Diversity of Soil Gamasine Mites (Acari: Mesostigmata: Gamasina) in an Area of Natural Vegetation and Cultivated Areas of the Cerrado Biome in Northern Brazil. Diversity 2020, 12, 331. https://doi.org/10.3390/d12090331
Brandão de Azevedo E, Henrique Azevedo L, Moreira GF, Santos FAd, Carvalho MAFd, Sarmento RdA, de Campos Castilho R. Diversity of Soil Gamasine Mites (Acari: Mesostigmata: Gamasina) in an Area of Natural Vegetation and Cultivated Areas of the Cerrado Biome in Northern Brazil. Diversity. 2020; 12(9):331. https://doi.org/10.3390/d12090331
Chicago/Turabian StyleBrandão de Azevedo, Emiliano, Letícia Henrique Azevedo, Grazielle Furtado Moreira, Fábio Araújo dos Santos, Marcos Alberto Francisco de Carvalho, Renato de Almeida Sarmento, and Raphael de Campos Castilho. 2020. "Diversity of Soil Gamasine Mites (Acari: Mesostigmata: Gamasina) in an Area of Natural Vegetation and Cultivated Areas of the Cerrado Biome in Northern Brazil" Diversity 12, no. 9: 331. https://doi.org/10.3390/d12090331
APA StyleBrandão de Azevedo, E., Henrique Azevedo, L., Moreira, G. F., Santos, F. A. d., Carvalho, M. A. F. d., Sarmento, R. d. A., & de Campos Castilho, R. (2020). Diversity of Soil Gamasine Mites (Acari: Mesostigmata: Gamasina) in an Area of Natural Vegetation and Cultivated Areas of the Cerrado Biome in Northern Brazil. Diversity, 12(9), 331. https://doi.org/10.3390/d12090331






