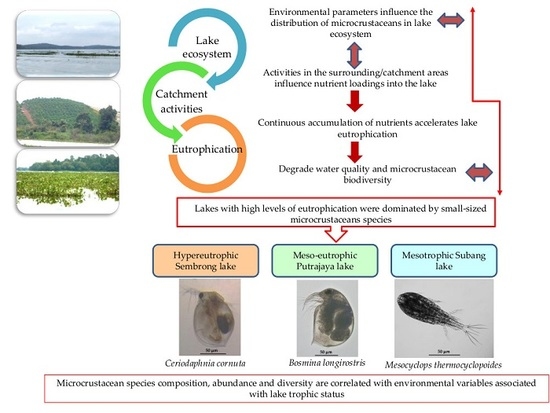
Planktonic Microcrustacean Community Structure Varies with Trophic Status and Environmental Variables in Tropical Shallow Lakes in Malaysia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Rainfall Data, Field Sampling and Laboratory Analyses
2.3. Data Analyses
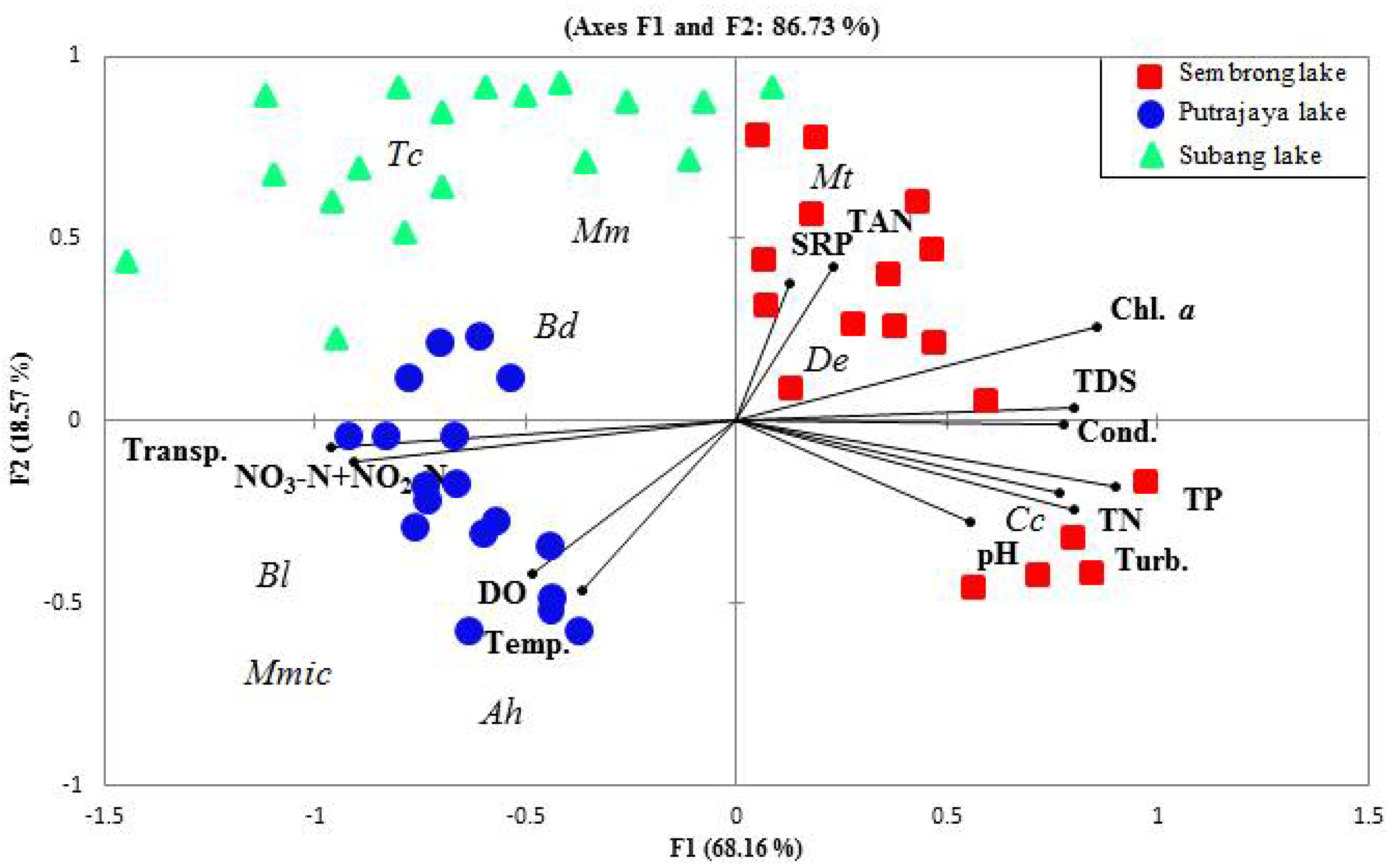
3. Results
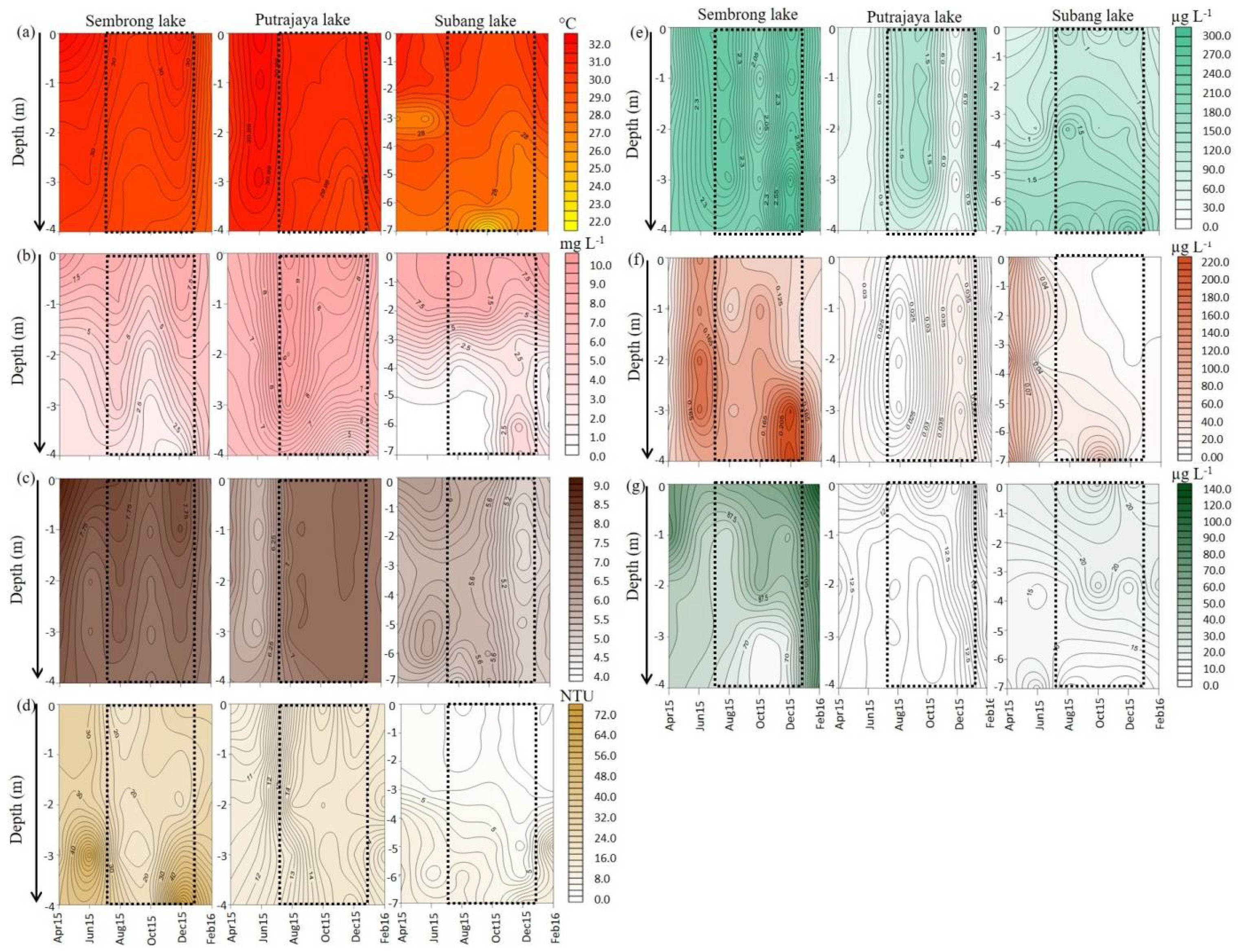
3.1. Rainfall, Physical and Chemical Parameters and Lake Trophic Status
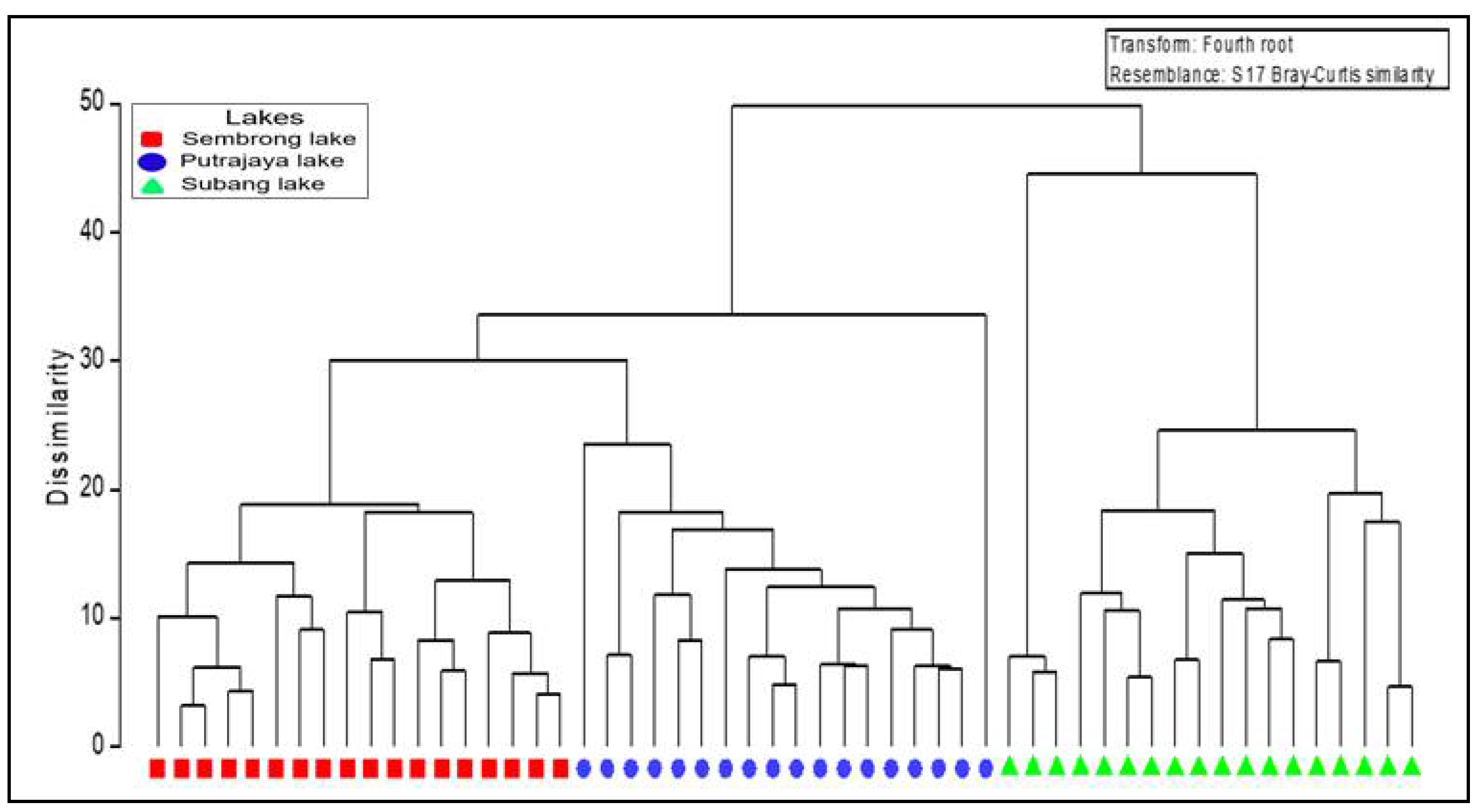
3.2. Microcrustacean Species Composition, Density and Biodiversity
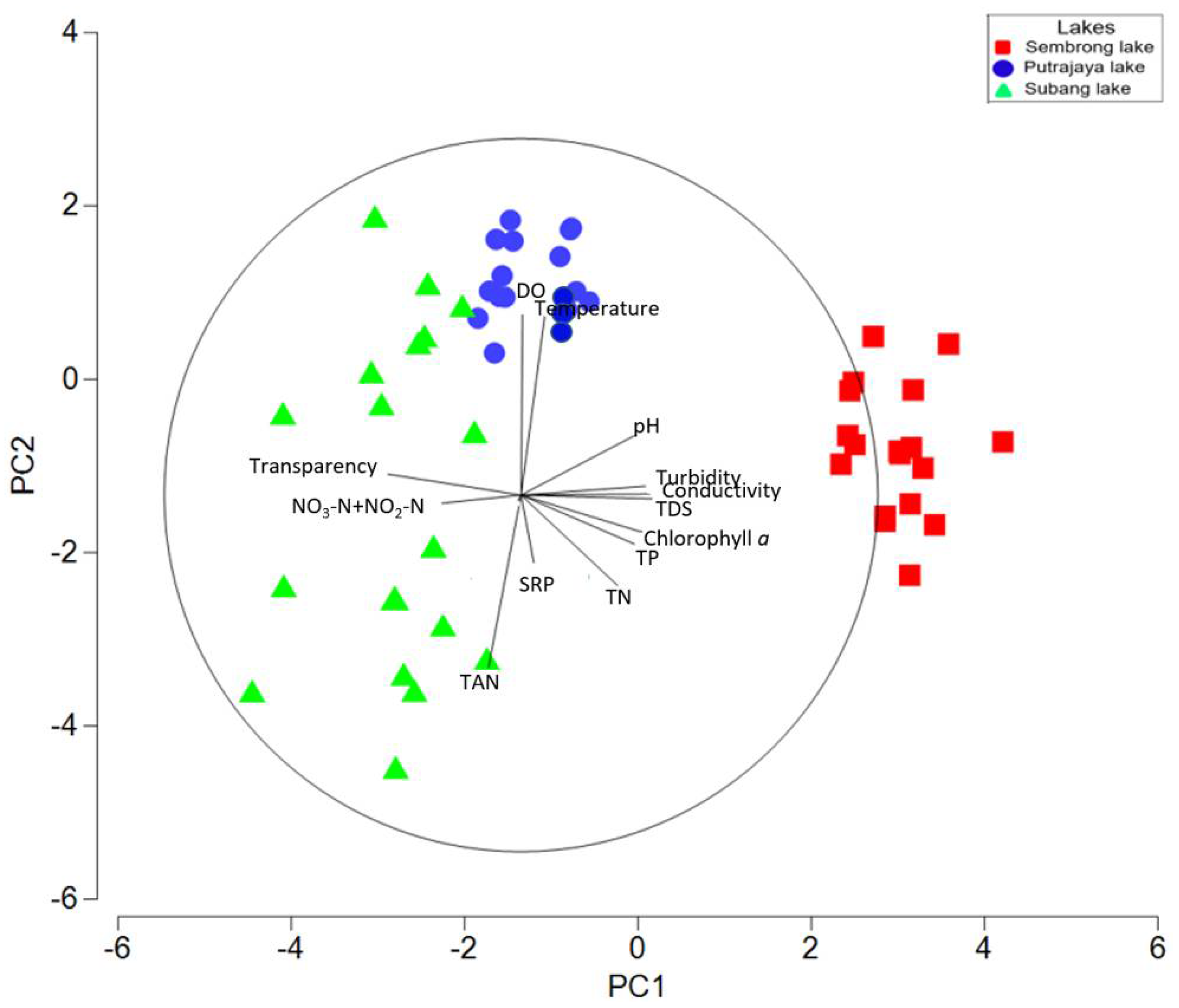
3.3. Relationship between Microcrustacean and Physical and Chemical Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hulot, F.D.; Lacroix, G.; Loreau, M. Differential responses of size-based functional groups to bottom–up and top–down perturbations in pelagic food webs: A meta-analysis. Oikos 2014, 123, 1291–1300. [Google Scholar] [CrossRef]
- Maciej, K.; Piotr, Z.; Magdalena, G.; Jolanta, E.K.; Joanna, K.; Irina, F. Effect of eutrophication and humification on nutrient cycles and transfer efficiency of matter in freshwater food webs. Hydrobiologia 2020, 847, 2521–2540. [Google Scholar] [CrossRef]
- Šorf, M.; Brandl, Z.; Znachor, P.; Vašek, M. Different effects of planktonic invertebrate predators and fish on the plankton community in experimental mesocosms. Ann. Limnol. 2014, 50, 71–83. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Deng, D.; Zhou, Z. Temporal and spatial variations in rotifer correlations with environmental factors in Shengjin Lake, China. Environ. Sci. Pollut. Res. 2016, 23, 8076–8084. [Google Scholar] [CrossRef] [PubMed]
- Stamou, G.; Katsiapi, M.; Moustaka-Gouni, M.; Michaloudi, E. Trophic state assessment based on zooplankton communities in Mediterranean lakes. Hydrobiologia 2019, 844, 83–103. [Google Scholar] [CrossRef]
- Sodré, E.d.O.; Bozelli, R.L. How planktonic microcrustaceans respond to environment and affect ecosystem: A functional trait perspective. Int. Aquat. Res. 2019, 11, 207–223. [Google Scholar] [CrossRef]
- Gillooly, J.F.; Brown, J.H.; West, G.B.; Savage, V.M.; Charnov, E.L. Effects of size and temperature on metabolic rate. Science 2001, 293, 2248–2251. [Google Scholar] [CrossRef]
- Gillooly, J.F.; Charnov, E.L.; West, G.B.; Savage, V.M.; Brown, J.H. Effects of size and temperature on developmental time. Nature 2002, 417, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Derry, A.M.; Arnott, S.E. Zooplankton community response to experimental acidification in boreal shield lakes with different ecological histories. Can. J. Fish. Aquat. 2007, 64, 887–898. [Google Scholar] [CrossRef]
- David, T.E.; James, J.P.; Michael, R.R. Relationship between environmental conditions and zooplankton community structure during summer hypoxia in the northern Gulf of Mexico. J. Plankton Res. 2012, 34, 602–613. [Google Scholar] [CrossRef]
- Elser, J.J.; Gudex, L.; Kyle, M.; Ishikawa, T.; Urabe, J. Effects of zooplankton on nutrient availability and seston C: N: P stoichiometry in inshore waters of Lake Biwa, Japan. Limnology 2001, 2, 91–100. [Google Scholar] [CrossRef]
- Špoljar, M.; Tomljanović, T.; Lalić, I. Eutrophication impact on zooplankton community: A shallow lake approach. Holist. Approach Environ. 2011, 1, 131–142. [Google Scholar]
- Domingues, R.B.; Anselmo, T.P.; Barbosa, A.B.; Sommer, U.; Galvão, H.M. Light as a driver of phytoplankton growth and production in the freshwater tidal zone of a turbid estuary. Estuar. Coast. Shelf Sci. 2011, 91, 526–535. [Google Scholar] [CrossRef]
- Davies-Colley, R.J.; Smith, D.G. Turbidity suspended sediment, and water clarity: A review. J. Am. Water Resour. Assoc. 2001, 37, 1085–1101. [Google Scholar] [CrossRef]
- Sendacz, S.; Caleffi, S.; Santos-Soares, J. Zooplankton biomass of reservoirs in different trophic conditions in the state of São Paulo, Brazil. Braz. J. Biol. 2006, 66, 337–350. [Google Scholar] [CrossRef]
- Paidere, J.; Dimante-Deimantovica, I.; Griņko, O.; Brakovska, A.; Brūvere, I. Applicability of zooplankton community study for ecological quality of salmonid water lakes in Latvia during summer. Acta Biol. 2012, 61 (Suppl. S3), 65–81. [Google Scholar]
- Peretyatko, A.; Teissier, S.; Symoens, J.J.; Triest, L. Phytoplankton biomass and environmental factors over a gradient of clear to turbid peri-urban ponds. Aquat. Conserv. 2007, 17, 584–601. [Google Scholar] [CrossRef]
- Paerl, H.W.; Xu, H.; Hall, N.S.; Rossignol, K.L.; Joyner, A.R.; Zhu, G.; Qin, B. Nutrient limitation dynamics examined on a multi-annual scale in Lake Taihu, China: Implications for controlling eutrophication and harmful algal blooms. J. Freshw. Ecol. 2015, 30, 5–24. [Google Scholar] [CrossRef]
- Chislock, M.F.; Doster, E.; Zitomer, R.A.; Wilson, A.E. Eutrophication: Causes, consequences, and controls in aquatic ecosystems. Nat. Educ. Knowl. 2013, 4, 10. [Google Scholar]
- Adibah, S.; Yusoff, F.M.; Ismail, I.S.; Toda, T. Reduced reproductive capacity in Moina micrura Kurz, 1875 exposed to toxic Microcystis spp. Asian Fish. Sci. 2020, 33, 42–49. [Google Scholar] [CrossRef]
- Di Genaro, A.C.; Sendacz, S.; Moraes, M.D.A.B.; Mercante, C.T.J. Dynamics of cladocera community in a tropical hypereutrophic environment (Garças Reservoir, São Paulo, Brazil). J. Water Resour. Prot. 2015, 7, 379–388. [Google Scholar] [CrossRef][Green Version]
- Tõnno, I.; Agasild, H.; Kõiv, T.; Freiberg, R.; Nõges, P.; Nõges, T. Algal diet of small-bodied crustacean zooplankton in a cyanobacteria-dominated eutrophic lake. PLoS ONE 2016, 11, e0154526. [Google Scholar] [CrossRef] [PubMed]
- Hisatugo, K.F.; Mansano, A.S.; Hayashi, L.H.; Regali-Seleghim, M.H. Ingestion of bacteria in a eutrophic subtropical reservoir pond with food web mainly controlled by zooplankton grazing. Limnologica 2014, 44, 98–106. [Google Scholar] [CrossRef]
- Work, K.A.; Havens, K.E. Zooplankton grazing on bacteria and cyanobacteria in a eutrophic lake. J. Plankton Res. 2003, 25, 1301–1306. [Google Scholar] [CrossRef]
- Perbiche-Neves, G.; Portinho, J.L.; Ferreira, R.A.R. Increases in microcrustaceans (Cladocera and Copepoda) associated with phytoplankton peaks in tropical reservoirs. Trop Ecol. 2016, 57, 523–532. [Google Scholar]
- Ventelä, A.M.; Wiackowski, K.; Moilanen, M.; Saarikari, V.; Vuorio, K.; Sarvala, J. The effect of small zooplankton on the microbial loop and edible algae during a cyanobacterial bloom. Freshw. Biol. 2002, 47, 1807–1819. [Google Scholar] [CrossRef]
- Umi, W.A.D.; Yusoff, F.M.; Aris, A.Z.; Sharip, Z. Rotifer community structure in tropical lakes with different environmental characteristics related to ecosystem health. J. Environ. Biol. 2018, 39, 795–807. [Google Scholar] [CrossRef]
- Lorenzen, C.J. Determination of chlorophyll and pheo-pigments: Spectrophotometric equations. Limnol. Oceanogr. 1967, 12, 343–346. [Google Scholar] [CrossRef]
- Kitamura, H.; Ishitani, H.; Kuge, Y.; Nakamoto, M. Determination of nitrate in freshwater and seawater by a hydrazine reduction method. Jpn. J. Water Pollut. Res. 1982, 5, 35–42. [Google Scholar] [CrossRef]
- Jeffries, D.S.; Dieken, F.P.; Jones, D.E. Performance of the autoclave digestion method for total phosphorus analysis. Water Res. 1979, 13, 275–279. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewate, 21st ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- Carlson, R.E. A trophic state index for lakes. Limnol. Oceanogr. 1977, 22, 361–369. [Google Scholar] [CrossRef]
- Lee, W.Y.; McAlice, B.J. Sampling variability of marine zooplankton in a tidal estuary. Estuar. Coast. Mar. Sci. 1979, 8, 565–582. [Google Scholar] [CrossRef]
- Lai, H.C.; Fernando, C.H. The freshwater calanoida (crustacea: Copepoda) of Singapore and Peninsular Malaysia. Hydrobiologia 1978, 61, 113–127. [Google Scholar] [CrossRef]
- Fernando, C.H.; Ponyi, J.E. The freeliving freshwater cyclopoid Copepoda (Crustacea) of Malaysia and Singapore. Hydrobiologia 1981, 78, 113–123. [Google Scholar] [CrossRef]
- Idris, B.A.G. Freshwater Zooplankton of Malaysia (Crustacea: Cladocera); Universiti Pertanian Malaysia Press: Serdang, Malaysia, 1983. [Google Scholar]
- Shield, R.J. A Guide to Identification of Rotifers, Cladocerans and Copepods from Australian Inland Waters; Co-operative Research Centre for Freshwater Ecology: Albury, Australia, 1995. [Google Scholar]
- Alekseev, V.R.; Haffner, D.G.; Vaillant, J.J.; Yusoff, F.M. Cyclopoid and calanoid copepod biodiversity in Indonesia. J. Limnol. 2013, 72, 245–274. [Google Scholar] [CrossRef]
- Sinev, A.Y.; Yusoff, F.M. Cladocera (Crustacea: Branchiopoda) of Sabah state in Borneo Island, Malaysia. Zootaxa 2015, 4000, 581–591. [Google Scholar] [CrossRef]
- Sinev, A.Y.; Yusoff, F.M. New data on Cladocera (Crustacea: Branchiopoda) of Sabah State, Borneo Island, Malaysia. Zootaxa 2018, 4438, 362–372. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. A further biodiversity index applicable to species lists: Variation in taxonomic distinctness. Mar. Ecol. Prog. Ser. 2001, 216, 265–278. [Google Scholar] [CrossRef]
- Ter Braak, C.J.; Verdonschot, P.F. Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat. Sci. 1995, 57, 255–289. [Google Scholar] [CrossRef]
- Tang, K.H.D. Climate change in Malaysia: Trends, contributors, impacts, mitigation and adaptations. Sci. Total Environ. 2019, 650, 1858–1871. [Google Scholar] [CrossRef]
- Baharim, N.B.; Yusop, Z.; Yusoff, I.; Tahir, W.Z.W.M.; Askari, M.; Othman, Z.; Abidin, M.R.Z. The relationship between heavy metals and trophic properties in Sembrong Lake, Johor. Sains Malays. 2016, 45, 43–53. [Google Scholar]
- Wu, Z.; Lai, X.; Zhang, L.; Cai, Y.; Chen, Y. Phytoplankton chlorophyll a in Lake Poyang and its tributaries during dry, mid-dry and wet seasons: A 4-year study. Knowl. Manag. Aquat. Ecosyst. 2014, 6, 13. [Google Scholar] [CrossRef]
- Sharip, Z.; Zaki, A.T.; Shapai, M.A.; Suratman, S.; Shaaban, A.J. Lakes of Malaysia: Water quality, eutrophication and management. Lakes Reserv. Res. Manag. 2014, 19, 130–141. [Google Scholar] [CrossRef]
- Meor Hussain, M.A.F.; Ahyaudin, A.; Amir Shah, R.; Shah, M. The structure and dynamics of net-zooplankton communities of the littoral versus limnetic zone of a typical embayment in a small flow through tropical reservoir. J. Biosci. 2002, 13, 23–34. [Google Scholar]
- Shah, A.S.R.M.; Ismail, J.; Latief, D.; Omar, W.M.W. The spatial structure of zooplankton communities of Pedu Reservoir, Malaysia. Wetland Sci. 2012, 10, 423–428. [Google Scholar]
- Shabdin, M.L.; Ismail, N.; ak Chukong, N.; Yusoff, N.K.; Ngau, H.T. Freshwater zooplankton of Bakun dam Sarawak, Malaysia. Asian J. Biol. Life Sci. 2014, 3, 120–124. [Google Scholar]
- Ismail, A.H.; Adnan, A.A.M. Zooplankton composition and abundance as indicators of eutrophication in two small man-made lakes. Trop. Life Sci. Res. 2016, 27, 31–38. [Google Scholar] [CrossRef]
- Pinto-Coelho, R.; Pinel-Alloul, B.; Méthot, G.; Havens, K.E. Crustacean zooplankton in lakes and reservoirs of temperate and tropical regions: Variation with trophic status. Can. J. Fish Aquat. Sci. 2005, 62, 348–361. [Google Scholar] [CrossRef]
- Dejen, E.; Vijverberg, J.; Nagelkerke, L.A.; Sibbing, F.A. Temporal and spatial distribution of microcrustacean zooplankton in relation to turbidity and other environmental factors in a large tropical lake (L. Tana, Ethiopia). Hydrobiologia 2004, 513, 39–49. [Google Scholar] [CrossRef]
- Okogwu, O.I.; Nwani, C.D.; Ugwumba, A.O. Seasonal variations in the abundance and biomass of microcrustaceans in relation to environmental variables in two shallow tropical lakes within the cross river floodplain, Nigeria. Acta Zool. Litu. 2009, 19, 205–215. [Google Scholar] [CrossRef]
- Chang, C.W.; Shiah, F.K.; Wu, J.T.; Miki, T.; Hsieh, C.H. The role of food availability and phytoplankton community dynamics in the seasonal succession of zooplankton community in a subtropical reservoir. Limnologica 2014, 46, 131–138. [Google Scholar] [CrossRef]
- Josué, I.I.; Cardoso, S.J.; Miranda, M.; Mucci, M.; Ger, K.A.; Roland, F.; Marinho, M.M. Cyanobacteria dominance drives zooplankton functional dispersion. Hydrobiologia 2019, 831, 149–161. [Google Scholar] [CrossRef]
- Offem, B.O.; Ayotunde, E.O.; Ikpi, G.U.; Ada, F.B.; Ochang, S.N. Plankton-based assessment of the trophic state of three tropical lakes. J. Environ. Prot. 2011, 2, 304–315. [Google Scholar] [CrossRef]
- Oberhaus, L.; Gélinas, M.; Pinel-Alloul, B.; Ghadouani, A.; Humbert, J.F. Grazing of two toxic Planktothrix species by Daphnia pulicaria: Potential for bloom control and transfer of microcystins. J. Plankton Res. 2007, 29, 827–838. [Google Scholar] [CrossRef]
- Agasild, H.; Nõges, T. Cladoceran and rotifer grazing on bacteria and phytoplankton in two shallow eutrophic lakes: In situ measurement with fluorescent microspheres. J. Plankton Res. 2005, 27, 1155–1174. [Google Scholar] [CrossRef]
- Chen, F.; Xie, P. The effects of fresh and decomposed Microcystis aeruginosa on cladocerans from a subtropic Chinese lake. J. Freshw. Ecol. 2003, 18, 97–104. [Google Scholar] [CrossRef]
- Wylie, J.L.; Currie, D.J. The relative importance of bacteria and algae as food sources for crustacean zooplankton. Limnol. Oceanogr. 1991, 36, 708–728. [Google Scholar] [CrossRef]
- Gophen, M.; Cavari, B.Z.; Berman, T. Zooplankton feeding on differentially labelled algae and bacteria. Nature 1974, 247, 393–394. [Google Scholar] [CrossRef]
- Starling, F.D.R. Comparative study of the zooplankton composition of six lacustrine ecosystems in Central Brazil during the dry season. Rev. Bras. Biol. 2000, 60, 101–111. [Google Scholar] [CrossRef][Green Version]
- Duigan, C.A.; Reid, S.; Monteith, D.T.; Bennion, H.; Seda, J.M.; Hutchinson, J. The past, present and future of Llangorse Lake—A shallow nutrient-rich lake in the Brecon Beacons National Park, Wales, UK. Aquat. Conserv. 1999, 9, 329–341. [Google Scholar] [CrossRef]
- Wang, S.; Xie, P.; Wu, S.; Wu, A. Crustacean zooplankton distribution patterns and their biomass as related to trophic indicators of 29 shallow subtropical lakes. Limnologica 2007, 37, 242–249. [Google Scholar] [CrossRef]
- Guevara, G.; Lozano, P.; Reinoso, G.; Villa, F. Horizontal and seasonal patterns of tropical zooplankton from the eutrophic Prado Reservoir (Colombia). Limnologica 2009, 39, 128–139. [Google Scholar] [CrossRef]
- Diel, P.; Kiene, M.; Martin-Creuzburg, D.; Laforsch, C. Knowing the enemy: Inducible defences in freshwater zooplankton. Diversity 2020, 12, 147. [Google Scholar] [CrossRef]
- Sampaio, E.V.; Rocha, O.; Matsumura-Tundisi, T.; Tundisi, J.G. Composition and abundance of zooplankton in the limnetic zone of seven reservoirs of the Paranapanema River, Brazil. Braz. J. Biol. 2002, 62, 525–545. [Google Scholar] [CrossRef]
- Bini, L.M.; da Silva, L.C.F.; Velho, L.F.M.; Bonecker, C.C.; Lansac-Tôha, F.A. Zooplankton assemblage concordance patterns in Brazilian reservoirs. Hydrobiologia 2008, 598, 247–255. [Google Scholar] [CrossRef]
- Deng, D.; Xie, P.; Zhou, Q.; Yang, H.; Guo, L.; Geng, H. Field and experimental studies on the combined impacts of cyanobacterial blooms and small algae on crustacean zooplankton in a large, eutrophic, subtropical, Chinese lake. Limnology 2008, 9, 1–11. [Google Scholar] [CrossRef]
- Guo, N.; Xie, P. Development of tolerance against toxic Microcystis aeruginosa in three cladocerans and the ecological implications. Environ. Pollut. 2006, 143, 513–518. [Google Scholar] [CrossRef]
- Paes, T.A.S.V.; Costa, I.A.S.D.; Silva, A.P.C.; Eskinazi-Sant’Anna, E.M. Can microcystins affect zooplankton structure community in tropical eutrophic reservoirs? Braz. J. Biol. 2016, 76, 450–460. [Google Scholar] [CrossRef][Green Version]
- Lampert, W. Laboratory studies on zooplankton-cyanobacteria interactions. N. Z. J. Mar. Freshw. Res. 1987, 21, 483–490. [Google Scholar] [CrossRef]
- Sun, X.; Tao, M.; Qin, B.; Qi, M.; Niu, Y.; Zhang, J.; Ma, Z.; Xie, P. Large-scale field evidence on the enhancement of small-sized cladocerans by Microcystis blooms in Lake Taihu, China. J. Plankton Res. 2012, 34, 853–863. [Google Scholar] [CrossRef]
- Degans, H.; De Meester, L. Top-down control of natural phyto-and bacterioplankton prey communities by Daphnia magna and by the natural zooplankton community of the hypertrophic Lake Blankaart. Hydrobiologia 2002, 479, 39–49. [Google Scholar] [CrossRef]
- Cerbin, S.; Balayla, D.J.; Van de Bund, W.J. Small-scale distribution and diel vertical migration of zooplankton in a shallow lake (Lake Naardermeer, The Netherlands). Hydrobiologia 2003, 491, 111–117. [Google Scholar] [CrossRef]
- Nogueira, M.G.; Reis Oliveira, P.C.; Tenorio de Britto, Y. Zooplankton assemblages (Copepoda and Cladocera) in a cascade of reservoirs of a large tropical river (SE Brazil). Limnetica 2008, 27, 151–170. [Google Scholar]
- Turner, J.T.; Tester, P.A. Zooplankton feeding ecology: Bacterivory by metazoan microzooplankton. J. Exp. Mar. Biol. Ecol. 1992, 160, 149–167. [Google Scholar] [CrossRef]
- Locke, A. Zooplankton responses to acidification: A review of laboratory bioassays. Water Air Soil Pollut. 1991, 60, 135–148. [Google Scholar] [CrossRef]
- Walseng, B.; Yan, N.D.; Schartau, A.K. Littoral microcrustacean (Cladocera and Copepoda) indicators of acidification in Canadian Shield lakes. AMBIO A J. Hum. Environ. 2003, 32, 208–213. [Google Scholar] [CrossRef]
- Caroni, R.; Irvine, K. The potential of zooplankton communities for ecological assessment of lakes: Redundant concept or political oversight? In Biology and Environment, Proceedings of the Royal Irish Academy; Royal Irish: Dublin, Ireland, 2010; pp. 35–53. [Google Scholar]
- Duigan, C.A.; Kovach, W.L. Relationships between littoral microcrustacea and aquatic macrophyte communities on the Isle of Skye (Scotland), with implications for the conservation of standing waters. Aquat. Conserv. 1994, 4, 307–331. [Google Scholar] [CrossRef]
- Berge, T.; Daugbjerg, N.; Andersen, B.B.; Hansen, P.J. Effect of lowered pH on marine phytoplankton growth rates. Mar. Ecol. Prog. Ser. 2010, 416, 79–91. [Google Scholar] [CrossRef]
- Richmond, C.; Marcus, N.H.; Sedlacek, C.; Miller, G.A.; Oppert, C. Hypoxia and seasonal temperature: Short-term effects and long-term implications for Acartia tonsa Dana. J. Exp. Mar. Biol. Ecol. 2006, 328, 177–196. [Google Scholar] [CrossRef]
- Karpowicz, M.; Ejsmont-Karabin, J.; Kozłowska, J.; Feniova, I.; Dzialowski, A.R. Zooplankton community responses to oxygen stress. Water 2020, 12, 706. [Google Scholar] [CrossRef]
- Doubek, J.P.; Campbell, K.L.; Doubek, K.M.; Hamre, K.; Lofton, M.; McClure, R.P.; Ward, N.K.; Carey, C.C. The effects of hypolimnetic anoxia on the diel vertical migration of freshwater crustacean zooplankton. Ecosphere 2018, 9, e02332. [Google Scholar] [CrossRef]
- Vanni, M.J. Freshwater zooplankton community structure: Introduction of large invertebrate predators and large herbivores to a small species community. Can. J. Fish Aquat. Sci. 1988, 45, 1758–1770. [Google Scholar] [CrossRef]
- Aka, M.; Pagano, M.; Saint-Jean, L.; Arfi, R.; Bouvy, M.; Cecchi, P.; Corbin, D.; Thomas, S. Zooplankton variability in 49 shallow tropical reservoirs of Ivory Coast (West Africa). Int. Rev. Hydrobiol. 2000, 85, 491–504. [Google Scholar] [CrossRef]



| Parameters | Sembrong Lake | Putrajaya Lake | Subang Lake | |||
|---|---|---|---|---|---|---|
| Mean ± SE | Range | Mean ± SE | Range | Mean ± SE | Range | |
| Water temperature (°C) | 29.70 ± 0.31 b | 28.42–30.48 | 30.53 ± 0.22 a | 30.05–31.44 | 28.78 ± 0.22 b | 28.10–29.32 |
| Dissolved oxygen (mg L−1) | 5.27 ± 0.44 b | 3.19–6.27 | 7.50 ± 0.37 a | 6.87–9.22 | 4.08 ± 0.17 b | 3.16–4.63 |
| pH | 7.52 ± 0.22 a | 6.81–8.33 | 6.81 ± 0.28 a | 5.53–7.31 | 5.10 ± 0.15 b | 4.57–5.55 |
| Turbidity (NTU) | 28.42 ± 2.90 a | 18.36–36.63 | 13.16 ± 0.75 b | 10.58–14.86 | 4.61 ± 0.45 c | 2.89–6.30 |
| Total dissolved solid (mg L−1) | 116.10 ± 8.65 a | 95.42–151.27 | 59.53 ± 1.97 b | 54.02–67.44 | 25.40 ± 3.41 c | 19.65–41.98 |
| Conductivity (µS cm−1) | 179.03 ± 13.35 a | 147.08–232.73 | 98.97 ± 3.28 b | 89.23–109.25 | 33.63 ± 1.73 c | 27.62–40.01 |
| Water transparency (cm) | 30.8 ± 0.9 b | 24.3–36.8 | 106.4 ± 1.2 a | 95.8–110.0 | 116.6 ± 5.05 a | 95.2–152.7 |
| Total phosphorus (µg L−1) | 140.23 ± 8.51 a | 90.18–180.89 | 30.19 ± 15.9 b | 17.90–39.50 | 24.16 ± 9.41 b | 14.90–40.65 |
| Soluble reactive phosphorus (µg L−1) | 40.06 ± 10.7 a | 10.90–100.45 | 20.83 ± 0.90 b | 10.88–20.66 | 40.22 ± 5.18 a | 10.65–70.15 |
| Total nitrogen (µg L−1) | 226.08 ± 76.4 a | 192.15–274.60 | 117.80 ± 28.8 b | 40.66–218.90 | 126.56 ± 45.20 b | 94.50–171.88 |
| Nitrate-N + Nitrite-N (µg L−1) | 43.33 ± 10.91 a | 20.22–66.54 | 32.12 ± 19.13 b | 28.08–44.15 | 26.28 ± 18.56 b | 19.05–40.05 |
| Total ammonium nitrogen (µg L−1) | 80.19 ± 31.42 b | 13.42–144.20 | 35.64 ± 10.55 c | 25.10–56.22 | 160.28 ± 33.40 a | 97.22–260.82 |
| Chlorophyll a (µg L−1) | 97.19 ± 4.70 a | 64.94–142.61 | 5.53 ± 0.65 b | 1.10–9.96 | 4.64 ± 1.12 b | 1.15–7.57 |
| Rainfall (mm) | 178.87 ± 20.12 b | 107.80–328.20 | 139.90 ± 15.87 b | 48.60–261.80 | 301.27 ± 32.09 a | 68.80–427.80 |
| Depth (m) | 3.90 ± 0.14 b | 2.90–5.10 | 3.30 ± 0.20 b | 2.00–5.00 | 7.04 ± 0.29 a | 5.50–9.10 |
| Indices | Sembrong Lake | Putrajaya Lake | Subang Lake |
|---|---|---|---|
| TSI Water transparency | 77.10 | 59.12 | 47.73 |
| TSI Total phosphorus | 74.35 | 51.77 | 49.61 |
| TSI Chlorophyll a | 75.50 | 47.57 | 45.65 |
| CTSI | 71.11 | 53.34 | 47.67 |
| Classification | Hypereutrophic | Meso-eutrophic | Mesotrophic |
| Variables | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| Water temperature | 0.06 | 0.52 | −0.06 | 0.12 |
| Dissolved oxygen (DO) | −0.01 | 0.56 | −0.19 | −0.12 |
| pH | 0.36 | 0.02 | 0.02 | −0.23 |
| Turbidity | 0.32 | 0.22 | −0.01 | −0.16 |
| Total dissolved solid (TDS) | 0.37 | 0.01 | 0.01 | −0.17 |
| Conductivity | 0.35 | 0.01 | 0.32 | 0.01 |
| Water transparency | −0.38 | 0.03 | 0.06 | −0.16 |
| Total phosphorus (TP) | 0.34 | −0.13 | 0.07 | 0.16 |
| Soluble reactive phosphorus (SRP) | 0.03 | −0.21 | −0.84 | −0.32 |
| Total nitrogen (TN) | 0.28 | −0.24 | 0.01 | −0.33 |
| Nitrate-N + Nitrite-N (NO3-N + NO2-N) | −0.22 | −0.02 | 0.38 | −0.76 |
| Total ammonium nitrogen (TAN) | −0.07 | −0.50 | 0.05 | 0.19 |
| Chlorophyll a | 0.35 | −0.05 | −0.02 | 0.04 |
| Eigenvalues | 6.43 | 2.72 | 1.08 | 0.84 |
| % Variation | 49.40 | 20.90 | 8.30 | 6.50 |
| Cum. % Variation | 49.40 | 70.30 | 78.6 | 85.10 |
| Group (Lakes) | R Significance Level (%) | p (%) |
|---|---|---|
| Sembrong lake, Putrajaya lake | 0.88 | 0.1 |
| Putrajaya lake, Subang lake | 0.89 | 0.1 |
| Sembrong lake, Subang lake | 0.87 | 0.1 |
| Species | Sembrong Lake | Putrajaya Lake | Subang Lake | |||
|---|---|---|---|---|---|---|
| Av. Sim | Contrib % | Av. Sim | Contrib % | Av. Sim | Contrib % | |
| Bosmina longirostris | 18.20 | 22.03 | ||||
| Ceriodaphnia cornuta | 25.74 | 30.67 | 15.39 | 18.63 | ||
| Mesocyclops thermocyclopoides | 18.20 | 21.69 | 13.57 | 16.43 | 29.39 | 40.22 |
| Mongolodiaptomus malaindosinensis | 17.42 | 20.76 | 13.35 | 16.16 | ||
| Thermocyclops crassus | 22.66 | 31.02 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umi, W.A.D.; Yusoff, F.M.; Aris, A.Z.; Sharip, Z.; Sinev, A.Y. Planktonic Microcrustacean Community Structure Varies with Trophic Status and Environmental Variables in Tropical Shallow Lakes in Malaysia. Diversity 2020, 12, 322. https://doi.org/10.3390/d12090322
Umi WAD, Yusoff FM, Aris AZ, Sharip Z, Sinev AY. Planktonic Microcrustacean Community Structure Varies with Trophic Status and Environmental Variables in Tropical Shallow Lakes in Malaysia. Diversity. 2020; 12(9):322. https://doi.org/10.3390/d12090322
Chicago/Turabian StyleUmi, Wahidah Ahmad Dini, Fatimah Md Yusoff, Ahmad Zaharin Aris, Zati Sharip, and Artem Y. Sinev. 2020. "Planktonic Microcrustacean Community Structure Varies with Trophic Status and Environmental Variables in Tropical Shallow Lakes in Malaysia" Diversity 12, no. 9: 322. https://doi.org/10.3390/d12090322
APA StyleUmi, W. A. D., Yusoff, F. M., Aris, A. Z., Sharip, Z., & Sinev, A. Y. (2020). Planktonic Microcrustacean Community Structure Varies with Trophic Status and Environmental Variables in Tropical Shallow Lakes in Malaysia. Diversity, 12(9), 322. https://doi.org/10.3390/d12090322