Effect of Casuarina Plantations Inoculated with Arbuscular Mycorrhizal Fungi and Frankia on the Diversity of Herbaceous Vegetation in Saline Environments in Senegal
Abstract
1. Introduction
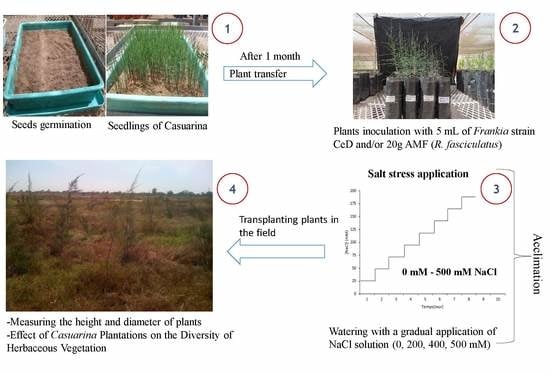
2. Materials and Methods
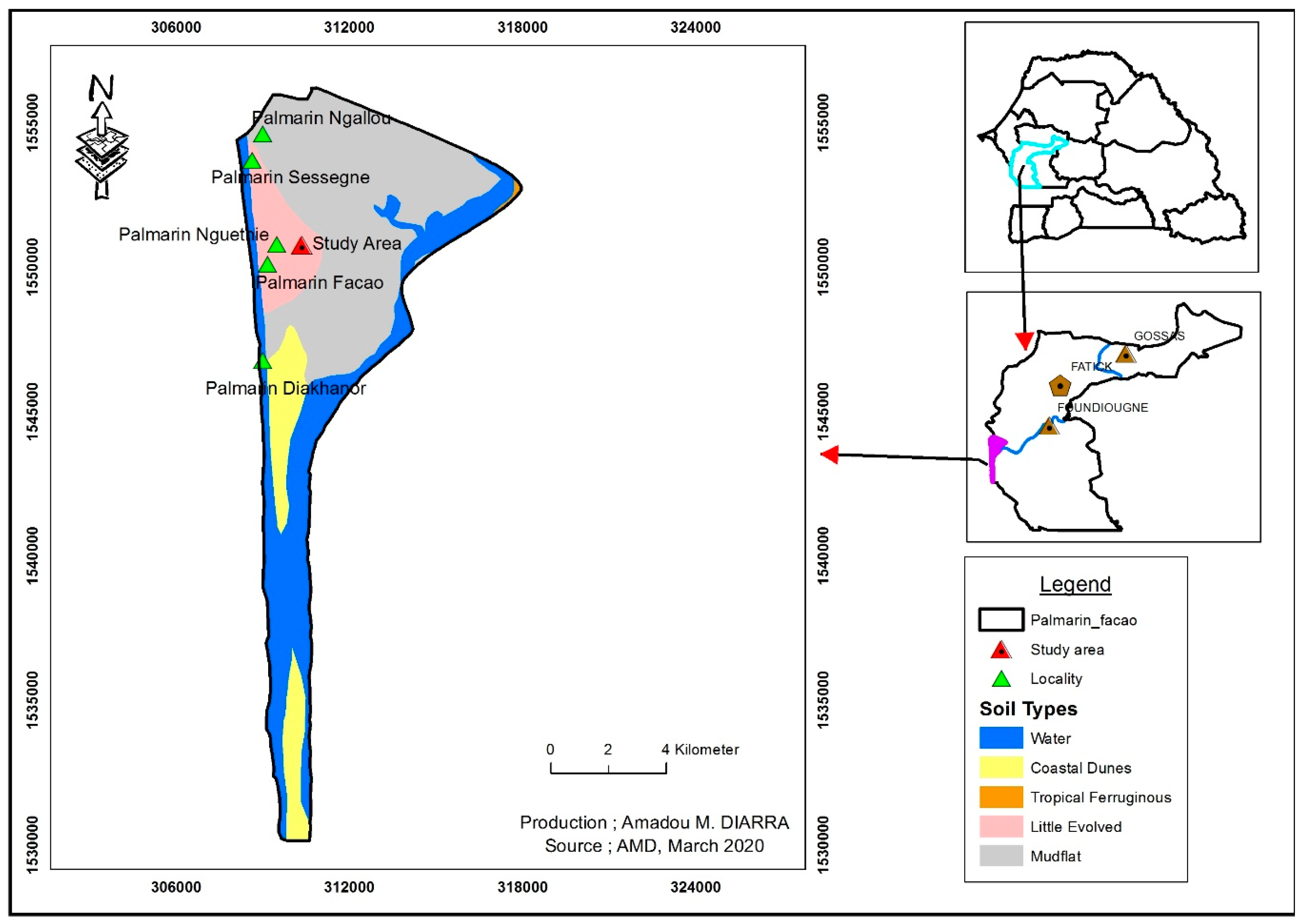
2.1. Field Study Site
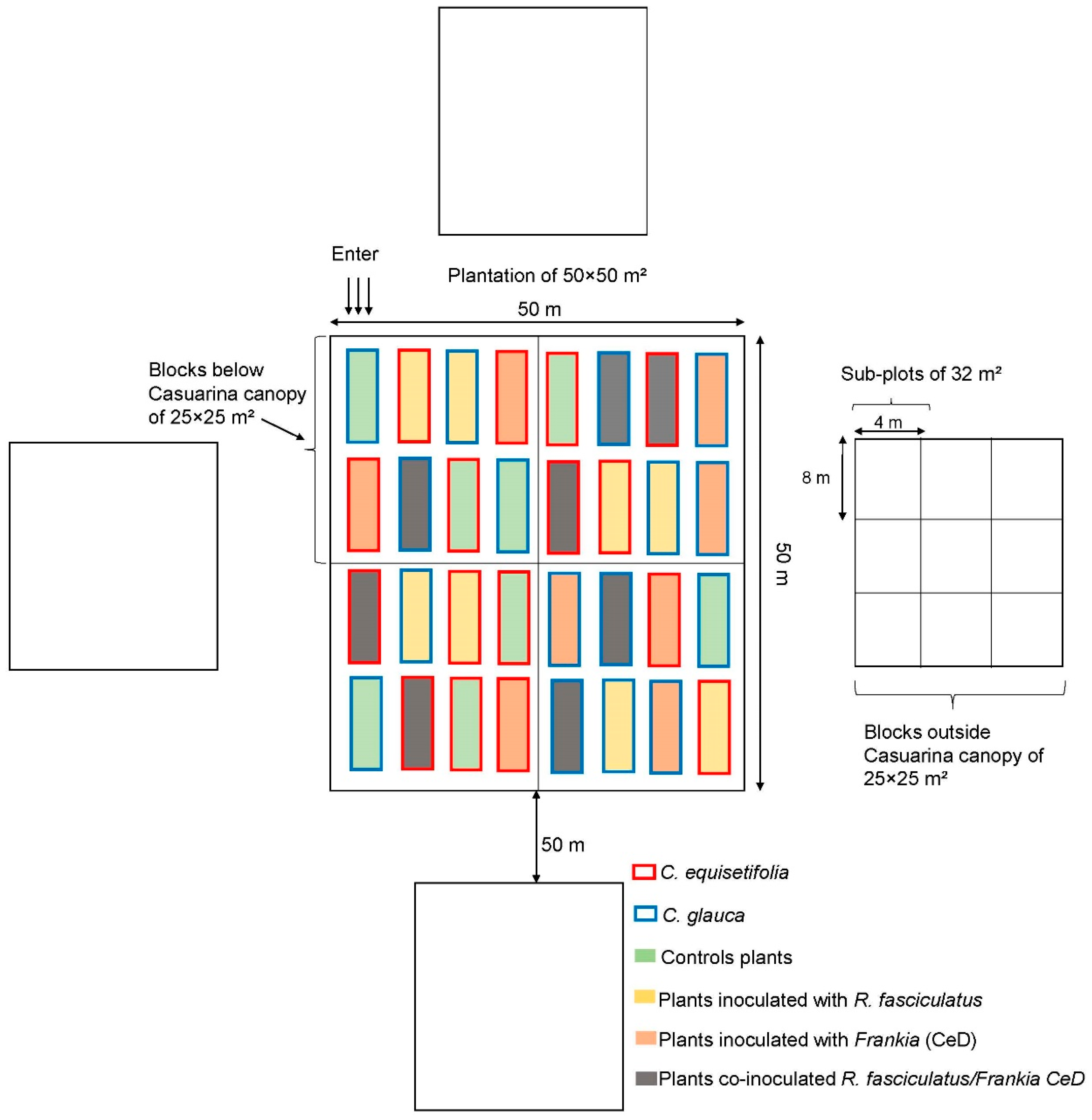
2.2. Experimentation in the Field
2.3. Physicochemical Characterization of the Site
2.4. Diversity of Herbaceous Vegetation
2.5. Diversity and Regularity Indices
2.5.1. Shannon–Weaver Index
2.5.2. Evenness Measure
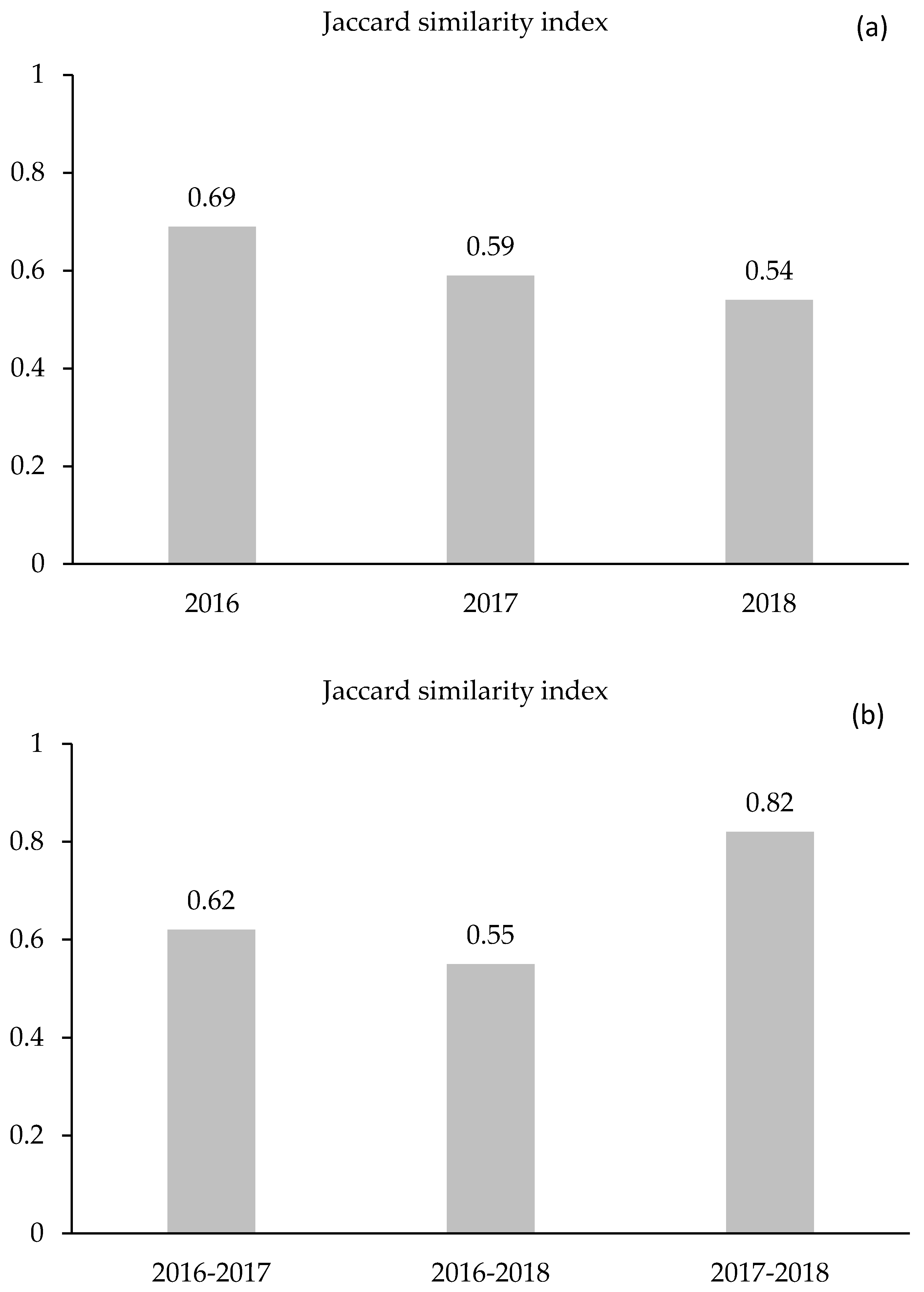
2.5.3. Jaccard’s Similarity Index
2.5.4. Specific Presence Contribution (S.P.C.)
2.6. Biomass
Specific Contribution Biomass (Csib)
2.7. Statistical Analysis
3. Results
3.1. Physicochemical Characteristics of Soil
3.2. Effect of Inoculation with AMF/Frankia on the Growth of C. equisetifolia and C. glauca Planted in the More Saline Area in Palmarin
3.3. Effect of the Casuarina Plantation on Species Diversity and Specific Contribution below and outside Their Canopy
3.4. Coverage, Specific Richness, and Diversity Index below and outside the Casuarina Canopy
3.5. Effect of Casuarina Species on Herbaceous Biomass in Saline Conditions
3.6. Specific Contribution of Dry Biomass below and outside the Casuarina Canopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

| Height | Collar Diameter | Survival Rate | Specific Contribution Presence | Dry Biomass | |
|---|---|---|---|---|---|
| Species | ** | ns | * | ns | ns |
| Inoculation | *** | ns | ** | ns | ns |
| Species× Inoculation | ns | ns | ns | ns | ns |
References
- Qadir, M.; Schubert, S. Degradation processes and nutrient constraints in sodic soils. Land Degrad. Dev. 2002, 13, 275–294. [Google Scholar] [CrossRef]
- Diadhiou, Y.B.; Ndour, A.; Niang, I.; Niang-Fall, A. Étude comparative de l’évolution du trait de côte sur deux flèches sableuses de la Petite Côte (Sénégal): Cas de Joal et de Djiffère. Norois 2016, 25–42. [Google Scholar] [CrossRef]
- Agence National de la Statistique et de la Démographie (ANSD). Recensement Générale de la Population et de l’Habitat (RGPH). 1988. Available online: http://www.ansd.sn/ressources/rapports/Rapport_RGPH_88.pdf (accessed on 7 February 2020).
- Agence National de la Statistique et de la Démographie (ANSD). Recensement Général de la Population et de l’Habitat, de l’Agriculture et de l’Elevage (RGPHAE). 2013. Available online: http://www.ansd.sn/ressources/rapports/Rapport-definitif-RGPHAE2013.pdf (accessed on 7 February 2020).
- Bleibaum, F. Case Study Senegal: Environmental Degradation and Forced Migration. In Environment, Forced Migration and Social Vulnerability; Afifi, T., Jäger, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Potgieter, L.J.; Richardson, D.M.; Wilson, J.R.U. Casuarina cunninghamiana in the Western Cape, South Africa: Determinants of naturalisation and invasion, and options for management. South Afr. J. Bot. 2014, 92, 134–146. [Google Scholar] [CrossRef]
- Djighaly, P.I.; Diagne, N.; Ngom, M.; Ngom, D.; Hocher, V.; Fall, D.; Diouf, D.; Laplaze, L.; Svistoonoff, S.; Champion, A. Selection of arbuscular mycorrhizal fungal strains to improve Casuarina equisetifolia L. and Casuarina glauca Sieb. tolerance to salinity. Ann. For. Sci. 2018, 75, 72. [Google Scholar] [CrossRef]
- Sayed, W.F. Improving Casuarina growth and symbiosis with Frankia under different soil and environmental conditions—Review. Folia Microbiol. 2011, 56, 1–9. [Google Scholar] [CrossRef]
- Diagne, N.; Diouf, D.; Svistoonoff, S.; Kane, A.; Noba, K.; Franche, C.; Bogusz, D.; Duponnois, R. Casuarina in Africa: Distribution, role and importance of arbuscular mycorrhizal, ectomycorrhizal fungi and Frankia on plant development. J. Environ. Manag. 2013, 128, 204–209. [Google Scholar] [CrossRef]
- Duro, N.; Batista-Santos, P.; da Costa, M.; Maia, R.; Castro, I.V.; Ramos, M.; Ramalho, J.C.; Pawlowski, K.; Máguas, C.; Ribeiro-Barros, A. The impact of salinity on the symbiosis between Casuarina glauca Sieb. ex Spreng, and N₂-fixing Frankia bacteria based on the analysis of Nitrogen and Carbon metabolism. Plant Soil 2016, 398, 327–337. [Google Scholar] [CrossRef]
- Hanin, M.; Ebel, C.; Ngom, M.; Laplaze, L.; Masmoudi, K. New Insights on Plant Salt Tolerance Mechanisms and Their Potential Use for Breeding. Front. Plant Sci. 2016, 7, 708. [Google Scholar] [CrossRef]
- Abeer, H.; Abd_Allah, E.F.; Alqarawi, A.; Egamberdieva, D. Induction of salt stress tolerance in cowpea [Vigna unguiculata (L.) Walp.] by arbuscular mycorrhizal fungi. Legum. Res. Int. J. 2015, 38, 579–588. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.; Kim, K.; Subramanian, P.; Senthilkumar, M.; Anandham, R.; Sa, T.-M. Arbuscular mycorrhizal fungi and associated bacteria isolated from salt-affected soil enhances the tolerance of maize to salinity in coastal reclamation soil. Agric. Ecosyst. Environ. 2016, 231, 233–239. [Google Scholar] [CrossRef]
- Zhong, C.; Zhang, Y.; Chen, Y.; Jiang, Q.; Chen, Z.; Liang, J.-F.; Pinyopusarerk, K.; Franche, C.; Bogusz, D. Casuarina research and applications in China. Symbiosis 2009, 50, 107–114. [Google Scholar] [CrossRef]
- Fall, F.; Le Roux, C.; Bâ, A.M.; Fall, D.; Bakhoum, N.; Faye, M.N.; Kane, A.; Ndoye, I.; Diouf, D. The rhizosphere of the halophytic grass Sporobolus robustus Kunth hosts rhizobium genospecies that are efficient on Prosopis juliflora (Sw.) DC and Vachellia seyal (Del.) P.J.H. Hurter seedlings. Syst. Appl. Microbiol. 2019, 42, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, K.C.; Karaoz, U.; Hanson, C.A.; Santee, C.A.; Bradford, M.A.; Treseder, K.; Wallenstein, M.D.; Brodie, E.L. Differential Growth Responses of Soil Bacterial Taxa to Carbon Substrates of Varying Chemical Recalcitrance. Front. Microbiol. 2011, 2, 94. [Google Scholar] [CrossRef] [PubMed]
- Diem, H.G.; Dommergues, Y. The isolation of Frankia from nodules of Casuarina. Can. J. Bot. 1983, 61, 2822–2825. [Google Scholar] [CrossRef][Green Version]
- Ngom, M.; Gray, K.; Diagne, N.; Oshone, R.; Fardoux, J.; Gherbi, H.; Hocher, V.; Svistoonoff, S.; Laplaze, L.; Tisa, L.S.; et al. Symbiotic Performance of Diverse Frankia Strains on Salt-Stressed Casuarina glauca and Casuarina equisetifolia Plants. Front. Plant Sci. 2016, 7, 1. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Pflanzensoziologie: Grundzüge der Vegetationskunde. Mit 168 Abb. Biologische Studienbücher (Geschlossen); Springer: Berlin/Heidelberg, Germany, 1928; ISBN 978-3-662-02056-2. [Google Scholar]
- Gillet, F. La Phytosociologie Synusiale Intégrée. Guide Méthodologique; Université de Neuchâtel, Institut de Botanique: Neuchâtel, Switzerland, 2000; 68p. [Google Scholar]
- Berhaut, J. La Flore illustrée du Sénégal. Préf. de L. Sédar Senghor. J. D’agriculture Traditionnelle Botanique Appliquée 1974, 21, 269–270. [Google Scholar]
- Lebrun, J.-P.; Stork, A.L. Enumération des Plantes à fleurs d’Afrique Tropicale—Volume 1: Généralités et Annonaceae à Pandaceae; Conservatoire et Jardin botaniques de la Ville de Genève: Geneva, Switzerland, 1991; ISBN 978-2-8277-0108-7. [Google Scholar]
- Lebrun, J.-P.; Stork, A.L. Enumération des Plantes à Fleurs d’Afrique Tropicale. Volume II. Chrysobalanaceae à Apiaceae; Conservatoire et Jardin botaniques de la Ville de Genève: Geneva, Switzerland, 1992; ISBN 978-2-8277-0109-4. [Google Scholar]
- Rougerie, G.S. Frontier et D. Pichod-Viale, Écosystèmes: Structure, fonctionnement, évolution. Annales Géographie 1992, 101, 343–344. [Google Scholar]
- Daget, P.; Poissonet, J. Une méthode d’analyse phytosociologique des prairies. Critères d’application. Ann. Agron. 1972, 22, 5–41. [Google Scholar]
- Rusch, G.; Armas, C.; Diouf, M.; Zapata, P.; Fall, D.; Casanoves, F.; Diémé, J.; Ibrahim, M.; Declerck, F.; Pugnaire, F. The importance of environmental gradients and tree functional attributes on tree-understory interactions in seasonally dry tropical agroforestry systems. In Proceedings of the Final Conference: “The Role of Functional Diversity for Ecosystem Services in Multi-Functional Agroforestry”, Trondheim, Norway, 23–25 May 2013. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Boil. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Engelmoer, D.J.P.; Behm, J.E.; Kiers, E.T. Intense competition between arbuscular mycorrhizal mutualists in anin vitroroot microbiome negatively affects total fungal abundance. Mol. Ecol. 2013, 23, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.S.; Shanan, N.T.; Massoud, O.N.; Swelim, D.M. Improving salinity tolerance of Acacia saligna (Labill.) plant by arbuscular mycorrhizal fungi and Rhizobium inoculation. Afr. J. Biotechnol. 2012, 11, 1259–1266. [Google Scholar] [CrossRef]
- Ashrafi, E.; Zahedi, M.; Razmjoo, J. Co-inoculations of arbuscular mycorrhizal fungi and rhizobia under salinity in alfalfa. Soil Sci. Plant Nutr. 2014, 60, 619–629. [Google Scholar] [CrossRef]
- Ren, C.-G.; Bai, Y.-J.; Kong, C.-C.; Bian, B.; Xie, Z. Synergistic Interactions Between Salt-tolerant Rhizobia and Arbuscular Mycorrhizal Fungi on Salinity Tolerance of Sesbania cannabina Plants. J. Plant Growth Regul. 2016, 35, 1098–1107. [Google Scholar] [CrossRef]
- Bar, Y.; Apelbaum, A.; Kafkafi, U.; Goren, R. Relationship between chloride and nitrate and its effect on growth and mineral composition of avocado and citrus plants. J. Plant Nutr. 1997, 20, 715–731. [Google Scholar] [CrossRef]
- Mishra, A.; Sharma, S.D.; Khan, G.H. Rehabilitation of degraded sodic lands during a decade of Dalbergia sissoo plantation in Sultanpur District of Uttar Pradesh, India. Land Degrad. Dev. 2002, 13, 375–386. [Google Scholar] [CrossRef]
- Trites, M.; Bayley, S.E. Vegetation communities in continental boreal wetlands along a salinity gradient: Implications for oil sands mining reclamation. Aquat. Bot. 2009, 91, 27–39. [Google Scholar] [CrossRef]
- Ågren, G.I.; Wetterstedt, J.Å.M.; Billberger, M.F.K. Nutrient limitation on terrestrial plant growth—Modeling the interaction between nitrogen and phosphorus. New Phytol. 2012, 194, 953–960. [Google Scholar] [CrossRef]
- Uma, M.; Saravanan, T.; Rajendran, K. Growth, litterfall and litter decomposition of Casuarina equisetifolia in a semiarid zone. J. Trop. For. Sci. 2014, 26, 125–133. [Google Scholar]
- Hata, K.; Kato, H.; Kachi, N. Leaf litter of the invasive Casuarina equisetifolia decomposes at the same rate as that of native woody species on oceanic islands but releases more nitrogen. Weed Res. 2012, 52, 542–550. [Google Scholar] [CrossRef]
- Diagne, N.; Djighaly, P.; Ngom, M.; Prodjinoto, H.; Ngom, D.; Hocher, V.; Fall, D.; Diouf, D.; Nambiar-Veetil, M.; Sy, M.; et al. Rehabilitation of Saline Lands Using Selected Salt-Tolerant Casuarina-Microorganisms Combinations. In Proceedings of the 5th International Casuarina Meeting, Chennai, India, 3–7 February 2014. [Google Scholar]
- Dagar, J.C.; Minhas, P. (Eds.) Agroforestry for the Management of Waterlogged Saline Soils and Poor-Quality Waters. Advances in Agroforestry; Springer: Dordrecht, The Netherlands, 2016; ISBN 978-81-322-2657-4. [Google Scholar] [CrossRef]
- Akhter, J.; Mahmood, K.; Malik, K.; Ahmed, S.; Murray, R. Amelioration of a saline sodic soil through cultivation of a salt-tolerant grass Leptochloa fusca. Environ. Conserv. 2003, 30, 168–174. [Google Scholar] [CrossRef]
- Malik, K.A.; Bilal, R.; Mehnaz, S.; Rasul, G.; Mirza, M.; Ali, S. Association of nitrogen-fixing, plant-growth-promoting rhizobacteria (PGPR) with kallar grass and rice. Plant Soil 1997, 194, 37–44. [Google Scholar] [CrossRef]
- Ravindranath, N.H. Mitigation and adaptation synergy in forest sector. Mitig. Adapt. Strat. Glob. Chang. 2007, 12, 843–853. [Google Scholar] [CrossRef]
- Grouzis, M.; Akpo, L.E. Influence d’Acacia raddiana sur la structure et le fonctionnement de la strate herbacée dans le Ferlo sénégalais. In Un Arbre au Désert; Le Floc’H, É., Ed.; IRD Éditions; OpenEdition: Marseille, France, 2003; pp. 249–262. [Google Scholar]


| Analysis | pH | Salinity | Nitrogen Kjeldahl | Carbon Organic | Phosphorus Total |
|---|---|---|---|---|---|
| ‰ | mg/kg | g/kg | mg/kg | ||
| Before planting | 5.3 a | 0.45 a | 155.75 b | 2.15 b | 42.25 b |
| 3 years after planting (below Casuarina canopy) | 5.95 a | 0.28 b | 275 a | 3.3 a | 170 a |
| Species | Treatments | Survival Rate (%) | Height (cm) | Collar Diameter (cm) | |
|---|---|---|---|---|---|
| 6 months after planting | C. equisetifolia | Control | 85 b | 65.14 de | 5.45 a |
| Frankia | 80 b | 68.94 cde | 6.13 a | ||
| Rf | 76.67 b | 60.51 e | 6.05 a | ||
| Rf + Frankia | 100 a | 73.67 bcd | 5.15 a | ||
| C. glauca | Control | 73.4 b | 78.51 abc | 5.75 a | |
| Frankia | 93.34 a | 79.10 abc | 6.02 a | ||
| Rf | 90 a | 81.98 ab | 5.51 a | ||
| Rf + Frankia | 86.67 ab | 88.00 a | 6.21 a | ||
| Species | . | *** | ns | ||
| Inoculation | ** | ** | ns | ||
| Species*Inoculation | ns | ns | ns | ||
| 2 years after | C. equisetifolia | Control | 30 c | 107.81 c | 12.26 a |
| Frankia | 55 b | 111.77 c | 13.87 a | ||
| Rf | 47.5 b | 168.13 ab | 13.45 a | ||
| Rf + Frankia | 50 b | 172.26 ab | 13.49 a | ||
| C. glauca | Control | 55 b | 144.40 b | 11.30 a | |
| Frankia | 67.5 a | 141.29 b | 12.31 a | ||
| Rf | 45 b | 181.05 a | 15.00 a | ||
| Rf + Frankia | 67.5 a | 166.98 ab | 12.30 a | ||
| Species | * | * | ns | ||
| Inoculation | ** | *** | ns | ||
| Species*Inoculation | . | . | ns | ||
| 3 years after | C. equisetifolia | Control | 30 b | 125.63 c | 13.55 a |
| Frankia | 55 a | 127.45 c | 14.07 a | ||
| Rf | 47.5 a | 180.13 ab | 14.86 a | ||
| Rf + Frankia | 50 a | 186.88 ab | 14.63 a | ||
| C. glauca | Control | 55 a | 152.77 bc | 12.97 a | |
| Frankia | 57 a | 163.29 abc | 15.33 a | ||
| Rf | 45 a | 196.50 a | 16.52 a | ||
| Rf + Frankia | 57.5 a | 192.38 a | 15.40 a | ||
| Species | . | * | ns | ||
| Inoculation | * | *** | ns | ||
| Species*Inoculation | ns | . | ns |
| Family | Genera | Species | BCC | OCC | ||||
|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2016 | 2017 | 2018 | |||
| Poaceae | Dactyloctenium | Dactyloctenium aegyptium (L.) Willd | 0.64 | 2.96 | 2.12 | 6.03 | 3.40 | 3.87 |
| Schisachyrium | Schizachyrium compressa (K. Schum.) | 3.21 | 2.59 | 5.30 | - | - | - | |
| Schizachyrium rupestre (Stapf.) Stapf | - | - | - | 0.86 | 2.04 | 1.66 | ||
| Chloris | Chloris prieurii Kunth | 3.85 | 3.33 | 3.53 | 3.45 | 3.40 | 5.52 | |
| Chloris barbata (L.) Sw. | - | 2.96 | 2.12 | - | - | - | ||
| Pennisetum | Pennisetum polystachion (L.) Schul. | - | 3.70 | 3.53 | 2.59 | 4.76 | 4.97 | |
| Eragrostis | Eragrostis tenella (L.) Beauv. | 5.13 | 2.96 | 1.41 | - | - | - | |
| Eragrostis tremula Hochst. Ex Steud. | 3.85 | 4.07 | 2.12 | 5.17 | 4.76 | 4.97 | ||
| Eragrostis aspera (Jacq.) Nees. | 3.21 | 3.70 | 1.77 | - | - | - | ||
| Paspalum | Paspalum vaginatum (L.) | 4,49 | 3.33 | 3.53 | - | - | - | |
| Aristida | Aristida funiculata Trin. & Rupr. | - | 2.96 | 3.53 | 5.17 | 5.44 | 1.10 | |
| Aristida mutabilis Trin. & Rupr. | - | 1.11 | 2.47 | - | - | - | ||
| Digitaria | Digitaria horizontalis Willd. | 3.85 | 2.96 | 2.47 | 1.72 | 6.12 | 6.08 | |
| Brachiaria | Brachiaria lata C.E. Hubb. | - | 0.37 | 0.71 | - | - | - | |
| Sporobolus | Sporobolus robustus Kunth | 5.77 | 8.15 | 7.07 | 3.45 | 6.80 | 3.87 | |
| Malvaceae | Hibiscus | Hibiscus rostellatus (Guill. & Perr.) | 0.64 | 1.48 | 1.41 | - | - | - |
| Hibiscus asper Hook. | 3.21 | 3.33 | 7.42 | 3.45 | 2.72 | - | ||
| Corchorus | Corchorus tridens L. | - | - | 0.71 | - | - | - | |
| Sida | Sida alba L. | 4.49 | - | - | 3.45 | 5.44 | 3.87 | |
| Cyperaceae | Fuirena | Fuirena ciliaris (L.) Roxb. | 2.56 | - | - | 0.86 | 0.68 | 5.52 |
| Cyperus | Cyperus esculentus L. | 1.92 | 4.07 | 2.47 | - | 4.76 | 3.31 | |
| Cyperus bulbosus Vahl. | 3.21 | 2.22 | 1.06 | 5.17 | 4.08 | 2.76 | ||
| Amaranthaceae | Amaranthus | Amaranthus greacizans L. | 0.64 | 3.70 | 3.89 | 6.03 | 2.04 | 2.21 |
| Philoxerus | Philoxerus vermicularis (L.) Sm. | 8.33 | 3.33 | 8.13 | 17.24 | 16.33 | 12.71 | |
| Convolvulaceae | Ipomoea | Ipomoea coptica Willd. | 1.28 | 0.74 | 1.77 | 5.17 | 2.04 | 3.87 |
| Ipomoea sinuata Ort. | - | - | 3.18 | - | - | - | ||
| Fabaceae | Indigofera | Indigofera berhautiana JB. Gillett. | - | - | 1.06 | - | - | 1.66 |
| Indigofera linifolia (Lf) Retz. | - | - | 1.06 | - | - | 2.21 | ||
| Alysicarpus | Alysicarpus ovalifolius Schum.& Thonn. | 1.92 | 3.33 | 1.77 | - | - | 2.21 | |
| Amaryllidaceae | Pancratium | Pancratium trianthum Herb. | - | 1.48 | 3.18 | - | - | - |
| Nyctaginaceae | Boerhavia | Boerhavia repens L. | 3.21 | 4.07 | 3.53 | 3.45 | 3.40 | 3.87 |
| Plantaginaceae | Scoparia | Scoparia dulcis L. | - | 3.70 | - | 5.17 | 0.68 | 1.10 |
| Asteraceae | Sphaeranthus | Sphaeranthus senegalensis DC. | 7.05 | 4.44 | 1.77 | 2.59 | 5.44 | 3.87 |
| Aizoaceae | Sesuvium | Sesuvium portulacastrum (L.) L. | 14.10 | 8.89 | 3.53 | 7.76 | 9.52 | 7.18 |
| Acanthaceae | Hygrophila | Hygrophila senegalensis (Nees) T.A | 8.33 | 7.78 | 7.77 | 5.17 | 5.44 | 6.63 |
| Rubiaceae | Spermacoce | Spermacoce verticillata L. | 5.13 | 2.22 | 3.18 | 6.03 | 0.68 | 4.97 |
| Sterculiaceae | Melochia | Melochia corchorifolia L. | - | - | 0.71 | - | - | - |
| BCC | OCC | |||||
|---|---|---|---|---|---|---|
| Years | 2016 | 2017 | 2018 | 2016 | 2017 | 2018 |
| Coverage (%) | 48.28 b | 67.48 a | 71.95 a | 37.57 b | 43.26 b | 45.03 b |
| Specific richness | 24 b | 29 ab | 33 a | 21 b | 22 b | 24 b |
| Shannon index | 1.9 b | 2.26 a | 2.39 a | 1.67 c | 1.76 c | 2.05 b |
| Evenness | 0.41 | 0.46 | 0.47 | 0.38 | 0.39 | 0.44 |
| BCC | OCC | |||||
|---|---|---|---|---|---|---|
| Csib | Csib | |||||
| Species | 2016 | 2017 | 2018 | 2016 | 2017 | 2018 |
| Dactylotenium aegyptium (L.) Willd | 3.12 | 1.44 | 1.97 | 2.46 | 2.56 | 1.90 |
| Schizachirium compressa (K. Schum.) Stapf | 1.78 | 1.65 | 3.63 | - | - | - |
| Schisachyrium rupestre (Stapf) Stapf | - | - | - | 1.87 | 1.21 | 0.56 |
| Chloris prieuriiKunt | 10.79 | 8.21 | 1.14 | 5.32 | 4.56 | 4.64 |
| Chloris barbata (L.) Sw. | - | 10.29 | 5.43 | - | - | - |
| Pennisetum polystachion (L.) Schul. | - | 2.30 | 5.99 | 3.97 | 4.75 | 3.08 |
| Eragrostis tenella (L.) Beauv. | 4.24 | 2.01 | 3.41 | - | - | - |
| Eragrostis tremula Hochst. Ex Steud. | 5.53 | 2.73 | 2.68 | 3.77 | 2.41 | 5.84 |
| Eragrostis aspera (Jacq.) Nees. | 3.48 | 4.44 | 3.30 | - | - | - |
| Paspalum vaginatum (L.) | 1.22 | 5.64 | 2.79 | - | - | - |
| Aristida funiculata Trin. & Rupr. | - | 2.19 | 1.83 | 5.24 | 3.05 | 3.27 |
| Aristida mutabilis Trin. & Rupr. | - | 1.22 | 4.04 | - | - | - |
| Digitaria horizontalis Willd. | 2.59 | 2.48 | 1.43 | 3.10 | 3.84 | 3.70 |
| Brachiaria lata (Schumach.) C.E. Hubb. | - | 2.80 | 2.38 | - | - | - |
| Sporobolus robustus Kunth. | 12.97 | 4.40 | 7.80 | 4.45 | 8.07 | 4.21 |
| Hibiscus rostellatus (Guill. & Perr.) | 2.28 | 0.61 | 4.65 | - | - | - |
| Hibiscus asper Hook. | - | - | 0.83 | - | - | - |
| Corchorus tridens L. | 0.58 | 1.13 | 2.88 | 2.82 | 3.17 | - |
| Sida alba L. | 0.89 | - | - | 4.17 | 3.35 | 4.96 |
| Fuirena ciliaris (L.) Roxb. | 0.53 | - | - | 3.34 | 3.77 | 3.10 |
| Cyperus esculentus L. | 2.01 | 2.16 | 2.39 | - | 2.68 | 3.38 |
| Cyperus bulbosus Vahl. | 2.23 | 1.72 | 2.65 | 2.30 | 3.09 | 1.85 |
| Amaranthus greacizans L. | 0.94 | 1.69 | 1.20 | 3.89 | 3.77 | 2.68 |
| Philoxerus vermicularis (L.) Sm. | 9.31 | 9.59 | 4.36 | 8.42 | 8.82 | 5.46 |
| Alysicarpus ovalifolius Schum. & Thonn. | 1.35 | 0.74 | 0.43 | - | - | 1.42 |
| Pancratium trianthum Herb. | - | 2.17 | 3.80 | - | - | - |
| Boerhavia repens L. | 0.91 | 0.43 | - | 1.43 | 2.15 | 0.91 |
| Scoparia dulcis L. | - | 1.37 | 0.97 | 3.97 | 4.67 | 3.91 |
| Sphaeranthus senegalensis DC. | 2.18 | 1.44 | - | 3.02 | 2.26 | 3.11 |
| Sesuvium portulacastrum (L.) L. | 13.35 | 12.22 | 6.57 | 16.44 | 11.99 | 7.61 |
| Hygrophila senegalensis (Nees)T. Anderson | 14.67 | 9.18 | 6.54 | 12.91 | 10.06 | 7.88 |
| Spermacoce verticillata L. | 1.24 | 2.23 | 4.01 | 2.66 | 2.00 | 2.20 |
| Ipomoea coptica Willd. | 1.80 | 1.51 | 2.35 | 4.45 | 7.76 | 2.98 |
| Ipomoea sinuata Ort. | - | - | 2.39 | - | - | - |
| Indigofera berhautiana JB. Gillett. | - | - | 1.44 | - | - | 5.01 |
| Indigofera linifolia (Lf) Retz. | - | - | 1.23 | - | - | 5.01 |
| Melochia corchorifolia L. | - | - | 1.10 | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djighaly, P.I.; Ngom, D.; Diagne, N.; Fall, D.; Ngom, M.; Diouf, D.; Hocher, V.; Laplaze, L.; Champion, A.; Farrant, J.M.; et al. Effect of Casuarina Plantations Inoculated with Arbuscular Mycorrhizal Fungi and Frankia on the Diversity of Herbaceous Vegetation in Saline Environments in Senegal. Diversity 2020, 12, 293. https://doi.org/10.3390/d12080293
Djighaly PI, Ngom D, Diagne N, Fall D, Ngom M, Diouf D, Hocher V, Laplaze L, Champion A, Farrant JM, et al. Effect of Casuarina Plantations Inoculated with Arbuscular Mycorrhizal Fungi and Frankia on the Diversity of Herbaceous Vegetation in Saline Environments in Senegal. Diversity. 2020; 12(8):293. https://doi.org/10.3390/d12080293
Chicago/Turabian StyleDjighaly, Pape Ibrahima, Daouda Ngom, Nathalie Diagne, Dioumacor Fall, Mariama Ngom, Diégane Diouf, Valerie Hocher, Laurent Laplaze, Antony Champion, Jill M. Farrant, and et al. 2020. "Effect of Casuarina Plantations Inoculated with Arbuscular Mycorrhizal Fungi and Frankia on the Diversity of Herbaceous Vegetation in Saline Environments in Senegal" Diversity 12, no. 8: 293. https://doi.org/10.3390/d12080293
APA StyleDjighaly, P. I., Ngom, D., Diagne, N., Fall, D., Ngom, M., Diouf, D., Hocher, V., Laplaze, L., Champion, A., Farrant, J. M., & Svistoonoff, S. (2020). Effect of Casuarina Plantations Inoculated with Arbuscular Mycorrhizal Fungi and Frankia on the Diversity of Herbaceous Vegetation in Saline Environments in Senegal. Diversity, 12(8), 293. https://doi.org/10.3390/d12080293