Abstract
The study of tardigrade diversity in Mexico is at early stage of development, to date, 56 extant species have been reported. To identify the tardigrade fauna associated with mosses in the Iztaccíhuatl volcano, we performed a systematic sampling along an altitudinal and multi-habitat gradient. A total of 57 moss samples were collected, 233 adults, 20 exuviae, and 40 free-laid tardigrade eggs were extracted from them. Five species were identified, and three putative species were determined. Diphascon mitrense and Minibiotus sidereus represents new records for Mexico and North America, while Adropion scoticum is a new record for Mexico. Additionally, one new species, Minibiotus citlalium sp. nov. was discovered; it resembles to Min. constellatus, Min. sidereus and Min. pentannulatus by the presence of a similar distribution pattern of star-shaped pores in the dorsal cuticle arranged in 11 transverse rows, which become double in the segments of the legs I–III, and by a very large star-shaped pore (5–6 tips) on each leg of the fourth pair. Minibiotus citlalium sp. nov. differs from other Minibiotus species mainly by macroplacoid length sequence, presence of both small and large star-shaped pores on the external surface on all legs, and by egg processes with inconspicuous ornamentation.
1. Introduction
Tardigrades are ubiquitous micrometazoans present in every biome on Earth, as they can be found in marine, freshwater, and terrestrial habitats [1]. Ecological studies dedicated to tardigrades are scarce, especially those that depend on replicated quantitative samples [2] (pp. 163–210). A patch distribution has been suggested even in apparently identical microhabitats. Therefore, a high number of samples (from hundreds to more than 1000) are necessary to reduce the standard error of population estimates and to obtain valid data for testing ecological hypotheses [2,3,4,5]. Tardigrade taxonomy is currently the subject of investigations, most of them relying on increasingly complex molecular datasets and extensive taxonomic contexts [6,7,8]; nonetheless, the taxonomic studies on Mexican tardigrades are at early stage. These include exploration, intensive collections, detailed observations, description, and denomination of species [9]. Previous studies on tardigrades in Mexico have recorded and described species from occasional and unsystematic collection events, which in turn have yielded scattered records in very few locations with more than a third of the country remaining unexplored [10].
Currently, 56 limno-terrestrial tardigrade species have been recorded in Mexico; however, one of them (Hypsibius pallidus Thulin, 1911) has no records of locality [11]. The remaining inhabit five of the seven ecoregions described for the country [12] (pp. 87–108). Seven species have been documented in at least two of the five ecoregions [10,13,14,15,16,17]: 1 in deserts of North America [15,18,19], 9 in great plains [15], 12 in warm-humid forests [10,16,20], 10 in warm-dry forest [10,13,14], and 31 in temperate mountains [14,15,16,17,19,21,22].
The temperate mountains, volcanoes, and high sierras of the Trans-Mexican Volcanic Belt (TMVB) belong to one of those ecoregions and are located between 19° and 20° N in central Mexico. TMVB includes the highest peaks in the country, with elevations up to 5650 m asl [23], and eight types of landscapes from sub-humid to arid [24] (pp. 39–55). These features lead to the recognition of the TMVB as a centre of diversification, endemism, and biogeographic transition for a wide variety of taxa [25].
Sierra Nevada is located within the central region of the TMVB, between the states of Puebla and Mexico, with the Popocatépetl and the Iztaccíhuatl volcanoes as main peaks. The predominant landscapes are temperate and semi-cold plains, hillsides, and sierras, while vegetation consists of pine and oak forests, with fragments of thorn scrub and low montane rainforest [24] (pp. 39–55). This kind of landscape with humid, temperate, and cold conditions, from low to high altitudes, provides suitable habitats for many species of tardigrades [26,27,28]. To our knowledge, the tardigrades from the Sierra Nevada have only been studied previously in 1972 [14], at the Popocatépetl volcano, with eight species recorded. These are (following the current taxonomic nomenclature and the system of the genera names abbreviation presented by Perry et. al. [29]): Echiniscus kerguelensis Richters, 1904, Milnesium tardigradum Doyère, 1840, Macrobiotus echinogenitus Richters, 1904, Macrobiotus furcatus Ehrenberg, 1859, Macrobiotus hufelandi C.A.S. Schultze, 1834, Ramazottius baumanni Ramazzotti, 1962, Ramazottius oberhaeuseri Doyère, 1840, and Pilatobius nodulosus Ramazzotti, 1957.
Most of these records are considered doubtful and must be confirmed taxonomically. For example, Macrobiotus hufelandi, Ramazzottius oberhaeuseri, and Milnesium tardigradum were amongst the earliest formally described tardigrade species. Under these names plenty of species were registered worldwide and were also subsequently reported as terrestrial cosmopolitan eutardigrades in the literature [30]. The most recently revision of these species complex indicates the presence of cryptic species; therefore, the previously published records must be corroborated, including Beasley [14]. Other case, corresponding to Mexican record of Macrobiotus echinogenitus, was published before the development of rigorous standards of specific diagnosis by Guidetti and Bertolani [31]. Therefore, previous records must be considered doubtful unless confirmed [32].
Access to this volcano was restricted by the Mexican federal government in 1994, due to increased volcanic activity. Therefore, further studies in this area have not been performed. On the other hand, even though the Iztaccíhuatl volcano is nearby and accessible from Popocatépetl, to date no tardigrades from this region were reported.
The goal of the present study was to explore the tardigrade fauna, using a systematic sampling in the Iztaccíhuatl volcano, along an altitudinal (2700–4500 m asl) and multi-habitat gradient, moss growing on rocks, tree bark, and soil; across Pinus L., 1753, Cupressus L., 1753, and Quercus L., 1753; mixed forest, and Abies religiosa (Kunth) Scheleschetendahl et Chamisso, 1830; and Pinus hartwegii Lindley, 1839 forest, scrub, and alpine tundra. The study includes a description of the new eutardigrade species Minibiotus citlalium sp. nov. Diphascon mitrense Pilato, Binda and Qualtieri, 1999 and Minibiotus sidereus Pilato, Binda and Lisi, 2003 represent new records for Mexico and North America, while Adropion scoticum (Murray, 1905) is a new record for Mexico.
2. Materials and Methods
2.1. Study Site
Fifty-seven moss samples were collected in the southwestern slope of Iztaccíhuatl volcano (Sierra Nevada, Trans Mexican Volcanic Belt; Figure 1a) in January 2018. The mosses were sampled in 12 geographical stations (S1–S12) distributed along an altitudinal gradient (2700–4500 m asl; Figure 1b) and in at least five different vegetation types, including: Pinus-Cupressus-Quercus mixed forest; Abies religiosa, and Cupressus sp. forest; Pinus hartwegii forest; alpine scrub; and alpine tundra (Figure 1c, Table 1). In each station, different moss morphotypes were identified and a square of 2 × 2 cm was sampled from each moss cushion and characterized depending on the collecting site, vegetation, and substrate type (rock, tree bark, and soil).
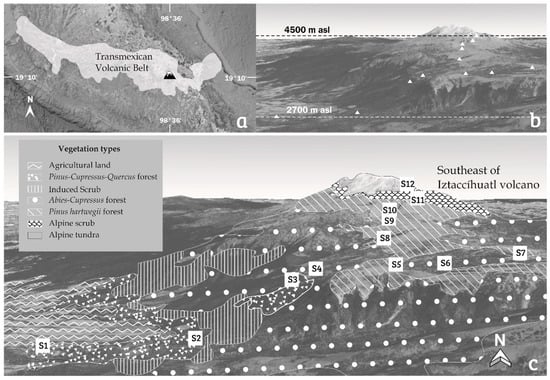
Figure 1.
Map of the study area. (a) Trans Mexican Volcanic Belt (TMVB), Mexico; (b) altitudinal gradient sampled (2700–4500 m asl); and (c) geographical stations (S1–S12) with vegetation types.

Table 1.
Station, sample number, geographic coordinates, vegetation, and substrate type, of the mosses sampled in the southwestern slope of Iztaccíhuatl volcano.
2.2. Sample Processing
Each moss sample was stored inside a paper envelope and dried. In the laboratory, the samples were rehydrated with 20 mL of tap water for 48 h. Later, samples were shaken and rinsed, and the supernatant were filtered using two stacked sieves with a decreasing mesh diameter (100 μm and 74 μm). The retained contents on the 74 μm sieve were washed into a Petri dish for examination under a stereoscopic microscope using dark field illumination at 45 × magnification.
All specimens for light microscopy were mounted individually onto microscope slides in Heinze polyvinyl alcohol (PVA) medium. Observations and photographs were taken using phase contrast microscopy (PCM) (ZEISS Axioskop with digital camera Axiocam ERC 55); for each species, images were recorded at successive focal depths and automatically combined into a single sharp image (i.e., focus stacking).
2.3. Morphometrics and Morphological Nomenclature
Structures were measured provided their orientations were suitable; body length was measured from the anterior to the posterior end of the body, excluding the hind legs. Terminology for the structures within the bucco-pharyngeal apparatus follows that from Pilato [33,34,35], Dastych [36], Kaczmarek and Michalczyk [37]. Macroplacoid length sequence is presented according to Kaczmarek et. al. [38]. Claws of Hypsibioidea were measured with the protocols as indicated in Beasley et. al. [21], whereas claws of Macrobiotoidea were measured with the protocols as indicated in Kaczmarek and Michalczyk [37]. The pt ratio (which is the ratio of the length of a given structure to the length of the buccal tube), is expressed as a percentage [33] and its values are always provided in italics. This was done to distinguish them from absolute measurements in micrometers. Morphometric data were compiled using the Parachela Excel template version 1.6, which is available from the Tardigrada Register (http://www.tardigrada.net/register/submit.htm) [39].
Species were identified using the keys provided by Ramazzotti and Maucci [11], Claxton [40], Fontoura and Pilato [41], and the original descriptions/redescriptions [35,42,43,44,45,46,47,48,49,50,51,52,53,54].
2.4. Description of Minibiotus citlalium sp. nov.
The identity of specimens of genus Minibiotus was based on the description of Schuster et. al. [55] amended by Claxton [40]; both included in the definition of this genus by Pilato and Binda [56]. The characters observed in our specimens and diagnostic of this genus were presence of bucco-pharyngeal apparatus of the Macrobiotus type, Minibiotus variant: an antero-ventral mouth, a rigid, short and narrow buccal tube, short ventral lamina (pt < 62), stylet supports inserted at 73% or less of the buccal tube length, pharyngeal apophyses and placoids present, stylet furcae typically shaped, short macroplacoid row length (pt < 42), and claws of the hufelandi type with lunules present. The identity of the Minibiotus citlalium sp. nov. was based on a character matrix of all described species of genus Minibiotus, which was built from the information contained in the original published descriptions. The characters used for the matrix were eyespot pigmentation, cuticular sculpturing, presence/absence of pores, pore shape, arrangement of pores, and pore size. Furthermore, the specimens were compared with the original descriptions of the most similar species: Minibiotus vinciguerrae Binda and Pilato, 1992; Min. sidereus Pilato et. al., 2003; Min. constellatus Michalczyk and Kaczmarek, 2003; Min. eichhorni Michalczyk and Kaczmarek, 2004; Min. pseudostellarus Roszkowska, Stec, Ciobanu, and Kaczmarek, 2016; and Min. pentannulatus Londoño, Daza, Lisi, and Quiroga, 2017. Given that Min. sidereus, Min. constellatus and Min. pentannulatus present a similar star-shaped pore sculpture pattern, compared to Minibiotus citlalium sp. nov., we requested photographs of these species to the authors of their descriptions for the comparison of their diagnostic characteristics.
To obtain more specimens and eggs of Minibiotus citlalium sp. nov. new moss samples were collected from the same stations and substrates (Table 1) where Minibiotus spp. were found (XX, Table 1). The samples were processed following the method described by Stec et. al. [57]. Ornamented eggs were individually placed in Petri dishes with mineral water at room temperature while waiting upon hatching.
Hatched specimens and eggs were used for light microscopy and processed as previously described; the eggs were measured based on Kaczmarek and Michalczyk [37]. For further refinement of initial observations, a subset of these material from the new species were processed for scanning electron microscopy (SEM). Briefly, specimens were first boiled in absolute ethanol and transferred to cold absolute ethanol. This was repeated three times. Then they were boiled again in absolute ethanol until complete evaporation. Finally, the specimens were mounted on metal plates and covered with gold. Specimens were examined in a Hitachi Scanning Electron Microscope S-2469N.
The description of Minibiotus citlalium sp. nov. was based on 16 animals and three eggs, one of them still hatching. In the description of Min. citlalium sp. nov. the diagnosis of the species and the differential diagnosis are included; both contain ranges of variation for some characters, while for others, the specific information obtained from the descriptions or from the images of the holotypes shown in the respective descriptions is included.
Information about diagnostic characters was consulted with the authors of the most similar species [58] (G. Pilato for Min. sidereus, Ł. Michalczyk for Min. constellatus, and R. Londoño for Min. pentannulatus pers. comm.)
Size Effect on Morphometric Data
Measurement and pt index of 29 morphological characters of animals corresponding to type series of Minibiotus citlalium sp. nov. are showed in Table 2. Because pt index only satisfactorily eliminates body size effects for isometric traits but do not eliminates from allometric ones [59,60]; we evaluate growth relation (isometric vs. allometric) in the continuous traits respect to body size in Minibiotus citlalium sp. nov., following the methodology proposed by Bartels et. al. [60]. The effect of “body size”, measured as buccal tube length (BTL), respect to 26 of 29 continuous body traits was evaluated, by mean a linear regression in each (Supplementary Material Table S1). Regressions were performed from log transformed values, omitting certain individuals if their orientation was unsuitable. The isometric or allometric trend was determinate in each trait, comparing the slope of each linear regression with a slope of 1; to do this, we performed t-tests (t = (b)1)/SE of the slope, df = n)20) [61]. Regression analysis were carried out using PAST ver. 4.03 [62].

Table 2.
Measurements and pt values of selected morphological structures of Minibiotus citlalium sp. nov. mounted in polivinil lactofenol medium. The individuals measured correspond to serial type (N- number of specimens/structures measured; RANGE refers to the smallest and the largest structure among all measured specimens; and SD—standard deviation).
We provide supporting data for growth trends, as well the slope (b) and the Y intercept (a) for each trait analyzed. It has been suggested that pt index for each body trait should be reported together with the slope (b) and the Y intercept (a), both obtained from the regression of logBS (body size) vs. logY (Y = body trait). Whit these parameters Thorpe’s normalization can be performed yield any quantitative trait size-independent [60].
3. Results
Tardigrades were observed in 32 out of the 57 samples examined (ca. 56%). In total, 233 tardigrades, 20 exuviae, and 40 free-laid eggs were found. Only 13 eggs were identified at a specific level. The tardigrades belong to seven genera within three eutardigrade families: Calohypsibiidae: Calohypsibius cf. ornatus (n = 5 individuals); Hypsibiidae: Diphascon mitrense (n = 2), Diphascon pingue (n = 11), Hypsibius cf. microps (n = 7), Hypsibius cf. pallidus (n = 10), Adropion scoticum (n = 51), and Pilatobius nodulosus (n = 32); and Macrobiotidae: Macrobiotus spp. (n = 24), Minibiotus sidereus (n = 50), and Minibiotus citlalium sp. nov. (n = 40).
Figures of habitus, buccal apparatus, claws, and specific details of cuticle for each species are presented in Supplementary Material (Figures S1–S7).
Taxonomic Accounts
Family: Calohypsibiidae Pilato, 1969 [63].
Calohypsibius cf. ornatus [64].
Material examined: five specimens XLV (5).
Remarks: The specimens correspond to the key proposed for the genus by Michalczyk and Kaczmarek [65] and the most recent revision of Calohypsibiidae by Gąsiorek et. al. [8]. We identified the presence of eight transverse lines of dorsal spines; however, we could not assign the specimens to Calohypsibius ornatus sensu stricto, because this taxon has a high intraspecific variability. The presence of several species has been suggested under this name but there is no detailed redescription [8,65]. Calohypsibius ornatus sensu lato has been reported from North America, in Greenland, Canada, and USA [66], and from South America, in Bolivia, Argentina, and Colombia [67].
Family: Hypsibiidae Pilato, 1969 [63].
Subfamily: Diphasconinae Diphasconinae Dastych, 1992 [68].
Genus: Diphascon Plate, 1888 [69].
Diphascon mitrense Pilato, Binda, and Qualtieri, 1999 [48].
Material examined: IV (1) and X (1).
Remarks: The specimens correspond well to the key proposed by Fontoura and Pilato [41]. Diphascon mitrense belongs to the pingue-species group, which is a complex of 10 morphologically homogeneous species [41].
This species has been only recorded in Argentina [67]; therefore, the specimens described in this study is the first record from Mexico and North America.
Diphascon pingue (Marcus, 1936) [70] sensu lato
Material examined: IV (1), IX (1), X (2), XXXI (3), XXXIII (1), and XXXVI (3).
Remarks: It is believed that Diphascon pingue sensu lato has a cosmopolitan distribution [71]. This species has been reported from North America in Greenland, Canada, and USA [66]; from Central America in Costa Rica [72]; and from South America, in Argentina, Bolivia, Brazil, and Ecuador [67]. From Mexico, it has been recently reported in temperate mountains of Sierra Madre Oriental [15].
Subfamily: Hypsibiinae Pilato, 1969 [63].
Genus: Hypsibius Ehrenberg, 1848 [73].
Hypsibius spp.
Remarks: Based on the characters: absence of cuticular bars on legs I–III, the pt of stylet support insertion point, the external and posterior primary claw branches length, the pt of the external buccal tube width, and the pt of the septulum, and following the key by Gąsiorek et. al. [44], Hypsibius dujardini (Doyère, 1840) (Figure S3a–c) and Hypsibius exemplaris Gąsiorek, Stec, Morek, and Michalczyk, 2018 (Figure S3d–f), were recognized. However, few specimens were found, which prevents a comparison of its morphological variation for an identification. Both species are a member of the dujardini-species group.
Hypsibius dujardini sensu lato is a species group [44] with an apparent global distribution [71]; it has been reported from North America, in Canada, Greenland, and USA [66]; from Central America in Costa Rica [72]; and from South America, in Argentina, Bolivia, Chile and Uruguay [67]. On the other hand, Hys. exemplaris has only been recorded from England [44].
Hypsibius cf. microps.
Material examined: IX (1), XVI (1), XXXI (3), and XXXVI (2).
Remarks: All collected specimens showed a short claw base and therefore the primary branches seemed to be attached closely to the base of the secondary claw (Figure S3i), characters that allow to differentiate this species from Hypsibius pallidus Thulin, 1911, (amended by Kaczmarek and Michalczyk, 2009) [42]. However, they differ in the ranges of other body measurements (body length, buccal tube length, macroplaoid 1 length, and base and branches of claw 3) provided in the re-description of Hyspsibius microps Thulin, 1928 (amended by Kaczmarek and Michalczyk, 2009) [42]. Additionally, we observed presence of the minute dot-like septula at the end of the placoids row (Figure S3h). A robust morphometric analysis is necessary to evaluate the morphological variation of this species and confirm the specific identity of the specimens.
Hypsibius microps is a member of convergens species group [42,44]. This species sensu lato is largely Holarctic [71] and has been recorded from North America, in Canada, Greenland, and USA [66]; from Central America in Costa Rica [72]; and from South America, in Argentina, Brazil and Uruguay [67].
Hypsibius cf. pallidus.
Material examined: IX (2), XXXI (3), XXXIII (1), XXXV (1), and XXXVI (3).
Remarks: All collected specimens show a long claw base and the primary branches seem to be connected at a high level of the secondary claw (Figure S3l), characters that allow to differentiate this species from Hys. microps [42]. However, they differ in the ranges of other body measurements (body length, macroplacoid 1 length, macroplacoid row length, and base and branches of claw 1 and 4) provided in the redescription of this species by the same authors. Additionally, we observed presence of the minute dot-like septula at the end of the placoids row (Figure S3k). A morphometric analysis is necessary to evaluate the morphological variation of this species and confirm the specific identity of the specimens.
Hypsibius pallidus is a member of convergens species group [42,44]; this species sensu lato has been reported from North America, in Canada, Greenland, USA and Mexico [11,66]; and from South America, in Argentina, Bolivia and Chile [67].
Subfamily: Itaquasconinae Rudescu, 1964 [74].
Genus: Adropion Pilato, 1987 [34].
Adropion scoticum [75] (Murray, 1905) sensu lato.
Material examined: XXIX (41), XXXI (2), XL (3), XLII (2), and XLVII (3).
Remarks: The collected specimens showed the characters proposed by Murray [75] and Li and Liu [76]. Adropion scoticum sensu lato is a cosmopolitan complex of remarkably similar species, which needs an integrative taxonomic review [66]. Additionally, there is no known type material of Adr. scoticum sensu stricto [66,77]; therefore, a redescription is required. This species has been reported from North America, in Canada, Greenland and USA [66]; from Central America in Costa Rica [72]; and from South America in Argentina, Bolivia, Brazil, Chile, Colombia, Peru, and Uruguay [67]. In this study, we present a new record from Mexico, which expands its distribution into North America.
Subfamily: Pilatobiinae Bertolani, Guidetti, Marchioro, Altiero, Rebecchi, and Cesari, 2014 [7].
Genus: Pilatobius Bertolani, Guidetti, Marchioro, Altiero, Rebecchi, and Cesari, 2014 [7].
Pilatobius nodulosus (Ramazzotti, 1957) [78].
Material examined: XX (7), XXX (2), XXXI (7), XXXIII (4), XXXIV (4), and XXXV (8).
Remarks: All collected specimens display the characters mentioned for this species in Ramazzotti [78]. Pilatobius nodulosus has been recorded from North America, in Canada, USA, and Mexico [66,72]. The Mexican record corresponds to Beasley [14], in the Popocatépetl volcano, near the tree line in open pine forest, and in temperate mountains in northern Mexico [15].
Family: Macrobiotidae Thulin, 1928 [79].
Genus: Macrobiotus Schultze, 1834 [80].
Macrobiotus spp.
Material examined: IV (2), XV (1), XLV (2), XLVI (7), XLIX (1), L (1), and LI (10).
Remarks: Based on the revision of the hufelandi group by Kaczmarek and Michalczyk [37], two groups of specimens were recognized based on the presence of two types of oral cavity armature (OCA): the maculatus and the patagonicus types, in the maculatus morphotype only the third band of teeth could be observed (Figure S6b,c), while in the patagonicus morphotype the second and the third bands of teeth can be recognized (Figure S6f,g). Within both groups, the specimens showed conspicuous morphological differences (Figure S6a,e), in the sculpture of the cuticle, size of the claws, and presence and ornamentation of lunules (Figure S6d,h). This suggests the occurrence of more than one species sampled.
Species identification can only be made through observation of the eggs; unfortunately, the eggs found could not be identified at a specific level, due to their poor condition.
Genus: Minibiotus Schuster, 1980 [55].
Minibiotus sidereus Pilato, Binda and Lisi, 2003 [49].
Material examined: XX (4), XXX (4), XXXIII (5 and 5 eggs), XL (10), XLVIII (15), LIII (7 and 5 eggs), and LIV (6).
Remarks: All collected specimens show the characters of Min. sidereus proposed by Pilato et. al. [49]. Cuticle with three types pores (“pearls”, sensu Pilato et. al. [49]): circular, elliptical, and star-shaped pores (Figure S7b–d). The largest star-shaped pores are visible on the head and on the legs; prominent large star-shaped pores, with many arms (5–7) are present on each leg of the fourth pair, which should be noted as a specific character (Figure S7g).
Minibiotus sidereus has only been recorded in Ecuador [49]; therefore, the specimens described in this study are the first record from Mexico and North America.
Minibiotus citlalium sp. nov. Dueñas-Cedillo and García-Román
(Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8 and Figure 9 Table 2, Table 3 and Table 4)
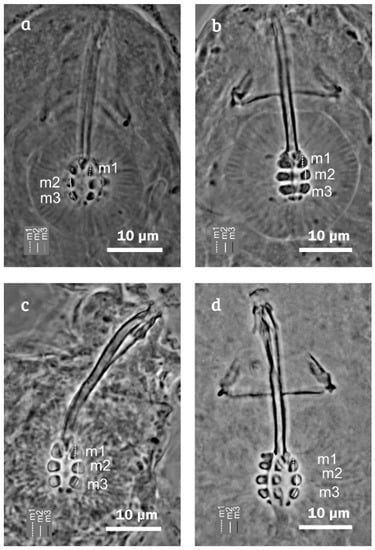
Figure 2.
Minibiotus citlalium sp. nov. bucco-pharyngeal apparatus. (a) Paratype 2 (dorsal view, phase contrast microscopy (PCM)); (b) paratype 11 (dorsal view, PCM); (c) paratype 10 (dorsal view, PCM); and (d) paratype 12 (dorsal view, PCM), m1 macroplacoid 1, m2 macroplacoid 2, and m3 macroplacoid 3.
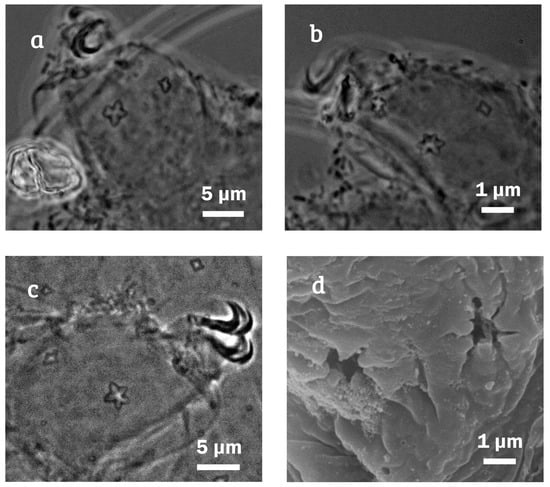
Figure 3.
Minibiotus citlalium sp. nov. pair of star-shaped pores, one of them conspicuously larger. (a) Leg II, paratype 1 (PCM); (b) leg III, paratype 1 (PCM); (c) leg II, paratype 11 (PCM); and (d) leg III with both star-shaped pores on external surface (SEM).
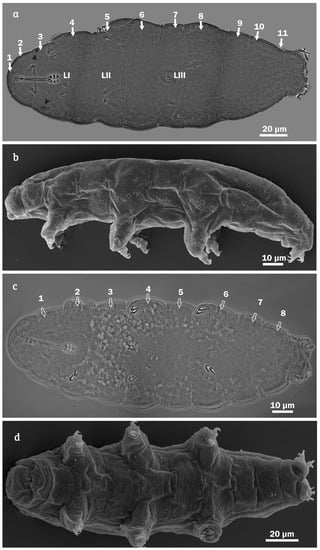
Figure 4.
Minibiotus citlalium sp. nov. habitus. (a) Dorsal view, showing 11 transverse rows of pores (holotype, PCM), full arrows indicate the 11 transverse rows of pores (dorsal view); LI leg I, LII leg II, LIII leg III, black arrowhead indicate eyes spots; (b) lateral view (SEM); (c) ventral view, showing eight transverse rows of pores (holotype, PCM), empty arrows indicate the eight transverse rows of pores (ventral view); and (d) ventral view (SEM).
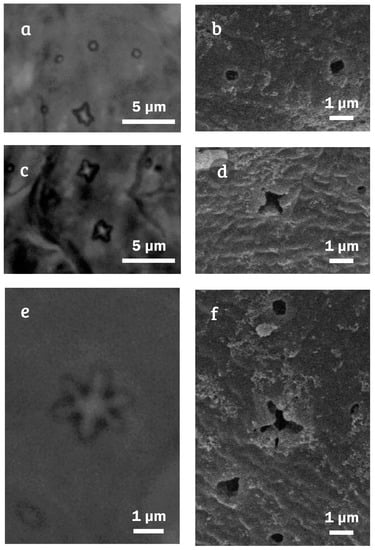
Figure 5.
Minibiotus citlalium sp. nov. pores type on cuticle: (a) dorsal cuticle with rounded pores (holotype, PCM); (b) dorsal cuticle with rounded pores, with (SEM); (c) dorsal cuticle with multi-lobated (4 tips) pores (holotype, PCM); (d) dorsal cuticle with multi-lobated (4 tips) pores (SEM); (e) dorsal cuticle with star-shaped (5–6 tips) pores (holotype, PCM); and (f) dorsal cuticle with star-shaped (5–6 tips) pores (SEM).
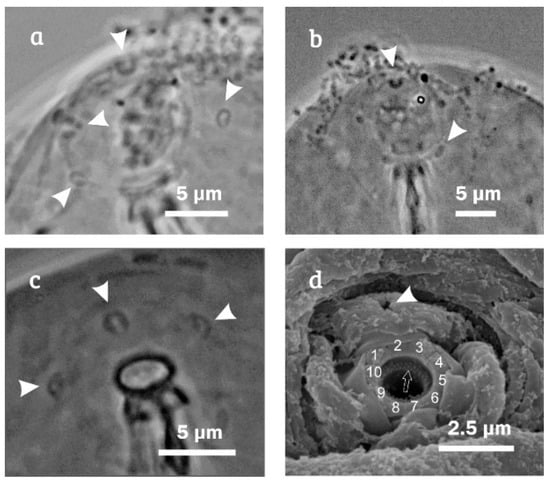
Figure 6.
Minibiotus citlalium sp. nov. detail of the buccal opening with five pores around mouth: (a) paratype 15 (PCM); (b) paratype 13 (PCM); (c) holotype (PCM), white arrowhead indicates the buccal pores; and (d) detail of the buccal opening (ventral view, SEM) showing a buccal pore, the peribuccal papulae are show numbered 1 to 10, empty arrow indicates the teeth.
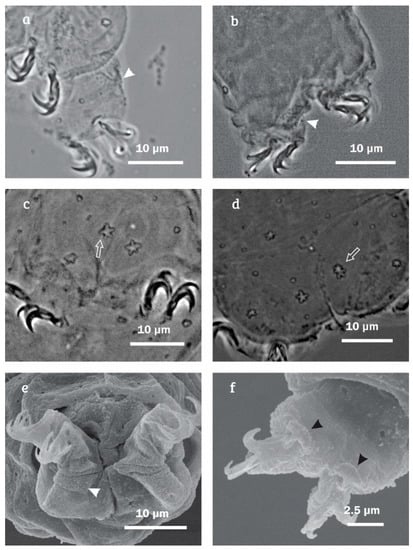
Figure 7.
Minibiotus citlalium sp. nov. detail of legs IV: (a) granulation (paratype 14, PCM); (b) granulation (paratype 5, PCM); (c) star-shaped pores (paratype 13, PCM; (d) star-shaped pores (holotype, PCM, empty arrow indicate the pair of star-shaped pores); (e) granulation on dorsal surface (SEM, white arrowheads indicate granulation); and (f) claw IV with smooth lunules (SEM, black arrowheads).
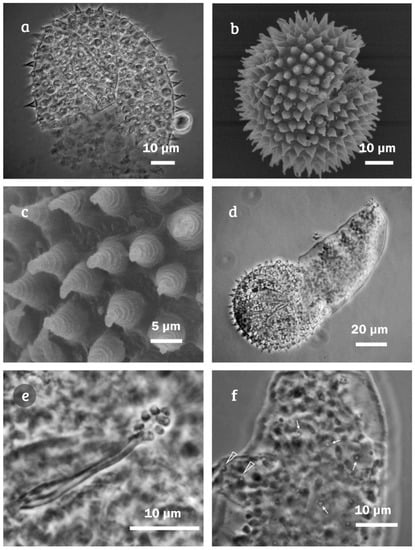
Figure 8.
Minibiotus citlalium sp. nov. eggs: (a) egg surface (PCM); (b) egg surface (SEM); (c) egg processes showing four annulations (SEM); (d) hatching specimen (PCM); (e) detail of the embryo showing buccal apparatus; and (f) detail of cuticle of hatching specimen, arrows indicates a star-shaped pores, a pair of star-shaped pores, one of them conspicuously larger.
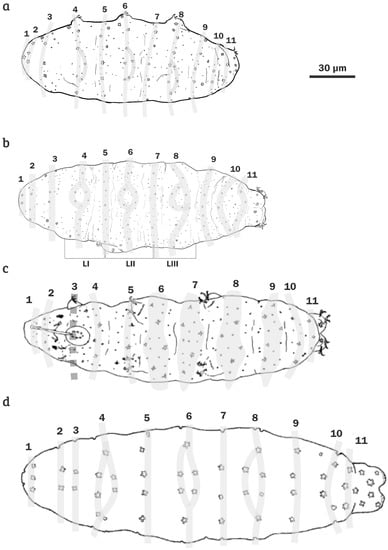
Figure 9.
A semi-schematic drawing of a dorsal positioned animal showing a pattern of the dorsal cuticle of star-shaped pores arranged in 11 transverse rows (Arabic numbers), which become double in the segments of the legs I–III: (a) Min. citlalium sp. nov.; (b) Min. constellatus; (c) Min. sidereus; and (d) Min. pentannulatus. LI = segment of leg I, LII: segment of leg II, and LIII = segment of leg III.

Table 3.
Number of star-shaped pores of four and five tips, the most abundant, on dorsal cuticle of Minibiotus citlalium sp. nov. mounted in polivinil lactofenol medium. The individuals measured correspond to type series (N—number of specimens measured, RANGE refers to the smallest and the largest structure among all measured specimens; and SD—standard deviation).

Table 4.
Measurements [in µm] of selected morphological structures of eggs of Minibiotus citlalium sp. nov. mounted in polivinil lactofenol medium (N—number of eggs/structures measured, RANGE—refers to smallest and largest structure among all measured specimens; and SD—standard deviation).
Diagnosis
Macroplacoid length sequence 1 ˃ 2 = 3 (Figure 2a–d); abundant pores (rounded and star-shaped), more than 250 on the entire dorsal cuticular surface. Each leg with two star-shaped pores on external surface, one of them conspicuously larger; the larger ones three times larger than the smaller ones (Figure 3a–d). Under PCM egg processes with inconspicuous ornamentation, and under SEM egg processes with four or five barely visible annulations (see egg section).
Type Locality
Iztaccíhuatl volcano, 19°05′14″ N, 98°40′03″ W, ca. 3498 m asl, Abies religiosa and Cupressus lusitanica Mill. forest, Cañada El Paraje, moss on tree bark, January 2018, coll. Dueñas-Cedillo and Armendáriz-Toledano.
Type Material and Type Repository
Holotype (slide: TAR/001), 15 paratypes (slides: TAR/002–TAR/16) and 14 simplex specimens. Holotype and nine paratypes (hatched animals) and two eggs (one of them hatching) were deposited in the Colección Nacional de Insectos, Departamento de Zoología, Instituto de Biología, Universidad Nacional Autónoma de México. Additionally, six paratypes and one egg were deposited in Laboratorio de Ecología, Departamento de Zoología, Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional.
Etymology
The specific epithet citlalium is a substantive in genitive that refers to the Náhuatl word citlali, meaning star, due to the presence of star-shaped pores throughout the body. The Náhuatl is an original language spoken by ancient Mexicans in central Mexico from the fifth century AD to present.
Description of Holotype
Measurements and statistics are shown in Table 2. Animals: Body length 142.4 μm [507] (Figure 4), eye spots visible in three specimens after fixation in PVA medium (Figure 4a). The entire cuticle is smooth and exhibits numerous pores (including the legs) with variable shape (Figure 4), such as rounded (Figure 5a,b), multi-lobated (4 tips) (Figure 5c,d) and star-shaped (5–6 tips) (Figure 5e,f). In the holotype, up to 278 pores on the entire dorsal cuticular surface were observed (Figure 4a). Additionally, pores increasing in abundance from anterior (48 pores) to the posterior (116 pores) portions of the body (Figure 4a,b); 83% of the pores correspond to rounded, 10% to multi-lobated (4 tips), and 7% to star-shaped (5–6 tips); the abundance of last two pores types are from 6–10 and from 2–10 respectively (Table 3). The rounded ones are smaller (0.41–0.86 µm; Figure 5a,b) than the multi-lobated (0.92–2.38 µm; Figure 5c,d) or star-shaped ones (2.19–3.5 µm; Figure 5e,f). The star-shaped pores are larger at the cephalic and caudal regions of the body, these star-shaped pores are arranged in the cuticle in 11 transverse rows, which become double in the segments of the legs I–III (Figure 4). In the ventral region, the star-shaped pores are less numerous than in the dorsal region and are distributed in eight transversal rows from segment of legs I up to before legs IV (Figure 4c,d).
Bucco-pharyngeal apparatus of the Minibiotus type, with five pores around the mouth and ten peribuccal papulae (Figure 6a–c). Oval pharyngeal bulb with triangular apophyses (near to first macroplacoid and about the same size), three macroplacoids and a small microplacoid. Macroplacoid shapes were drop-shaped (m1), granular (m2), and granular or almost quadrangular (m3); macroplacoid length sequence 1 ˃ 2 = 3 (Figure 2; Table 2). The oral cavity armature is composed of two bands of teeth, which are not visible under PCM and at least the first band of teeth is well visible under SEM (Figure 6d).
Legs show no cuticular bars and other thickenings (Figure 4); each leg with both small and large star-shaped pores on external surface, the larger ones three times larger than the smaller ones (Figure 3). Fine granulation exclusive on legs IV, on dorsal surface (Figure 7a,b,e) and a large star-shaped pore (5–6 tips) present in each leg (Figure 7c,d), with diameter of 2.4 µm in the holotype. Claws are short and robust, like the hufelandi type with conspicuous accessory points (Figure 7a–f). Lunules under all claws with smooth margins (Figure 7f).
Eggs. (measurements and statistics in Table 4): freely laid, colorless, spherical, smooth surface (Figure 8a,b) under PCM processes with inconspicuous ornamentation, only visible in few instances; if visible, it showed between three to four annulations (Figure 8a), under SEM processes with four annulations, five in some cases barely visible (Figure 8c); processes longer than width (37–57%), tipped processes flexible (1.53–2.08 µm, Figure 8a,b), and entire base process (Figure 8c). Among the eggs, one from a specimen hatching was observed (Figure 8d). On this individual, the oral apparatus and the star-shaped pores were observed (Figure 8e), as well as a pair of star-shaped pores, one of them conspicuously, on external surface of the legs III (Figure 8f), both diagnostic characters of Minibiotus citlalium sp. nov.
Differential diagnosis.
By the presence of star-shaped pores in the cuticle, Minibiotus citlalium sp. nov (Figure 9a) is similar to M. pseudostellarus, M. eichhorni, M. constellatus, M. sidereus and Min. pentannulatus, but differs from the first two species by the presence of eleven transverse rows of star-shaped pores, which are also present in M. constellatus (Figure 9b), M. sidereus (Figure 9c) and Min. pentannulatus (Figure 9d). The star-shaped pores in M. pseudostellarus are randomly distributed [81], and in M. eichhorni are arranged in six transverse rows [82]. The new species differs specifically from M. constellatus, M. sidereus, and M. pentannulatus by two attributes, (1) different macroplacoid sequence 1 ˃ 2 = 3, and (2), each leg (I–III) with two star-shaped pores on external surface, one of them conspicuously larger; the larger ones three times larger than the smaller ones. In M. constellatus the macroplacoid sequence is 1 ˃ 2 ˃ 3, in M. sidereus and M. pentannulatus is 1 ˃ 2 < 3; in M. constellatus each leg with almost two star-shaped pores of similar size, in M. sidereus about three star-shaped pores in each leg, and in M. pentannulatus almost four star-shaped pores in each leg. Additionally, Min. citlalium sp. nov. differs from:
(1) Minibiotus constellatus, only recorded from Peru [51], by absence of granulation on legs I–III (granulation present on all legs in M. constellatus).
(2) Minibiotus sidereus, only recorded from Ecuador [49], by shorter placoid row (5.9–9.0 μm (26.5–33.4) in M. citlalium sp. nov. vs. 9.6 μm (36.4) in M. sidereus); smaller star-shaped pores of legs IV (2.9 μm in M. citlalium sp. nov. vs. 6.4 μm in M. sidereus); smaller diameter egg including processes (57.4–66.0 μm in M. citlalium sp. nov. vs. 73–83.5 μm in M. sidereus) and excluding them (48.8–60.8 μm in M. citlalium sp. nov. vs. 62–69 μm in M. sidereus); shorter processes (4.4 μm in M. citlalium sp. nov. vs. up to 9.5 μm in M. sidereus), inconspicuous annulations or absent on most processes. When these are present, 3–4 can be recognized in M. citlalium sp. nov., while 6–7 conspicuous annulations are present on the most processes in M. sidereus.
(3) Minibiotus pentannulatus, recorded from Colombia [54] and Tanzania [83] by the pattern of star-shaped pores arranged in 11 transverse rows (double in the segments of the legs I–III) was present in all specimens, which ranged from 142.0–250.0 µm in length, whereas in M. pentannulatus the pattern of star-shaped pores can only be recognized in the smaller animals from 144–203 µm. Minibiotus citlalium sp. nov. also differ from M. pentannulatus by the pt of some claws, as follows: of the claw 1, by lower pt of the external primary branch (11.8–20.7 in M. citlalium sp. nov. vs. 21.3–24.8 in Min. pentannulatus); of the claw 2, by lower pt of the internal primary branch (14.8–21.5 in M. citlalium sp. nov. vs. 22.1–26.0) in M. pentannulatus, also of the claw 2 by lower pt of the internal secondary branch (10.3–17.9 in Min. citlalium sp. ov. vs. 18.0–21.2 in M. pentannulatus); and of claw 4 by lower pt of the anterior primary branch (14.0–25.8 in M. citlalium sp. nov. vs. 26.2–29.2 in M. pentannulatus), also of claw 4 by lower pt of the posterior primary branch (13.2–26.3 in M. citlalium sp. nov. vs. 27.4–28.9 in M. pentannulatus); egg with smaller diameter with processes (60.9 μm in M. citlalium sp. nov. vs. 74.5 μm in M. pentannulatus), and by the pattern of annulations in the processes (see comparison with M. sidereus), 4 to 6 conspicuous annulations are present on the processes in M. pentannulatus.
Stec et. al. [83] registered a population of Minibiotus pentannulatus in Tanzania; these authors add characters to those proposed by Londoño et. al. [54] for M. pentannulatus. These characters also support the differences between this species and M. citlalium sp. nov. These characters are granulation on legs I to IV, differ from M. citlalium sp. nov. which only presents granulation on legs IV (Figure 7a–c); M. pentannulatus presents up to three star-shaped pores of different size, on external surface of legs I–III, the smaller star-shaped pore always present in the centre of the above mentioned granulation patch, meanwhile M. citlalium sp. nov. present a pair of star-shaped pores (one smaller than the other one on external surface of legs I– III), and both pores are above smooth cuticle (Figure 3); and in M. pentannulatus a cuticular fold with a pore in the centre is present just above the mouth opening and visible well under PCM (only in laterally positioned specimens) and under SEM; meanwhile, in M. citlalium sp. nov. five pores around the mouth are present (Figure 6).
Size effect on morphometric data.
Linear regressions of 19 out of 26 continuous body traits of Minibiotus citlalium sp. nov. showed significant correlation with BTL (Supplementary Material Table S1), most of them with r2 values higher than 0.8. Placoid lengths were uncorrelated with BTL. Of the 19 correlated traits, t-tests from slopes supported that 12 were isometric (slope value not significantly different from 1) and seven were allometric (slope value significantly different from 1) (SM Table S1), the relationship between the trait (Y) and BTL (=body size) (i.e., b-slope) and theoretical values of traits adjusted to mean BTL (Y-intercept) of this species are provided in SM Table S1.
4. Discussion
As noted in the introduction, the tardigrades of Mexico require more extensive taxonomic studies, and currently, only a few faunistic surveys have been published (e.g., [7,13,14,15,16,17,18,19,20,21,22]). Most of the previous studies have provided an inventory of tardigrade fauna but from non-systematic samplings. Moreover, the number and size of samples and collection sites were rarely mentioned.
In the present study, from a systematic sampling of 57 moss samples, along an altitudinal gradient, including three moss substrates and four vegetation types, five tardigrade species were recorded (Dip mitrense, Dip. pingue, Adr scoticum, Pil. nodulosus, Min sidereus), three putative species could be associated (i.e., records with insufficient data to complete the identification: Cal cf. ornatus, Hys cf. microps, Hys. cf. pallidus, and Macrobiotus spp.), and a new taxon Min. citlalium sp. nov., was described. For each of these taxa, description and illustrations were provided.
Geographic records
Our study confirms the presence of Diphascon pingue and Pilatobius nodulosus species previously recorded in Mexico and provides three new records in the country expanding their distribution in America and in some cases connecting the previous records among North, Central and South America. The presence of Dip. mitrense, Min. sidereus, and Adr. scoticum in Iztaccíhuatl volcano constitute three new Tardigrada records from Mexico, the first two species also new records for North America. Diphascon mitrense and Min. sidereus had only been recorded in their respective type localities, Argentina, and Ecuador [48,49]; therefore, this study provides the second record in America for both taxa, expanding their distribution to North America. On the other hand, Adr. scoticum record connects the previous records in North America [66], with Central and South America [67,72]. Iztaccíhuatl records of Diphascon pingue and Pil. nodulosus demonstrate their occurrence in Mexico, since both taxa have been already recorded from Nuevo León state, [14,15] and in the Sierra Nevada in Popocatépetl volcano [14], respectively.
Additionally, the majority of examined species (Cal. cf. ornatus, Hys. cf. microps, Hys. cf. pallidus) belong to taxonomically difficult groups, or in some cases to species complexes as Hys. dujardini and Dip. pingue. These species groups and complex groups display high levels of morphological intra-specific variation from apparently wide geographic ranges in America and other areas around the world [66,67,72], which makes it difficult to interpret the records of this survey in a geographical context. Further studies considering both morphological and molecular data will clarify the taxonomic identity and distribution of their members.
Tardigrades community.
As mentioned above, Popocatépetl and Iztaccíhuatl volcanoes are the main physiographic elements of the Sierra Nevada; they present similar environmental conditions, landscapes, climate, seasonal rain regime, and same vegetation types [24]. Given these common characteristics and geographic proximity of both volcanoes, a similar species composition pattern between Iztaccíhuatl and that recorded previously for Popocatepetl [14] was expected. Although we found an equivalent number of species in both volcanoes, eight in Popocatépetl (Echiniscus kerguelensis, Minesium tardigradum, Macrobiotus echinogenitus, Mac. furcatus, Mac. hufelandi, Ramazottius baumanni, Ram. oberhaeuseri, and Pilatobius nodulosus), and five and three putative species in Iztaccíhuatl volcano, the species composition was dramatically different, because only one species, Pil. nodulosus, was present in both volcanoes. In the present study, Pil. nodulosus was found in moss growing on tree bark and soil, from 3400 to 4000 m asl, across the Abies religiosa and Pinus hartwegii forests. In the Popocatépetl volcano, it was found in the lichen Pseudevernia intensa (Nyl.) Hale and W. L. Culb., growing on bark at 4000 m asl, near the timberline in open Pinus hartwegii forest. This species has already been registered in Pinus and Abies forests, in soil, lichen, leaf litter, and moss growing on bark, and soil in North America [66,71]). Unfortunately, biogeographic or biological comparisons cannot be made because most of the records documented for the Popocatépetl are currently considered doubtful by other authors, as they were supported by a single collection event and the identification was based on the original descriptions available, which in turn was vague and barely detailed [10,14,37,84,85,86]. Despite that the taxa recorded in the Popocatépetl have been found in different regions of the world [71], recent studies based on morphological and molecular data have shown that they belong to taxonomically difficult species complexes [37,44,87,88,89].
To adequately compare the tardigrades from both volcanoes, it would be necessary to carry out an appropriate sampling in the Popocatépetl volcano and include both morphological and molecular data. This will allow the clarification of its tardigrade diversity and to propose additional ecological studies that include environmental variables to understand the differences in tardigrade communities, since it is common that tardigrade assemblages display ecological structure across mountain ranges. For example, in the Sierra de Guadarrama (Spain), Guil et. al. [27] found that the richness and abundance of tardigrades were associated with environmental variables at both macro (altitude, vegetation structure, climate, and soil characteristics) and micro (leaf litter type and moss weight) scales.
Systematic considerations.
Minibiotus citlalium sp. nov. share with Min. constellatus, Min. sidereus and Min. pentannulatus abundant star-shaped pores in anterior and posterior parts of the body, the distribution pattern of these ornamentations in dorsal cuticle arranged in eleven rows, and similar morphology of eggshell with ringed processes. They also share a holotropical distribution in American and African continent: Min. constellatus recorded in Peru, Min. sidereus in Ecuador and Mexico, and Min. pentannulatus in Colombia and Tanzania. The above characteristics support that these taxa conform a species-group, within which Minibiotus citlalium sp. nov. and Min. sidereus are the most similar in morphology; they present larger stars in the anterior and posterior regions of the body, show a clearly larger pair of stars in the fourth pair of legs, as well as a very similar eggshell. Furthermore, in the present study both species were found in the same samples, so apparently, they also share habitat characteristics. A phylogenetic reconstruction via molecular markers will allow help to clarify their evolutionary relationships.
Allometry in morphometric traits of Min. citlalium sp. nov.
In Eutardigrada taxonomy, many continuous traits display correlations with body size [90,91,92]. A considerably proportion of these characters grow proportionally with this trait (i.e., isometric traits), while in others the growth is not proportional (i.e., allometric ones), which in turn makes them unsuitable for taxonomic purposes [93]. As in other Eutardigrada members that display many allometric quantitative traits with respect to BTL [60], our regression analyses of morphometric data from Min. citlalium sp. nov., support that seven traits were allometric and 11 traits were isometric relative to BTL (SM Table S1), indicating that this trend is extensive in eutardigrades.
Ratios (pt indexes) are widely used to eliminate body size effects [33,92,93]; however, their use only successfully eliminate this effects in traits that increase proportionally to body size, but not in those that increase unproportionally (i.e., allometric ones) [59]. To overcome this problem, in this study a protocol [60] that provide parameters from regressions was performed, to obtain the slope (b) and Y intercept (a) in each analyzed trait. This in turn will allow to perform Thorpe normalization and to obtain size-effect free traits, independently of their correlation trend respect to body size (isometric or allometric).
5. Conclusions
Based on a systematic sampling across a multi-habitat gradient in a temperate mountain of the Trans Mexican Volcanic Belt, we found five tardigrade species, three putative species, one record to genus level, and a new species Min. citlalium sp. nov., which we described. Of them, three are new records for Mexico: Dip. mitrense, Adr. scoticum and Min. sidereus (the first two new for North America). This raises the current number of tardigrade taxa in Mexico from 56 to 61, and in the temperate mountains of the Trans Mexican Volcanic Belt from 8 to 13.
Supplementary Materials
The following are available online at https://www.mdpi.com/1424-2818/12/7/271/s1. Figure S1. Calohypsibius cf. ornatus. a—habitus (latero-dorsal view), showing eight transversal parallel rows of cuticular spines (sp spines, rw row); b—buccal apparatus of the Calohypsbius type, with one bend in the posterior portion. Figure S2. Diphascon mitrense and Dip. pingue. a—Dip. mitrense habitus, b—bucco-pharyngeal apparatus, c—Dip. pingue habitus, and d—bucco-pharyngeal apparatus (buccal tube length, macroplacoid row, first macroplacoid, m2 s macroplacoid, m3 third macroplacoid, and white arrow indicates thickening drop shape). The length of the buccal tube is expressed as a percentage on a vertical line. It also stands for length comparison to other structures. Figure S3. Hypsibius cf. dujardini, Hys. cf. exemplaris, Hys. cf. microps and Hys. cf. pallidus. a—Hys. cf. dujardini habitus, b—buccal apparatus, c—claws IV; d—Hys. cf. exemplaris habitus, e—buccal apparatus, f—claws III; g—Hys. cf. microps habitus, h—buccal apparatus, i—claws IV; j—Hys. cf. pallidus habitus, k—buccal apparatus, and l—claws IV. The white arrow indicates the minute dot-like septula at the end of the placoids row. Figure S4. Adropion scoticum. a—habitus, b—bucco-pharyngeal apparatus, c—claws I–II; d—claws III, and e—claws IV. (ph pharynx length, m1 first macroplacoid, m2 s macroplacoid, m3 third macroplacoid, cI claws I, cII claws II, cIII claws III, cIV claws IV, and white arrow indicates cuticular bars on legs I–III). Figure S5. Pilatobius nodulosus. a—habitus, b—bucco-pharyngeal apparatus, c—posterior part of the body. (ph pharynx length, m1 first macroplacoid, m2 s macroplacoid, m3 third macroplacoid, tb tubercles, and white arrow indicates thickening drop shape). Figure S6. Macrobiotus sp. a—habitus, b—buccal apparatus, c—oral cavity armature maculatus type, d—claws IV of hufelandi type; Macrobiotus sp. e—habitus, f—buccal apparatus, g—oral cavity armature patagonicus type, h—claws IV of hufelandi type. White arrow indicates II and III band of teeth. Figure S7. Minibiotus sidereus. a—habitus (dorsal view), b—cuticular small circular pores, c—large star-shaped pores with 3–8 arms (4–5 most common), d—big star-shaped pore in each leg of fourth pair, e—buccal apparatus of Minibiotus type, macroplacoid length sequence 1 ˃ 2 < 3, f—cuticular bar on leg III, g,h—egg, i processes. (m1 first macroplacoid, m2 s macroplacoid, m3 third macroplacoid, white arrow indicates cuticular bar). Table S1. Results of linear regressions of morphometric characters measured in relation to buccal tube length in Minibiotus citlalium sp. nov. Data log–log transformed. N = sample size; b = slope; a * = Y intercept; r2 = coefficient of correlation; p (r2) = probability associated to “r”; t = Student’s T test for slopes; p(t) = probability that b differs from slope of 1; and traits with p < 0.05 are allometric and are indicated in bold.
Author Contributions
Conceptualization, F.A.-T. and E.A.R.; methodology, A.D.-C., E.M.-M., J.G.-R., F.A.-T., and E.A.R.; software, A.D.-C., J.G.-R., and F.A.-T.; validation, A.D.-C., F.A.-T., and E.A.R.; formal analysis, A.D.-C., J.G.-R., and F.A.-T.; investigation, A.D.-C., J.G.-R., and F.A.-T.; resources, F.A.-T. and E.A.R.; data curation, A.D.-C. and F.A.-T.; writing—original draft preparation, A.D.-C., J.G.-R., E.M.-M., and F.A.T.; writing—review and editing, F.A.-T. and E.A.R.; visualization, A.D.-C., J.G.-R., and F.A.-T.; supervision, F.A.-T. and E.A.R.; project administration, F.A.-T. and E.A.R.; and funding acquisition, F.A.-T. and E.A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by 2018SIP-IPN, 20195761 and by Annual Operating budget IB-UNAM 2019.
Acknowledgments
We thank to Carlos Briones and Monsterrat Cervantes for field assistance in collecting samples. We also thank Pedro Mercado Ruaro from the Laboratorio de Botánica Estructural, for phase contrast photographs, and to María Berenit Mendoza Gárfias from Laboratorio de Microscopía Electrónica for the SEM micrographs, both from the Laboratorio Nacional de Biodiversidad (LaNaBio), Instituto de Biología. Universidad Nacional Autónoma de México. We thank Joseph Heras, Andres Aguilar and Łukasz Kaczmarek for valuable comments on earlier versions of the manuscript. A.D.-C. (496686) and J.G.-R. (617368) were Consejo Nacional de Ciencia y Tecnología fellowships. All the biological material studied, was collected using the license for scientific collection, for researchers and scientific collectors linked to research institutions FAUT-0352” (Secretaria de Medio Ambiente y Recursos Naturales—SEMARNAT).
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Nelson, D.R.; Marley, N.J. The biology and ecology of lotic Tardigrada. Freshw. Biol. 2000, 44, 93–109. [Google Scholar] [CrossRef]
- Nelson, D.R.; Bartels, P.J.; Guil, N. Tardigrade Ecology. In Water Bears: The Biology of Tardigrades; Schill, R.O., Ed.; Springer Nature: Basel, Switzerland, 2018; pp. 163–210. [Google Scholar]
- Nelson, D.R. Current status of the Tardigrada: Evolution and ecology. Integr. Comp. Biol. 2002, 42, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.A. Small-scale spatial distribution variability in terrestrial tardigrade populations. Hydrobiologia 2006, 558, 133–139. [Google Scholar] [CrossRef]
- Degma, P.; Katina, S.; Sabatovicová, L. Horizontal distribution of moisture and Tardigrada in a single moss cushion. J. Zool. Syst. Evol. Res. 2011, 49, 71–77. [Google Scholar] [CrossRef]
- Bertolani, R.; Rebecchi, L.; Giovannini, I.; Cesari, M. DNA barcoding an integrative taxonomy of Macrobiotus hufelandi C.A.S. Schultze 1834, the first tardigrade species to be described, and some related species. Zootaxa 2011, 2997, 19–36. [Google Scholar] [CrossRef]
- Bertolani, R.; Guidetti, R.; Marchioro, T.; Altiero, T.; Rebecchi, L.; Cesari, M. Phylogeny of Eutardigrada: New molecular data and their morphological support lead to the identification of new evolutionary lineages. Mol. Philogenet. Evol. 2014, 76, 110–126. [Google Scholar] [CrossRef]
- Gąsiorek, P.; Morek, W.; Stec, D.; Blagden, B.; Michalczyk, Ł. Revisiting Calohypsibiidae and Microhypsibiidae: Fractonotus Pilato, 1998 and its phylogenetic position within Isohypsibiidae (Eutardigrada: Parachela). Zoosystema 2019, 41, 71–89. [Google Scholar] [CrossRef]
- Mayo, S.J.; Allkin, R.; Baker, W.; Blagoderov, V.; Brake, I.; Clark, B.; Govaerts, R.; Godfray, C.; Haigh, A.; Hand, R.; et al. Alpha e-taxonomy: Responses from the systematics community to the biodiversity crisis. Kew Bull. 2008, 63, 1–16. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Diduszko, D.; Michalczyk, Ł. New records of Mexican Tardigrada. Rev. Mex. Biodivers. 2011, 82, 1324–1327. [Google Scholar] [CrossRef]
- Ramazzotti, G.; Maucci, W. Il Phylum Tardigrada. Terza edizione riveduta e corretta. Mem. Ist. Ital. Idrobiol. Dott. Marco Marchi 1983, 41, 1–1012. [Google Scholar]
- Challenger, A.; Soberón, J. Los ecosistemas terrestres, In Capital Natural de Mexico, Vol. I: Conocimiento Actual de la Biodiversidad; Koleff, P., Sarukhán, J.R., Eds.; Conabio: Mexico City, Mexico, 2018; pp. 87–108. [Google Scholar]
- Heinis, F. Beitrag zur Kenntnis der zentral americanischen Moosfauna. Rev. Suisse Zool. 1911, 19, 253–266. [Google Scholar] [CrossRef]
- Beasley, C.W. Some tardigrades from Mexico. Southwest. Nat. 1972, 17, 21–29. [Google Scholar] [CrossRef]
- Moreno-Talamantes, A.; Roszkowska, M.; García-Aranda, M.A.; Flores-Maldonado, J.J.; Kaczmarek, Ł. Current knowledge on Mexican tardigrades with a description of Milnesium cassandrae sp. nov. (Eutardigrada: Milnesiidae) and discussion on the taxonomic value of dorsal pseudoplates in the genus Milnesium Doyère, 1840. Zootaxa 2019, 4691, 501–524. [Google Scholar] [CrossRef] [PubMed]
- Pilato, G.; Lisi, O. Notes of some tardigrades from southern Mexico with descriptions of three new species. Zootaxa 2006, 1236, 53–68. [Google Scholar] [CrossRef]
- Schuster, R.O. Tardigrada from the Barranca del Cobre, Sinaloa y Chihuahua, Mexico. Biol. Soc. Wash. 1971, 84, 213–224. [Google Scholar]
- May, R.M. Nouveau genre et espéce de tardigrade du Mexique: Haplomacrobiotus hermosillensis. Bull. Soc. Zool. France 1948, 73, 95–97. [Google Scholar]
- Moreno-Talamantes, A.; Roszkowska, M.; Ríos-Guayasamín, P.; Flores-Maldonado, J.J.; Kaczmarek, Ł. First record of Dactylobiotus parthenogeneticus Bertolani, 1982 (Eutardigrada: Murrayidae) in Mexico. Check List 2015, 11, 1723. [Google Scholar] [CrossRef]
- Pérez-Pech, W.A.; Anguas-Escalante, A.; Cutz-Pool, L.Q.; Guidetti, R. Doryphoribius chetumalensis sp. nov. (Eutardigrada: Isohypsibiidae) a new tardigrade species discovered in an unusual habitat of urban areas of Mexico. Zootaxa 2017, 4344, 347–352. [Google Scholar] [CrossRef]
- Beasley, C.W.; Kaczmarek, Ł.; Michalczyk, Ł. Doryphoribius mexicanus, a new species of Tardigrada Eutardigrada: Hypsibiidae from Mexico North America. Biol. Soc. Wash. 2008, 121, 34–40. [Google Scholar] [CrossRef]
- León-Espinosa, G.A.; Moreno-Talamantes, A.; Rodríguez-Almaraz, G.A. Ositos de agua (Tardigrada) de México: Los famosos desconocidos. Biol. Soc. 2019, 2, 61–70. Available online: https://issuu.com/biologiaysociedad/docs/biologiaysociedadn4/61 (accessed on 1 January 2020).
- Ferrari, L. Avances en el conocimiento de la Faja Volcánica Transmexicana durante la última década. Boletín de la Sociedad Geológica Mexicana 2000, 53, 84–92. [Google Scholar] [CrossRef]
- Cantellano de Rosas, E. Reconocimiento espacial de los paisajes. In Biodiversidad de la Faja Volcánica Transmexicana; Vega-Luna, I., Morrone, J.J., Espinosa, D., Eds.; Universidad Nacional Autónoma de Mexico; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Mexico City, Mexico, 2007; pp. 39–55. [Google Scholar]
- Morrone, J.J. Hacia una síntesis biogeográfica de Mexico. Rev. Mex. Biodivers. 2005, 76, 207–252. [Google Scholar] [CrossRef]
- Dastych, H. The tardigrada of Poland. Monogr Fauny Polski 1988, 16, 1–255. [Google Scholar]
- Guil, N.; Hortal, J.; Sánchez-Moreno, S.; Machordon, A. Effects of macro and micro-environmental factors on the species richness of terrestrial tardigrade assemblages in an Iberian mountain environment. Landsc. Ecol. 2009, 24, 375–390. [Google Scholar] [CrossRef]
- Nelson, D.R.; Bartels, P.J. “Smoky Bears”-Tardigrades of the Great Smoky Mountains National Park. Southwest. Nat. 2007, 6, 229–238. [Google Scholar] [CrossRef]
- Perry, E.; Miller, W.R.; Kaczmarek, Ł. Recommended abbreviations for the names of genera of the phylum Tardigrada. Zootaxa 2019, 4608, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Stec, D.; Morek, W.; Gąsiorek, P.; Michalczyk, Ł. Unmasking hidden species diversity within the Ramazzottius oberhaeuseri complex, with an integrative redescription of the nominal species for the family Ramazzottiidae (Tardigrada: Eutardigrada: Parachela). Syst. Biodivers. 2018, 16, 357–376. [Google Scholar] [CrossRef]
- Guidetti, R.; Bertolani, R. Tardigrade taxonomy: An updated check list of the taxa and a list of characters for their identification. Zootaxa 2005, 845, 1–46. [Google Scholar] [CrossRef]
- Meyer, H.A. The Terrestrial and Freshwater Tardigrada of Northeastern North America, with New Records from Maine. Notes Northeast. Nat. 2011, 4, 534–541. [Google Scholar] [CrossRef]
- Pilato, G. Analisi di nuovi caratterinello studio degli Eutardigradi. Animalia 1981, 8, 51–57. [Google Scholar]
- Pilato, G. Revision of the genus Diphascon Plate, 1889, with remarks on the subfamily Itaquasconinae (Eutardigrada, Hypsibiidae). In Biology of Tardigrades. Selected Symposia and Monographs; Bertolani, R., Ed.; Mucchi, U.Z.I.: Modena, Italy, 1987; Volume 1, pp. 337–357. [Google Scholar]
- Pilato, G.; Binda, M.G. A comparison of Diphascon (D.) alpinum Murray, 1906, D. (D.) chilenense Plate, 1889 and D. (D.) pingue Marcus, 1936 Tardigrada, and description of a new species. Zoologischer Anzeiger 1997, 236, 181–185. [Google Scholar]
- Dastych, H. A new species of the genus Macrobiotus Schultze, 1834 from Iles Kerguélen, the sub-Artic (Tardigrada). Mitteilungen Aus Dem Hamburgischen Zoologischen Mus. Und Institut 2002, 99, 11–27. [Google Scholar]
- Kaczmarek, Ł.; Michalczyk, Ł. The Macrobiotus hufelandi group (Tardigrada) revisited. Zootaxa 2017, 4363, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, Ł.; Cytan, J.; Zawierucha, K.; Diduszko, D.; Michalczyk, Ł. Tardigrades from Peru (South America), with descriptions of three new species of Parachela. Zootaxa 2014, 3790, 357–379. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, Ł.; Kaczmarek, Ł. The Tardigrada Register: A comprehensive online data repository for tardigrade taxonomy. J. Limnol. 2013, 72, 175–181. [Google Scholar] [CrossRef]
- Claxton, S.K. A revision of the genus Minibiotus Tardigrada: Macrobiotidae with descriptions of new species from Australia. Rec. Aust. Mus. 1998, 50, 125–160. [Google Scholar] [CrossRef]
- Fontoura, P.; Pilato, G. Diphascon (Diphascon) faialense sp. nov. a new species of Tardigrada Eutardigrada, Hypsibiidae from the Azores and a key to the species of the D. pingue group. Zootaxa 2007, 1589, 47–55. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Michalczyk, Ł. Redescription of Hypsibius microps Thulin, 1928 and H. pallidus Thulin, 1911 Eutardigrada: Hypsibiidae based on the type material from the Thulin collection. Zootaxa 2009, 2275, 60–68. [Google Scholar] [CrossRef]
- Gąsiorek, P.; Zawierucha, K.; Stec, D.; Michalczyk, Ł. Integrative redescription of a common Arctic water bear Pilatobius recamieri Richters, 1911. Polar Biol. 2017, 40, 2239–2252. [Google Scholar] [CrossRef]
- Gąsiorek, P.; Stec, D.; Morek, W.; Michalczyk, Ł. An integrative redescription of Hypsibius dujardini Doyère, 1840, the nominal taxon for Hypsibioidea Tardigrada: Eutardigrada. Zootaxa 2018, 44151, 45–75. [Google Scholar] [CrossRef]
- Binda, M.G.; Pilato, G. Minibiotus furcatus, nuova posizione sistematica per Macrobiotus furcatus Ehrenberg, 1985 e descrizione di due nouve specie (Eutardigrada). Catania 1992, 19, 111–120. [Google Scholar]
- Pilato, G.; Binda, M.G. Two new species of Diphascon (Eutardigrada) from New South Wales. N. Z. J. Zool. 1998, 25, 171–174. [Google Scholar] [CrossRef][Green Version]
- Pilato, G.; Binda, M.G. Three new species of Diphascon of the pingue group Eutardigrada, Hypsibiidae from Antarctica. Polar Biol. 1999, 21, 335–342. [Google Scholar] [CrossRef]
- Pilato, G.; Binda, M.G.; Qualtieri, F. Diphascon mitrense, new species of eutardigrade from Tierra del Fuego. Boll. Sedute Accad. Gioenia Sci. Nat. Catania 1999, 31, 101–105. [Google Scholar]
- Pilato, G.; Binda, M.G.; Lisi, O. Remarks on some species of tardigrades from South America with description of Minibiotus sidereus n. sp. Zootaxa 2003, 195, 1–8. [Google Scholar] [CrossRef]
- Michalczyk, Ł.; Kaczmarek, Ł. A description of the new tardigrade Macrobiotus reinhardti (Eutardigrada: Macrobiotidae, harmsworthi group) with some remarks on the oral cavity armature within the genus Macrobiotus Schultze. Zootaxa 2003, 331, 1–24. [Google Scholar] [CrossRef]
- Michalczyk, Ł.; Kaczmarek, Ł. Minibiotus constellatus, new species of Tardigrada from Peru (Eutardigrada: Macrobiotidae). Genus 2003, 14, 295–305. [Google Scholar]
- Miller, W.R.; McInnes, S.J.; Bergstrøm, D.M. Tardigrades of the Australian Antarctic: Hypsibius heardensis (Eutardigrada: Hypsibiidae: Dujardini group) a new species from sub-Antarctic Heard Island. Zootaxa 2005, 1022, 57–64. [Google Scholar] [CrossRef]
- Dastych, H. Redescription and revalidation of the Sub-Antarctic tardigrade Hypsibius murrayi (Richters, 1907) based on the rediscovered type material (Tardigrada, Panarthropoda). Entomol. Heute 2018, 30, 95–115. [Google Scholar]
- Londoño, R.; Daza, A.; Lisi, O.; Quiroga, S. New species of waterbear Minibiotus pentannulatus (Tardigrada: Macrobiotidae) from Colombia. Rev. Mex. Biodivers. 2017, 88, 807–814. [Google Scholar] [CrossRef]
- Schuster, R.O.; Nelson, D.R.; Grigarick, A.A.; Christenberry, D. Systematic criteria of Eutardigrada. Trans. Am. Microsc. Soc. 1980, 99, 284–303. [Google Scholar] [CrossRef]
- Pilato, G.; Binda, G. Definition of families, subfamilies, genera and subgenera of the Eutardigrada, and keys to their identification. Zootaxa 2010, 2404, 1–54. [Google Scholar] [CrossRef]
- Stec, D.; Smolak, R.; Kaczmarek, Ł.; Michalczyk, Ł. An integrative description of Macrobiotus paulinae sp. nov. (Tardigrada: Eutardigrada: Macrobiotidae: Hufelandi group) from Kenya. Zootaxa 2015, 4052, 501–526. [Google Scholar] [CrossRef] [PubMed]
- Pilato, G.; (Catania University, Catania, Italy); Michalczyk, Ł.; (Jagiellonian University, Kraków, Poland); Londoño, R.; (Magdalena University, Santa Marta, Colombia). Personal communication, 2019.
- Lleonart, J.; Salat, J.; Torres, G.J. Removing allometric effects of body size in morphological analysis. J. Theor. Biol. 2000, 205, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Bartels, P.J.; Nelson, D.R.; Exline, R.P. Allometry and the removal of body size effects in the morphometric analysis of tardigrades. J. Zool. Syst. Evol. Res. 2011, 49, 17–25. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice Hall: Englewood Cliffs, NJ, USA, 2008; pp. 1–929. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 1 June 2020).
- Pilato, G. Evoluzione e nuova sistemazione degli Eutardigrada. Boll. Zool. 1969, 36, 327–345. [Google Scholar] [CrossRef]
- Richters, F. Beitrage zur Kenntnis der Fauna der Umgebung von Frankfurt am Main. In Beitra ge zur Kenntnis der Fauna der Umgebung von Frankfurt a. M.; 1900; pp. 21–44. Available online: https://archive.org/details/cbarchive_105587_beitrgezurkenntnisderfaunaderu1900 (accessed on 8 July 2020).
- Michalczyk, Ł.; Kaczmarek, Ł. The first record of the genus Calohypsibius Thulin, 1928 (Eutardigrada: Calohypsibiidae) from Chile (South America) with a description of a new species Calohypsibius maliki. N. Z. J. Zool. 2005, 32, 287–292. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Michalczyk, Ł.; McInnes, S.J. Annotated zoogeography of non-marine Tardigrada. Part III: North America and Greenland. Zootaxa 2016, 4203, 1–249. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Michalczyk, Ł.; McInnes, S.J. Annotated zoogeography of non-marine Tardigrada. Part II: South America. Zootaxa 2015, 3923, 1–107. [Google Scholar] [CrossRef] [PubMed]
- Dastych, H. Paradiphascon manningi gen. n. sp. n., a new water-bear from South Africa, with the erecting of a new subfamily Diphasconinae (Tardigrada). Mitteilungen Aus Dem Hamburgischen Zoologischen Mus. Und Institut 1992, 89, 125–139. [Google Scholar]
- Plate, L. Beiträge zur Naturgeschichte der Tardigraden. Zoologische Jahrbücher Abteilung für Anatomie Und Ontogenie Der Tiere 1888, 3, 487–550. [Google Scholar] [CrossRef]
- Marcus, E. Tardigrada. In Das Tierreich; Schulze, F.E., Kükenthal, W., Heider, K., Eds.; Walter de Gruyter: Berlin/Leipzig, Germany, 1936; Volume 66, pp. 1–340. [Google Scholar]
- McInnes, S.J. Zoogeographic distribution of terrestrial/freshwater tardigrades from current literature. J. Nat. Hist. 1994, 28, 257–352. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Michalczyk, Ł.; McInnes, S.J. Annotated zoogeography of non-marine Tardigrada. Part I: Central America. Zootaxa 2014, 3763, 1–62. [Google Scholar] [CrossRef] [PubMed]
- Ehrenberg, C.G. Fortgesetzte Beobachtungen über jetzt herrschende atmospharische mikroscopische, etc. mit Nachtrag und Novarum Specierum Diagnosis. Akad. Wiss. Berl. 1848, 13, 370–381. [Google Scholar]
- Rudescu, L. Tardigrada. Fauna Republicii Populare Romine. Bucuresti 1964, 4, 1–398. [Google Scholar]
- Murray, J. The Tardigrada of the Scottish Lochs. Trans. R. Soc. Edinb. 1905, 41, 677–698. [Google Scholar] [CrossRef]
- Li, X.C.; Liu, Y. A new subspecies of the genus Diphascon and two new records of Tardigrada (Eutardigrada: Hypsibiidae, Macrobiotidae) from China. Acta Zootaxon. Sin. 2005, 30, 309–313. [Google Scholar]
- Morgan, C.I. An Annotated Catalogue of Tardigrada in the Collections of the Royal Scottish Museum, Edinburgh; Museum Information Series; Royal Scottish: Glasgow, Scotland; Natural History: Edinburg, Scotland, 1977; Volume 5, pp. 1–29. ISSN 0307-5036. [Google Scholar]
- Ramazzotti, G. Due nuove specie di Tardigradi extra–europei. Atti Soc. Ital. Sci. Nat. Mus. Civ. Stor. Nat. Milano 1957, 96, 188–191. [Google Scholar]
- Thulin, G. Über die Phylogenie und das System der Tardigraden. Hereditas 1928, 11, 207–266. [Google Scholar] [CrossRef]
- Schultze, K.A.S. Macrobiotus Hufelandii, Animal e Crustaceorum Classe Novum, Reviviscendi Post Diuturnam Asphyxiam et Ariditatem Potens; Curths, C.A., Ed.; Apud Carolus Curths: Berlin, Germany, 1834; pp. 165–169. [Google Scholar]
- Roszkowska, M.; Stec, D.; Ciobanu, D.A.; Kaczmarek, Ł. Tardigrades from Nahuel Huapi National Park (Argentina South America) with descriptions of two new Macrobiotidae species. Zootaxa 2016, 4105, 243–260. [Google Scholar] [CrossRef]
- Michalczyk, Ł.; Kaczmarek, Ł. Minibiotus eichhorni sp. nov., a new species of eutardigrade (Eutardigrada: Macrobiotidae) from Peru. Ann. Zool. 2004, 54, 673–676. [Google Scholar]
- Stec, D.; Kristensen, R.M.; Michalczyk, Ł. An integrative description of Minibiotus ioculator sp. nov. from the Republic of South Africa with notes on Minibiotus pentannulatus Londoño et. al., 2017 (Tardigrada: Macrobiotidae). Zoologischer Anzeiger 2020, 286, 117–134. [Google Scholar] [CrossRef]
- Marcus, E. Tardigrada. In Bronn’s Klassen und Ordnungen Des Tierreichs; Akademische Verlagsgesellschaft: Leipzig, Germany, 1929; Volume 5, pp. 1–608. [Google Scholar]
- Ramazzotti, G. Il phylum Tardigrada. Memorie Dell’istituto Italiano Idrobiologia de Marchi 1962, 14, 1–595. [Google Scholar]
- Ramazzotti, G. Il Phylum Tardigrada (1° Supplemento). Memorie Dell’istituto Italiano Di Idrobiologia 1965, 19, 101–212. [Google Scholar]
- Michalczyk, Ł.; Wełnicz, W.; Frohme, M.; Kaczmarek, Ł. Redescriptions of three Milnesium Doyère, 1840 taxa (Tardigrada: Eutardigrada: Milnesiidae), including the nominal species for the genus. Zootaxa 2012, 3154, 1–20. [Google Scholar] [CrossRef]
- Michalczyk, Ł.; Wełnicz, W.; Frohme, M.; Kaczmarek, Ł. Corrigenda of Zootaxa, 3154:1–20 Redescriptions of three Milnesium Doyère, 1840 taxa (Tardigrada: Eutardigrada: Milnesiidae), including the nominal species for the genus. Zootaxa 2012, 3393, 66–68. [Google Scholar] [CrossRef]
- Meyer, H.A.; Domingue, M.N. Minibiotus acadianus (Eutardigrada: Macrobiotidae), a new species of Tardigrada from southern Louisiana, U.S.A. West. N. Am. Nat. 2011, 71, 38–43. [Google Scholar] [CrossRef]
- Kihm, J.; Kim, S.; McInnes, S.J.; Zawierucha, K.; Rho, H.S.; Kang, P.; Park, T.S. Integrative description of a new Dactylobiotus (Eutardigrada: Parachela) from Antarctica that reveals an intraspecific variation in tardigrade egg morphology. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Pelufo, J.R.; Rocha, A.M.; Pelufo, M.C. Species diversity and morphometrics of tardigrades in a medium–size city in the Neotropical Region: Santa Rosa (La Pampa, Argentina). Anim. Biodivers. Conserv. 2007, 30, 43–51. [Google Scholar]
- Pilato, G.; Costa, G.; Conti, E.; Binda, M.G.; Lisi, O. Morphometric analysis of some metric characters of two Macrobiotus species (Eutardigrada, Macrobiotidae). J. Limnol. 2007, 66, 26–32. [Google Scholar] [CrossRef]
- Corruccini, R.S. Allometry correction in taximetrics. Syst. Zool. 1972, 21, 375–383. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).