Abstract
Bromeliad phytotelmata are habitats for different organisms and models for ecological studies. Although poorly known, these environments are widely distributed in tropical ecosystems, harboring cosmopolitan and endemic species. Here, we investigated the diversity of the eukaryotic community in bromeliad phytotelmata considering the influence of altitude. We randomly sampled three bromeliad individuals (twice per season over one year) at four altitudinal strata (20 m, 400 m, 910 m, and 915 m) through a mountain range in southern Brazil. Species richness of phytotelmata community was higher at intermediate altitude while community-wide multivariate analyses revealed differences in phytotelmata communities at each height. Winter was the season with highest community richness, but a peak in summer was observed. Diversity partitioning in different spatial components showed that gamma diversity decreased linearly with altitude, whereas alpha diversity peaked at intermediate altitudes, and beta diversity decreased with height. The relative importance of the components of beta diversity showed different patterns according to the altitude: turnover was more important at intermediate and lower levels, while higher altitude communities were more nested. Our results indicate that differences in height affect diversity patterns of bromeliad phytotelmata communities, which were more diverse at lower altitudes in comparison with more homogeneous communities at higher levels.
1. Introduction
Bromeliads (Bromeliaceae) are one of the most representative groups of plants in the Neotropical region, with a great taxonomic, ecological, and morphological diversity [1]. Brazil is the Neotropical country with the highest degree of diversity and endemism of this family [2]. These plants are key players in enhancing the biological diversity in the neotropics, as their structural complexity provides habitat for terrestrial, semi-aquatic, and aquatic fauna [3]. Their leaves are arranged in rosettes, and in their bases, the accumulation of water and organic matter, promotes an environment for several organisms [4], emphasizing the importance of these plants in the expansion of biological diversity [5]. Bromeliad phytotelmata can accumulate up to 50 L of suspended water per hectare, being important freshwater habitats, and contributing to the nutrient cycling [6,7].
The aquatic community inhabiting bromeliads phytotelmata are very diverse, from bacteria and small protozoa, microcrustaceans, and oligochaetes, to insect larvae and tadpoles of anurans [8,9,10,11]. The phytotelmata colonization process seems to be complex, involving a series of interconnected events, such as active and passive dispersion, arrival, and the process of establishment of the organism [12]. Frank and Lounibos [13] compared this process of colonization with islands, dividing the organisms into phytotelm-specialists (highly selective colonization) and non-specialists (random colonization). The phytotelm communities are exposed to stressful environmental conditions, such as nutrient restriction and water deficit during drought periods. These factors lead to the development of survival mechanisms for long-term community maintenance. The environment formed by the bromeliad leaves can be complex and end up providing a variety of compartments and ecological gradients [14]. This microenvironment not only benefits the community of aquatic organisms and bromeliad, but also provides shelter, protection, and sources of water and food for birds and mammals. In this way, phytotelmata in bromeliads expands the availability of resources and habitats in the ecosystems they are inserted [15]. The majority of studies on aquatic invertebrates in bromeliads have focused on cataloguing species not previously known in phytotelmata [1]. However, some ecological studies have contributed to the understanding of this habitat, e.g., [16,17,18].
Mountain ranges provide interesting environments and natural experiments for studying how the diversity of organisms varies according to altitude [19]. Although the inverse relationship between altitude and species diversity has been accepted as a general pattern for some time, e.g., [20], there is also evidence that diversity can be higher at intermediate elevation levels. The decreasing area and isolation of mountaintops, as well as the low rates of colonization and the high rates of extinction, may be responsible for the decrease in diversity at higher elevations, and the consequent diversity peak at intermediate elevations [21]. In tropical humid mountains, species richness generally decreases with increasing altitude, with the main factors involving: habitat reduction, atmospheric pressure, air temperature, and increased solar radiation [22,23,24]. Phytotelmata have been models for taxonomic and ecological studies due to the high diversity found in these small ecosystems, and because the abundance of plant-held water in nature [3]. Although these patterns are noticeable for many groups, few studies investigate altitudinal differences in bromeliad phytotelmata communities [1,14]. In these studies, there was a tendency of decreasing abundance with increasing altitude, with a peak of specific richness observed at intermediate levels. Rahbek [25] has suggested this pattern of a peak of species richness at intermediate altitudes, where he analyzed that not all species presents a monotonic decline with altitude.
Studies investigating altitudinal differences in phytotelm communities generally use species richness and abundance analysis to verify patterns and significant differences. Few studies have explored the different components of spatial diversity in phytotelm communities, and even fewer explored their response to altitudinal gradients. Jocque and Field [1] found correlations between the turnover component of beta diversity with altitude, meaning that elevational gradients increase the substitution of species between communities. Other studies, like Richardson et al. [26], verified the influence of post-hurricane disturbances for two decades on phytotelmata communities, resulting in decline of alpha and gamma diversities due to the loss of bromeliad individuals, as well as an increase in rare species in the community composition. Busse et al. [27] used beta diversity to verify changes in community structure over a canopy gradient, and nestedness values demonstrated that communities in phytotelmata more exposed to sunlight were subsets of a more diverse community present in forested areas.
The aim of this study was to investigate how phytotelmata community diversity responds to an elevational range in southern Brazil, using ciliates, arthropods, and rotifers as community descriptors. We hypothesized that (i) different altitudes would present discrete community compositions; (ii) community diversity for all spatial levels would decrease as altitude increases; and (iii) the turnover component of beta diversity (i.e., substitution of species between communities) would increase with altitude.
2. Materials and Methods
Study area: the study was conducted along an altitudinal gradient in Atlantic forest areas located in the southern part of a mountain range, in Rio Grande do Sul state, Brazil (29°39′41′’ S and 50°12′59′’ W, 29°29′46′’ S and 50°20′00′’ W) between October 2017 and September 2018. Four sampling sites were selected (Figure 1) according to the bromeliads distribution along the altitudinal gradient: Maquiné (~20 m); Garapiá River (~400 m) and two forest remnants areas in Pró-Mata (~910 m and 915 m). Eight sampling campaigns were carried out, two in each season: spring, summer, fall, and winter.
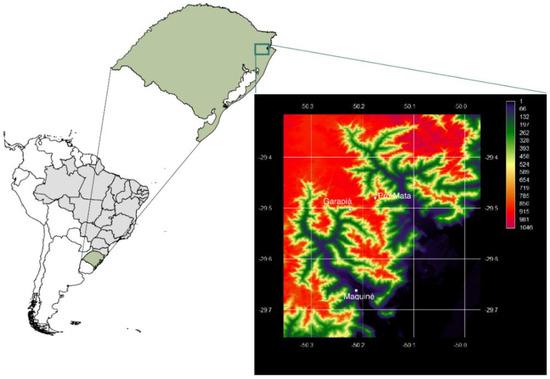
Figure 1.
Sample sites at different altitudes in Serra Geral, Rio Grande do Sul, Brazil.
Maquiné city (29°35′ S 50°16′ W), the sampling point at sea level, is located in the northeast region of Rio Grande do Sul state, in the Maquiné river basin. The area of the Maquiné River has approximately 546 km2 and is inserted in a transition between the slopes of Serra Geral and the coastal plain, with steep altitude gradients. This feature determines a landscape with well-defined plains and mountains along the entire Maquiné river valley [28]. In the sampling site, the vegetation is characterized by a regenerating Atlantic Forest with the presence of growing trees, as well as shrubs. Most of the trees had open canopies, but some presented a very high, well-built treetop. The Garapia River (29°30′’S 50°14′), the point at intermediate altitude, is located in a trail inside the valley. It presents an initial stretch along an access route to a waterfall, within a forest with secondary vegetation. The bromeliads were located in a forest with a slightly more advanced stage of conservation, but still impacted by human activity. The vegetation cover is similar to the observed at 20 m, with the presence of well-developed trees and some shrub plants. The canopy closure was similar to Maquiné sampling site, where the light partially hits the forest ground.
The high altitude sampling site (29°29′ S 50°21′) was located at the Federal Protected Area. The study site is a private protected área (reserva Particular de Patrimônio Natural—RPPN Pró-Mata) owned by the Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), in São Francisco de Paula. This area comprises a mosaic of ecosystems, with Atlantic Rainforests intertwined by natural grasslands, including expressive biological diversity. At 910 m, the vegetation surrounding was composed by shrub plants and high trees, occurring near a lake. The Atlantic forest at this location has a mix with the Araucaria forest, with these plants located near the sampling bromeliads. At 915 m, the bromeliads were located at a much more developed forest, with high presence of trees, epiphytes, and shrubs.
According to Köppen’s classification, the climate in the study region is mesothermic, with undefined dry season and hot summer (Cfa—subtropical humid climate), with mean annual precipitation of 1608 mm. Detailed data on temperature and precipitation at the sampling sites can be observed in Table 1.

Table 1.
Annual precipitation and temperature in Maquiné and Garapiá–Rio Grande do Sul–Brazil. (IGRA–Instituto Rio Grandense do Arroz) and RPPN Pró-Mata.
The study area is inserted in the Neotropical phytogeographic region [29]. The global scale biogeographic classification developed by the World Wildlife Fund (WWF) [30] classifies the rainforest that reaches the RPPN-Pró-Mata from its slopes in Serra do Mar coastal forest. This biogeographic unit comprises the coastal rain forests that cover the mountain ranges, from Rio de Janeiro to Rio Grande do Sul states, including lowland, submontana, montana, and altomontana formations, subject to high rainfall. With a high precipitation, we can assure a more efficient sample, once we depend of tank bromeliads filled with water.
Bromeliad species: at each sampling site, one species of bromeliad was sampled per altitude, since the same species did not occur at the sampled altitude range: cisterns of Vriesea gigantea Gaudich were sampled in Maquiné, Vriesea incurvata Gaudich in Garapiá River, and Vriesea platynema Gaudich and Vriesea friburgensis Gaudich in Pró-Mata (at 910 and 915 m, respectively). All bromeliad individuals sampled had similar size (nearly 45 cm high and 50 cm in diameter) to assure that size of the bromeliad would not influence the community composition.
Sampling standardization: For each bromeliad species, three individuals were randomly sampled in every campaign. These individuals, at each sampling site, were at least 5 m apart. Fifteen milliliters of the phytotelm water were collected using a sterile Pasteur pipette and placed in Falcon tubes. Eight milliliters of each sample were kept refrigerated (~6–10 °C) for qualitative analysis, and 7 ml were fixed with acid Lugol (10%), for abundance estimation. For the quantitative analyses, one milliliter of each fixed sample was analyzed using a Sedgewick Rafter counting cell chamber. Three replicates of the quantitative samples were counted and the average of organisms present in each bromeliad sampled was obtained.
Taxa determination: the eukaryotic community was observed in vivo using an optic microscope (Olympus, Tokyo, Japan), and taxonomic determination was performed using specific literature [8,31,32,33]. Morphological analysis of the morphospecies present in the samples focused on ciliates, rotifers and arthropods, and identification was performed at the most specific taxonomic level. In addition, Oligochaeta, Platyhelminthes, and Nematoda were also analyzed as components of the community.
Data analysis: data were organized in a community matrix of communities (four sites at different altitudes, three bromeliad individuals in each site, one sample per season for each individual) described by mean values of eukaryotic taxa abundance. We tested the influence of altitude and seasonality on community species richness using additive multiple regression models. In these models, we included richness as the dependent variable, and altitude and sampling season as independent variables. We built four independent models: total community richness and separately for arthropods, ciliates, and rotifers. We estimated the significance of the independent variables with Analyses of Variance, and tested all pairwise contrasts within each independent variable for significant models with permutational post-hoc tests, corrected for multiple comparisons [34]. We tested the effect of the altitude on community composition with a one-way multivariate analysis of variance (MANOVA) with permutation (9999 resampling iterations), considering the altitudinal stratum as a factor [35]. This analysis was carried out with function adonis2 of package ‘vegan’ [36,37], and pairwise comparisons between altitudinal strata were tested with package ‘RVAideMemoire’ [38]. To illustrate the distribution of the communities across the altitudinal gradient, we performed a Constrained Correspondence Analysis (CCA) [39], using the altitude as constraining factor for the ordination. We tested the significance of the CCA model with a permutational analysis of variance. These analyses were also performed with package ‘vegan’, and the results were plotted in the two-dimensional ordination space with package ‘ggord’ [39]. We evaluated the association between individual species and the different altitudes using the indicator species approach, in which the strength of association between species and a given vector of group membership is computed, and a p-value for each association is generated via bootstrap resampling. We carried out this analysis with package ‘indicspecies’ [40], using the IndVal index [41]. We explored eukaryotic diversity in three different spatial levels [42], and the raw data matrix described above was summarized according to each level: alpha (cistern level, resulting in 12 communities, three per altitudinal stratum), gamma (altitude level, resulting in four communities, one per altitudinal stratum) and beta (variation of species composition between communities at the alpha level). In addition, we estimated the contribution of each beta diversity component: spatial turnover and nestedness [43]. Diversity partitioning analyses were carried out with package ‘vegan’, using Sørensen dissimilarity index to calculate overall beta diversity and the nestedness component, whereas the turnover component was calculated with Simpson dissimilarity. We plotted diversity profiles using Hill numbers [44] in order to compare the different altitudes regarding multiple diversity indexes at the same time [45]. Profiles were constructed separately for the gamma and alpha spatial levels.
3. Results
A total of 45 morphospecies distributed among six taxonomic groups were found at different sampled altitudes. The most representative phyla were: Ciliophora, Arthropod, Rotifera, Annelida, Platyhelminthes, and Nematoda. The list of morphospecies found in the tanks of Vriesea spp. at different altitudes is shown in Table S1, in Supplementary Material. A total of 35 morphospecies were found in phytotelmata at intermediate altitude, followed by 32 at lower altitude. At higher altitudes (910 m and 915 m), 28, and 30 morphospecies were observed, respectively. Ciliophora was the phylum with the highest number of morphospecies (24), where the majority were hymenostome ciliates (Lambornella trichoglossa, Tetrahymena sp. and Glaucoma sp.), followed by Euplotes sp., and Halteria grandinella. Peritrich ciliates (Rhabdostyla, Vorticella, and Lagenophrys) were also present in the samples, attached to other invertebrates and to organic matter. Rotifer morphospecies found in phytotelmata communities belong to Philodinidae family (bdelloids), with different body shapes and swimming modes. Crustaceans, insects, and hydracharinids composed the Arthropoda community. Harpacticoid copepods (Atheyella fuhrmani) and the ostracod genus Elphidium were present at all altitudes sampled. Insects were characterized by mosquito and beetle larvae, belonging to Chironomidae, Culicidae, Thaumaleidae, Ceratopogonidae and Psychodidae (Diptera families), and Scirtidae (Coleoptera). The genus Dero and an unidentified group represented Oligochaeta morphospecies (2). Two morphospecies of flatworms were found in all samples. Nematodes, although very abundant in quantitative samples, were rarely observed in live samples, probably due to damage resulting from sampling.
Overall community species richness was higher at 400 m elevation. However, variation of richness within each of the three most important groups was high and did not follow a common pattern. We found a seasonal variation in species richness considering mean values for each season, with highest values found in autumn and winter samples. Seasonal variation within each altitudinal stratum was high for most altitudes and organisms (Figure 2, Table 2).
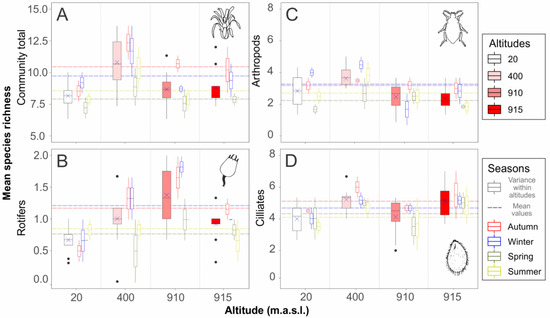
Figure 2.
Species richness of phytotelmata community at different altitudes along four sampling seasons. Mean richness for the whole community (A) and separately for Rotifers (B), Arthropods (C), and Ciliates (D). Wide black-lined boxes: pooled variation comprising all sampling seasons within each altitude, with mean richness values indicated by blue crosses. Narrow colored boxes: seasonal richness variation within each altitude. Dashed colored lines: mean richness values per season, pooled values for all altitudes. See Table 2 for test results. V. gigantea at sea level (20 m); V. incurvata at intermediate altitude (400 m); V. friburgensis and V. platynema at high altitudes (910 and 915 m, respectively).

Table 2.
Relationships between richness descriptors of phytotelmata community and altitude and sampling season (spr—Spring, aut—Autumn, sum—Summer, win—Winter). Results from multiple regression models that included richness descriptors as dependent variables and season + altitude as independent variables. Significant models and independent predictors (analysis of variance) in bold. Significant pairwise differences between categories of the independent variables derived from permutational post-hoc tests.
The Constrained Correspondence Analysis revealed different community compositions for the lower, intermediate, and higher altitudes. Plots from the higher altitudes (910–915 m) were mostly overlapped (Figure 3). The ordination pattern was corroborated by a multivariate analysis of variance, which resulted in compositions of different communities considering the altitude factor (MANOVA; R2 = 0.2454 m F = 4.7714, p = 0.001). Pairwise comparisons between altitudes resulted in significant differences in all pairs (p < 0.01), with the exception of 910–915 m (p = 0.252). In addition, the analysis of indicator species resulted in at least one indicator per altitude (Table 3; also plotted in Figure 3).
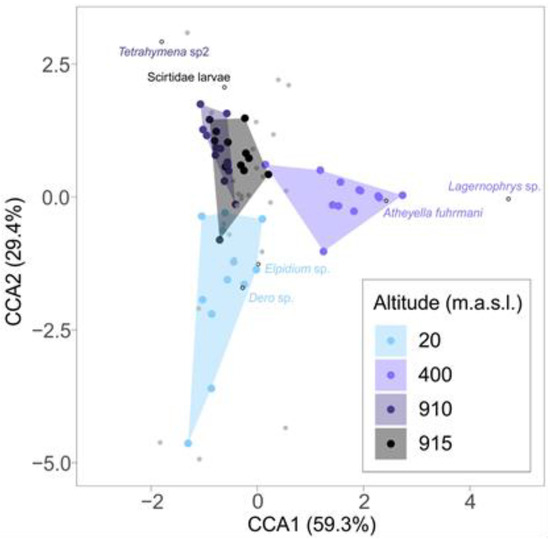
Figure 3.
Ordination scatterplot resulting from a Constrained Correspondence Analysis of eukaryotic communities present in bromeliad cisterns at four different altitudes. Percentages of the variation explained by each ordination axis are indicated parenthetically. Different colors (polygons and points) represent different altitudes. Gray points represent eukaryotic species. Hollow points indicated the position in the ordination space of taxa selected in the indicator species analysis (see methods and results). Indicator species’ name colors match the altitude they were related to.

Table 3.
Indicator species per altitude, with the statistical significance and strength of association described by the ‘IndVal’ index.
The diversity profiles calculated with Hill numbers revealed different patterns for different spatial components of diversity. Considering the gamma level (altitude), diversity decreased as altitude increased (Figure 4A). However, this pattern was not recovered considering the alpha (cistern) level, which showed the diversity peak at intermediate altitudes, with overlapping profiles for the remaining altitudes (Figure 4B). Beta diversity, similarly to the pattern shown for gamma, decreased as altitude increased. In addition, the contribution of the different components of beta diversity varied according to the altitude level: turnover was more important in lower and intermediate altitudes, whereas nestedness was more important in higher altitudes (Table 4).
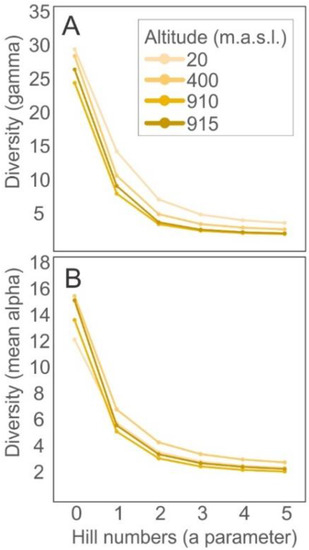
Figure 4.
Diversity profiles built with the Hill series of eukaryotic communities present in bromeliad cisterns at four different altitudes. Data summarized according to the spatial component of diversity: altitude level, or gamma component (A) and cistern level, showing mean values for the alpha component (B).

Table 4.
Spatial components of the diversity of eukaryotic communities present in bromeliad cisterns in four different altitudes. Alpha (cistern level; mean values ± standard deviation) and gamma (pooled to the altitude level) diversities were calculated with Gini-Simpson diversity index. Beta diversity calculated with Sørensen dissimilarity index. Beta diversity components (turnover and nestedness) are expressed as raw values and proportional contribution (%).
4. Discussion
Three patterns of species distribution for tropical and non-tropical elevational ranges considering altitudes from below 500 m to above 1500 m have been described so far [25,46]. These patterns can be classified as unimodal, monotonic decline, and an increasing of species richness curve at intermediate elevations, declining at high altitudes. In the case of bromeliad communities, studies developed by Richardson [14,47] and Jocque and Field [1], showed that they presented a peak of specific richness and animal biomass at intermediate altitudes. A similar result is observed here where the specific richness and alpha diversity peaked at intermediate altitude. According to Richardson [47], bromeliad phytotelmata from forests up to 300 m present high levels of phosphorus (P), potassium (K), calcium (Ca), and manganese (Mn) from canopy debris and leaf decomposition, with these parameters decreasing at higher altitudes. These nutrients are important for cellular physiology and animal structure of many groups, and may be influencing faunal richness and abundance at intermediate height [48].
In an ecological context, some factors may explain an increase in diversity of bromeliads cisterns at intermediate altitudes. One of these drivers is related to the probable increase in immigration and colonization of cisterns, in comparison with higher altitudes [12]. The greater availability of species can increase functional complementarity within the community, such as the replacement of top predators by competitive pressure. At intermediate elevations, we found a high diversity of Thaumaleidae, Culicidae and Chironomidae mosquito larvae. Interestingly, these arthropod groups play significant roles at top-down processes in the phytotelmata community [49]. Moreover, Psychodidae and Ceratopogonidae larvae were present at low and middle altitudes, enhancing diversity of top-predators over an elevational gradient. At higher altitudes, only Culicidae and Chironomidae families composed the community. Primary and secondary consumers, such as filter feeders, bacterivorous, and heterotrophic groups increase diversity up to intermediate altitudes, and then decrease at higher altitudes. A rich ciliate assemblage was present in the lower strata, with genera Metopus, Spirostomum, and Holosticha occurring only at 20 m, while Discomorphella and Lagenophrys occurred at 400 m. All these ciliates are primary consumers, filtering organic matter, and linking biomass energy to secondary consumers and top predators (copepods, beetles, and mosquito larvae) [50,51]. A third morphospecies of a Philodinidae rotifer, which is also a primary consumer, occurred only at 400 m.
Studies that test seasonality in phytotelmata communities have already been explored [3]. Differences in abundance and species richness were verified by different authors in wet and dry seasons, and between summer and winter [52,53,54,55]. However, there are few studies that combined seasonality with other ecological factors, such as altitude. Our results on phytotelmata species richness showed that the eukaryotic community in bromeliad tanks is affected by different seasons. In the total community and in the main sampled groups (arthropods, ciliate, and rotifers), there are some differences in seasonal patterns. In all groups, especially considering the total community and the rotifers, a peak of richness occurred in winter and autumn seasons, at all sampled heights. On the other hand, Montero [55], studying seasonality in macrofauna assemblages of phytotelmata, found that winter showed the lowest community richness and abundance. In our study, arthropods and ciliates presented highest richness during the winter, although at some altitudes there was also a greater peak during summer (Figure 2). We believe that this result may be due to constant rainfalls throughout autumn and winter, a characteristic of our subtropical climate. The input of water may stimulate eukaryotes to excyst (rotifers and ciliates) or finish the diapause process (arthropod larvae), enhancing the biological diversity in phytotelmata.
A key factor for success in phytotelmata communities is the mode and rate of dispersal of their inhabitants. Becker and Camargo [56] have suggested that species of amphibians are potential dispersers of Elphidium sp. (Ostracoda) and Dero sp. (Naididae/Oligochaeta). In an experimental study, these authors found individuals of Elphidium and Dero attached to the skin of Xenohyla truncata (Hylidae/Anura), and suggested that these invertebrates may use their host as a disperser agent. Moreover, the same genus of ostracod was attached to the skin of Leptodeira sp., a snake collected from the tanks of Aechmea nudicaulis in Barra de Maricá, which may also act in the dispersion of these crustaceans [56]. Dero and Elphidium were present in all bromeliad individuals sampled in the present study, which could suggest that dispersal events may be occurring. Since the sampled bromeliads were located in forest areas that harbor frogs and snakes, these organisms may be acting as phoretic agents of dispersion. Epibiotic relationships observed between aquatic invertebrates and peritrichs in this study reflect a dispersion mode for the epibiont, which is carried by its hosts throughout other cisterns [57]. Phoresy may occur with greater intensity at intermediate altitudes in this study, since it was the only strata where an epibiotic relationship between Lagenophrys and ostracods was observed. In this specific case, we suggest that “hyperphoresy” it is occurring, where Lagenophrys can hitchhike with Elphidium ostracods, which in turn may be transported to other bromeliads by serpents and amphibians. Another epibiotic relationship, between mites and Rhabdostyla, was present at 910 m, but its occurrence was rare since the epibionts (Rhabdostyla) seem to affect their host movement, and consequently, their own dispersal capacity.
Organisms that live in bromeliad water need to cope with unpredictable cycles of abundance and shortage of water, as well as abundance and scarcity of food. Several organisms are capable of dispersion when the conditions are deteriorating, but those that cannot disperse easily generally are able to encyst or enter dormant stages until suitable conditions are restored. When food is scarce, many species of ciliates can change the body size to be able to graze on a different prey category [58]. For example, Foissner et al. [8] found that several species of ciliates from tank bromeliads could switch from a small, bacteriophagous, microstome morphotype to a large, predatory, macrostome lifestyle. This switch is probably due to strong competition that these communities undergo at certain times. In the present study, an unidentified species of tetrahymenid ciliate occurred in a macrostome stage that fed on Paramecium caudatum, and in a microstome stage that preyed upon bacteria. The presence of these different morphotypes suggest that the sampled bromeliads have fluctuations in food availability which could be due competition among species present in the phytotelmata. The ciliate assemblage found in the sampled bromeliads included genera reported from soil samples (e.g., Colpoda) as well as limnetic ciliates that are very common in freshwater environments (e.g., Tetrahymena, Halteria, Pleuronema, Vorticella) demonstrating that phytotelmata are composed by a mixture assemblage of soil and freshwater organisms as suggested by Foissner [8].
Indicator species analysis pointed out morphospecies that presented strong association with sampled altitudes. For example, Dero sp. (Oligochaeta) and Elphidium sp. (Ostracode) were associated with lower altitudes, while the peritrich ciliate Lagenophrys sp. (attached to Elphidium ostracods) and the harpacticoid copepod Atheyella fuhrmani represented the intermediate altitude (400 m). Other animals may disperse these organisms, with phoresy potentially representing a key point on increasing diversity and turnover of species at lower and intermediate altitudes [56,59]. At higher altitudes, an unidentified ciliate in the Tetrahymenidae family showed a correlation with 910 m altitude, while Scirtidae larvae (Coleoptera) is the indicator species at 915 m. Scirtidae larvae inhabiting water-filled tree holes scrape the surfaces of leaf litter, and mosquito larvae consume their feces, which is likely to occur in bromeliad phytotelmata as well [50]. Watts [60], in a review of Macrohelodes, a genus in Scirtidae family, reported the presence of three species at intermediate and higher altitudes from a mountain range in Queensland (Australia), suggesting the existence of a complex of beetle species from this family occurring in closed forests on mountaintops. Additionally, a new genus of Scirtidae (Anticyphon) was described inhabiting high altitudes of Andean cloud forests [61]. Relationships between altitude and Tetrahymenidae species are lacking in the literature, although they often exhibit a cosmopolitan distribution between spatial varieties [8].
Few studies used a spatial diversity partitioning approach in the context of phytotelmata communities. Richardson et al. [26] verified how diversity changed in bromeliad communities post hurricane disturbance events in 1989 and 1998. The decline in alpha and gamma diversity may have involved the loss of rarer species, as expected from metapopulation theory. Jocque and Field [1] found a correlation between the turnover component of beta diversity and altitude, analyzing aquatic communities of phytotelmata. Our results indicated that species turnover was the dominant driver of beta diversity at lower and intermediate altitudes, i.e., there was a high rate of species substitution between bromeliad cisterns in lower altitudes. Conversely, the importance of the nestedness component increased in higher altitudes, i.e., species composition of bromeliad cisterns from these altitudes were in part subsets of the more species-rich cisterns, which ultimately means a more homogeneous metacommunity in comparison with lower and intermediate altitudes. This pattern was corroborated by the community composition analyses as following: (i) all pairwise composition comparisons between altitudes resulted in significant differences, with the exception of the high-altitude communities; and (ii) high-altitude communities showed a high degree of overlap and uniformity (i.e., smaller polygons, or short distances between sampling units and centroids) in the ordination space, as opposed to low- and intermediate-altitude communities, which were clearly segregated showing higher dispersion.
In our study, Gamma diversity (γ), which comprised pooled values of all cisterns sampled in each strata, showed an inverse relationship with altitude, i.e., a monotonic decrease of diversity as altitude increased. This pattern corroborates the idea that elevational gradients act as species filters, with a tendency of phytotelmata communities to be more homogeneous towards higher altitudes. Considering these results, bromeliad phytotelmata communities seem to be similar to typical patterns for other animals, with a monotonic decline. Rahbeck [25] related at least 18 species among plants, vertebrates, and invertebrates that exhibit this pattern of species richness among an altitudinal range. Some insects, like ants and phanaeinae dung-beetles, have shown a tendency to decrease diversity with increasing altitude [62,63]. Factors such as resource availability and productivity were described as predictors of diversity patterns, which in altitudinal gradients ultimately produce the monotonic decline of diversity with increasing elevation [20,64]. The isolation and reduced area of mountaintops, as well as low rates of colonization, invasion, and high extinction rates, may also contribute to explain this pattern, found for many species [21].
Diversity responses to altitude found in our study seem to be complex, exhibiting specific patterns depending on scale and community descriptor. Specific richness peaked at intermediate altitudes, reflecting a hump-shaped pattern [25]. Arthropods and ciliate communities seem to present this pattern, which corroborates Rahbek’s [25] hypothesis that not all taxa exhibit a uniform and monotonic response to altitude. Richardson [14], and Jocque and Field [1], found this pattern analyzing specific richness of aquatic invertebrates in bromeliad tanks over an altitudinal gradient. For certain taxa and regions, there is a monotonic richness decrease with increasing altitudes. This pattern has received empirical support for mammals, spiders, ants, and plants [65,66,67,68]. In the case of microorganisms, studies have shown that they exhibit not only monotonically decreasing diversity when moving from low to high elevations, but also an increasing diversity at intermediate altitudes [69,70]. One explanation for a mid elevational peak includes “mid elevational condensation zones” (ecotones), where species from intermediate altitudes can be specific to their level, or a mixture of species from higher and lower altitude [25]. This elevational pattern, however, is less consistent for species with small ranges, suggesting that environmental factors may be more clearly accounted for when constraints on domain boundaries are loosened [70].
Richardson [14] also found that alpha diversity of phytotelmata communities declined as elevation increased, considering an altitude range between approximately 600 m to 1070 m in montane rain forests. In our study, this range comprises forests from 20 m to above 900 m, and alpha diversity was higher at intermediate altitudes. Considering that Richardson [14] lower levels of sampling corresponded to middle altitudes, the results of that study supports the higher values of alpha diversity found in our study at intermediate altitude. Possibly, these areas configured the “mid elevational condensation zones” described by Rahbeck [25], where low- and high-altitude “specialists” may be found, in addition to the characteristic community observed at intermediate altitudes. Collwell and Lees [71], assumed that natural boundaries can limit species distributions in varying degrees, explaining the mid domain effect with geometric theory. Considering a mountain range, geometric boundary constraints naturally result in the increasing overlap of species ranges at midpoint of the mountain.
On the other hand, considering regional diversity (γ), aquatic invertebrates inhabiting bromeliad phytotelmata tend to decrease community diversity as altitude increases. These results support the idea proposed by Brown [20] and Stevens [21]. They suggested that when considering phytotelmata community as a mix of taxonomic groups, and analyzing not only specific richness, but also their abundance and different diversity index, patterns seem to follow an inverse correlation with altitude (monotonic decline). Several factors could be responsible for this pattern, among them reduction of area, changes in physical-chemical proprieties and resource availability may be related to this uniform decrease of diversity [49]. Results on beta diversity corroborated these interpretations, with low and mid elevations showing higher turnover values, amplifying phytotelmata communities with more substitution of species. At higher altitudes, a more homogeneous and nested community represents limitations of dispersal and colonization.
5. Conclusions
Our results bring novel empirical evidence that phytotelmata community structure and composition respond to altitudinal gradients. Interpreting diversity responses to altitudinal differences seems to be complex. Considering bromeliad phytotelmata as ecosystems, the altitudinal gradient can affect differently each studied group. In a broader view, our analysis demonstrated that altitude-increasing act as species filter, where foothills and lower levels present more species substitution and higher diversity, while mountaintops harbor more homogeneous and nested communities. Although response patterns to altitude variations have been unknown for many organisms and ecosystems, we bring evidence to the bulk of this theory using bromeliad phytotelmata communities as descriptors, a system that is as ubiquitous in tropical systems as it is poorly studied. Further studies on the responses of these systems to altitudinal and other spatial gradients should include other dimensions of biodiversity, such as functional and phylogenetic approaches, as well as potential abiotic drivers, to access in-depth processes related to the assembly of these communities.
Supplementary Materials
The following are available online at https://www.mdpi.com/1424-2818/12/9/326/s1, Table S1: List of morphospecies found in the phytotelmata of Vriesea gigantean, Vriesea incurvata, Vriesea platynema, and Vriesea friburgensis during the study period.
Author Contributions
Conceptualization, E.M. and L.R.P.U.; Methodology, E.M. and L.R.P.U.; Data collection, E.M.; Data Analyses, E.M. and P.M.A.F.; Writing—Original draft preparation, E.M.; Writing—Review and editing, L.R.P.U. and P.M.A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external fund.
Acknowledgments
We would like to thank RPPN Pró-Mata/PUCRS and Daniel (Águas do Garapiá) for allowing access to their proprieties for our research. E.M. was supported by a fellowship granted by CNPq (Conselho Nacional de Desenvolvimento Cientifico e Tecnologico).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jocque, M.; Field, R. Aquatic invertebrate communities in tank bromeliads: How well do classic ecological patterns apply? Hydrobiologia 2014, 730, 153–166. [Google Scholar] [CrossRef]
- Martinelli, G.; Vieira, C.M.; Gonzalez, M.; Leitman, P.; Piratininga, A.; Da Costa, A.F.; Forzza, R.C. Bromeliaceae da mata atlântica brasileira: Lista de espécies, distribuição e conservação. Rodriguésia 2008, 59, 209–258. [Google Scholar] [CrossRef]
- Kitching, R.L. Food Webs and Container. In The Natural History and Ecology of Phytotelmata; Cambridge University Press: Cambridge, UK, 2000; 448p. [Google Scholar]
- Goffredi, S.K.; Jang, G.E.; Haroon, M.F. Transcriptomics in the tropics: Total RNA-based profiling of Costa Rican bromeliad-associated communities. Comput. Struct. Biotechnol. J. 2014, 13, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Tews, J.; Brose, U.; Grimm, V.; Tielborger, K.; Wichmann, M.C.; Schwager, M.; Jeltsch, F. Animal species diversity driven by habitat heterogeneity/diversity: The importance of key stone structures. J. Biogeogr. 2004, 31, 79–92. [Google Scholar] [CrossRef]
- Nadkarni, N.M. An ecological overview and checklist of vascular epiphytes in the Monteverde cloud forest reserve, Costa Rica. Brenesia 1986, 24, 55–632. [Google Scholar]
- Nadkarni, N.M.; Matelson, T.J. Bird use of epiphyte resources in neotropical trees. Condor 1989, 91, 891. [Google Scholar] [CrossRef]
- Foissner, W.; Struder-Kypke, M.; Van Der Staay, G.W.M.; Moon-Van Der Staay, S.Y.; Hackstein, J.H.P. Endemic ciliates (Protozoa, Ciliophora) from brazilian tank bromeliads (Bromeliaceae): A combined morphological, molecular and ecological study. Eur. J. Protistol. 2003, 39, 365–372. [Google Scholar] [CrossRef]
- Bacigalupo, A.; A Segura, J.; García, A.; Hidalgo, J.; Galuppo, S.; Cattan, P.E. First finding of Chagas disease vectors associated with wild bushes in the Metropolitan Region of Chile. Rev. Médica Chile 2006, 134, 1230–1236. [Google Scholar]
- Martinson, G.O.; Werner, F.A.; Scherber, C.; Conrad, R.; Corre, M.D.; Flessa, H.; Wolf, K.; Klose, M.; Gradstein, S.R.; Veldkamp, E. Methane emissions from tank bromeliads in neotropical forests. Nat. Geosci. 2010, 3, 766–769. [Google Scholar] [CrossRef]
- Whittaker, R.H.; Niering, W.A. Vegetation of the Santa Catarina Mountains, Arizona. V. biomass, production, and diversity along the elevation gradient. Ecology 2015, 56, 771–790. [Google Scholar] [CrossRef]
- Maguire, B. Phytotelmata: Biota and community structure determination in plant-held waters. Annu. Rev. Ecol. Syst. 1971, 2, 439–464. [Google Scholar] [CrossRef]
- Frank, J.H.; Lounibos, L.P. Phytotelmata: Swamps or islands? Fla. Èntomol. 1987, 70, 14. [Google Scholar] [CrossRef]
- Richardson, B.A. The bromeliad microcosm and the assess-ment of faunal diversity in a neotropical forest. Biotropica 1999, 31, 321–336. [Google Scholar] [CrossRef]
- Benzing, D.H. Bromeliaceae: Profile of an Adaptative Radiation; Cambridge University Press: Cambridge, UK, 2000; 675p. [Google Scholar]
- Armbruster, P.; Hutchinson, R.A.; Cotgreave, P. Factors influencing community structure in a South American tank bromeliad fauna. Oikos 2002, 96, 225–234. [Google Scholar] [CrossRef]
- Jabiol, J.; Corbara, B.; Dejean, A.; Céréghino, R. Structure of aquatic insect communities in tank-bromeliads in an East-Amazonian rainforest in French Guiana. For. Ecol. Manag. 2009, 257, 351–360. [Google Scholar] [CrossRef]
- Brouard, C.; D’Alche-Buc, F.; Szafranski, M. Semi-supervised penalized output kernel regression for link prediction. In Proceedings of the 28th International Conference on Machine Learning (ICML), Bellevue, WA, USA, 28 June–2 July 2011; pp. 593–600. [Google Scholar]
- Kageyama, P.Y.; Gandara, F.B. Restauração e conservação de ecossistemas tropicais. In Métodos de Estudos em Biologia da Conservação e Manejo de Vida Silvestre, 2nd ed.; Cullen Júnior, L., Rudan, R., Valladares-Padua, C., Eds.; UFPR: Paraná, Brazil, 2000; pp. 383–394. [Google Scholar]
- Brown, J.H. Species diversity. Analytical biogeography. In An Integrated Approach to the Study of Animal and Plant Distribution; Myers, A.A., Giller, P.S., Eds.; Springer: Dordrecht, Germany, 1988; pp. 57–89. [Google Scholar]
- Stevens, G.C. The elevational gradient in altitudinal range: An extension of rapoport’s latitudinal rule to altitude. Am. Nat. 1992, 140, 893–911. [Google Scholar] [CrossRef]
- Körner, C. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef]
- McCain, C.M. Global analysis of bird elevational diversity. Glob. Ecol. Biogeogr. 2009, 18, 346–360. [Google Scholar] [CrossRef]
- McCain, C.M. Global analysis of reptile elevational diversity. Glob. Ecol. Biogeogr. 2010, 541–553. [Google Scholar] [CrossRef]
- Rahbek, C. The elevational gradient of species richness: A uniform pattern? Ecography 1995, 18, 200–205. [Google Scholar] [CrossRef]
- Richardson, M.J.; Richardson, B.A.; Srivastava, D.S. The Stability of invertebrate communities in bromeliad phytotelmata in a rain forest subject to hurricanes. Biotropica 2015, 47, 201–207. [Google Scholar] [CrossRef]
- Busse, A.; Antiqueira, P.A.P.; Neutzling, A.S.; Wolf, A.M.; Romero, G.Q.; Petermann, J. Different in the dark: The effect of habitat characteristics on community composition and beta diversity in bromeliad microfauna. PLoS ONE 2018, 13, e0191426. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, C.H.; Troian, L.C.; Guterrez, L.M.; Magalhães, R.G. Diagnóstico Socioeconômico e Ambiental do Município de Maquiné—RS: Perspectivas para um Desenvolvimento Rural Sustentável; Relatório de Pesquisa, ANAMA—PGDR/UFRGS—Prefeitura Municipal de Maquiné: Porto Alegre, Brazil, 2000; 56p. [Google Scholar]
- Cabrera, A.L.; Willink, A. Biogeografia de América Latina. Monografia 13, Série de Biología; Organización de los Estados Americanos (OEA): Washington, DC, USA, 1973; p. 120. [Google Scholar]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Umderwood, E.C. Terrestrial ecoregions of the world: A new map of life on Earth. Bioscience 2001, 51, 933–939. [Google Scholar] [CrossRef]
- Patterson, D.J.; Hedley, S. Free-living Freshwater Protozoa—A Colour Guide; ASM Press: Boca Ratón, FL, USA, 2003; p. 223. [Google Scholar]
- Pinho, L.C. Diptera. In Guia on-line: Identificação de larvas de Insetos Aquáticos do Estado de São Paulo; Froehlich, C.G., Ed.; USP: São Paulo, Brazil, 2008; Available online: http://sites.ffclrp.usp.br/aguadoce/guiaonline (accessed on 5 April 2017).
- Brinkhurst, R.O.; Wetzel, M.J. Aquatic Oligochaeta of the world: Supplement. A catalogue of new freshwater species, descriptions, and revisions. Can. Tech. Report Hydrogr. Ocean Sci. 1984, 44, 1–101. [Google Scholar]
- Bretz, F.; Hothorn, T.; Westfall, P. Multiple Comparisons Using R; CRC Press: Boca Raton, FL, USA, 2010; 193p. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package, R package version: 1.15-4; R Project Institute for Statistics and Mathematics, WU Wirtschaftsuniversität Wien: Wien, Austria; Available online: http://CRAN.R-project.org/package=vegan (accessed on 15 August 2011).
- Hervé, M. RVAideMemoire: Testing and Plotting Procedures for Biostatistics. R package version 0.9-75. 2020. Available online: https://CRAN.R-project.org/package=RVAideMemoire (accessed on 25 July 2020).
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012; 618p. [Google Scholar]
- Beck, M.W. Ggord: Ordination Plots with Ggplot2. R Package Version 1.1.4. 2019. Available online: https://rdrr.io/github/fawda123/ggord/ (accessed on 26 July 2020).
- De Cáceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2009, 19, 134–143. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning abundance-based multiple-site dissimilarity into components: Balanced variation in abundance and abundance gradients. Methods Ecol. Evol. 2016, 8, 799–808. [Google Scholar] [CrossRef]
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Chao, A.; Chiu, C.-H.; Jost, L. Unifying species diversity, phylogenetic diversity, functional diversity, and related similarity and differentiation measures through hill numbers. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 297–324. [Google Scholar] [CrossRef]
- Rahbek, C. The relationship among area, elevation, and regional species richness in neotropical birds. Am. Nat. 1997, 149, 875–902. [Google Scholar] [CrossRef] [PubMed]
- Richardson, B.A.; Richardson, M.J.; Scatena, F.N.; McDowell, W.H. Effects of nutrient availability and other elevational changes on bromeliad populations and their invertebrate communities in a humid tropical forest in Puerto Rico. J. Trop. Ecol. 2000, 16, 167–188. [Google Scholar] [CrossRef]
- Emsley, J. Nature’s Building Blocks: An A-Z Guide to the Elements; Oxford University Press: Oxford, UK, 2001; p. 538. [Google Scholar]
- Kitching, R.L. Foodwebs Inphytotelmata: “Bottom-up” and “top-down” explanations for community structure. Annu. Rev. Èntomol. 2001, 46, 729–760. [Google Scholar] [CrossRef] [PubMed]
- Lounibos, L.P.; Frank, J.H. Insects and allies associated with bromeliads: A review. Terr. Arthropod Rev. 2009, 1, 125–153. [Google Scholar] [CrossRef]
- Cotgreave, P.; Hill, M.J.; Middleton, D.A.J. The relationship between body size and population size in bromeliad tank faunas. Biol. J. Linn. Soc. 1993, 49, 367–380. [Google Scholar] [CrossRef]
- Liria, J. Fauna fitotelmata en las bromelias Aechmea fendleri André y Hohenbergia stellata Schult del Parque Nacional San Esteban, Venezuela. Rev. Per. Biol. 2007, 14, 33–38. [Google Scholar]
- Pereira, D.L.V.; Neiss, U.G.; Ferreira-Keppler, R. Distribuição de Paravelia recens (Drake & Harris, 1935) (Hemíptera, Heteroptera, Veliidae) em Guzmania brasiliensis Ule, 1907 (Bromeliaceae) na Reserva Florestal Adolpho Ducke, Amazonas, Brasil. Acta Amaz. 2007, 37, 147–150. [Google Scholar] [CrossRef]
- Lopez, L.C.S.; Iglesias Rios, R. Phytotelmata community distribution in tanks of shaded and sun-exposed terrestrial bromeliads from restinga vegetation. Selbyana 2001, 22, 219–224. [Google Scholar]
- Montero, G.; Feruglio, C.; Barberis, I.M. The Phytotelmata and foliage macrofauna assemblages of a bromeliad species in different habitats and seasons. Insect Conserv. Divers. 2010, 3, 92–102. [Google Scholar] [CrossRef]
- Becker, V.O.; Camargo, A.J.A. Frogs and snakes as phoretic dispersal agents of bromeliad ostracods (Limnocytheridae: Hpjdum) and annelids (Naididae: Dero). Biotropica 1999, 31, 705–708. [Google Scholar]
- Cabral, A.F.; Dias, R.J.P.; Utz, L.R.P.; Alves, R.G.; Agosto, M.D. Spatial and temporal occurrence of Rhabdostyla cf. chironomi Kahl, 1933 (Ciliophora, Peritrichia) as an epibiont on chironomid larvae in a lotic system in the neotropics. Hydrobiologia 2010, 644, 351–359. [Google Scholar] [CrossRef]
- Clegg, J.S. Cryptobiosis—A peculiar state of biological organization. Comp. Biochem. Physiol. Part. B Biochem. Mol. Boil. 2001, 128, 613–624. [Google Scholar] [CrossRef]
- Green, A.J.; Figuerola, J.; Green, A. Recent advances in the study of long-distance dispersal of aquatic invertebrates via birds. Divers. Distrib. 2005, 11, 149–156. [Google Scholar] [CrossRef]
- Watts, C.H.S. Revision of the Genus Macrohelodes (Scirtidae: Coleoptera; Insecta). Trans. R. Soc. South. Aust. 2010, 134, 19–52. [Google Scholar] [CrossRef]
- Ruta, R. Anticyphon gen. nov., a new genus of Scirtidae (Coleoptera: Scirtoidea) inhabiting high altitude Andean cloud forests and páramo formation. Zootaxa 2016, 4175, 301–318. [Google Scholar] [CrossRef]
- Kwon, T.-S.; Kim, S.-S.; Chun, J.H. Pattern of ant diversity in Korea: An empirical test of Rapoport’s altitudinal rule. J. Asia-Pacific Èntomol. 2014, 17, 161–167. [Google Scholar] [CrossRef]
- Herzog, S.K.; Hamel-Leigue, A.C.; Larsen, T.H.; Mann, D.J.; Soria-Auza, R.W.; Gill, B.D.; Edmonds, W.D.; Spector, S. Elevational distribution and conservation biogeography of phanaeine dung beetles (Coleoptera: Scarabaeinae) in Bolivia. PLoS ONE 2013, 8, e64963. [Google Scholar] [CrossRef]
- Begon, M.; Harper, J.L.; Townsend, C.R. Ecology: Individuals, Populations, and Communities, 3rd ed.; Blackwell Science: Cambridge, MA, USA, 1996; p. 1068. [Google Scholar]
- McCain, C.M. The mid-domain effect applied to elevational gradients: Species richness of small mammals in Costa Rica. J. Biogeogr. 2003, 31, 19–31. [Google Scholar] [CrossRef]
- Chaladze, G.; Otto, S.; Tramp, S. A spider diversity model for the Caucasus Ecoregio. J. Insect Cons 2014, 18, 407–416. [Google Scholar] [CrossRef]
- Chaladze, G. Climate-based model of spatial pattern of the species richness of ants in Georgia. J. Insect Conserv. 2012, 16, 791–800. [Google Scholar] [CrossRef]
- Grytnes, J.-A. Species-richness patterns of vascular plants along seven altitudinal transects in Norway. Ecography 2003, 26, 291–300. [Google Scholar] [CrossRef]
- Bryant, J.A.; Lamanna, C.; Morlon, H.; Kerkhoff, A.J.; Enquist, B.J.; Green, J.L. Microbes on mountainsides: Contrasting elevational patterns of bacterial and plant diversity. Proc. Natl. Acad. Sci. USA 2008, 105, 11505–11511. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, H.; Bezemer, T.M.; Hao, Z. Drivers of bacterial beta diversity in two temperate forests. Ecol. Res. 2015, 31, 57–64. [Google Scholar] [CrossRef]
- Colwell, R.; Lees, D.C. The mid-domain effect: Geometric constraints on the geography of species richness. Trends Ecol. Evol. 2000, 15, 70–76. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).