The Biodiversity of Demodecid Mites (Acariformes: Prostigmata), Specific Parasites of Mammals with a Global Checklist and a New Finding for Demodex sciurinus
Abstract
1. Introduction
2. Materials and Methods
2.1. Detection of Demodecidae in Sciurus vulgaris
2.2. Literature Review—The Checklist Structure, Biogeographic and Parasitological Data Analysis
3. Results
3.1. A New Record of Demodex sciurinus
3.2. Biodiversity and Geographic Distribution of Demodecidae Mites
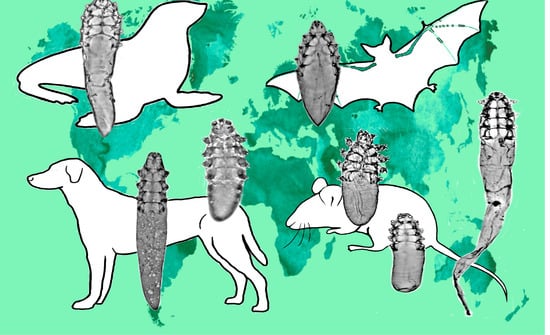
3.3. Demodecidae Parasitism and Relationships with Hosts
4. Discussion
4.1. Demodecidae Biodiversity Analysis in the Light of Taxonomic Identification Problems
4.2. State and Perspectives for the Study on Geographic Distribution
4.3. Host-Parasite Relationships
| Demodecid Mites | Host Species (Ordo, Family) | Occurrence | Comments to the Status of Demodecid Mite Species |
|---|---|---|---|
| Apodemodex cornutus Bukva, 1996 | Neomys anomalus Cabrera, 1907 (Soricomorpha, Soricidae) | Czech Republic loc. class. [48] | Valid |
| Demodex acutipes Bukva and Preisler, 1988 | Cervus elaphus Linnaeus, 1758 (Artiodactyla, Cervidae) | Czech Republic loc. class. [49], Poland [50] | Valid |
| Demodex aelleni Fain, 1960 | Myotis daubentonii (Kuhl, 1817) (Chiroptera, Vespertilionidae) | Switzerland loc. class. [51] | Valid |
| Demodex agrarii Bukva, 1994 | Apodemus agrarius (Pallas, 1771) (Rodentia, Muridae) | Poland [46,52], Slovak Republic loc. class. [53] | Valid |
| Demodex ailuropodae Xu, Xie, Liu, Zhou and Shi, 1986 | Ailuropoda melanoleuca (David, 1869) (Carnivora, Ursidae) | China, zoological garden ex situ [54] | Valid |
| Demodex antechini Nutting and Sweatman, 1970 | Antechinus stuartii Macleay, 1841 (Dasyuromorphia, Dasyuridae) | Australia loc. class. [55] | Valid |
| Demodex apodemi Hirst, 1918 | Apodemus agrarius (Rodentia, Muridae) | Poland [52], Russia [56] | Valid; described by Hirst [57], next considered as a subspecies D. arvicolae var. apodemi [15], and verified by Izdebska [16] as D. apodemi; specimens from A. agrarius, probably belongs to the separate species |
| Apodemus sylvaticus (Linnaeus, 1758) (Linnaeus, 1758) | Great Britain loc. class [15,57], Poland [58], Russia [56] | ||
| Demodex araneae Nutting, 1950 | Ateles sp. (Primates, Atelidae) | Nutting [39] after Nutting [59] | Nom. nud.; description not published within the meaning of the ICZN |
| Demodex aries Desch, 1986 | Ovis aries Linnaeus, 1758 (Artiodactyla, Bovidae) | New Zealand loc. class [60], Czech Republic [61] | Valid |
| Demodex artibei Vargas, Bassols, Desch, Quintero and Polaco, 1995 | Artibeus aztecus K. Andersen, 1906 (Chiroptera, Phyllostomidae) | Mexico loc. class. [62] | Valid |
| Demodex arvicolae Zschokke, 1888 | Microtus agrestis (Linnaeus, 1761) (Rodentia, Cricetidae) | Astrahan/Europe on the border with Asia [56], Europe loc. class. [15] | Valid; the record from the M. arvalis is questionable; maybe D. microti was found |
| Microtus arvalis (Pallas, 1778) (Rodentia, Cricetidae) | Astrahan/Europe on the border with Asia [56] | ||
| Demodex aurati Nutting, 1961 | Mesocricetus auratus (Waterhouse, 1839) (Rodentia, Cricetidae) | Described and finding in laboratory animals, e.g., ex situ [37,63,64,65,66,67,68,69] | Valid |
| Demodex auricularis Izdebska, Rolbiecki and Fryderyk, 2014 | Apodemus sylvaticus (Rodentia, Muridae) | Poland loc. class. [58] | Valid |
| Demodex bandicotae Izdebska, Rolbiecki, Morand and Ribas, 2017 | Bandicota indica (Beschstein, 1800) (Rodentia, Muridae) | Laos loc. class. [17] | Valid |
| Demodex bantengi Firda, Nutting and Sweatman, 1987 | Bos javanicus d’Alton, 1823 (Artiodactyla, Bovidae) | Bali loc. class. [70] | Valid |
| Demodex bicaudatus Kniest and Lukoschus, 1981 | Macroglossus minimus (E. Geoffroy, 1810) (Chiroptera, Pteropodidae) | Australia loc. class. [71] | Valid |
| Demodex bisonianus Kadulski and Izdebska, 1996 | Bison bonasus (Linnaeus, 1758) (Artiodactyla, Bovidae) | Poland loc. class. [72,73,74,75,76,77,78] | Valid |
| Demodex bonapartei Nutting, 1950 | Mustela erminea cicognanii Bonaparte, 1838 (Carnivora, Mustelidae) | Nutting [39] after Nutting [59] | Nom. nud.; description not published within the meaning of the ICZN |
| Demodex bovis Stiles, 1892 (redescription Desch and Nutting 1971) | Bos taurus Linnaeus, 1758 (Artiodactyla, Bovidae) | Probably cosmopolitan, e.g., s. loc. [79], Egypt [80], Ethiopia [81], Nigeria [82], Sudan [83,84,85], Canada [86], USA [87], Argentina [88], Colombia [89], India [90], Mongolia [91], New Zealand [92,93], Czech Republic [94], Germany [95], Hungary [96], Italy [97], Poland [78,98] | Valid; European bison - an accidental finding in a closed farm |
| Bos taurus indicus Linnaeus, 1758 (Artiodactyla, Bovidae) [B. indicus given by the Authors [99] is actually B. t. indicus] | Brazil [99] | ||
| Bison bonasus (Artiodactyla, Bovidae) | Poland, in the breeding condition ex situ [74,77] | ||
| Demodex brevis Akbulatova, 1963 (redescription, Desch and Nutting 1972) | Homo sapiens Linnaeus, 1758 (Primates, Hominidae) | Cosmopolitan, e.g., USA [100,101,102], China [103,104], Australia [105], New Zealand [93], Poland [106], Russia loc. class. [107], Turkey [108,109,110] | Valid |
| Demodex buccalis Bukva, Vítovec and Vlček 1985 | Myodes glareolus (Schreber, 1780) (Rodentia, Cricetidae) | Czech Republic loc. class [111], Poland [112] | Valid |
| Demodex caballi (Railliet, 1895) (redescription, Desch and Nutting 1978) | Equus caballus Linnaeus, 1758 (Perissodactyla, Equidae) | Probably cosmopolitan, e.g., s. loc. [113], USA [114,115], New Zealand [116] | Valid |
| Demodex cafferi Nutting and Guilfoy, 1979 | Syncerus caffer (Sparrman, 1779) (Artiodactyla, Bovidae) | Botswana loc. class. [117], Republic of South Africa [118,119] | Valid |
| Demodex canis (Leydig, 1859) (redescription, Nutting and Desch, 1978) | Canis lupus familiaris Linnaeus, 1758 (Carnivora, Canidae) | Probably cosmopolitan, e.g., s. loc. [120], USA [121], Colombia [122], Cuba [123], India [124,125,126], Nepal [127], Pakistan [128], Thailand [129], New Zealand [93,130], Bangladesh [131], Poland [2,8,38,132,133], Russia [134], Turkey [135] | Valid |
| Demodex caprae Railliet, 1895 | Capra hircus Linnaeus, 1758 (Artiodactyla, Bovidae) | Probably cosmopolitan, e.g., Ethiopia [136], China [137], New Zealand [93], Czech Republic [138], France loc. class. [113], Poland [139], Switzerland [113,140] | Valid |
| Demodex carolliae Desch, Lebel, Nutting and Lukoschus, 1971 | Carollia perspicillata (Linnaeus, 1758) (Chiroptera, Phyllostomidae) | Republic of Suriname loc. class. [141] | Valid |
| Demodex castoris Izdebska, Fryderyk and Rolbiecki, 2016 | Castor fiber Linnaeus, 1758 (Rodentia, Castoridae) | Poland loc. class. [142] | Valid |
| Demodex cati Megnin, 1877 (redescription, Desch and Nutting, 1979) | Felis catus Linnaeus, 1758 (Carnivora, Felidae) | Probably cosmopolitan, e.g., s. loc. [143], USA [144,145], Brazil [146], New Zealand [116], Bulgaria [147], Germany [148], Great Britain [15], Italy [149], Poland [132], Spain [150] | Valid |
| Demodex caviae Bacigalupo and Roveda, 1954 | Cavia porcellus (Linnaeus, 1758) (Rodentia, Caviidae) | Described and finding in laboratory animals, e.g., ex situ [151,152,153] | Valid |
| Demodex cervi Prietsch, 1886 | Rusa unicolor (Kerr, 1792) (Artiodactyla, Cervidae) | Germany ex situ [154] | Valid; hom. for D. cervi sensu Kutzer and Grünberg, 1972 (see D. kutzeri) |
| Demodex chiropteralis Hirst, 1921 | Plecotus auritus (Linnaeus, 1758) (Chiroptera, Vespertilionidae) | Great Britain loc. class. [155], Poland [12] | Valid |
| Demodex conicus Izdebska and Rolbiecki, 2015 | Mus musculus Linnaeus, 1758 (Rodentia, Muridae) | Poland loc. class. [156] | Valid |
| Demodex cornei: Izdebska and Rolbiecki, 2018 | Canis lupus familiaris Linnaeus, 1758 (Carnivora, Canidae) | Probably cosmopolitan, Poland loc. class. [2] | Valid; records before 2018 have uncertain status, except Izdebska [8] and Izdebska and Fryderyk [38] |
| Demodex corniculatus Izdebska, 2012 | Apodemus flavicollis (Melchior, 1834) (Rodentia, Muridae) | Poland loc. class. [1,16] | Valid |
| Demodex criceti Nutting and Rauch, 1958 | Mesocricetus auratus (Waterhouse, 1839) (Rodentia, Cricetidae) | Described and finding in laboratory animals, e.g., ex situ [37,65,66,68,69,157] | Valid |
| Demodex cricetuli Hurley and Desch, 1994 | Cricetulus migratorius (Rodentia, Cricetidae) | Described in laboratory animals ex situ [158] | Valid |
| Demodex cuniculi Pfeiffer, 1903 | Oryctolagus cuniculus (Linnaeus, 1758) (Lagomorpha, Leporidae) | In the breeding condition, e.g., China [15], Great Britain loc. class. [15,159] | Valid |
| Demodex cyonis Morita, Ohmi, Kiwaki, Ike and Nagata, 2018 | Canis lupus familiaris Linnaeus, 1758 (Carnivora, Canidae) | Japan loc. class. [160] | Valid |
| Demodex dasypodi Desch and Stewart, 2002 | Dasypus novemcinctus Linnaeus, 1758 (Cingulata, Dasypodidae) | USA loc. class. [161] | Valid |
| Demodex desmodi Desch, 1994 | Desmodus rotundus (E. Geoffroy, 1810) (Chiroptera, Phyllostomidae) | Republic of Suriname loc. class. [162] | Valid |
| Demodex erinacei Hirst, 1917 | Erinaceus europaeus Linnaeus, 1758 (Erinaceomorpha, Erinaceidae) | Great Britain loc. class. [163] | Valid |
| Demodex erminae Hirst, 1919 | Mustela erminea Linnaeus, 1758 (Carnivora, Mustelidae) | New Zealand [93], Great Britain loc. class. [15] | Valid |
| Demodex equi Railliet, 1895 | Equus caballus Linnaeus, 1758 (Perissodactyla, Equidae) | Probably cosmopolitan, e.g., s. loc. [113], USA [164], Great Britain [15], Poland [165] | Valid |
| Demodex felis | Felis catus Linnaeus, 1758 (Carnivora, Felidae) | [40] | Nom. nud. |
| Demodex flagellurus Bukva, 1985 | Mus musculus (Rodentia, Muridae) | Czech Republic loc. class. [166], Poland [167,168,169] | Valid |
| Demodex folliculorum (Simon, 1842) (redescription Desch and Nutting, 1972) | Homo sapiens (Primates, Hominidae) | Cosmopolitan, e.g., Algeria [170], Egypt [171], USA [100,101], China [103], India [172,173], Australia [105], New Zealand [93,130], Belgium [174], Croatia [175], Germany loc. class. [176], Great Britain [177], Greece [178], Iceland [179], Ireland [180], Poland [106], Turkey [108,109,110,181,182] | Valid |
| Demodex folliculorum sinensis Xie, Liu, Hsu and Hsu, 1982 | Homo sapiens (Primates, Hominidae) | China [183] | |
| Demodex foveolator Bukva, 1984 | Crocidura suaveolens (Pallas, 1811) (Soricomorpha, Soricidae) | Czech Republic loc. class. [184], Poland [18] | Valid |
| Demodex fusiformis Izdebska and Rolbiecki, 2015 | Mus musculus (Rodentia, Muridae) | Poland loc. class. [169] | Valid |
| Demodex gapperi Nutting, Emejuaiwe and Tisolel, 1971 | Myodes gapperi (Rodentia, Cricetidae) | USA loc. class. [185] | Valid |
| Demodex gatoi Desch and Stewart, 1999 | Felis catus Linnaeus, 1758 (Carnivora, Felidae) | USA loc. class. [186,187], Austria [188], Finland [189], Poland [44,132], Spain [150]) | Valid |
| Demodex ghanensis Oppong, Lee and Yasin, 1975 | Bos taurus (Artiodactyla, Bovidae) | Ghana loc. class. [190], Sudan [83] | Valid |
| Demodex glareoli Hirst, 1919 | Myodes glareolus (Rodentia, Cricetidae) | Great Britain loc. class. [15], Poland [112] | Valid; described by Hirst [15] as a subspecies D. arvicolae var. glareoli, than verified by Izdebska [112] as D. glareoli |
| Demodex gliricolens Hirst, 1921 | Arvicola amphibius (Linnaeus, 1758) (Rodentia, Cricetidae) | Great Britain loc. class. [155] | Valid |
| Demodex gracilentus Izdebska and Rolbiecki, 2013 | Apodemus agrarius (Rodentia, Muridae) | Poland loc. class. [46] | Valid |
| Demodex huttereri Mertens, Lukoschus and Nutting, 1983 | Apodemus agrarius (Rodentia, Muridae) | Germany loc. class. [191], Poland [192] | Valid |
| Demodex injai Desch and Hillier, 2003 | Canis lupus familiaris Linnaeus, 1758 (Carnivora, Canidae) | Probably cosmopolitan, USA loc. class. [193], Brazil [194], Spain [195], Poland [8,38] | Valid |
| Demodex intermedius Lukoschus, Mertesn, Nutting and Nadchatram, 1984 | Tupaia glis (Diard, 1820) (Scandentia, Tupaiidae) | Malaysia loc. class. [196] | Valid |
| Demodex kutzeri Bukva, 1987 | Alces alces (Linnaeus, 1758) (Atriodactyla, Cervidae) | Poland [197] | Valid; (=D. cervi sensu Kutzer and Grünberg, 1972; hom. for D. cervi Prietsch, 1886) |
| Capreolus capreolus (Linnaeus, 1758) (Artiodactyla, Cervidae) | Poland [198,199] | ||
| Cervus elaphus Linnaeus, 1758 (Artiodactyla, Cervidae) | Austria [200], Czech Republic loc. class. [154], Poland [198,201] | ||
| Cervus elaphus nelsoni Nelson, 1902 (Artiodactyla, Cervidae) | USA [202,203] | ||
| Cervus nippon pseudaxis Gervais, 1841 (Artiodactyla, Cervidae) | Berlin, zoological garden ex situ [154] | ||
| Dama dama (Linnaeus, 1758) | Poland [204] | ||
| Odocoileus hemionus hemionus (Rafinesque, 1817) (Artiodactyla, Cervidae) | USA [202,203] | ||
| Odocoileus virginianus (Zimmermann, 1780) (Artiodactyla, Cervidae) | USA [202] | ||
| Demodex lacrimalis Lukoschus and Jongman, 1974 | Apodemus sylvaticus (Rodentia, Muridae) | Italy [205], Netherlands loc. class. [205], Poland [206] | Valid |
| Demodex leucogasteri Hughes and Nutting, 1981 | Onychomys leucogaster (Wied-Neuwied, 1841) (Rodentia, Cricetidae) | USA loc. class. [207] | Valid |
| Demodex longior Hirst, 1918 | Apodemus sylvaticus (Rodentia, Muridae) | Great Britain loc. class. [15,57], Poland [46], Russia [56] | Valid |
| Demodex longissimus Desch, Nutting and Lukoschus, 1972 | Carollia perspicillata (Chiroptera, Phyllostomidae) | Republic of Suriname loc. class. [208] | Valid |
| Demodex lutrae Izdebska and Rolbiecki, 2014 | Lutra lutra (Linnaeus, 1758) (Carnivora, Mustelidae) | Poland loc. class. [3] | Valid |
| Demodex macaci Karjala, Desch and Starost, 2005 | Macaca mulatta (Zimmermann, 1780) (Primates, Cercopithecidae) | USA, laboratory colony ex situ [209] | Valid |
| Demodex macroglossi Desch, 1981 | Macroglossus minimus (Chiroptera, Pteropodidae) | Australia loc. class. [210] | Valid |
| Demodex marculus Izdebska and Rolbiecki, 2015 | Mus musculus (Rodentia, Muridae) | Poland loc. class. [169] | Valid |
| Demodex marsupiali Nutting, Lukoschus and Desch, 1980 | Didelphis marsupialis Linnaeus, 1758 (Didelphimorphia, Didelphidae) | Republic of Surinam loc. class. [211] | Valid |
| Demodex melanopteri Lukoschus, Jongman and Nutting, 1972 | Eptesicus brasiliensis melanopterus (Jentink, 1904) (Chiroptera, Vespertilionidae) [E. melanopterus given by the Authors [212] is actually E. b. melanopterus] | Republic of Suriname loc. class. [212] | Valid |
| Demodex melesinus Hirst, 1921 | Meles meles (Linnaeus, 1758) (Carnivora, Mustelidae) | Great Britain loc. class. [213], Poland [4] | Valid |
| Demodex merioni (=meriones) | Meriones spp. (Rodentia, Muridae) | Finding in laboratory animals, e.g., [36,37] | Nom. nud. |
| Demodex mexicanus Vargas, Bassols, Desch, Quintero and Polaco, 1995 | Corynorhinus mexicanus G. M. Allen, 1916 (Chiroptera, Vespertilionidae) | Mexico loc. class. [62] | Valid |
| Demodex microti Izdebska and Rolbiecki, 2013 | Microtus arvalis (Rodentia, Cricetidae) | Poland loc. class. [214] | Valid |
| Demodex mollis Izdebska, Rolbiecki, Fryderyk and Mierzyński, 2017 | Apodemus flavicollis (Rodentia, Muridae) | Poland loc. class. [1] | Valid |
| Demodex molossi Desch, Nutting and Lukoschus, 1972 | Molossus molossus (Pallas, 1766) (Chiroptera, Molossidae) | Republic of Suriname loc. class. [208] | Valid |
| Demodex muscardini Hirst, 1917 | Muscardinus avellanarius (Linnaeus, 1758) (Rodentia, Gliridae) | Armenia [56], Great Britain loc. class. [15,163] | Valid |
| Demodex musculi Oudemans, 1897 (redescription, Izdebska and Rolbiecki, 2015) | Mus musculus (Rodentia, Muridae) | Europe [15], ds loc. class. [215], Poland [167,169], Russia [56], Spain [216]; laboratory animals, e.g., ex situ [7,217,218] | Valid |
| Demodex myotidis | Myotis lucifugus lucifugus (Le Conte, 1831) (Chiroptera, Vespertilionidae) | Nutting [39] after Nutting [59] | Nom. nud.; description not published within the meaning of the ICZN |
| Myotis septentrionalis Trouessart, 1897 (Chiroptera, Vespertilionidae) | Nutting [39] after Di Benedetto [219] | ||
| Eptesicus fuscus (Beauvois, 1796) (Chiroptera, Vespertilionidae) | Nutting [39] after Di Benedetto [219] | ||
| Demodex mystacina Desch, 1989 | Mystacina tuberculata Gray, 1843 (Chiroptera, Mystacinidae) | New Zealand loc. class. [220] | Valid |
| Demodex nanus Hirst, 1918 (redescription Desch, 1987) | Rattus norvegicus (Berkenhout, 1769) (Rodentia, Muridae) | Great Britain [57], Poland [19,221,222], Russia [56]; laboratory animals ex situ [223] | Valid |
| Rattus rattus (Linnaeus, 1758) (Rodentia, Muridae) | New Zealand [223], Great Britain loc. class. [57], Russia [56] | ||
| Demodex neomydis Bukva, 1995 | Neomys anomalus (Soricomorpha, Soricidae) | Czech Republic loc. class. [224] | Valid |
| Demodex neoopisthosomae Desch, Lukoschus and Nadchatram, 1986 | Eonycteris spelaea (Dobson, 1871) (Chiroptera, Pteropodidae) | Malaysia loc. class. [225] | Valid |
| Demodex norvegicus Bukva, 1995 | Rattus norvegicus (Rodentia, Muridae) | Czech Republic loc. class. [226], Poland [19,221,222] | Valid |
| Demodex novazelandica Desch, 1989 | Mystacina tuberculata (Chiroptera, Mystacinidae) | New Zealand loc. class. [220] | Valid |
| Demodex nycticeii Desch, 1996 | Nycticeius humeralis (Rafinesque, 1818) (Chiroptera, Vespertilionidae) | USA loc. class. [227] | Valid |
| Demodex odocoilei Desch and Nutting, 1974 | Odocoileus virginianus (Artiodactyla, Cervidae) | USA loc. class. [228] | Valid |
| Odocoileus hemionus columbianus (Richardson, 1829) (Artiodactyla, Cervidae) | USA [229] | ||
| Demodex ovis Railliet, 1895 (redescription, Desch 1986) | Ovis aries (Artiodactyla, Bovidae) | s. loc. [113], Australia [60], New Zeland [60], Czech Republic [61], Israel [230], Poland [231] | Valid |
| Demodex peromysci Lambert, Lukoschus and Whitaker, 1983 | Peromyscus leucopus (Rafinesque, 1818) (Rodentia, Cricetidae) | USA loc. class. [232] | Valid |
| Demodex phocidi Desch, Dailey and Tuomi, 2003 | Phoca vitulina Linnaeus, 1758 (Carnivora, Phocidae) | USA, sealife center ex situ [233], Poland in situ [5] | Valid |
| Demodex phodopi Desch, Davis and Klompen, 2006 | Phodopus sungorus (Pallas, 1773) (Rodentia, Cricetidae) | Described in laboratory animals ex situ [234] | Valid |
| Demodex phylloides Csokor, 1879 | Sus scrofa scrofa Linnaeus, 1758 (Artiodactyla, Suidae) | Poland [235,236,237,238] | Valid |
| Sus scrofa domesticus Erxleben, 1777 (Artiodactyla, Suidae) | Probably cosmopolitan, e.g., Tanzania [239], Canada [240], USA [87], Brasil [241,242], New Zealand [93,130], historical Galicia loc. class. [243], Italy [244] | ||
| Demodex phyllostomatis Leydig, 1859 | Phyllostomus hastatus (Pallas, 1767) (Chiroptera, Phyllostomidae) | Republic of Suriname loc. class. [51,120] | Valid |
| Demodex plecoti Izdebska Rolbiecki, Mierzyński and Bidziński, 2019 | Plecotus auritus (Chiroptera, Vespertilionidae) | Poland loc. class. [10] | Valid |
| Demodex ponderosus Izdebska and Rolbiecki, 2014 | Rattus norvegicus (Rodentia, Muridae) | Poland loc. class. [222] | Valid |
| Demodex pseudaxis Schpringol’ts-Schmidt, 1937 | Cervus nippon hortulorum Swinhoe, 1864 (Artiodactyla, Cervidae) | Russia/Far east [245] | Valid; need verification |
| Demodex ratti Hirst, 1917 (redescription Bukva, 1995) | Rattus norvegicus (Rodentia, Muridae) | Czech Republic [226], Europe, s. loc. [15,246], Poland [19,221,222,246,247,248], Russia [56] | Valid |
| Demodex ratticola Bukva, 1995 | Rattus norvegicus (Rodentia, Muridae) | Czech Republic loc. class. [226], Poland [222,247,248] | Valid |
| Demodex rosus Bukva, Vítovec and Vlček, 1985 | Apodemus flavicollis (Rodentia, Muridae) | Czech Republic loc. class. [111], Poland [249] | Valid |
| Demodex sabani Desch, Lukoschus and Nadchatram, 1984 | Leopoldamys edwardsi (Thomas, 1882) (Rodentia, Muridae) | Malaysia [250] | Valid |
| Leopoldamys sabanus (Thomas, 1887) (Rodentia, Muridae) | Malaysia loc. class. [250] | ||
| Niviventer cremoriventer (Miller, 1900) (Rodentia, Muridae) | Malaysia [250] | ||
| Niviventer rapit (Bonhote, 1903) (Rodentia, Muridae) | Malaysia [250] | ||
| Rattus annandalei (Bonhote, 1903) (Rodentia, Muridae) | Malaysia [250] | ||
| Rattus tiomanicus (Miller, 1900) (Rodentia, Muridae) | Malaysia [250] | ||
| Sundamys muelleri (Jentink, 1879) (Rodentia, Muridae) | Malaysia [250] | ||
| Demodex saimiri Lebel and Nutting 1973 | Saimiri sciureus (Linnaeus, 1758) (Primates, Cebidae) | Biological supply houses ex situ [251] | Valid |
| Demodex sciurei Lebel, 1970 | Saimiri sciureus (Primates, Cebidae) | [252] | Nom. nud.; description not published within the meaning of the ICZN |
| Demodex sciurinus Hirst, 1923 | Sciurus vulgaris Linnaeus, 1758 (Rodentia, Sciuridae) | Great Britain loc. class. [45], Poland [present study] | Valid |
| Demodex sinocricetuli Desch and Hurley, 1997 | Cricetulus barabensis (Pallas, 1773) (Rodentia, Cricetidae) | laboratory animals ex situ [253] | Valid |
| Demodex soricinus Hirst, 1918 (redescription Bukva, 1993 | Plecotus auritus (Chiroptera, Vespertilionidae) | Great Britain [57,155] | Valid; P. auritus -probably wrong host record |
| Sorex araneus Linnaeus, 1758 (Soricomorpha, Soricidae) [S. vulgaris given by the Author [57] is actually S. araneus] | Czech Republic [254], Great Britain loc. class. [57], Poland [255] | ||
| Demodex spelaea Desch, Lukoschus and Nadchatram, 1986 | Eonycteris spelaea (Chiroptera, Pteropodidae) | Malaysia loc. class. [225] | Valid |
| Demodex suis (Kadlec, 1975) | Sus scrofa domesticus (Artiodactyla, Suidae) | s. loc. [256], s. loc. [257], Czech Republic [258] | Nom abort.; syn. D. phylloides |
| Demodex sungori Desch, Davis and Klompen, 2006 | Phodopus sungorus (Rodentia, Cricetidae) | Described in laboratory animals ex situ [234] | Valid |
| Demodex sylvilagi Maravelas, 1962 | Sylvilagus transitionalis (Bangs, 1895) (Lagomorpha, Leporidae) | Nutting [39] after Maravelas [259] | Nom. nud.; description not published within the meaning of the ICZN |
| Demodex talpae Hirst, 1921 | Talpa europaea Linnaeus, 1758 (Soricomorpha, Talpidae) | Great Britain loc. class. [155], Poland [260] | Valid |
| Demodex tauri Bukva, 1986 | Bos taurus (Artiodactyla, Bovidae) | Czech Republic loc. class. [261] | Valid |
| Demodex tigris Shi, Xie and Hsu, 1985 | Panthera tigris amoyensis (Hilzheimer, 1905) (Carnivora, Felidae) | China, zoological garden ex situ [262] | Valid |
| Demodex tortellinioides Desch and Holz, 2006 | Antechinus agilis Dickman, Parnaby, Crowther and King, 1998 (Dasyuromorphia, Dasyuridae) | Australia loc. class. [263] | Valid |
| Demodex transitionalis Moravelas, 1962 | Sylvilagus transitionalis (Bangs, 1895) (Lagomorpha, Leporidae) | Nutting [39] after Maravelas [259] | Nom. nud.; description not published within the meaning of the ICZN |
| Demodex uncii Desch, 1993 | Uncia uncia (Schreber, 1775) (Carnivora, Felidae) | USA, zoological garden ex situ Desch [264] | Valid |
| Demodex ursi Desch, 1995 | Ursus americanus Pallas, 1780 (Carnivora, Ursidae) | USA loc. class. [265,266] | Valid |
| Demodex vibrissae Izdebska, Rolbiecki and Fryderyk, 2016 | Mus musculus (Rodentia, Muridae) | Poland loc. class. [47] | Valid |
| Demodex zalophi Dailey and Nutting, 1979 | Zalophus californianus (Lesson, 1828) (Carnivora, Otariidae) | USA, Australia loc. class. [267] | Valid |
| Glossicodex musculi Izdebska and Rolbiecki, 2016 | Mus musculus (Rodentia, Muridae) | Poland loc. class. [11] | Valid |
| Ophthalmodex apodemi Bukva, Nutting and Desch, 1992 | Apodemus sylvaticus (Rodentia, Muridae) | Czech Republic loc. class. [268] | Valid |
| Ophthalmodex artibei Lukoschus and Nutting, 1979 | Artibeus lituratus (Chiroptera, Phyllostomidae) | Republic of Surinam loc. class. [269] | Valid |
| Ophthalmodex australiensis Woeltjes and Lukoschus, 1981 | Rhinonicteris aurantia (Gray, 1845) (Chiroptera, Hipposideridae) | Australia loc. class. [270] | Valid |
| Ophthalmodex carolliae Lukoschus, Woeltjes, Desch and Nutting, 1980 | Carollia perspicillata (Chiroptera, Phyllostomidae) | Republic of Surinam loc. class. [271] | Valid |
| Ophthalmodex juniatae Veal, Giesen and Whitaker, 1984 | Myotis lucifugus (Le Conte, 1831) (Chiroptera, Vespertilionidae) | USA loc. class. [272] | Valid |
| Ophthalmodex molossi Lukoschus, Woeltjes, Desch and Nutting, 1980 | Molossus molossus (Chiroptera, Molossidae) | Republic of Surinam loc. class. [271] | Valid |
| Ophthalmodex wilsoni Woeltjes and Lukoschus, 1981 | Vespadelus pumilus (Gray, 1841 (Chiroptera, Vespertilionidae) | Australia loc. class. [270] | Valid |
| Pterodex carolliae Lukoschus, Woeltjes, Desch and Nutting, 1980 | Carollia perspicillata (Chiroptera, Phyllostomidae) | Republic of Suriname loc. class. [273] | Valid |
| Rhinodex baeri Fain, 1959 | Galago moholi A. Smith, 1836 (Primates, Galagidae) | Rwanda loc. class. [274] | Valid |
| Soricidex dimorphus Bukva, 1982 | Sorex araneus Linnaeus, 1758 (Soricomorpha, Soricidae) | Czech Republic loc. class. [275,276], Poland [255] | Valid |
| Stomatodex cercarteti Desch, 1991 | Cercartetus nanus (Desmarest, 1818) (Diprotodontia, Burramyidae) | Australia loc. class. [277] | Valid |
| Stomatodex corneti Fain, 1960 | Valid | ||
| Stomatodex corneti corneti Fain, 1960 | Barbastella barbastellus (Schreber, 1774) (Chiroptera, Vespertilionidae) | Belgium loc. class. [51], Great Britain [278] | |
| Nycteris sp. (Chiroptera, Nycteridae) | Rwanda [51] | ||
| Stomatodex corneti myotis Fain, 1960 | Myotis dasycneme (Boie, 1825) (Chiroptera, Vespertilionidae) | Belgium [51] | |
| Myotis myotis (Borkhausen, 1797) (Chiroptera, Vespertilionidae) | Belgium [51] | ||
| Stomatodex galagoensis Fain, 1959 | Galago moholi A. Smith, 1836 (Primates, Galagidae) | Rwanda loc. class. [274] | Valid |
| Stomatodex rousetti Fain, 1960 | Rousettus aegyptiacus (Geoffroy, 1810) (Chiroptera, Pteropodidae) | Democratic Republic of the Congo loc. class. [51] | Valid |
| Character | ♂ (n = 8) | ♀ (n = 13) |
|---|---|---|
| Length of gnathosoma | 15 (13–18), SD 2 | 17 (15–18), SD 1 |
| Width of gnathosoma (at base) | 18 (15–23), SD 2 | 18 (14–20), SD 2 |
| Length of podosoma | 49 (43–55), SD 5 | 54 (50–60), SD 3 |
| Width of podosoma | 31 (28–35), SD 3 | 33 (30–38), SD 2 |
| Length of opisthosoma | 72 (55–90), SD 11 | 97 (75–125), SD 16 |
| Width of opisthosoma | 31 (25–36), SD 4 | 33 (30–38), SD 2 |
| Aedeagus | 20 (16–23), SD 3 | – |
| Vulva | – | 6 (4–8), SD 1 |
| Total length of body | 135 (120–158), SD 14 | 168 (143–193), SD 17 |
| Mammals Ordo | Mammals Species | Demodecid Mites |
|---|---|---|
| PRIMATES | Galago moholi | Rhinodex baeri Stomatodex galagoensis |
| Homo sapiens | Demodex brevis Demodex folliculorum | |
| RODENTIA | Apodemus agrarius | Demodex agrarii Demodex apodemi Demodex gracilentus Demodex huttereri |
| Apodemus flavicollis | Demodex corniculatus Demodex mollis Demodex rosus | |
| Apodemus sylvaticus | Demodex apodemi Demodex auricularis Demodex lacrimalis Demodex longior Ophthalmodex apodemi | |
| Mus musculus | Demodex conicus Demodex flagellurus Demodex fusiformis Demodex marculus Demodex musculi Demodex vibrissae Glossicodex musculi | |
| Rattus norvegicus | Demodex nanus Demodex norvegicus Demodex ponderosus Demodex ratti Demodex ratticola | |
| Mesocricetus auratus | Demodex aurati Demodex criceti | |
| Myodes glareolus | Demodex buccalis Demodex glareoli | |
| Phodopus sungorus | Demodex phodopi Demodex sungori | |
| SORICOMORPHA | Neomys anomalus | Apodemodex cornutus Demodex neomydis |
| Sorex araneus | Demodex soricinus Soricidex dimorphus | |
| CHIROPTERA | Carollia perspicillata | Demodex carolliae Demodex longissimus Ophthalmodex carolliae Pterodex carolliae |
| Eonycteris spelaea | Demodex neoopisthosomae Demodex spelaea | |
| Macroglossus minimus | Demodex bicaudatus Demodex macroglossi | |
| Molossus molossus | Demodex molossi Ophthalmodex molossi | |
| Mystacina tuberculata | Demodex mystacina Demodex novazelandica | |
| Plecotus auritus | Demodex chiropteralis Demodex plecoti | |
| CARNIVORA | Canis lupus familiaris | Demodex canis Demodex cornei Demodex cyonis Demodex injai |
| Felis catus | Demodex cati Demodex gatoi | |
| PERISSODACTYLA | Equus caballus | Demodex caballi Demodex equi |
| ARTIODACTYLA | Cervus elaphus | Demodex acutipes Demodex kutzeri |
| Cervus nippon | Demodex kutzeri Demodex pseudaxis | |
| Odocoileus hemionus | Demodex kutzeri Demodex odocoilei | |
| Odocoileus virginianus | Demodex kutzeri Demodex odocoilei | |
| Bos taurus | Demodex bovis Demodex ghanensis Demodex tauri | |
| Ovis aries | Demodex aries Demodex ovis |
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Izdebska, J.N.; Rolbiecki, L.; Fryderyk, S.; Mierzyński, Ł. Adult and immature stages of the new species of the genus Demodex (Acariformes: Demodecidae) with data on parasitism, topography, and topical specificity of demodecid mites in the yellow-necked mouse, Apodemus flavicollis (Rodentia: Muridae). J. Parasitol. 2017, 103, 320–329. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Rolbiecki, L. The status of Demodex cornei: Description of the species and developmental stages, and data on demodecid mites in the domestic dog Canis lupus familiaris. Med. Vet. Entomol. 2018, 32, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Izdebska, J.N.; Rolbiecki, L. Demodex lutrae n. sp. (Acari) in European otter Lutra lutra (Carnivora: Mustelidae) with data from other demodecid mites in carnivores. J. Parasitol. 2014, 100, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Izdebska, J.N.; Cierocka, K.; Rolbiecki, L.; Kozina, P.; Kołodziej-Sobocińska, M. Demodex melesinus (Acariformes: Demodecidae) – the forgotten European badger parasite, rediscovered after 100 years. Acta Parasitol. 2018, 63, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Izdebska, J.N.; Rolbiecki, L.; Cierocka, K.; Pawliczka, I. Demodex phocidi (Acariformes: Demodecidae) from Phoca vitulina (Carnivora: Phocidae) – the second observation in the world and a supplement to the species description. Oceanol. Hydrobiol. Stud. 2020, 49, 49–55. [Google Scholar] [CrossRef]
- Izdebska, J.N. New data of demodecosis hominis—Ethiology, pathogenesis and diagnosis. In Arthropods. Ecological and Pathological Aspects of Parasite-Host Relationships; Buczek, A., Błaszak, C., Eds.; Akapit: Lublin, Poland, 2010; pp. 137–145. [Google Scholar]
- Nashat, M.A.; Luchins, K.R.; Riedel, E.R.; Izdebska, J.N.; Lepherd, M.L.; Lipman, N.S. Characterization of Demodex musculi infestation, associated co-morbidities, and its topographical distribution in a mouse strain with defective adaptive immunity. Comp. Med. 2017, 67, 315–329. [Google Scholar]
- Izdebska, J.N. Demodex spp. (Acari, Demodecidae) and demodecosis in dogs: Characteristics, symptoms, occurrence. Bull. Vet. Inst. Puławy 2010, 54, 335–338. [Google Scholar]
- Izdebska, J.N. Adaptation to Parasitism in Skin Mites From The Demodecidae Family (Acari, Prostigmata). In Arthropods. Epidemiological Importance; Buczek, A., Błaszak, C., Eds.; Koliber: Lublin, Poland, 2006; pp. 31–36. [Google Scholar]
- Izdebska, J.N.; Rolbiecki, L.; Mierzyński, Ł.; Bidziński, K. Morphological and ontogenetic characteristics of Demodex plecoti sp. nov. (Acariformes: Demodecidae) from the brown long-eared bat Plecotus auritus (Chiroptera: Vespertilionidae), with comments on parasitism. Syst. Appl. Acarol. 2019, 24, 377–388. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Rolbiecki, L. A new genus and species of demodecid mites from the tongue of a house mouse Mus musculus: Description of adult and immature stages with data on parasitism. Med. Vet. Entomol. 2016, 30, 135–143. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Rolbiecki, L.; Mierzyński, Ł.; Bidziński, K. Demodecid mites (Acariformes, Demodecidae) in brown long-eared bat Plecotus auritus (Chiroptera, Vespertilionidae)—Second record in the world and systematic status of Demodex chiropteralis Hirst, 1921. Ann. Parasitol. 2018, 64, 109–113. [Google Scholar] [PubMed]
- Bochkov, A.V. A review of mites of the Parvorder Eleutherengona (Acariformes: Prostigmata)—Permanent parasites of mammals. Acarina 2009, 1, 1–149. [Google Scholar]
- Rather, P.A.; Hassan, I. Human Demodex mite: The versatile mite of dermatological importance. Indian J. Dermatol. 2014, 59, 60–66. [Google Scholar] [CrossRef]
- Hirst, S. Studies on Acari. No. 1. The genus Demodex, Owen; British Museum (Natural History): London, UK, 1919; pp. 1–44. [Google Scholar]
- Izdebska, J.N. A new Demodecidae species (Acari) from the yellow-necked mouse Apodemus flavicollis (Rodentia, Muridae)—Description with data on parasitism. J. Parasitol. 2012, 98, 1101–1104. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Rolbiecki, L.; Morand, S.; Ribas, A. A new species and new host record of Demodecidae (Acariformes: Prostigmata) associated with the bandicoot rat (Rodentia: Muridae) from Lao PDR with data on parasitism and a checklist of the demodecid mites of rodents. Syst. Appl. Acarol. 2017, 22, 1910–1923. [Google Scholar] [CrossRef]
- Cierocka, K.; Izdebska, J.N.; Rolbiecki, L. Demodex foveolator (Acariformes: Demodecidae) from Crocidura suaveolens (Soricomorpha: Soricidae)—The second observation worldwide, and a checklist of the demodecid mites of soricomorphs. Ann. Parasitol. 2019, 65, 329–332. [Google Scholar]
- Izdebska, J.N. Demodex spp. (Acari: Demodecidae) in brown rat (Rodentia: Muridae) in Poland. Wiad. Parazytol. 2004, 50, 333–335. [Google Scholar]
- Wilson, D.E.; Reeder, D.M. Mammals Species of the World: A Taxonomic and Geographic Reference, 3rd ed.; The Johns Hopkins University Press: Baltimore, MD, USA, 2005; pp. 1–2142. [Google Scholar]
- Taxonomic Information System (ITIS). Available online: http://www.itis.gov (accessed on 25 April 2020).
- Carpenter, J.W.; Freeny, J.C.; Patton, C.S. Occurrence of Demodex Owen 1843 on a white-tailed deer from Oklahoma. J. Wildl. Dis. 1972, 8, 112–114. [Google Scholar] [CrossRef]
- Hamir, A.N.; Snyder, D.E.; Hanlon, C.A.; Rupprecht, C.E. First Report of a Demodex sp. in raccoons (Procyon lotor). J. Wildl. Dis. 1993, 29, 139–141. [Google Scholar] [CrossRef]
- Waggie, K.S.; Marion, P.L. Demodex sp. in California ground squirrels. J. Wildl. Dis. 1997, 33, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Vestweber, J.G.; Ridley, R.K.; Nietfeld, J.C.; Wilkerson, M.J. Demodicosis in an American bison. Can. Vet. J. 1999, 40, 417–418. [Google Scholar] [PubMed]
- Vogelnest, L.J.; Vogelnest, L.; Mueller, R.S. An undescribed Demodex sp. and demodicosis in a captive koala (Phascolarctos cinereus). J. Zoo Wildl. Med. 2000, 31, 100–106. [Google Scholar]
- Gentes, M.L.; Proctor, H.; Wobeser, G. Demodicosis in a mule deer (Odocoileus hemionus hemionus) from Saskatchewan, Canada. J. Wildl. Dis. 2007, 43, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, S. Important ectoparasites of alpaca (Vicugna pacos). Acta Vet. Scand. 2010, 52 (Suppl. 1), S17. [Google Scholar] [CrossRef]
- Jimenez, I.A.; Odom, M.R.; Childs-Sanford, S.E.; Lucio-Forster, A.; Bowman, D.D. Lynxacarus and Demodex infestation in a captive jaguar (Panthera onca) in La Democracia, Belize. Vet. Rec. Case Rep. 2020, 8, e001037. [Google Scholar] [CrossRef]
- Zhao, Y.E.; Peng, Y.; Wang, X.I.; Wu, L.P.; Wang, M.; Yan, H.I.; Xiao, S.X. Facial dermatosis associated with Demodex: A case-control study. J. Zhejiang Univ. Sci. B 2011, 12, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Fourie, J.J.; Liebenberg, J.E.; Horak, I.G.; Taenzler, J.; Heckeroth, A.R.; Frénais, R. Efficacy of orally administered fluralaner (BravectoTM) or topically applied imidacloprid/moxidectin (Advocate®) against generalized demodicosis in dogs. Parasit. Vectors 2015, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yasine, A.; Kumsa, B.; Hailu, Y.; Ayana, D. Mites of sheep and goats in Oromia Zone of Amhara Region, North Eastern Ethiopia: Species, prevalence and farmers awareness. BMC Vet. Res. 2015, 11, 1–6. [Google Scholar] [CrossRef][Green Version]
- Six, R.H.; Becskei, C.; Mazaleski, M.M.; Fourie, J.J.; Mahabir, S.P.; Myers, M.R.; Slootmans, N. Efficacy of sarolaner, a novel oral isoxazoline, against two common mite infestations in dogs: Demodex spp. and Otodectes cynotis. Vet. Parasitol. 2016, 222, 62–66. [Google Scholar] [CrossRef]
- Yun, C.H.; Yun, J.H.; Baek, J.O.; Roh, J.Y.; Lee, J.R. Demodex mite density determinations by standardized skin surface biopsy and direct microscopic examination and their relations with clinical types and distribution patterns. Ann. Dermatol. 2017, 29, 137–142. [Google Scholar] [CrossRef]
- Salem, N.Y.; Abdel-Saeed, H.; Farag, H.S.; Ghandour, R.A. Canine demodicosis: Hematological and biochemical alterations. Vet. World EISSN 2020, 13, 68–72. [Google Scholar] [CrossRef]
- Wilkerson, J.D.; Berger, D.M.P. Parasites of Gerbils. In Flynn’s Parasites of Laboratory Animals; Baker, D.G., Ed.; Blackwell Publishing: Ames, IA, USA, 2007; pp. 413–420. [Google Scholar]
- Fehr, M.; Koestlinger, S. Ectoparasites in small exotic mammals. Vet. Clin. Exot. Anim. 2013, 16, 611–657. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Fryderyk, S. Diversity of three species of the genus Demodex (Acari, Demodecidae) parasitizing dogs in Poland. Pol. J. Environ. Stud. 2011, 20, 565–569. [Google Scholar]
- Nutting, W.B. Demodicidae—Status and prognostics. Acarologia 1964, 6, 441–454. [Google Scholar]
- Silbermayr, K.; Horvath-Ungerboeck, C.; Eigner, B.; Joachim, A.; Ferrer, L. Phylogenetic relationships and new genetic tools for the detection and discrimination of the three feline Demodex mites. Parasitol. Res. 2015, 114, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.; Sastre, N.; Ravera, I.; Altet, L.; Francino, O.; Bardagí, M.; Ferrer., L. Identification of a third feline Demodex species through partial sequencing of the 16S rDNA and frequency of Demodex species in 74 cats using a PCR assay. Vet. Dermatol. 2015, 26, 239–246. [Google Scholar] [CrossRef]
- Taffin, E.R.; Casaert, S.; Claerebout, E.; Vandekerkhof, T.J.; Vandenabeele, S. Morphological variability of Demodex cati in a feline immunodeficiency virus-positive cat. J. Am. Vet. Med. Assoc. 2016, 249, 1308–1312. [Google Scholar] [CrossRef]
- Jańczak, D.; Ruszczak, A.; Kaszak, I.; Gołąb, E.; Barszcz, K. Clinical aspects of demodicosis in veterinary and human medicine. Med. Weter. 2017, 73, 265–271. [Google Scholar]
- Jańczak, D.; Gołąb, E.; Borkowska-Bąkała, D.; Barszcz, K. Inwazja Demodex gatoi u kota rasy brytyjskiej krótkowłosej leczonego przewlekle lekami immunosupresyjnymi. Med. Weter. 2017, 73, 248–251. [Google Scholar]
- Hirst, S. On some new or little-known species of Acari. Proc. Zool. Soc. Lond. 1923, 2, 971–1000. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Rolbiecki, L. A new species of Demodex (Acari, Demodecidae) with data on topical specificity and topography of demodectic mites in the striped field mouse Apodemus agrarius (Rodentia, Muridae). J. Med. Entomol. 2013, 50, 1202–1207. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Rolbiecki, L.; Fryderyk, S. A new species of Demodex (Acari: Demodecidae) from the skin of the vibrissal area of the house mouse Mus musculus (Rodentia: Muridae), with data on parasitism. Syst. Appl. Acarol. 2016, 21, 1031–1039. [Google Scholar] [CrossRef]
- Bukva, V. Apodemodex cornutus gen. n. et sp. n. (Acari: Demodecidae): New genus and new species of the hair follicle mite from the Mediterranean water shrew, Neomys anomalus (Insectivora: Soricidae). Folia Parasitol. 1996, 43, 312–316. [Google Scholar]
- Bukva, V.; Preisler, J. Observations on the morphology of the hair follicle mites (Acari: Demodicidae) from Cervus elaphus L. 1758 including description of Demodex acutipes sp. n. Folia Parasitol. 1988, 35, 67–75. [Google Scholar]
- Izdebska, J.N.; Fryderyk, S. Demodex acutipes Bukva et Preisler, 1988 (Acari, Demodecidae)—A rare parasite of red deer (Cervus elaphus L.). Ann. Parasitol. 2012, 58, 161–166. [Google Scholar]
- Fain, A. Les acarines psoriques parasites des chauves-souris. XIII. La famille Demodecidae Nicolet. Acarologia 1960, 2, 80–87. [Google Scholar]
- Izdebska, J.N.; Cydzik, K. Occurrence of Demodex spp. (Acari, Demodecidae) in the striped field mouse Apodemus agrarius (Rodentia, Muridae) in Poland. Wiad. Parazytol. 2010, 56, 59–61. [Google Scholar]
- Bukva, V. Demodex agrarii sp. n. (Acari: Demodecidae) from cerumen and the sebaceous glands in the ears of the striped field mouse, Apodemus agrarius (Rodentia). Folia Parasitol. 1994, 41, 305–311. [Google Scholar]
- Xu, Y.; Xie, H.; Liu, S.; Zhou, Z.; Shi, X. A new species of the genus Demodex (Acariformes: Demodicidae). Acta Zool. Sin. 1986, 32, 163–167. [Google Scholar]
- Nutting, W.B.; Sweatman, G.K. Demodex antechini sp.nov. (Acari, Demodicidae) parasitic on Antechinus stuartii (Marsupialia). Parasitology 1970, 60, 425–429. [Google Scholar] [CrossRef]
- Bregetova, N.G.; Bulanova-Zahvatkina, E.M.; Volgin, V.I.; Dubinin, V.B.; Zahvatkin, A.A.; Zemskaâ, A.A.; Lange, A.B.; Pavlovskij, E.N.; Serdûkova, G.V.; Šluger, E.G. Kleŝi Gryzunov Fauny SSSR; Izdatel’stvo Akademii Nauk SSSR: Moskva/Leningrad, Russia, 1955; pp. 1–459. [Google Scholar]
- Hirst, S. XI.—On four new species of the genus Demodex, Owen. Ann. Mag. Nat. Hist. Ser. 9 1918, 2, 145–146. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Rolbiecki, L.; Fryderyk, S. Demodex auricularis sp. nov. (Acari: Demodecidae) from the ear canal of the European wood mouse Apodemus sylvaticus (Rodentia: Muridae). Int. J. Acarol. 2014, 40, 214–219. [Google Scholar] [CrossRef]
- Nutting, W.B. Studies on the genus Demodex Owen (Acari, Demodicoidea, Demodicidae). Ph.D. Thesis, Cornell University, Ithaca, NY, USA, 1950. [Google Scholar]
- Desch, C.E. Demodex aries sp. nov., a sebaceous gland inhabitant of the sheep, Ovis aries, and a redescription of Demodex ovis Hirst, 1919. N. Z. J. Zool. 1986, 13, 367–375. [Google Scholar] [CrossRef]
- Bukva, V. Three species of the hair follicle mites (Acai: Demodecidae) parasitizing the sheep, Ovis aries L. Folia Parasitol. 1990, 37, 81–91. [Google Scholar]
- Vargas, M.; Bassols, I.B.; Desch, C.E.; Quintero, M.T.; Polaco, O.J. Description of two new species of the genus Demodex Owen, 1843 (Acari: Demodecidae) associated with Mexican bats. Int. J. Acarol. 1995, 21, 75–82. [Google Scholar] [CrossRef]
- Nutting, W.B. Demodex aurati sp. nov. and D. criceti, ectoparasites of the golden hamster (Mesocricetus auratus). Parasitology 1961, 51, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Nutting, W.B.; Rauch, H. Distribution of Demodex aurati in the host (Mesocricetus auratus) skin complex. J. Parasitol. 1963, 49, 323–329. [Google Scholar] [CrossRef]
- Owen, D.; Young, C. The occurrence of Demodex aurati and Demodex criceti in the Syrian hamster (Mesocricetus auratus) in the United Kingdom. Vet. Rec. 1973, 17, 282–284. [Google Scholar] [CrossRef]
- Retnasabapathy, A.; Lourdusamy, D. Demodex aurati and Demodex criceti in the golden hamster (Mesocricetus auratus). Southeast. Asian J. Trop. Med. Public Health. 1974, 5, 460. [Google Scholar] [CrossRef]
- Cardoso, M.J.L.; Franco, S.R.V.S. Demodicosis in golden hamster (Mesocricetus auratus)—Case in Brasil. Ars Vet. 2003, 19, 126–128. [Google Scholar]
- Karaer, Z.; Kurtdede, A.; Ural, K.; Sari, B.; Cingi, C.C.; Karakurum, M.C.; Haydardedeoğlu, A.E. Demodicosis in a golden (Syrian) hamster (Mesocricetus auratus). Ankara Univ. Vet. Fak. Derg. 2009, 56, 227–229. [Google Scholar]
- Brosseau, G. Oral fluralaner as a treatment for Demodex aurati and Demodex criceti in a golden (Syrian) hamster (Mesocricetus auratus). Can. Vet. J. 2020, 61, 135–137. [Google Scholar] [PubMed]
- Firda, K.E.; Nutting, W.B.; Sweatman, G.K. Demodex bantengi n. sp. from Bos javanicus (D’alton) with notes on gross pathology (Acari: Demodicidae). Int. J. Acarol. 1987, 13, 227–231. [Google Scholar] [CrossRef]
- Kniest, F.M.; Lukoschus, F.S. Parasites of Western Australia. XIII. A new species of demodicid mite from the meibomian glands of the bat Macroglossus minimus. Rec. West. Aust. Mus. 1981, 9, 111–118. [Google Scholar]
- Kadulski, S.; Izdebska, J.N. Demodex bisonianus sp. nov. (Acari, Demodicidae) a new parasite of the bison (Bison bonasus L.). Wiad. Parazytol. 1996, 42, 103–110. [Google Scholar] [PubMed]
- Izdebska, J.N. Zmienność Adulti i Form Juwenilnych Demodex bisonianus (Acari, Demodecidae). In Stawonogi Pasożytnicze i Alergogenne; Buczek, A., Błaszak, C., Eds.; KGM: Lublin, Poland, 2000; pp. 47–56. [Google Scholar]
- Izdebska, J.N. The occurrence of parasitic arthropods in two groups of European bison in the Białowieża Primeval Forest. Wiad. Parazytol. 2001, 47, 801–804. [Google Scholar] [PubMed]
- Izdebska, J.N. Stawonogi pasożytnicze żubrów z Bieszczad. Sci. Messenger Lviv State Acad. Vet. Med. S.Z. Gzhytskyj 2001, 3, 208–211. [Google Scholar]
- Izdebska, J.N. European bison arthropod parasites from closed Polish breeding facilities. Acta Parasitol. 2001, 46, 135–137. [Google Scholar]
- Izdebska, J.N. Skin mites (Acari: Demodecidae, Psoroptidae and Sarcoptidae) of the European bison, Bison bonasus. Biol. Lett. 2006, 43, 169–174. [Google Scholar]
- Izdebska, J.N. Roztocze skórne (Acari: Demodecidae, Psoroptidae, Sarcoptidae) żubra na tle akarofauny ssaków kopytnych—Problemy specyficzności żywicielskiej i topicznej. In Rola Hodowli Ex Situ w Procesie Restytucji Zubra; Olech, W., Ed.; O.K.L.: Gołuchów, Poland, 2007; pp. 22–27. [Google Scholar]
- Stiles, C. On Demodex folliculorum var. bovis in American cattle. Can. Entomol. 1892, 24, 286–290. [Google Scholar] [CrossRef]
- Salib, F.A. First report of bovine demodicosis in native Egyptian cow. Biomed. J. Sci. Tech. Res. 2018, 11, 8424–8426. [Google Scholar] [CrossRef]
- Chanie, M.; Tadesse, S.; Bogale, B. Prevalence of bovine Demodicosisin Gondar Zuria District, Amhara Region, Northwest Ethiopia. Glob. Vet. 2013, 11, 30–35. [Google Scholar]
- Slingenbergh, J.; Mohammed, A.N.; Bida, S.A. Studies on bovine demodecosis in northern Nigeria. Specification and host parasite relationships. Vet. Q. 1980, 2, 90–94. [Google Scholar] [CrossRef]
- Abu-Samra, M.T.; Shuaib, Y.A. Pathology and pathogenesis of bovine skin and meibomian gland demodicosis. Rev. Elev. Med. Vet. Pays Trop. 2014, 67, 77–85. [Google Scholar] [CrossRef]
- Abu-Samra, M.T.; Shuaib, Y.A. Bovine demodicosis: Leather from the raw material to the finished product. J. Soc. Leather Techol. Chem. 2015, 99, 80–90. [Google Scholar]
- Abu-Samra, M.T.; Shuaib, Y.A. Morphometric and morphologic characteristics of Demodex bovis and Demodex ghanensis (Acarina: Trombidiformes: Demodicidae) isolated from cattle in the Sudan. J. Anim. Vet. Sci. 2017, 3, 25–33. [Google Scholar]
- Kennedy, M.J. Mange in cattle: Demodectic mange. Agri Facts 2001, 4, 1–2. [Google Scholar]
- Desch, D.E.; Nutting, W.B. Demodicids (Trombidiformes: Demodicidae) of medical and veterinary importance. In Proceedings of the 3d International Congress of Acarology, Prague, Czech Republic, 31 August–6 September 1971; pp. 499–505. [Google Scholar]
- Trucco, T.; Ahibe, H.; Arribillaga, M. Sarna demodécica (Demodex bovis) en terneros en la provincia de Entre Ríos. Descripción de un cuadro clínico. Vet. Arg. 2019, 36, 1–8. [Google Scholar]
- Cardona, J.; Vargas, M.; Perdomo, S. Descripción clínica de la demodicosis bovina (Demodex bovis) en Córdoba, Colombia. Rev. Investig. Vet. Perú 2013, 24, 125–129. [Google Scholar] [CrossRef]
- Reddy, B.S.; Sivajothi, S. Clinical management of demodicosis in Ongole cattle. J. Parasit. Dis. 2016, 40, 1311–1312. [Google Scholar] [CrossRef][Green Version]
- Matthes, H.F.; Bukva, V. Features of bovine demodecosis (Demodex bovis Stiles, 1892) in Mongolia: Preliminary observations. Folia Parasitol. 1993, 40, 154–155. [Google Scholar]
- Helson, G.A.H. Some arthropods affecting man and livestock in New Zealand. N. Z. Vet. J. 1956, 4, 11–18. [Google Scholar] [CrossRef]
- Nutting, W.B.; Kettle, P.R.; Tenquist, J.D.; Whitten, L.K. Hair follicle mites (Demodex spp.) in New Zealand. N. Z. J. Zool. 1975, 2, 219–222. [Google Scholar] [CrossRef]
- Bukva, V.; Vítovec, J.; Schandl, V. První nálezy demodikózy skotu v Československu. Vet. Med. Praha 1985, 30, 515–520. [Google Scholar] [PubMed]
- Hoffmann, G. Grössenunterschiede bei Demodex bovis (Acari). Angew. Parasitol. 1989, 30, 141–143. [Google Scholar]
- Nemeséri, L.; Széky, A. Die Rinderdemodikose. Berl. Munch. Tierarztl. Wochenschr. 1962, 16, 304–307. [Google Scholar]
- Villa, L.; Gazzonis, A.L.; Perlotti, C.; Zanzani, S.A.; Sironi, G.; Manfredi, M.T. First report of Demodex bovis infestation in bovine besnoitiosis co-infected dairy cattle in Italy. Parasitol. Int. 2020, 79. in press. [Google Scholar] [CrossRef]
- Grzywiński, L.; Kliszewski, E.; Piotrowski, R. Demodekoza bydła i jej zwalczanie. Wiad. Parazytol. 1986, 32, 585–590. [Google Scholar]
- de Araújo Wanderley, J.N.; Athayde, A.C.R.; de Moura, J.F.P.; Bezerra, L.R.; de Melo Vaz, A.F.; de Lima, E.Q.; de Oliveira, J.P.F.; Silva, W.W. Factors affecting occurrence of demodecosis by Demodex bovis in Sindhi cattle (Bos indicus). Trop. Anim. Health Prod. 2020, in press. [Google Scholar]
- Desch, C.; Nutting, W.B. Demodex folliculorum (Simon) and D. brevis Akbulatova of man: Redescription and reevaluation. J. Parasitol. 1972, 58, 169–177. [Google Scholar] [CrossRef]
- Sengbusch, H.G.; Hauswirth, J.W. Prevalence of hair follicle mites, Demodex folliculorum and D. brevis (Acari: Demodicidae), in a selected human population in Western New York, USA. J. Med. Entomol. 1986, 23, 384–388. [Google Scholar] [CrossRef]
- Gao, Y.Y.; Di Pascuale, M.A.; Li, W.; Liu, D.T.; Baradaran-Rafii, A.; Elizondo, A.; Kawakita, T.; Raju, V.K.; Tseng, S.C. High prevalence of Demodex in eyelashes with cylindrical dandruff. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3089–3094. [Google Scholar] [CrossRef]
- Zhao, Y.E.; Guo, N.; Wu, L.P. The effect of temperature on the viability of Demodex folliculorum and Demodex brevis. Parasitol. Res. 2009, 105, 1623–1628. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Ding, X.; Tseng, S.C.G. High prevalence of Demodex brevis infestation in Chalazia. Am. J. Ophthalmol. 2014, 157, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Nutting, W.B.; Green, A.C. Pathogenesis associated with hair follicle mites (Demodex spp.) in Australian Aborigines. Br. J. Dermatol. 1976, 94, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Izdebska, J.N.; Jankowski, Z. Demodex brevis and D. folliculorum (Actinedida, Demodecidae): Specific human parasites. A comparative study of the effectiveness of diagnostic methods involving autopsy. In Advances in Polish Acarology; Gabryś, G., Ignatowicz, S., Eds.; SGGW: Warszawa, Poland, 2006; pp. 128–136. [Google Scholar]
- Akbulatova, L.H. Demodekoza čeloveka. Vestnik Dermat. Venerol. 1963, 38, 34–42. [Google Scholar]
- Kaya, S.; Selimoglu, M.A.; Kaya, O.A.; Ozgen, U. Prevalence of Demodex folliculorum and Demodex brevis in childhood malnutrition and malignancy. Pediatr. Int. 2013, 55, 85–89. [Google Scholar] [CrossRef]
- Sari, Y.; Zeytun, E.; Doğan, S.; Karakurt, Y. Oküler dandrufflu hastalarda Demodex folliculorum ve D. brevis (Acari: Demodicidae) yaygınlığı ve yoğunluğu. Acarol. Stud. 2019, 1, 23–43. [Google Scholar]
- Zeytun, E.; Karakurt, Y. Prevalence and load of Demodex folliculorum and Demodex brevis (Acari: Demodicidae) in patients with chronic blepharitis in the province of Erzincan, Turkey. J. Med. Entomol. 2019, 8, 2–9. [Google Scholar] [CrossRef]
- Bukva, V.; Vítovec, J.; Vlček, M. Demodex rosus sp. n. and D. buccalis sp. n. (Acari: Demodicidae) parasitizing the upper digestive tract of rodents. Folia Parasitol. 1985, 32, 151–162. [Google Scholar]
- Izdebska, J.N.; Kozina, P.; Gólcz, A. The occurrence of Demodex spp. (Acari, Demodecidae) in bank vole Myodes glareolus (Rodentia, Cricetidae) with data on its topographical preferences. Ann. Parasitol. 2013, 59, 129–133. [Google Scholar]
- Railliet, A. Traité de Zoologie Médicale et Agricole; Asselin et Houzeau: Paris, France, 1895; pp. 1–1303. [Google Scholar]
- Desch, C.E.; Nutting, W.B. Redescription of Demodex caballi (= D. folliculorum var. equi Railliet, 1895) from the horse, Equus caballus. Acarologia 1978, 20, 235–240. [Google Scholar]
- Mathes, R.L.; Paige Carmichael, K.; Peroni, J.; Anthony Moore, P. Primary lacrimal gland adenocarcinoma of the third eyelid in a horse. Vet. Ophthalmol. 2011, 14, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Tenquist, J.D.; Charleston, W.A.G. An annotated checklist of ectoparasites of terrestrial mammals in New Zealand. J. R. Soc. N. Z. 2001, 31, 481–542. [Google Scholar] [CrossRef]
- Dräger, N.; Paine, G.D. Demodicosis in African buffalo (Syncerus caffer caffer) in Botswana. J. Wildl. Dis. 1980, 16, 521–524. [Google Scholar] [CrossRef]
- Nutting, W.B.; Guilfoy, F.M. Demodex cafferi n. sp. from the African buffalo, Syncerus cafer. Int. J. Acarol. 1979, 5, 9–14. [Google Scholar] [CrossRef]
- Wolhuter, J.; Bengis, R.G.; Reilly, B.K.; Cross, P.C. Clinical demodicosis in African buffalo (Syncerus caffer) in the Kruger National Park. J. Wildl. Dis. 2009, 45, 502–504. [Google Scholar] [CrossRef][Green Version]
- Leydig, F. Ueber Haarsackmilben und Krätzmilben. Arch. Naturgesch. 1859, 25, 338–354. [Google Scholar]
- Nutting, W.B.; Desch, C.E. Demodex canis: Redescription and reevaluation. Cornell Vet. 1978, 68, 139–149. [Google Scholar] [PubMed]
- Arroyo-Munive, Y.J.; Hincapié-Gutiérrez, L.C. Demodicosis generalizada canina tratada con Fluralaner: Reporte de un caso. Vet. Zoot. 2018, 12, 62–71. [Google Scholar]
- Guerra, Y.; Mencho, J.D.; Rodríguez Diego, J.G.; Marín, E.; Olivares, J.L. Demodex spp. en perros con demodicosis, en una región de Cuba. Rev. Salud Anim. 2010, 32, 37–41. [Google Scholar]
- Sivajothi, S.; Sudhakara Reddy, B.; Kumari, K.N.; Rayulu, V.C. Morphometry of Demodex canis and Demodex cornei in dogs with demodicosis in India. Int. J. Vet. Health Sci. Res. 2013, 1, 1–4. [Google Scholar]
- Sivajothi, S.; Sudhakara Reddy, B.; Rayulu, V.C. Demodicosis caused by Demodex canis and Demodex cornei in dogs. J. Parasit. Dis. 2015, 39, 673–676. [Google Scholar] [CrossRef]
- Veena, M.; Dhanalakshmi, H.; Kavitha, K.; Souza, P.E.D.; Puttalaksmamma, G.C. Morphological characterization of Demodex mites and its therapeutic management with neem leaves in canine demodicosis. J. Entomol. Zool. Stud. 2017, 5, 661–664. [Google Scholar]
- Sharma, S.; Pokharel, S. Diagnosis and therapeutic management of mixed Demodex and Sarcoptes mite infestation in dog. Acta Sci. Agric. 2019, 3, 163–166. [Google Scholar]
- Ashfaq, K.; Aqib, A.I.; Fakhar-e-Alam Kulyar, M.; Naeem, R.F.; Shoaib, M.; Bhutta, Z.A.; Tanveer, Q.; Asif, M. Alternative therapeutic approach to treat canine demodicosis. EC Vet. Sci. 2019, 4, 251–256. [Google Scholar]
- Sakulploy, R.; Sangvaranond, A. Canine demodicosis caused by Demodex canis and short opisthosomal Demodex cornei in Shi Tzu dog from Bangkok Metropolitan Thailand. Kasetsart Vet. 2010, 20, 27–35. [Google Scholar]
- Thomson, G.M. The Naturalisation of Animals and Plants in New Zealand; Cambridge University Press: Cambridge, UK, 1922; pp. 1–607. [Google Scholar]
- Ali, M.H.; Begum, N.; Azam, M.G.; Roy, B.C. Prevalence and pathology of mite infestation in street dogs at Dinajpur municipality area. J. Bangladesh Agril. Univ. 2011, 9, 111–119. [Google Scholar] [CrossRef]
- Izdebska, J.N. Demodecid mites (Acari, Actinedida) in carnivorous mammals (Mammalia, Carnivora) in Poland. In Arthropods. A Variety of Forms and Interactions; Buczek, A., Błaszak, C., Eds.; Koliber: Lublin, Poland, 2005; pp. 121–125. [Google Scholar]
- Pawełczyk, O.; Pająk, C.; Solarz, K. The risk of exposure to parasitic mites and insects occurring on pets in Southern Poland. Ann. Parasitol. 2016, 62, 337–344. [Google Scholar]
- Moskvina, T.V. Two morphologically distinct forms of Demodex mites found in dogs with canine demodicosis from Vladivostok, Russia. Acta Vet. Beogr. 2017, 67, 82–91. [Google Scholar] [CrossRef]
- Pekmezci, G.Z.; Pekmezci, D.; Bolukbas, C.S. Molecular characterization of Demodex canis (Acarina: Demodicidae) in domestic dogs (Canis familiaris). Kocatepe Vet. J. 2018, 11, 430–433. [Google Scholar] [CrossRef]
- Mulugeta, Y.; Yacob, H.T.; Ashenafi, H. Ectoparasites of small ruminants in three selected agro-ecological sites of Tigray Region, Ethiopia. Trop. Anim. Health Prod. 2010, 42, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.E.; Cheng, J.; Hu, L.; Ma, J.X. Molecular identification and phylogenetic study of Demodex caprae. Parasitol. Res. 2014, 113, 3601–3608. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, P.; Lukešová, D.; Skřivánek, M.; Hofírek, B.; Štursa, I. Privní nálezy demodikózy koz v České Republice. Vet. Med. (Praha) 1996, 41, 289–293. [Google Scholar]
- Kaba, J.; Fagasiński, A.; Krawiec, M. Nużyca u kóz. Życie Wet. 1999, 74, 24–28. [Google Scholar]
- Strabel, D.; Schweizer, G.; Gansohr, B.; Braun, U. Der einsatz von avermectinen bei zwei ziegen mit demodikose. Schweiz. Arch. Tierheilkd. 2003, 145, 585–587. [Google Scholar] [CrossRef]
- Desch, C.; Lebel, R.R.; Nutting, W.B.; Lukoschus, F. Parasitic mites of Surinam: I. Demodex carolliae sp. nov. (Acari: Demodicidae) from the fruit bat Carollia perspicillata. Parasitology 1971, 62, 303–308. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Fryderyk, S.; Rolbiecki, L. Demodex castoris sp. nov. (Acari: Demodecidae) parasitizing Castor fiber (Rodentia), and other parasitic arthropods associated with Castor spp. Dis. Aquat. Org. 2016, 118, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Megnin, P. Memoire sur le Demodex folliculorum Owen. J. Aanat. Physiol. 1877, 13, 97–122. [Google Scholar]
- Desch, C.; Nutting, W.B. Demodex cati Hirst 1919: A redescription. Cornell Vet. 1979, 69, 280–285. [Google Scholar]
- Bizikova, P. Localized demodicosis due to Demodex cati on the muzzle of two cats treated with inhalant glucocorticoids. Vet. Dermatol. 2014, 25, 222–225. [Google Scholar] [CrossRef]
- Valandro, M.A.; da Exaltação Pascon, J.P.; de Arruda Mistieri, M.L.; Gallina, T. Demodiciose felina por Demodex cati. Acta Sci. Vet. 2016, 44 (Suppl. 1), 1–4. [Google Scholar]
- Iliev, P.T.; Zhelev, G.; Ivanov, A.; Prelezov, P. Demodex cati and feline immunodeficiency virus co-infection in a cat. Bulg. J. Vet. Med. 2019, 22, 237–242. [Google Scholar] [CrossRef]
- Löwenstein, C.; Beck, W.; Bessmann, K.; Mueller, R.S. Feline demodicosis caused by concurrent infestation with Demodex cati and an unnamed species of mite. Vet. Rec. 2005, 157, 290–292. [Google Scholar] [CrossRef] [PubMed]
- Matricoti, I.; Maina, E. The use of oral fluralaner for the treatment of feline generalized demodicosis: A case report. J. Small Anim. Pract. 2017, 58, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Ortúñez, A.; Verde, M.T.; Navarro, L.; Real, L.; Vilela, C. Demodicosis felina: A propósito de tres casos clínicos. Clin. Vet. Peq. Anim. 2009, 29, 165–171. [Google Scholar]
- Bacigalupo, J.; Roveda, R.J. Demodex caviae n. sp. Rev. Med. Vet. 1954, 36, 149–153. [Google Scholar]
- Ballweber, L.R.; Harkness, J.E. Parasites of guinea pigs. In Flynn’s Parasites of Laboratory Animals; Baker, D.G., Ed.; Blackwell Publishing: Ames, IA, USA, 2007; pp. 421–449. [Google Scholar]
- Schönfelder, J.; Henneveld, K.; Schönfelder, A.; Hein, J.; Müller, R. Concurrent infestation of Demodex caviae and Chirodiscoides caviae in a guinea pig. A case report. Tierarztl. Prax. Kleintiere 2010, 38, 28–30. [Google Scholar]
- Bukva, V. Demodex kutzeri sp. n. (Acari: Demodicidae), an identical parasite of two species of deer, Cervus elaphus and C. nippon pseudaxis. Folia Parasitol. 1987, 34, 173–181. [Google Scholar]
- Hirst, S. On some new or little-known species of Acari, mostly parasitic in habit. Proc. Zool. Soc. Lond. 1921, 1, 357–378. [Google Scholar]
- Izdebska, J.N.; Rolbiecki, L. A new species of the genus Demodex Owen, 1843 (Acari: Demodecidae) from the ear canals of the house mouse Mus musculus L. (Rodentia: Muridae). Syst. Parasitol. 2015, 91, 167–173. [Google Scholar] [CrossRef]
- Nutting, W.B.; Rauch, H. Demodex criceti n. sp. (Acarina: Demodicidae) with notes on its biology. J. Parasitol. 1958, 44, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Hurley, R.J.; Desch, C.E. Demodex cricetuli: New species of hair follicle mite (Acari: Demodecidae) from the Armenian hamster, Cricetulus migratorius (Rodentia: Cricetidae). J. Med. Entomol. 1994, 31, 529–533. [Google Scholar] [CrossRef]
- Harvey, R.G. Demodex cuniculi in dwarf rabbits (Oryctolagus cuniculus). J. Small Anim. Pract. 1990, 31, 204–207. [Google Scholar] [CrossRef]
- Morita, T.; Ohmi, A.; Kiwaki, A.; Ike, K.; Nagata, K. A new stubby species of Demodectic mite (Acari: Demodicidae) from the domestic dog (Canidae). J. Med. Entomol. 2018, 55, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Desch, C.E.; Stewart, B. First description of a hair follicle mite from the host order Xenarthra: Demodex dasypodi n. sp. (Acari: Demodecidae) from the nine-banded armadillo, Dasypus novemcinctus Linnaeus, 1758 (Dasypodidae). Int. J. Acarol. 2002, 28, 169–174. [Google Scholar] [CrossRef]
- Desch, C.E. A new species of Demodex Owen, 1843 (Acari: Demodecidae) from the meibomian glands of the vampire bat Desmodus rotundus (E. Geoffroy, 1810) (Chiroptera: Phyllostomidae: Desmodontinae) from Surinam. Int. J. Acarol. 1994, 20, 39–43. [Google Scholar] [CrossRef]
- Hirst, S. LII.—On three new parasitic Acari. Ann. Mag. Nat. Hist. Ser. 8 1917, 20, 431–434. [Google Scholar] [CrossRef]
- Besch, E.D.; Griffiths, H.J. Demonstration of Demodex equi (Railliet, 1895) from a horse from Minnesota. J. Am. Vet. Med. Assoc. 1956, 128, 82–83. [Google Scholar]
- Izdebska, J.N. Symptomless Skin Mite Infestation of Horses in Northern Poland. In Advances in Polish Acarology; Gabryś, G., Ignatowicz, S., Eds.; SGGW: Warszawa, Poland, 2006; pp. 123–127. [Google Scholar]
- Bukva, V. Demodex flagellurus sp. n. (Acari: Demodicidae) from the preputial and clitoral glands of the house mouse, Mus musculus L. Folia Parasitol. 1985, 32, 73–81. [Google Scholar]
- Izdebska, J.N. Nowe gatunki Demodex spp. (Acari, Demodecidae) u Mus musculus w Polsce. Wiad. Parazytol. 2000, 46, 277–280. [Google Scholar]
- Izdebska, J.N.; Rolbiecki, L. Correlation between the occurrence of mites (Demodex spp.) and nematodes in the house mice (Mus musculus Linnaeus, 1758) in the Gdańsk urban agglomeration. Biol. Lett. 2006, 43, 175–178. [Google Scholar]
- Izdebska, J.N.; Rolbiecki, L. Two new species of Demodex (Acari: Demodecidae) with a redescription of Demodex musculi and data on parasitism in Mus musculus (Rodentia: Muridae). J. Med. Entomol. 2015, 52, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Yassine, M.; Thorraya, L.; Haiet, A.H. Demodex follicullorum mimicking fungal infection: A case report. J. Dermat. 2018, 3, 1–2. [Google Scholar] [CrossRef]
- Salem, D.A.; El-Shazly, A.; Nabih, N.; El-Bayoumy, Y.; Saleh, S. Evaluation of the efficacy of oral ivermectin in comparison with ivermectin–metronidazole combined therapy in the treatment of ocular and skin lesions of Demodex folliculorum. Int. J. Infect. Dis. 2013, 17, e343–e347. [Google Scholar] [CrossRef] [PubMed]
- Tandon, N.; Hati, A.K.; Thammayya, A. Demodex folliculorum in man. Rec. Zool. Surv. India 1980, 76, 139–142. [Google Scholar]
- Hallur, V.; Singh, G.; Rudramurthy, S.M.; Kapoor, R.; Chakrabarti, A. Demodex mite infestation of unknown significance in a patient with rhinocerebral mucormycosis due to Apophysomyces elegans species complex. J. Med. Microbiol. 2013, 62, 926–928. [Google Scholar] [CrossRef]
- Forton, F.; Seys, B. Density of Demodex folliculorum in rosacea: A case-control study using standardized skin-surface biopsy. Br. J. Dermatol. 1993, 128, 650–659. [Google Scholar] [CrossRef]
- Basta-Juzbašić, A.; Šubić, J.Š.; Ljubojević, S. Demodex folliculorum in development of dermatitis rosaceiformis steroidica and rosacea-related diseases. Clin. Dermatol. 2002, 20, 135–140. [Google Scholar] [CrossRef]
- Simon, G. Ueber eine in den kranken und normalen Haarsäcken des Menschen lebende Milbe. Arch. Anat. Physiol. Wissensch. Med. 1842, 11, 218–237. [Google Scholar]
- Spickett, S.G. Studies on Demodex folliculorum Simon (1842). I. Life history. Parasitology 1961, 51, 181–192. [Google Scholar] [CrossRef]
- Larios, G.; Alevizos, A.; Perimeni, D.; Rigopoulos, D.; Katsambas, A. Rosacea-like demodicidosis. Lancet Infect. Dis. 2008, 8, 804. [Google Scholar] [CrossRef]
- Gunnarsdottir, S.; Kristmundsson, A.; Freeman, M.A.; Bjornsson, O.M.; Zoega, G.M. Demodex folliculorum (hair follicle mite), a hidden cause of blepharitis. Laeknabladid 2016, 102, 231–235. [Google Scholar] [PubMed]
- Murphy, O.; O’Dwyer, V.; Lloyd-McKernan, A. The efficacy of tea tree face wash, 1, 2-Octanediol and microblepharoexfoliation in treating Demodex folliculorum blepharitis. Contact Lens Anterior Eye 2018, 41, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Eser, A.; Erpolat, S.; Kaygusuz, I.; Balci, H.; Kosus, A. Investigation of Demodex folliculorum frequency in patients with polycystic ovary syndrome. An. Bras. Dermatol. 2017, 92, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Doğan, S.; Doğan, S. An accidental finding of a demodectic mite on a slide: Demodex folliculorum (Simon) (Acari: Demodecidae). Syst. Appl. Acarol. 2019, 24, 962–964. [Google Scholar] [CrossRef]
- Xie, H.X.; Liu, S.L.; Hsu, Y.H.; Hsu, Y.C. Taxonomy of the family Demodicidae and a new subspecies (Acarina: Demodicidae). Acta Zootaxon. Sin. 1982, 7, 265–269. [Google Scholar]
- Bukva, V. Demodex foveolator sp. n. (Acari: Demodicidae), a new epidermis-dwelling parasite of Crocidura suaveolens (Pallas, 1821). Folia Parasitol. 1984, 31, 42–52. [Google Scholar]
- Nutting, W.B.; Emejuaiwe, S.O.; Tisdel, M.O. Demodex gapperi sp. n. (Acari: Demodicidae) from the red-backed vole, Clethrionomys gapperi. J. Parsitol. 1971, 57, 660–665. [Google Scholar] [CrossRef]
- Desch, C.E.; Stewart, T.B. Demodex gatoi: New species of hair follicle mite (Acari: Demodecidae) from the domestic cat (Carnivora: Felidae). J. Med. Entomol. 1999, 36, 167–170. [Google Scholar] [CrossRef]
- Short, J.; Gram, D. Successful treatment of Demodex gatoi with 10% Imidacloprid/1% Moxidectin. J. Am. Anim. Hosp. Assoc. 2016, 52, 68–72. [Google Scholar] [CrossRef]
- Silbermayr, K.; Joachim, A.; Litschauer, B.; Panakova, L.; Sastre, N.; Ferrer, L.; Horvath-Ungerboeck, C. The first case of Demodex gatoi in Austria, detected with fecal flotation. Parasitol. Res. 2013, 112, 2805–2810. [Google Scholar] [CrossRef] [PubMed]
- Saari, S.A.M.; Juuti, K.H.; Palojärvi, J.H.; Väisänen, K.M.; Rajaniemi, R.L.; Saijonmaa-Koulumies, L.E. Demodex gatoi - associated contagious pruritic dermatosis in cats—A report from six households in Finland. Acta Vet. Scand. 2009, 51, 1–8. [Google Scholar] [CrossRef]
- Oppong, E.N.W.; Lee, R.P.; Yasin, S.A. Demodex ghanensis sp. nov. (Acari, Demodicidae) parasitic on West African cattle. Ghana J. Sci. 1975, 15, 39–43. [Google Scholar]
- Mertens, L.A.J.M.; Lukoschus, F.S.; Nutting, W.B. Demodex huttereri spec. nov. (Acarina: Prostigmata: Demodicidae) from the meibomian glands of Apodemus agrarius (Rodentia: Muridae). Bonn. Zool. Beitr. 1983, 34, 489–498. [Google Scholar]
- Izdebska, J.N.; Rolbiecki, L.; Fryderyk, S. New data on distribution of Demodex huttereri Mertens, Lukoschus et Nutting, 1983 and topical specificity and topography of demodectic mites in striped field mouse Apodemus agrarius. Wiad. Parazytol. 2011, 57, 261–264. [Google Scholar]
- Desch, C.E.; Hillier, A. Demodex injai: A new species of hair follicle mite (Acari: Demodecidae) from the domestic dog (Canidae). J. Med. Entomol. 2003, 40, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Amaral Silva Sgarbossa, R.S.; Vieira Sechi, G.; Duarte Pacheco, B.; Buba Lucina, S.; Roberto Paulo, M.; dos Santos Monti, F.; Rodriges de Farias, M. The epidemiological and clinical aspects of Demodex injai demodicosis in dogs: A report of eight cases. Semin Cienc. Agrar. 2017, 38, 3387–3394. [Google Scholar] [CrossRef]
- Ordeix, L.; Bardagi, M.; Scarampella, F.; Ferrer, L.; Fondati, A. Demodex injai infestation and dorsal greasy skin and hair in eight wirehaired fox terrier dogs. Vet. Dermatol. 2009, 20, 267–272. [Google Scholar] [CrossRef]
- Lukoschus, F.S.; Mertens, L.J.A.M.; Nutting, W.B.; Nadchatram, M. Demodex intermedius sp. nov. (Acarina: Prostigmata: Demodicidae) from the meibomian glands of the tree-shrew Tupaia glis (Mammalia: Scandentia). Malay. Nat. J. 1984, 36, 233–245. [Google Scholar]
- Kadulski, S. Dalsze badania nad stawonogami pasożytniczymi łosia Alces alces w Polsce. Wiad. Parazytol. 1996, 42, 349–355. [Google Scholar]
- Kadulski, S. Ectoparasites of Cervidae in north-east Poland. Acta Parasitol. 1996, 41, 204–210. [Google Scholar]
- Izdebska, J.N.; Fryderyk, S. Demodex spp. (Acari, Demodecidae) in wild Artiodactyla in Poland. Acta Parasitol. 2000, 45, 163–164. [Google Scholar]
- Kutzer, E.; Grünberg, W. Demodikose beim Rothirsch (Cervus elaphus). Z. Parasitenkd. 1972, 40, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Izdebska, J.N.; Kozina, P.; Fryderyk, S. The occurrence of Demodex kutzeri Bukva, 1987 (Acari, Demodecidae) in red deer (Cervus elaphus L.) in Poland. Ann. Parasitol. 2013, 59, 85–88. [Google Scholar] [PubMed]
- Desch, C.E.; Andrews, J.J.; Baeten, L.A.; Holder, Z.; Powers, J.G.; Weber, D.; Ballweber, L.R. New records of hair follicle mites (Demodecidae) from North American Cervidae. J. Wildl. Dis. 2010, 46, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Palopoli, M.F.; Tra, V.; Matoin, K.; Mac, P.D. Evolution of host range in the follicle mite Demodex kutzeri. Parasitology 2017, 144, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Kadulski, S.; Szczurek, B. Ectoparasites on fallow deer, Dama dama (L.) in Pomerania, Poland. Acta Parasitol. 2004, 49, 80–86. [Google Scholar]
- Lukoschus, F.S.; Jongman, R.G.H. Demodex lacrimalis spec. nov. (Demodicidae: Trombidiformes) from the Meibomian glands of the European wood mouse Apodemus sylvaticus. Acarologia 1974, 16, 274–281. [Google Scholar]
- Izdebska, J.N.; Fryderyk, S. New data on the occurrence of Demodex lacrimalis (Acari, Demodecidae) of the wood mouse Apodemus sylvaticus (Rodentia, Muridae). Ann. UMCS Biol. 2012, 67, 7–11. [Google Scholar] [CrossRef][Green Version]
- Hughes, S.E.; Nutting, W.B. Demodex leucogasteri n. sp. from Onychomys leucogaster—With notes on its biology and host pathogenesis. Acarologia 1981, 22, 181–186. [Google Scholar]
- Desch, C.; Nutting, W.B.; Lukoschus, F.S. Parasitic mites of Surinam VII: Demodex longissimus n. sp. from Carollia perspicillata and D. molossi n. sp. from Molossus molossus (Demodicidae: Trombidiformes); meibomian complex inhabitants of Neotropical bats (Chiroptera). Acarologia 1972, 14, 35–53. [Google Scholar]
- Karjala, Z.; Desch, C.E.; Starost, M.F. First description of a new species of Demodex (Acari: Demodecidae) from rhesus monkey. J. Med. Entomol. 2005, 42, 948–952. [Google Scholar] [CrossRef]
- Desch, C.E. A new species of demodicid mite (Acari: Prostigmata) from Western Australia parasitic on Macroglossus minimus (Chiroptera: Pteropodidae). Rec. West. Aust. Mus. 1981, 9, 41–47. [Google Scholar]
- Nutting, W.B.; Lukoschus, F.S.; Desch, C.E. Parasitic mites of Surinam. XXXVII. Demodex marsupiali sp. nov. from Didelphis marsupialis: Adaptation to glandular habitat. Zool. Meded. Leiden 1980, 56, 83–92. [Google Scholar]
- Lukoschus, F.S.; Jongman, R.H.G.; Nutting, W.B. Parasitic mites of Surinam. XII. Demodex melanopteri sp. n. (Demodicidae: Trombidiformes) from the meibomian glands of the Neotropical bat Eptesicus melanopterus. Acarologia 1972, 14, 54–58. [Google Scholar]
- Hirst, S. On three new parasitic mites (Leptus, Schongastia, and Demodex). Ann. Mag. Nat. Hist. 1921, 7, 37–39. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Rolbiecki, L. Demodex microti n. sp. (Acari: Demodecidae) in Microtus arvalis (Pallas) (Rodentia, Cricetidae) with a checklist of the demodecid mites of cricetids. Syst. Parasitol. 2013, 86, 187–196. [Google Scholar] [CrossRef]
- Oudemans, A.C. List of Dutch Acari. 7th part: Acarididae Latr. 1806, and Phytoptidae Pagenst. 1861, with synonymical remarks end description of new species etc. Tijdschr. Entomol. 1897, 40, 250–269. [Google Scholar]
- Ventura, J.; Feliu, C.; Foronda, P.; Francino, O.; Sastre, N. First record of the presence of skin mites (Demodex musculi) in wild house mice from the Canary Islands (Spain). Int. J. Acarol. 2020, 46. in press. [Google Scholar]
- Smith, P.C.; Zeiss, C.J.; Beck, A.P.; Scholz, J.A. Demodex musculi infestation in genetically immunomodulated mice. Comp. Med. 2016, 66, 278–285. [Google Scholar]
- Nashat, M.A.; Ricart Arbona, R.J.; Lepherd, M.L.; Santagostino, S.F.; Livingston, R.S.; Riedel, E.R.; Lipman, N.S. Ivermectin-compounded feed compared with topical moxidectin–imidacloprid for eradication of Demodex musculi in laboratory mice. J. Am. Assoc. Lab. Anim. Sci. 2018, 57, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, S.A. The biology of Demodex myotidis sp.nov. (Acarina: Demodicidae) from three species of bats. Master’s Thesis, University of Massachusetts Amherst, Aherst, MA, USA, 1961. [Google Scholar]
- Desch, C.E. Two new species of Demodex (Acari: Demodicidae) from the New Zealand shorttailed bat, Mystacina tuberculata Gray, 1843 (Chiroptera: Mystacinidae). N. Z. J. Zool. 1989, 16, 221–230. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Rolbiecki, L. Występowanie Demodex spp. w Korelacji z Poziomem Zarażenia Helmintami Szczura Wędrownego Rattus norvegicus (Berk.) z Aglomeracji Miejskiej Trójmiasta. In Fauna Miast Europy Środkowej 21. Wieku; Indykiewiecz, P., Barczak, T., Eds.; LOGO: Bydgoszcz, Poland, 2004; pp. 581–584. [Google Scholar]
- Izdebska, J.N.; Rolbiecki, L. New species of Demodex (Acari: Demodecidae) with data on parasitism and occurrence of other demodecids of Rattus norvegicus (Rodentia, Muridae). Ann. Entomol. Soc. Am. 2014, 107, 740–747. [Google Scholar] [CrossRef]
- Desch, C.E. Redescription of Demodex nanus (Acari: Demodicidae) from Rattus norvegicus and R. rattus (Rodentia). J. Med. Entomol. 1987, 24, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Bukva, V. Demodex neomydis sp. n. (Acari: Demodecidae) from the hair follicles of the Mediterranean water shrew, Neomys anomalus (Insectivora: Soricidae). Folia Parasitol. 1995, 42, 299–306. [Google Scholar] [PubMed]
- Desch, C.E.; Lukoschus, F.S.; Nadchatram, M. Two new species of Demodex (Acari: Demodicidae) from the meibomian glands of the tropical Old Word bat, Eonycteris spelaea (Chiroptera). Int. J. Acarol. 1986, 12, 13–25. [Google Scholar] [CrossRef]
- Bukva, V. Demodex species (Acari: Demodecidae) parasitizing the brown rat, Rattus norvegicus (Rodentia): Redescription of Demodex ratti and description of D. norvegicus sp. n. and D. ratticola sp. n. Folia Parasitol. 1995, 42, 149–160. [Google Scholar]
- Desch, C.E. Demodex nycticeii: A new species of hair follicle mite (Acari: Demodecidae) from the evening bat, Nycticeius humeralis (Chiroptera: Vespertilionidae). Int. J. Acarol. 1996, 22, 187–191. [Google Scholar] [CrossRef]
- Desch, C.E.; Nutting, W.B. Demodex odocoilei sp. nov. from the white-tailed deer, Odocoileus virginianus. Can. J. Zool. 1974, 52, 785–789. [Google Scholar] [CrossRef]
- Bildfell, R.J.; Mertins, J.W.; Mortenson, J.A.; Cottam, D.F. Hair-loss syndrome in black-tailed deer of the Pacific Northwest. J. Wildl. Dis. 2004, 40, 670–681. [Google Scholar] [CrossRef][Green Version]
- Yeruham, I.; Rosen, S.; Hadani, A. Sheep demodecosis (Demodex ovis, Railliet, 1895) in Israel. Rev. Elev. Méd. Vét. Pays Trop. 1986, 39, 363–365. [Google Scholar]
- Kamyszek, F.; Wertejuk, M. Badania porównawcze ekstensywności zarażenia ektopasożytami owiec w regionach: Wielkopolskie i szczecińskie w latach 1971–1980. Wiad. Parazytol. 1983, 29, 335–341. [Google Scholar] [PubMed]
- Lombert, H.A.P.M.; Lukoschus, F.S.; Whitaker, J.O. Demodex peromysci, n. sp. (Acari: Prostigmata: Demodicidae), from the meibomian glands of Peromyscus leucopus (Rodentia: Cricetidae). J. Med. Entomol. 1983, 20, 377–382. [Google Scholar] [CrossRef]
- Desch, C.E.; Dailey, M.D.; Tuomi, P. Description of a hair follicle mite (Acari: Demodecidae) parasitic in the earless seal family Phocidae (Mammalia: Carnivora) from the harbor seal Phoca vitulina Linnaeus, 1758. Int. J. Acarol. 2003, 29, 231–235. [Google Scholar] [CrossRef]
- Desch, C.E.; Davis, S.L.; Klompen, H. Two new species of Demodex Owen, 1843, the hair follicle mites (Demodecidae), from the dzungarian hamster, Phodopus sungorus (Pallas, 1773) (Rodentia: Muridae). Int. J. Acarol. 2006, 32, 75–80. [Google Scholar] [CrossRef]
- Kadulski, S. Ectoparasites of the boar Sus scrofa L. in Poland. In Proceedings of the Fourth International Congress of Parasitology, A Conference of the World Federation of Parasitologists, Warszawa, Poland, 19–26 August 1978; pp. 211–212. [Google Scholar]
- Fryderyk, S. Pasożytnicze Acari dzika (Sus scrofa L.) z Pojezierza Pomorskiego. Wiad. Parazytol. 2000, 46, 163–168. [Google Scholar]
- Fryderyk, S.; Izdebska, J.N. Demodex phylloides (Acari, Demodecidae) as a specific parasite of Sus scrofa (Mammalia, Artiodactyla). Wiad. Parazytol. 2001, 47, 797–800. [Google Scholar]
- Izdebska, J.N.; Fryderyk, S. Zmienność morfologiczna Demodex phylloides (Acari, Demodecidae) u dzika (Sus scrofa). In Stawonogi w Medycynie; Buczek, A., Błaszak, C., Eds.; Liber: Lublin, Poland, 2002; pp. 25–34. [Google Scholar]
- Kabululu, M.L.; Ngowi, H.A.; Kimera, S.I.; Lekule, F.P.; Kimbi, E.C.; Johansen, M.V. Risk factors for prevalence of pig parasitoses in Mbeya Region, Tanzania. Vet. Parasitol. 2015, 15, 460–464. [Google Scholar] [CrossRef]
- Ramsay, W.R. On Demodex phylloides, (Csokor), in the skin of Canadian swine. Proc. Can. Inst. 1883, 1, 275–281. [Google Scholar]
- Silveira, R.L.; Ribeiro, R.B.; Cruz, A.C.M.; Almeida, L.G.; Carvalho, E.C.Q. Demodicose suínano norte do estado do Riode Janeiro: Relato de caso. Arq. Bras. Med. Vet. Zootec. 2012, 64, 555–558. [Google Scholar] [CrossRef]
- Bersano, J.G.; Mendes, M.C.; Duarte, F.C.; Del Fava, C.; de Oliveira, S.M.; Filha, E.S.; Pinheiro, E.S.; de Castro Nassar, A.F.; de Vasconcellos Bilynskyj, M.C.; Ogata, R.A.; et al. Demodex phylloides infection in swine reared in a periurban family farm located on the outskirts of the Metropolitan Region of São Paulo, Brazil. Vet. Parasitol. 2016, 230, 67–73. [Google Scholar] [CrossRef]
- Csokor, J. Ueber Haarsaekmilben und eine neue Varietät derselben bei Sehweinen, Demodex phylloides. Verh. Zool. Bot. Ges. Österr. 1879, 29, 419–450. [Google Scholar]
- Puccini, V.; Colella, G. Prima segnalazione per l’Italia sud-orientale di Demodex phylloides Csokor 1879 nel suino. Acta Med. Vet. 1975, 21, 65–72. [Google Scholar]
- Špringol’c Šmidt, A.I. Éktoparazity nekotoryh vidov dal’novostočnyh olenej. Vestn. Dal’nevostočnyj Fil. Akad. Nauk SSSR 1937, 26, 133–140. [Google Scholar]
- Hirst, S. Remarks on certain species of the genus Demodex, Owen (the Demodex of man, the horse, dog, rat, and mouse). Ann. Mag. Nat. Hist. Ser. 8 1917, 20, 232–235. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Rolbiecki, L. Demodectic mites of the brown rat Rattus norvegicus (Berkenhout, 1769) (Rodentia, Muridae) with a new finding of Demodex ratticola Bukva, 1995 (Acari, Demodecidae). Ann. Parasitol. 2012, 58, 71–74. [Google Scholar] [PubMed]
- Izdebska, J.N.; Rolbiecki, L. Topical structure and topography of Demodex spp. (Acari Demodecidae), in brown rat Rattus norvegicus (Rodentia, Muridae). In Arthropods. The Medical and Economic Importance; Buczek, A., Błaszak, C., Eds.; Akapit: Lublin, Poland, 2012; pp. 133–141. [Google Scholar]
- Izdebska, J.N.; Fryderyk, S. Recent data on Demodex rosus Bukva, Vitovec et Vlcek, 1985 (Acari, Demodecidae) from oral cavity of yellow-necked field mouse, Apodemus flavicollis (Rodentia, Muridae). Wiad. Parazytol. 2011, 57, 257–260. [Google Scholar]
- Desch, C.E.; Lukoschus, F.S.; Nadchatram, M. A new demodicid (Acari: Demodicidae) from the meibomian glands of Southeast Asian rats (Rodentia: Muridae). Trop. Biomed. 1984, 1, 55–62. [Google Scholar]
- Lebel, R.R.; Nutting, W.B. Demodectic mites of subhuman Primates I: Demodex saimiri sp. n. (Acari: Demodicidae) from the squirrel monkey, Saimiri sciureus. J. Parasitol. 1973, 59, 719–722. [Google Scholar] [CrossRef]
- Lebel, R.R. Demodex saimiri sp. nov. and Demodex sciurei sp. nov. ectoparasites of the squirrel monkey, Saimiri sciureus, with notes on demodicids from other subhuman primates. Master’s Thesis, University of Massachusetts, Amherst, MA, USA, 1970. [Google Scholar]
- Desch, C.E.; Hurley, R.J. Demodex sinocricetuli: New species of hair follicle mite (Acari: Demodecidae) from the Chinese form of the striped hamster, Cricetulus barabensis (Rodentia: Muridae). J. Med. Entomol. 1997, 34, 317–320. [Google Scholar] [CrossRef]
- Bukva, V. Redescription of Demodex soricinus (Acari: Demodecidae) from the sebaceous glands of Sorex araneus (Insectivora). Folia Parasitol. 1993, 40, 243–248. [Google Scholar]
- Izdebska, J.N. Species of Demodecidae (Acari, Actinedida), new for the fauna of Poland, in common shrew (Sorex araneus L.). Zool. Pol. 2004, 49, 47–51. [Google Scholar]
- Kaufmann, J. Parasitic Infections of Domestic Animals: A Diagnostic Manual; Birkhäuser Verlag: Basel, Switzerland, 1996; pp. 1–423. [Google Scholar]
- Mehlhorn, M. (Ed.) Encyclopedic Reference of Parasitology, 4th ed.; Springer Verlag: Berlin/Heidelberg, Germany, 2016; pp. 1–3084. [Google Scholar]
- Kadlec, V. Demodex suis. Vet. Med. 1975, 20, 441–443. [Google Scholar]
- Maravelas, G.K. Studies of Demodex sylvilagi n. sp. (Acarina: Demodicidae) from the New England cottontail Sylvilagus transitionalis Bangs with a description of Demodex transitionalis n. sp. Master’s Thesis, University of Massachusetts Amherst, Amherst, MA, USA, 1962. [Google Scholar]
- Izdebska, J.N.; Rolbiecki, L. New data on parasites of mole Talpa europaea (Mammalia, Insectivora) in northern Poland. Wiad. Parazytol. 2003, 49, 97–98. [Google Scholar]
- Bukva, V. Demodex tauri sp. n. (Acari: Demodicidae), a new parasite of cattle. Folia Parasitol. 1986, 33, 363–369. [Google Scholar]
- Shi, X.; Xie, H.; Hsu, Y. A new species of the genus Demodex (Acariformes: Demodicidae). Acta Zool. Sin. 1985, 10, 385–387. [Google Scholar]
- Desch, C.E.; Holz, P.H. Demodex tortellinioides n. sp. (Acari: Demodecidae) from the agile antechinus, Antechinus agilis Dickman, Parnaby, Crowther and King, 1998 (Marsupialia: Dasyuridae) in Australia. Int. J. Acarol. 2006, 32, 81–84. [Google Scholar] [CrossRef]
- Desch, C.E. A new species of hair follicle mite (Acari: Demodecidae) from the snow leopard, Panthera uncia (Schreber, 1775) (Felidae). Int. J. Acarol. 1993, 19, 63–67. [Google Scholar] [CrossRef]
- Desch, C.E. A new species of Demodex (Acari: Demodecidae) from the black bear of North America, Ursus americanus Pallas, 1780 (Ursidae). Int. J. Acarol. 1995, 21, 23–26. [Google Scholar] [CrossRef]
- Foster, G.W.; Cames, T.A.; Forrester, D.J. Geographical distribution of Demodex ursi in black bears from Florida. J. Wildl. Dis. 1998, 34, 161–164. [Google Scholar] [CrossRef]
- Dailey, M.D.; Nutting, W.B. Demodex zalophi sp. nov. (Acari: Demodicidae) from Zalophus californianus, the California sea lion. Acarologia 1979, 21, 423–428. [Google Scholar]
- Bukva, V.; Nutting, W.B.; Desch, C.E. Description of Ophthalmodex apodemi sp.n. (Acari: Demodecidae) from the ocular area of Apodemus sylvaticus (Rodentia: Muridae) with notes on pathogenicity. Int. J. Acarol. 1992, 18, 269–276. [Google Scholar] [CrossRef]
- Lukoschus, F.S.; Nutting, W.B. Parasitic mites of Surinam. XIII. Ophthalmodex artibei gen. nov., spec. nov. (Prostigmata: Demodicidae) from Artibeus lituratus with notes on pathogenesis. Int. J. Acarol. 1979, 5, 299–304. [Google Scholar] [CrossRef]
- Woeltjes, A.G.W.; Lukoschus, F.S. Parasites of Western Australia. XIV. Two new species of Ophthalmodex Lukoschus and Nutting (Acarina: Prostigmata: Demodicidae) from the eyes of bats. Rec. West. Aust. Mus. 1981, 9, 307–313. [Google Scholar]
- Lukoschus, F.S.; Woeltjes, A.G.; Desch, C.E.; Nutting, W.B. Parasitic mites of Surinam XXXV: Two new Ophthalmodex spp. (O. carolliae, O. molossi: Demodicidae) from the bats Carollia perspicillata and Molossus molossus. Int. J. Acarol. 1980, 6, 45–50. [Google Scholar] [CrossRef]
- Veal, R.A.; Giesen, K.M.T.; Whitaker, J.O. A new species of the genus Ophthalmodex Lukoschus & Nutting 1979 (Prostigmata: Demodicidae) from Myotis lucifugus (Chiroptera: Vespertilionidae). Acarologia 1984, 25, 347–350. [Google Scholar]
- Lukoschus, F.S.; Woeltjes, A.G.W.; Desch, C.E.; Nutting, W.B. Parasitic mites of Surinam XX: Pterodex carolliae gen. nov., spec. nov. (Demodicidae) from the fruit bat, Carollia perspicillata. Int. J. Acarol. 1980, 6, 9–14. [Google Scholar] [CrossRef]
- Fain, A. Deux nouveaux genres d’Acariens vivant dans l’épaisseur des muqueuses nasale et buccale chez un Lémurien. Bull. Ann. Soc. R. Belg. Entomol. 1959, 95, 263–273. [Google Scholar]
- Bukva, V. Soricidex dimorphus g. n., sp. n. (Acari, Demodicidae) from the common shrew, Sorex araneus. Folia Parasitol. 1982, 40, 343–349. [Google Scholar]
- Bukva, V. Sexual dimorphism in the hair follicle mites (Acari: Demodecidae): Scanning electron microscopy of Soricidex dimorphus. Folia Parasitol. 1993, 40, 71–79. [Google Scholar]
- Desch, C.E. A new Australian species of Stomatodex Fain (Acari: Demodecidae) from the oesophagus of Cercartetus nanus (Desmarest) (Marsupialia). J. Aust. Entomol. Soc. 1991, 30, 239–241. [Google Scholar] [CrossRef]
- Baker, A.S.; Craven, J.C. Checklist of the mites (Arachnida: Acari) associated with bats (Mammalia: Chiroptera) in the British Isles. Syst. Appl. Acarol. 2003, 14, 1–20. [Google Scholar] [CrossRef]
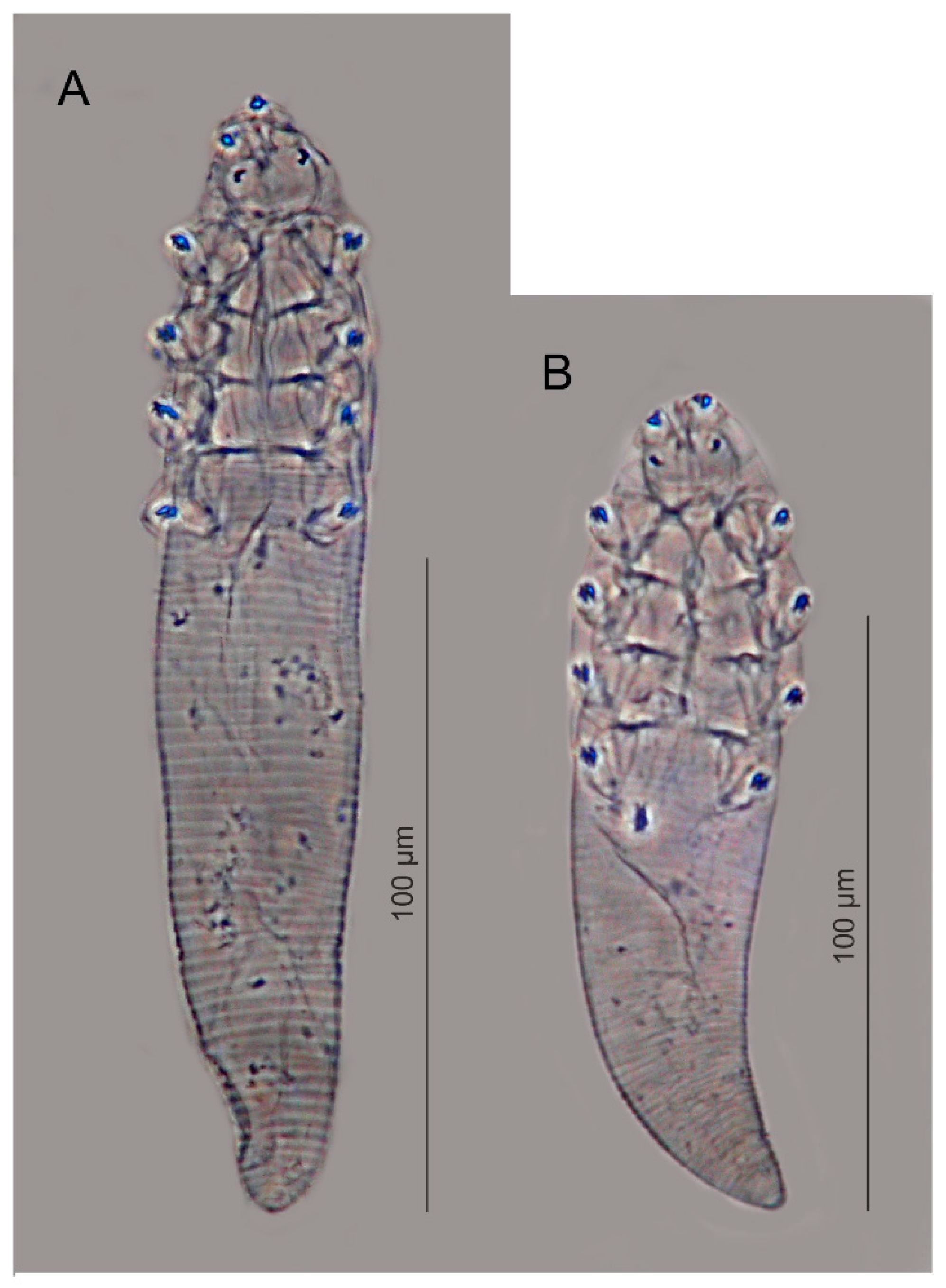
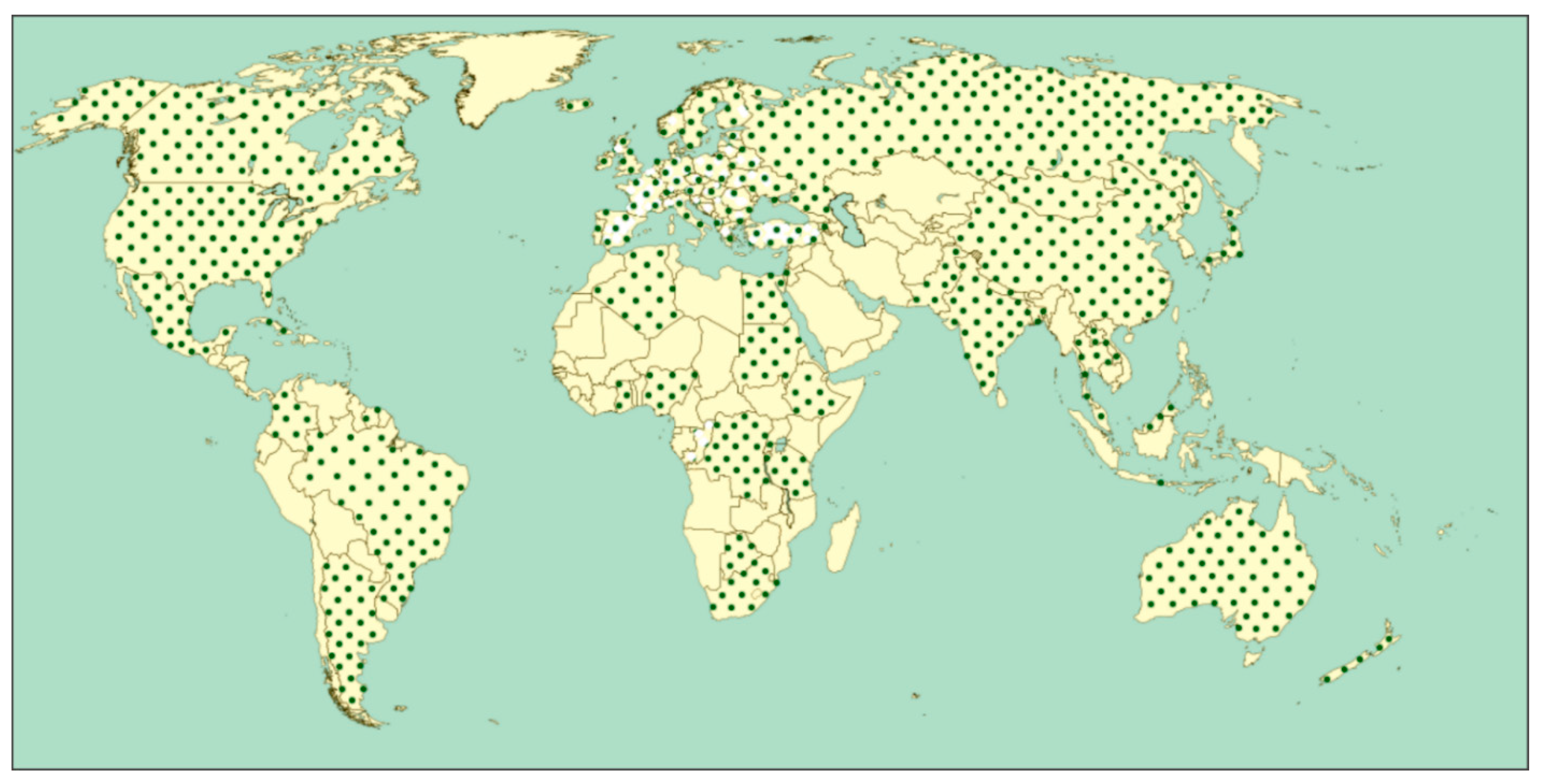
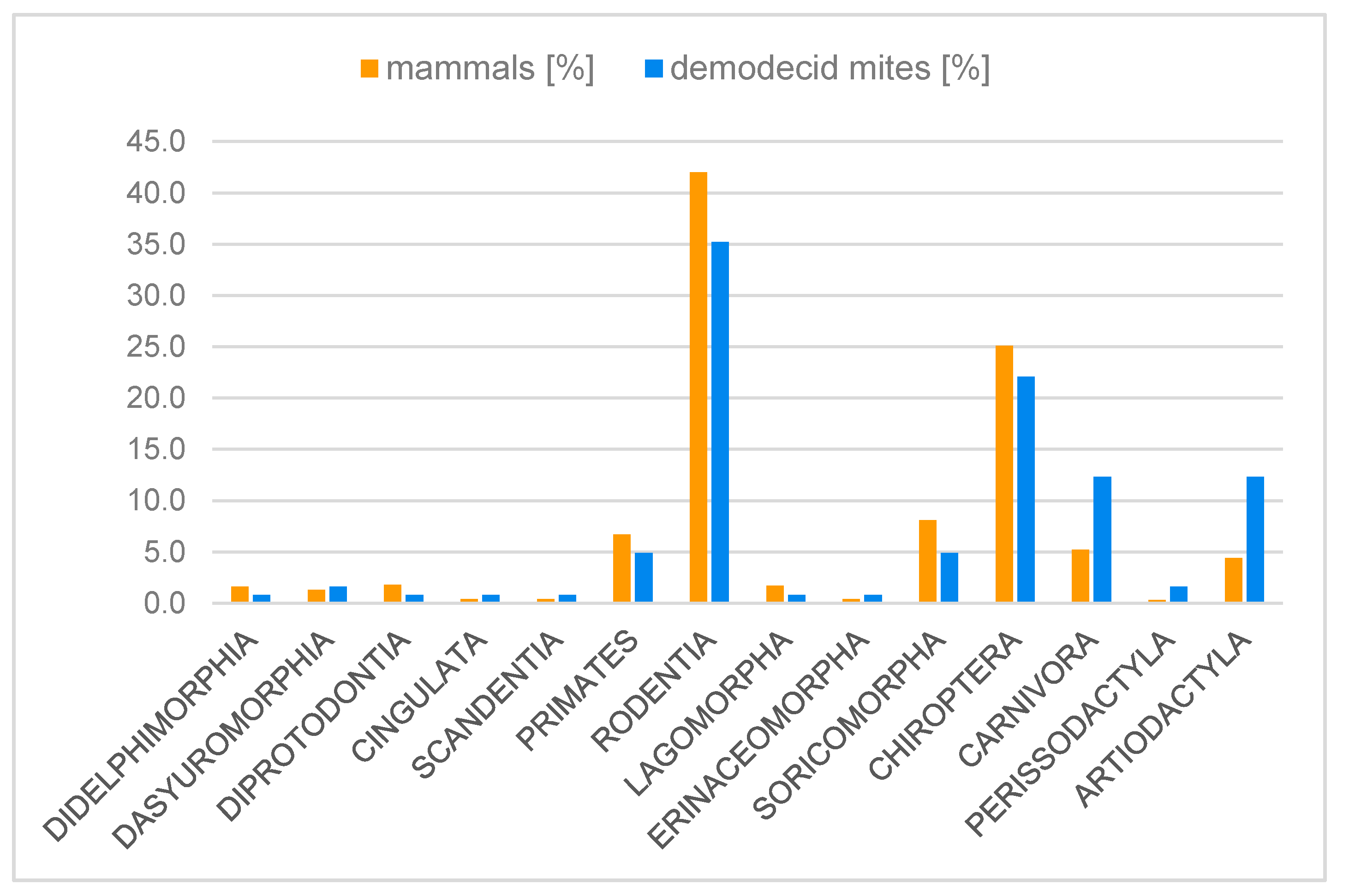
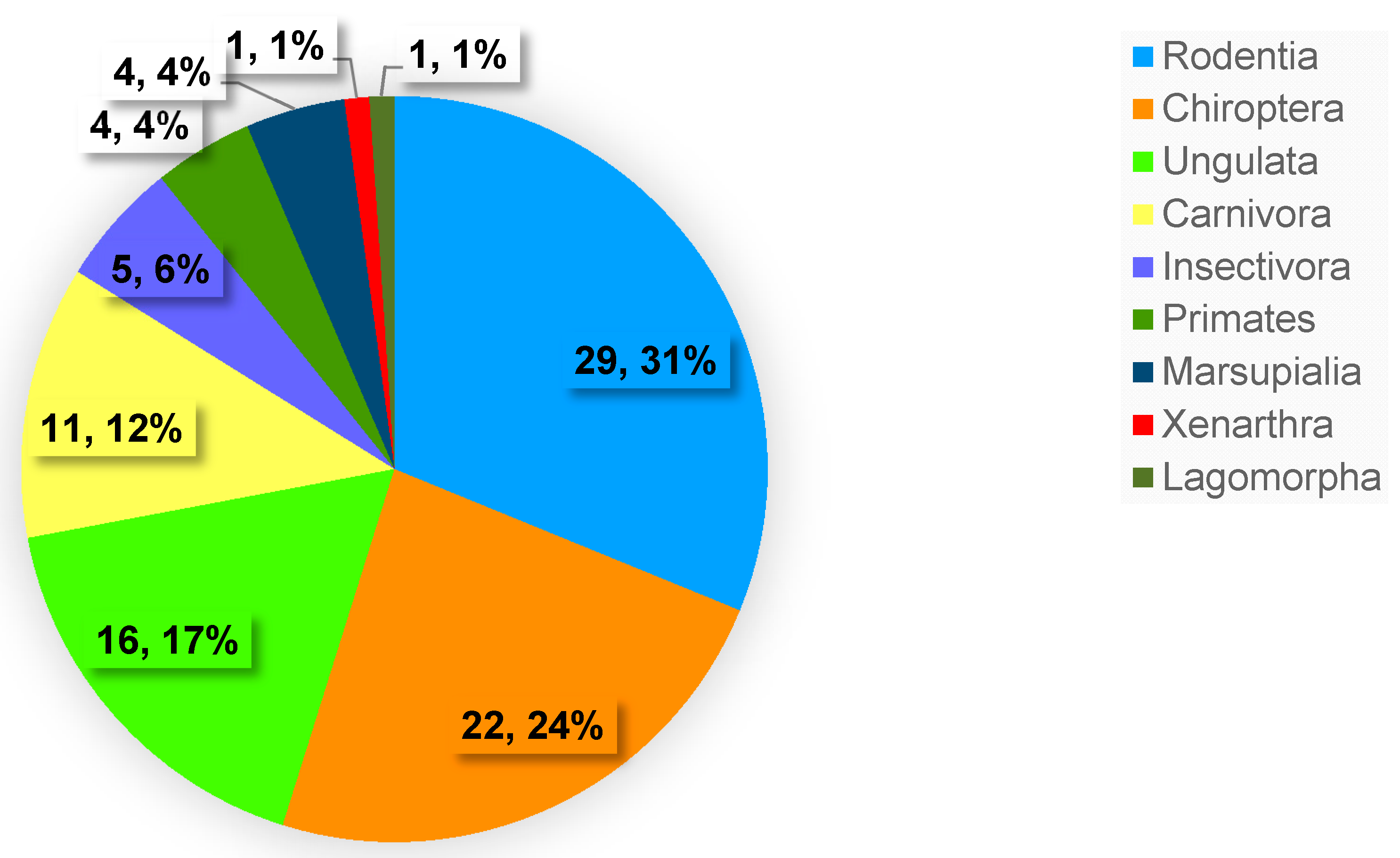
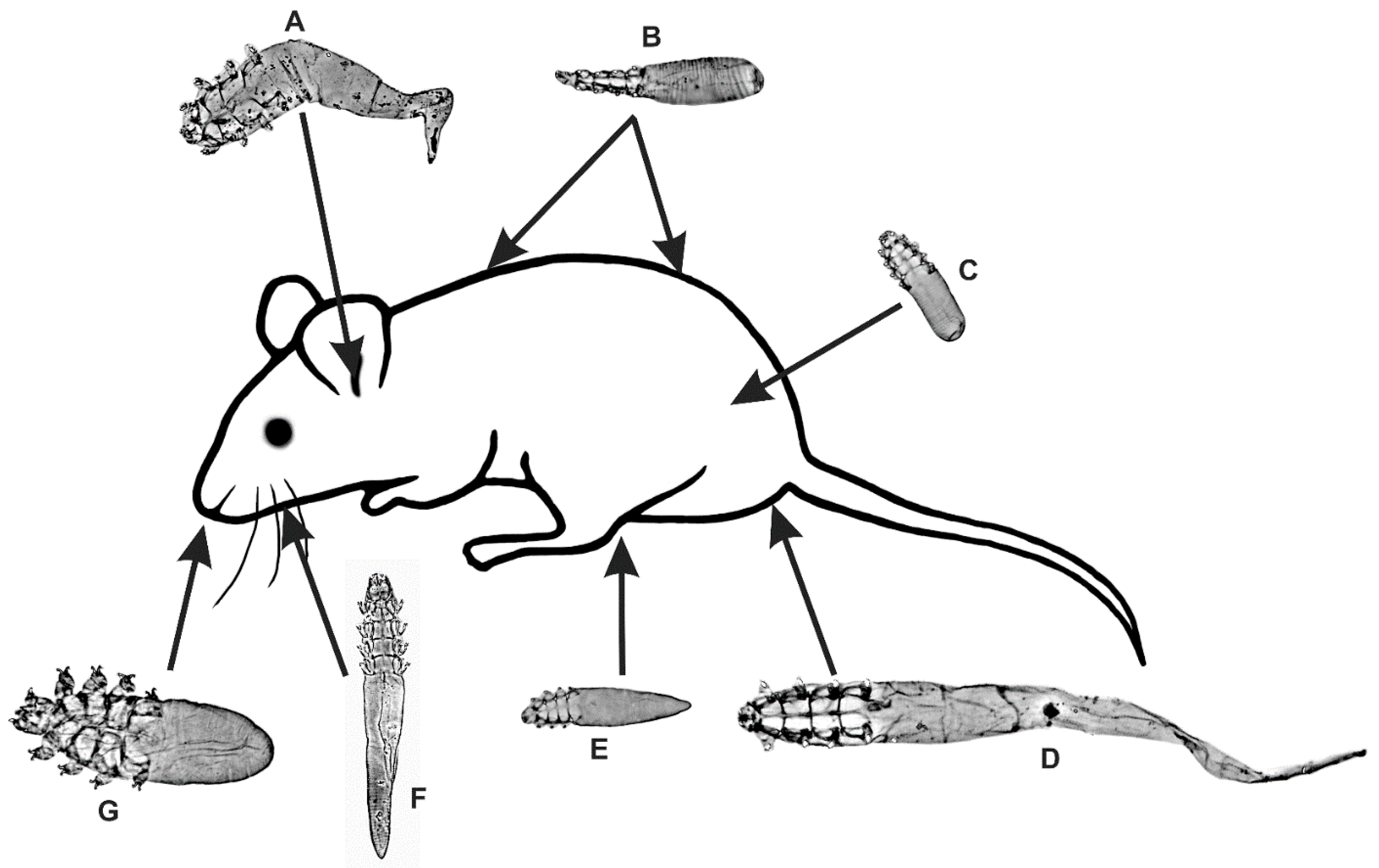
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izdebska, J.N.; Rolbiecki, L. The Biodiversity of Demodecid Mites (Acariformes: Prostigmata), Specific Parasites of Mammals with a Global Checklist and a New Finding for Demodex sciurinus. Diversity 2020, 12, 261. https://doi.org/10.3390/d12070261
Izdebska JN, Rolbiecki L. The Biodiversity of Demodecid Mites (Acariformes: Prostigmata), Specific Parasites of Mammals with a Global Checklist and a New Finding for Demodex sciurinus. Diversity. 2020; 12(7):261. https://doi.org/10.3390/d12070261
Chicago/Turabian StyleIzdebska, Joanna N., and Leszek Rolbiecki. 2020. "The Biodiversity of Demodecid Mites (Acariformes: Prostigmata), Specific Parasites of Mammals with a Global Checklist and a New Finding for Demodex sciurinus" Diversity 12, no. 7: 261. https://doi.org/10.3390/d12070261
APA StyleIzdebska, J. N., & Rolbiecki, L. (2020). The Biodiversity of Demodecid Mites (Acariformes: Prostigmata), Specific Parasites of Mammals with a Global Checklist and a New Finding for Demodex sciurinus. Diversity, 12(7), 261. https://doi.org/10.3390/d12070261