Historical and Contemporary Diversity of Galaxiids in South America: Biogeographic and Phylogenetic Perspectives
Abstract
1. Introduction
2. Phylogenetic Relationship of South American Galaxiids
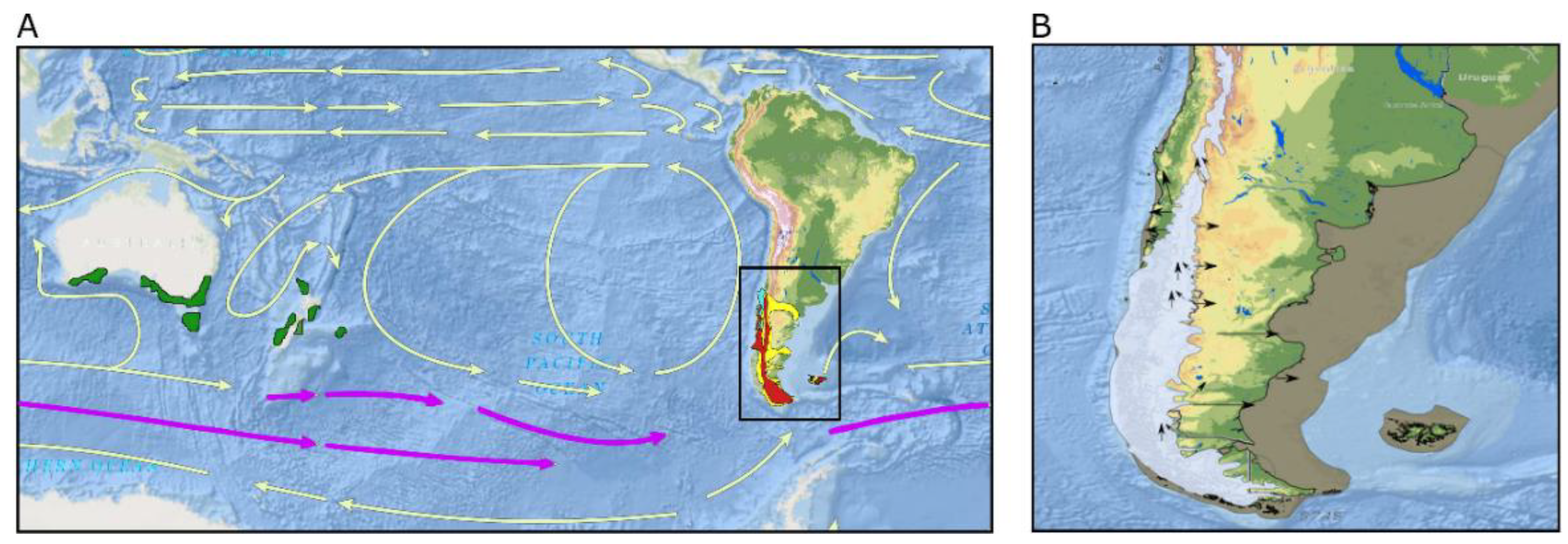
3. Phylogeography and Landscape Genetics Studies on Galaxiid Fishes
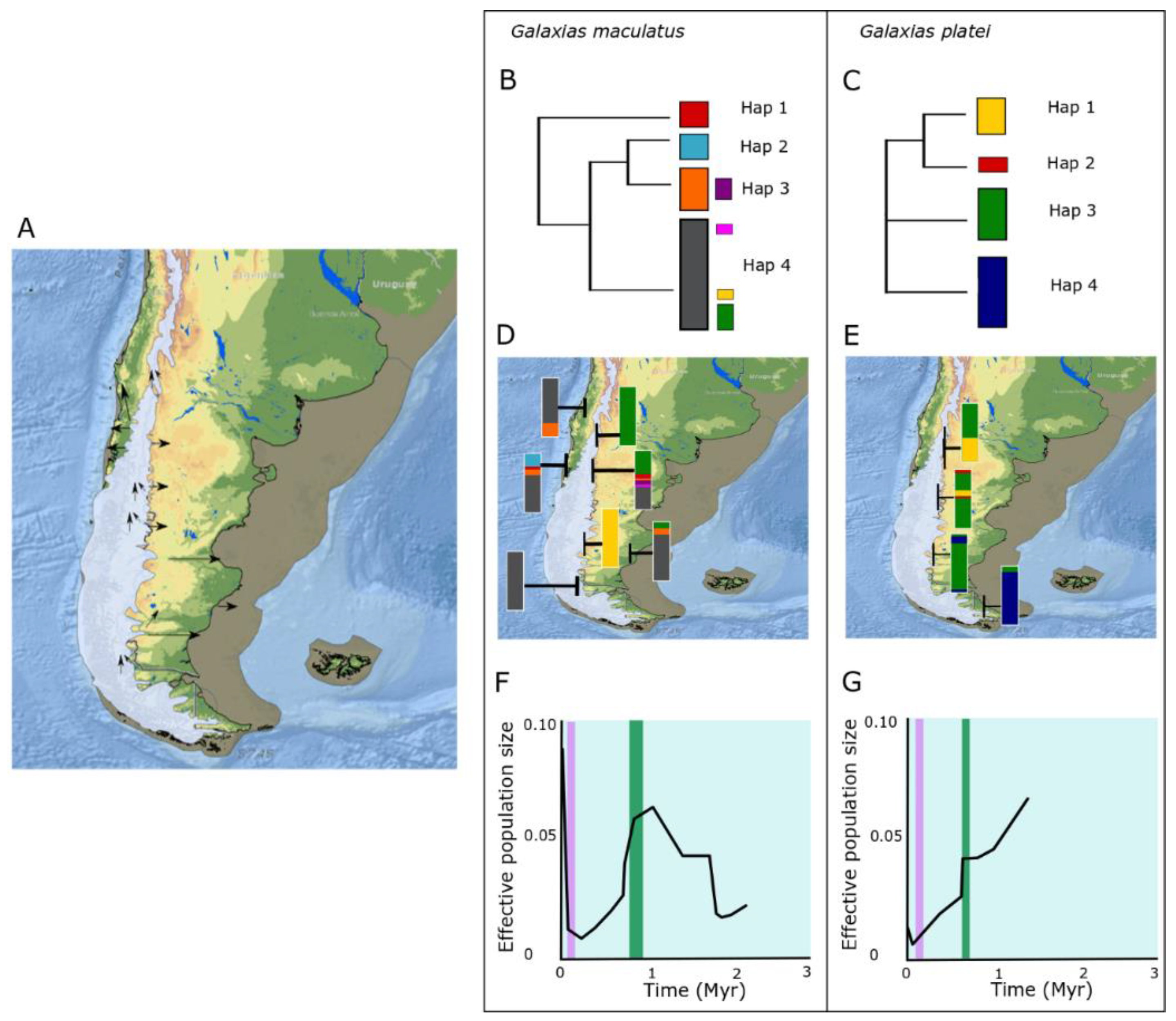
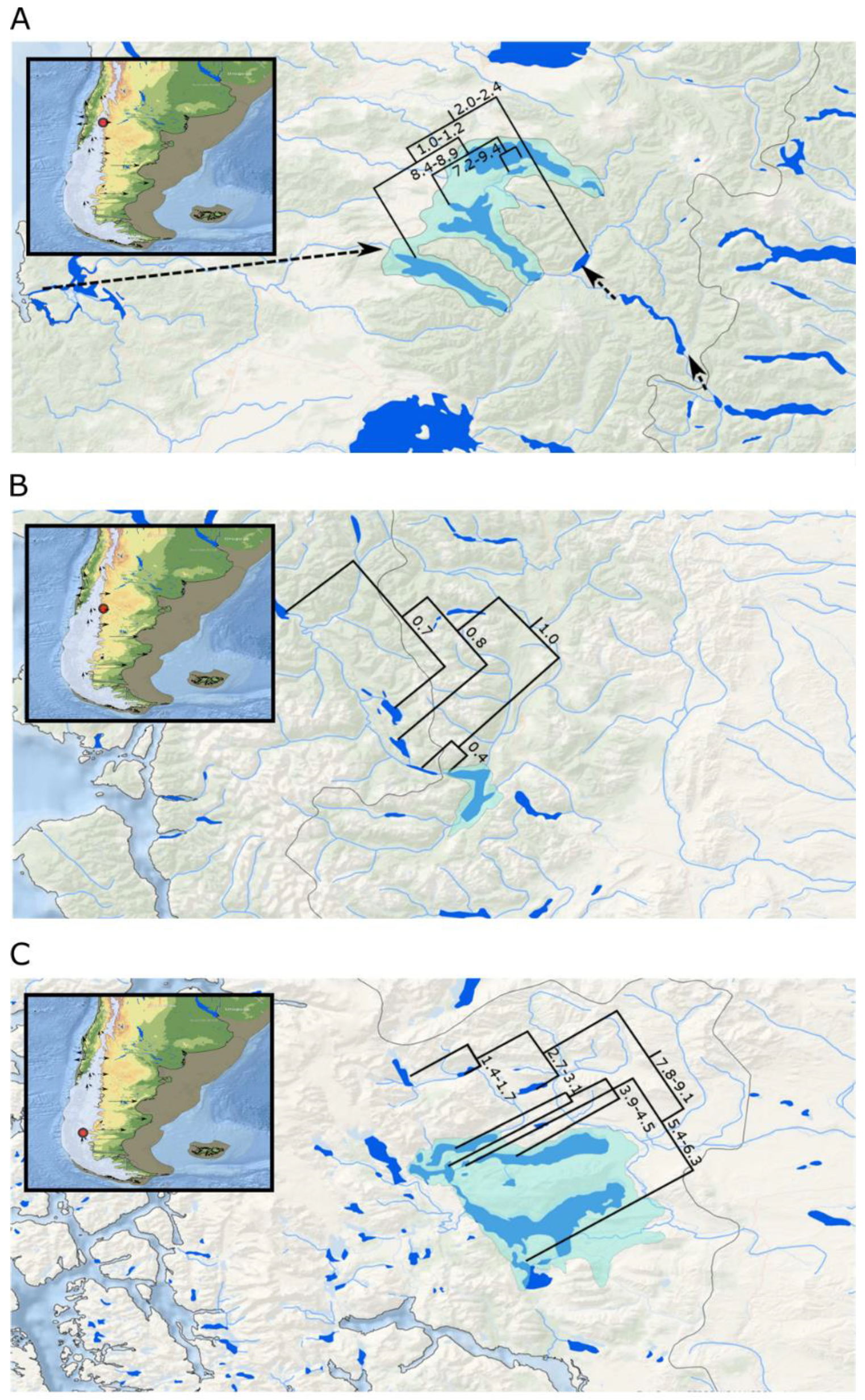
3.1. Galaxias maculatus
3.2. Galaxias platei
3.3. Aplochiton
4. Discussion
4.1. Phylogenetic Position of South American Galaxiids
4.2. Genetic Diversity: The Role of Contemporary Impacts of Human Activity
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cussac, E.V.; Habit, E.; Ciancio, J.; Battini, M.; Rossi, C.R.; Barriga, J.P.; Baigún, C.; Crichigno, S. Freshwater fishes of Patagonia: Conservation and fisheries. J. Fish Biol. 2016, 89, 1068–1097. [Google Scholar] [CrossRef] [PubMed]
- Habit, E.; Piedra, P.; Ruzzante, D.E.; Walde, S.J.; Belk, M.C.; Cussac, V.E.; Gonzalez, J.; Colin, N. Changes in the distribution of native fishes in response to introduced species and other anthropogenic effects. Glob. Ecol. Biogeogr. 2010, 19, 697–710. [Google Scholar] [CrossRef]
- Cavallotto, J.L.; Violante, R.A.; Hernández-Molina, F.J. Geological aspects and evolution of the Patagonian Continental Margin. Biol. J. Linn. Soc. 2011, 103, 346–362. [Google Scholar] [CrossRef][Green Version]
- Ponce, J.F.; Rabassa, J.; Coronato, A.; Borromei, A.M. Paleographic evolution of the Atlantic coast of Pampa and Patagonia from the Last Glacial Maximum to the Middle Holocene. Biol. J. Linn. Soc. 2011, 103, 363–379. [Google Scholar] [CrossRef]
- Delgado, L.M.; Górski, K.; Habit, E.; Ruzzante, D.E. The effects of diadromy and its loss on genomic divergence: The case of amphidromous Galaxias maculatus populations. Mol. Ecol. 2019, 28, 5217–5231. [Google Scholar] [CrossRef] [PubMed]
- Carrea, C.; Cussac, V.E.; Ruzzante, D.E. Genetic and phenotypic variation among Galaxias maculatus populations reflects contrasting landscape effects between northern and southern Patagonia. Freshw. Biol. 2013, 58, 36–49. [Google Scholar] [CrossRef]
- Rojo, J.H.; Fernández, D.A.; Figueroa, D.E.; Boy, C.C. Phenotypic and genetic differentiation between diadromous and landlocked puyen Galaxias maculatus. J. Fish Biol. 2020, 96, 956–967. [Google Scholar] [CrossRef]
- Boy, C.C.; Morriconi, E.; Calvo, J. Reproduction in puyen, Galaxias maculatus (Pisces: Galaxiidae), in the southernmost extreme of distribution. J. Appl. Ichthyol. 2007, 23, 547–554. [Google Scholar] [CrossRef]
- Boy, C.C.; Pérez, A.F.; Fernández, D.A.; Calvo, J.; Morriconi, E.R. Energy allocation in relation to spawning and overwintering of a diadromous Puyen (Galaxias maculatus) population in the southernmost limit of the species distribution. Polar Biol. 2008, 32, 9–14. [Google Scholar] [CrossRef]
- Montoya, G.; Jara, A.; Solis-Lufi, K.; Colin, N.; Habit, E. First stages of the life cycle in native fish from the San Pedro River (Valdivia River Basin, Chile). Gayana 2012, 76, 86–100. [Google Scholar]
- Cussac, V.; Cervellini, P.; Battini, M. Intralacustrine movements of Galaxias maculatus and Odontesthes hatcheri during their early life history. Environ. Biol. Fish. 1992, 35, 141–148. [Google Scholar] [CrossRef]
- McDowall, R.M. Galaxias maculatus (Jenyns)—The New Zealand whitebait. Mar. Dep. Fish. Res. Bull. 1968, 2, 1–84. [Google Scholar]
- McDowall, R.M.; Robertson, D.A.; Saito, R. Occurrence of galaxiid larvae and juveniles in the sea. N. Z. J. Mar. Freshw. Res. 1975, 9, 1–9. [Google Scholar] [CrossRef]
- McDowall, R.M.; Charteris, S. The possible adaptive advantages of terrestrial egg deposition in some fluvial diadromous galaxiid fishes (Teleostei: Galaxiidae). Fish. Fish. 2006, 7, 153–164. [Google Scholar] [CrossRef]
- Mercer, M.; Searle, C.P.; Cifuentes, R.; Habit, E.; Belk, C.M. Morphometric response of Galaxias maculatus (Jenyns) to lake colonization in Chile. Diversity 2020, 12, 219. [Google Scholar] [CrossRef]
- Dunn, R.N.; O’Brien, K.L.; Burridge, P.C.; Closs, P.G. Morphological convergence and divergence in Galaxias fishes in lentic and lotic habitats. Diversity 2020, 12, 183. [Google Scholar] [CrossRef]
- Macchi, P.J.; Cussac, V.E.; Alonso, M.F.; Denegri, M.A. Predation relationships between introduced salmonids and the native fish fauna in lakes and reservoirs in northern Patagonia. Ecol. Freshw. Fish 1999, 8, 227–236. [Google Scholar] [CrossRef]
- Milano, D.; Cussac, V.E.; Macchi, P.J.; Ruzzante, D.E.; Alonso, M.F.; Vigliano, P.H.; Denegri, M.A. Predator associated morphology in Galaxias platei in Patagonian lakes. J. Fish Biol. 2002, 61, 138–156. [Google Scholar] [CrossRef]
- Milano, D.; Ruzzante, D.E.; Cussac, V.E.; Macchi, P.J.; Ferriz, R.A.; Barriga, J.P.; Aigo, J.C.; Lattuca, M.E.; Walde, S.J. Latitudinal and ecological correlates of morphological variation in Galaxias platei (Pisces, Galaxiidae) in Patagonia. Biol. J. Linn. Soc. 2006, 87, 69–82. [Google Scholar] [CrossRef]
- Belk, M.C.; Habit, E.; Ortiz-Sandoval, J.J.; Sobenes, C.; Combs, E.A. Ecology of Galaxias platei in a depauperate lake. Ecol. Freshw. Fish. 2014, 23, 615–621. [Google Scholar] [CrossRef]
- Milano, D.; Barriga, P.J. Reproductive aspects of Galaxias platei (Pisces, Galaxiidae) in a deep lake in North Patagonia. Mar. Freshw. Res. 2018, 69, 1379–1388. [Google Scholar] [CrossRef]
- Vera-Escalona, I.; Senthivasan, S.; Habit, E.; Ruzzante, D.E. Past, present, and future of a freshwater fish metapopulation in a threatened landscape. Conserv. Biol. 2018, 32, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Barrantes, M.E.; Lattuca, M.E.; Vanella, F.A.; Fernández, D.A. Thermal ecology of Galaxias platei (Pisces, Galaxiidae) in South Patagonia: Perspectives under a climate change scenario. Hydrobiologia 2017, 802, 255–267. [Google Scholar] [CrossRef]
- Ortiz-Sandoval, J.; Górski, K.; Sobenes, C.; González, J.; Manosalva, A.; Elgueta, A.; Habit, E. Invasive trout affect trophic ecology of Galaxias platei in Patagonian lakes. Hydrobiologia 2017, 790, 201–212. [Google Scholar] [CrossRef]
- Berra, T.M.; Ruiz, V.H. Rediscovery of Galaxias globiceps Eigenmann from southern Chile. Trans. Am. Fish. Soc. 1994, 123, 595–600. [Google Scholar] [CrossRef]
- Murillo, H.V.; Ruiz, V.H. El puye Galaxias globiceps eigenmann 1927 (osteichthyes: Galaxiidae): ¿una especie en peligro de extincion? Gayana 2002, 66, 191–197. [Google Scholar] [CrossRef]
- Correa-Araneda, F.; De Los Rios, P.; Habit, E. Presence of the red jollytail, Brachygalaxias bullocki (Regan, 1908) (Galaxiformes: Galaxiidae), in freshwater forested wetlands from Chile. Rev. Chil. Hist. Nat. 2014, 87, 20. [Google Scholar] [CrossRef][Green Version]
- Dyer, B. Systematic review and biogeography of the freshwater fishes of Chile. Estud. Oceanol. 2000, 19, 77–98. [Google Scholar]
- Alo, D.; Correa, C.; Samaniego, H.; Krabbenhoft, C.A.; Turner, T.F. Otolith microchemistry and diadromy in Patagonian river fishes. PeerJ 2019, 7, e6149. [Google Scholar] [CrossRef]
- Cussac, V.; Ortubay, S.; Iglesias, G.; Milano, D.; Lattuca, M.E.; Barriga, J.P.; Battini, M.; Gross, M. The distribution of South American galaxiid fishes: The role of biological traits and post-glacial history. J. Biogeogr. 2004, 31, 103–121. [Google Scholar] [CrossRef]
- Maldonado-Márquez, A.; Contador, T.; Rendoll-Cárcamo, J.; Moore, S.; Pérez-Troncoso, C.; Gomez-Uchida, D.; Harrod, C. Southernmost distribution limit for endangered Peladillas (Aplochiton taeniatus) and non-native coho salmon (Oncorhynchus kisutch) coexisting within the Cape Horn biosphere reserve, Chile. J. Fish. Biol. 2020, 96, 1495–1500. [Google Scholar]
- Alo, D.; Correa, C.; Arias, C.; Cardenas, L. Diversity of Aplochiton fishes (Galaxiidea) and the taxonomic resurrection of A. marinus. PLoS ONE 2013, 8, e71577. [Google Scholar] [CrossRef] [PubMed]
- Elgueta, A.; González, J.; Ruzzante, D.E.; Walde, S.J.; Habit, E. Trophic interference by Salmo trutta on Aplochiton zebra and Aplochiton taeniatus in southern Patagonian lakes. J. Fish. Biol. 2013, 82, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Zemlak, S.T.; Habit, E.M.; Walde, S.J.; Battini, M.A.; Adams, E.D.; Ruzzante, D.E. Across the southern Andes on fin: Glacial refugia, drainage reversals and a secondary contact zone revealed by the phylogeographical signal of Galaxias platei in Patagonia. Mol. Ecol. 2008, 17, 5049–5061. [Google Scholar] [CrossRef]
- Zemlak, S.T.; Habit, E.M.; Walde, S.J.; Carrea, C.; Ruzzante, D.E. Surviving historical Patagonian landscapes and climate: Molecular insights from Galaxias maculatus. BMC Evol. Biol. 2010, 10, 67. [Google Scholar] [CrossRef]
- Zemlak, S.T.; Walde, S.J.; Habit, E.M.; Ruzzante, D.E. Climate-induced changes to the ancestral population size of two Patagonian galaxiids: The influence of glacial cycling. Mol. Ecol. 2011, 20, 5280–5294. [Google Scholar] [CrossRef]
- Habit, E.; Gonzalez, J.; Ruzzante, D.E.; Walde, S.J. Native and introduced fish species richness in Chilean Patagonian lakes: Inferences on invasion mechanisms using salmonid-free lakes. Divers. Distrib. 2012, 18, 1153–1165. [Google Scholar] [CrossRef]
- Vera-Escalona, I.; Habit, E.; Ruzzante, D.E. Echoes of a distant time: Effects of historical processes on contemporary genetic patterns in Galaxias platei in Patagonia. Mol. Ecol. 2015, 24, 4112–4128. [Google Scholar] [CrossRef]
- Vera-Escalona, I.; Habit, E.; Ruzzante, D.E. Invasive species and postglacial colonization: Their effects on the genetic diversity of a Patagonian fish. Proc. R. Soc. B 2019, 286, 20182567. [Google Scholar] [CrossRef]
- Waters, M.J.; Burridge, C.P. Extreme Intraspecific Mitochondrial DNA Sequence Divergence in Galaxias maculatus (Osteichthys: Galaxiidae), One of the world’s most widespread Freshwater fish. Mol. Phylogenet. Evol. 1999, 11, 1–12. [Google Scholar] [CrossRef]
- Waters, M.J.; López, J.A.; Wallis, G.P. Molecular phylogenetics and biogeography of galaxiid fishes (Osteichthyes: Galaxiidae): Dispersal, vicariance, and the position of Lepidogalaxias salamandroides. Syst. Biol. 2000, 49, 777–795. [Google Scholar] [CrossRef] [PubMed]
- Burridge, P.C.; McDowall, R.M.; Craw, D.; Wilson, M.V.; Waters, J.M. Marine dispersal as a pre-requisite for Gondwanan vicariance among elements of the galaxiid fish fauna. J. Biogeogr. 2012, 39, 306–321. [Google Scholar] [CrossRef]
- Vanhaecke, D.; Garcia de Leaniz, G.; Gajardo, G.; Thomas, T.; Consuegra, S. Metapopulation dynamics of a diadromous galaxiid fish and potential effects of salmonid aquaculture. Freshw. Biol. 2012, 57, 1241–1252. [Google Scholar] [CrossRef]
- González-Wevar, C.; Salinas, P.; Hüne, M.; Segovia, N.; Vargas-Chacoff, L.; Oda, E.; Poulin, E. Contrasting genetic structure and diversity of Galaxias maculatus (Jenyns, 1848) along the Chilean coast: Stock identification for fishery management. J. Hered. 2015, 106, 439–447. [Google Scholar] [CrossRef]
- González-Wevar, A.C.; Salinas, P.; Hüne, M.; Segovia, N.I.; Vargas-Chacoff, L.; Astorga, M.; Cañete, J.I.; Poulin, E. Phylogeography in Galaxias maculatus (Jenyns, 1848) along two biogeographical provinces in the Chilean coast. PLoS ONE 2015, 10, e0131289. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Jarabo, I.; Gonzalez-Wevar, C.A.; Oyarzun, R.; Fuentes, J.; Poulin, E.; Bertran, C.; Vargas-Chacoff, L. Isolation driven divergence in osmoregulation in Galaxias maculatus (Jenyns, 1848) (Actinopterygii: Osmeriformes). PLoS ONE 2019, 11, e0154766. [Google Scholar] [CrossRef]
- Victoriano, F.P.; Vera, I.; Olmos, V.; Dib, M.; Insunza, B.; Muñoz-Ramírez, C.; Montoya, R.; Jara, A.; Habit, E. Patrones idiosincráticos de diversidad genética de peces nativos del Río San Pedro (Cuenca del Río Valdivia), un sistema de la región glaciada del sur de Chile. Gayana 2012, 76, 01–09. [Google Scholar] [CrossRef][Green Version]
- Victoriano, P.; Muñoz Ramírez, C.P.; Canales-Aguirre, C.B.; Vera-Escalona, I.; Burgos-Careaga, T.; Muñoz Mendoza, C.; Habit, E.N. Contrasting evolutionary responses in two codistributed species of Galaxias (Pisces, Galaxiidae) in a river from the glaciated range in Southern Chile. Royal Soc. Open Sci. 2020, 7, 200632. [Google Scholar] [CrossRef]
- Carrea, C.; Barriga, J.P.; Cussac, V.E.; Ruzzante, D.E. Genetic and phenotypic differentiation among Galaxias maculatus populations in a Patagonian postglacial lake system. Biol. J. Linn. Soc. 2012, 107, 368–382. [Google Scholar] [CrossRef][Green Version]
- Ruzzante, D.E.; Walde, S.J.; Gosse, J.C.; Cussac, V.E.; Habit, E.; Zemlak, T.S.; Adams, E.D. Climate control on ancestral population dynamics: Insight from Patagonian fish phylogeography. Mol. Ecol. 2008, 17, 2234–2244. [Google Scholar] [CrossRef]
- Vera-Escalona, I.; Habit, E.; Ruzzante, D.E. The complete mitochondrial genome of the freshwater fish Galaxias platei and a comparison with other species of the genus Galaxias (faraway, so close?). Mitochondrial DNA A 2017, 28, 176–177. [Google Scholar] [CrossRef] [PubMed]
- Vanhaecke, D.C.; Garcia de Leaniz, G.; Gajardo, J.; Dunham, G.; Giannico; Consuegra, S. Genetic signatures of historical dispersal of fish threatened by biological invasions: The case of galaxiids in South America. J. Biogeogr. 2015, 42, 1942–1952. [Google Scholar] [CrossRef]
- Zattara, E.E.; Premoli, A.C. Genetic structuring in Andean landlocked populations of Galaxias maculatus: Effects of biogeographic history. J. Biogeogr. 2005, 32, 5–14. [Google Scholar] [CrossRef]
- Smith, D.; Harrison, S.; Firth, C.R.; Jordan, J.T. The early Holocene sea level rise. Quat. Sci. Rev. 2011, 30, 1846–1860. [Google Scholar] [CrossRef]
- Turchetto-Zolet, A.; Pinheiro, E.; Salgueiro, F.; Palma–Silva, C. Phylogeographical patterns shed light on evolutionary process in South America. Mol. Ecol. 2013, 22, 1193–1213. [Google Scholar] [CrossRef]
- Rabassa, J.; Coronato, A.; Martínez, O. Late Cenozoic Glaciations in Patagonia and Tierra del Fuego: An updated review. Biol. J. Linn. Soc. 2011, 103, 316–335. [Google Scholar] [CrossRef]
- Sérsic, A.N.; Cosacov, A.; Cocucci, A.A.; Johnson, L.A.; Pozner, R.; Avila, L.J.; Sites, J.W., Jr.; Morando, M. Emerging phylogeographical patterns of plants and terrestrial vertebrates from Patagonia. Biol. J. Linn. Soc. 2011, 103, 475–494. [Google Scholar] [CrossRef]
- Berra, T.M.; Crowley, L.; Ivantsoff, W.; Fuerst, P.A. Galaxias maculatus: An explanation of its biogeography. Mar. Freshw. Res. 1996, 47, 845–849. [Google Scholar] [CrossRef]
- Fraser, C.I.; Nikula, R.; Ruzzante, D.E.; Waters, J.M. Poleward bound: Biological impacts of Southern Hemisphere glaciations. Trends Ecol. Evol. 2012, 27, 462–471. [Google Scholar] [CrossRef]
- Ruzzante, D.E.; Simons, A.P.; McCracken, G.R.; Habit, E.; Walde, S.J. Multiple drainage reversal episodes and glacial refugia in a Patagonian fish revealed by sequenced microsatellites. Proc. R. Soc. Lond. B 2020, 287, 20200468. [Google Scholar] [CrossRef]
- McDowall, M.R. Fishes of the family Aplochitonidae. J. R. Soc. N. Z. 1971, 1, 31–52. [Google Scholar] [CrossRef]
- McDowall, R.; Nakaya, K. Morphological divergence in the two species of Aplochiton Jenyns (Salmoniformes: Aplochitonidae): A generalist and a specialist. Copeia 1988, 1, 233–236. [Google Scholar] [CrossRef]
- Wallis, G.; Wallis, L.; Waters, J. What, if anything, is Galaxias vulgaris? 20 years of research on galaxiid speciation. N. Z. Sci. Rev. 2009, 66, 21–25. [Google Scholar]
- Barriga, P.J.; Battini, M.A.; García-Asorey, M.; Carrea, C.; Macchi, P.J.; Cussac, V.E. Intraspecific variation in diet, growth, and morphology of landlocked Galaxias maculatus during its larval period: The role of food availability and predation risk. Hydrobiologia 2012, 679, 27–41. [Google Scholar] [CrossRef]
- Boy, C.; Pérez, A.; Lattuca, M.; Calvo, J.; Morriconi, E. Reproductive biology of Galaxias maculatus (Jenyns 1842) in the Río Ovando, a high-latitude environment in southernmost Patagonia, Argentina. J. Appl. Ichthyol. 2009, 25, 661–668. [Google Scholar] [CrossRef]
- Campos, H. Population studies of Galaxias maculatus (Jenyns) (Osteichthys: Galaxiidae) in Chile with reference to the number of vertebrae. Stud. Neotrop. Fauna Env. 1974, 9, 55–76. [Google Scholar] [CrossRef]
- Ruzzante, E.D.; Walde, S.J.; Cussac, V.E.; Dalebout, M.L.; Seibert, J.; Ortubay, S.; Habit, E. Phylogeography of the Percichthyidae (Pisces) in Patagonia: Roles of orogeny, glaciation, and volcanism. Mol. Ecol. 2006, 15, 2949–2968. [Google Scholar] [CrossRef]
- Ruzzante, D.E.; Walde, S.J.; Macchi, P.J.; Alonso, M.; Barriga, J.P. Phylogeography and phenotypic diversification in the Patagonian fish Percichthys trucha: The roles of Quaternary glacial cycles and natural selection. Biol. J. Linn. Soc. 2011, 103, 514–529. [Google Scholar] [CrossRef][Green Version]
- Correa, C.; Bravo, A.P.; Hendry, A.P. Reciprocal trophic niche shifts in native and invasive fish: Salmonids and galaxiids in Patagonian lakes. Freshw. Biol. 2012, 57, 1769–1781. [Google Scholar] [CrossRef]
- Eby, A.L.; Roach, W.J.; Crowder, L.B.; Stanford, J.A. Effects of stocking-up freshwater food webs. Trends Ecol. Evol. 2006, 21, 576–584. [Google Scholar] [CrossRef]
- Habit, E.; González, J.; Ortiz-Sandoval, J.; Elgueta, A.; Sobenes, C. Efectos de la invasión de salmónidos en ríos y lagos de Chile. Rev. Ecosistemas 2015, 24, 43–51. [Google Scholar] [CrossRef]
- Anderson, E.P.; Jenkins, C.N.; Heilpern, S.; Maldonado-Ocampo, J.A.; Carvajal-Vallejos, F.M.; Encalada, A.C.; Rivadeneira, J.F.; Hidalgo, M.; Cañas, C.M.; Ortega, H.; et al. Fragmentation of Andes-to-Amazon connectivity by hydropower dams. Sci. Adv. 2018, 4, eaao1642. [Google Scholar] [CrossRef] [PubMed]
- Díaz, G.; Arriagada, P.; Górski, K.; Link, O.; Karelovic, B.; Gonzalez, J.; Habit, E. Fragmentation of Chilean Andean rivers: Expected effects of hydropower development. Rev. Chil. Hist. Nat. 2019, 92, 1. [Google Scholar]
- Habit, E.; García, A.; Díaz, G.; Arriagada, P.; Link, O.; Parra, O.; Thoms, M. River science and management issues in Chile: Hydropower development and native fish communities. River Res. Appl. 2019, 35, 489–499. [Google Scholar] [CrossRef]
- Valenzuela-Aguayo, F.; McCracken, G.R.; Manosalva, A.; Habit, E.; Ruzzante, D.E. Human-induced habitat fragmentation effects on connectivity, diversity, and population persistence of an endemic fish, Percilia irwini, in the Biobío River basin (Chile). Evol. Appl. 2020, 13, 794–807. [Google Scholar] [CrossRef]
- Becker, L.A.; Crichigno, S.A.; Cussac, V.E. Climate change impacts on freshwater fishes: A Patagonian perspective. Hydrobiologia 2018, 816, 21–38. [Google Scholar] [CrossRef]
| Genus | Species | DNA Sequences | Microsatellites | SNPs |
|---|---|---|---|---|
| Galaxias | maculatus | [35,36,40,41,42,43,44,45,46,47,48] | [6,43,49] | [5] |
| platei | [34,36,42,47,48,50,51] | [22,38,39] | ||
| globiceps | - | - | - | |
| Aplochiton | taeniatus | [32,43,47,52] | [32,52] | |
| zebra | [32,41,43,47,52] | [32,52] | ||
| marinus | [32] | [32] | ||
| Brachygalaxias | bullocki | [42] | ||
| gothei | [42] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vera-Escalona, I.; Delgado, M.L.; Habit, E.; Ruzzante, D.E. Historical and Contemporary Diversity of Galaxiids in South America: Biogeographic and Phylogenetic Perspectives. Diversity 2020, 12, 304. https://doi.org/10.3390/d12080304
Vera-Escalona I, Delgado ML, Habit E, Ruzzante DE. Historical and Contemporary Diversity of Galaxiids in South America: Biogeographic and Phylogenetic Perspectives. Diversity. 2020; 12(8):304. https://doi.org/10.3390/d12080304
Chicago/Turabian StyleVera-Escalona, Iván, M. Lisette Delgado, Evelyn Habit, and Daniel E. Ruzzante. 2020. "Historical and Contemporary Diversity of Galaxiids in South America: Biogeographic and Phylogenetic Perspectives" Diversity 12, no. 8: 304. https://doi.org/10.3390/d12080304
APA StyleVera-Escalona, I., Delgado, M. L., Habit, E., & Ruzzante, D. E. (2020). Historical and Contemporary Diversity of Galaxiids in South America: Biogeographic and Phylogenetic Perspectives. Diversity, 12(8), 304. https://doi.org/10.3390/d12080304





