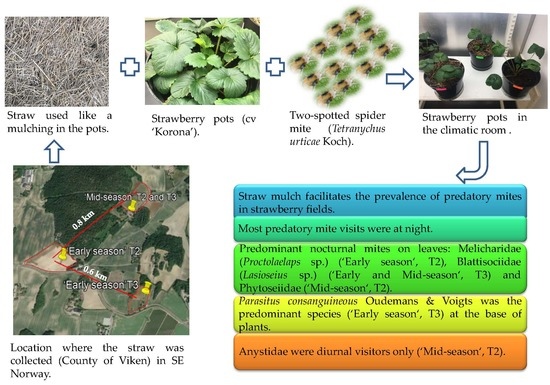
Cereal Straw Mulching in Strawberry—A Facilitator of Plant Visits by Edaphic Predatory Mites at Night?
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
2.2. Preparing Experimental Pots and Plants
2.3. Sampling Straw Mulch and Soil in the Field
2.4. Berlese Funnel Extraction of Mites
2.5. Observation and Sampling of Mites on Marked Leaflets during Experiments
2.6. Extraction of Mites from the Whole Plant (End of Experiments)
2.7. Statistical Analysis
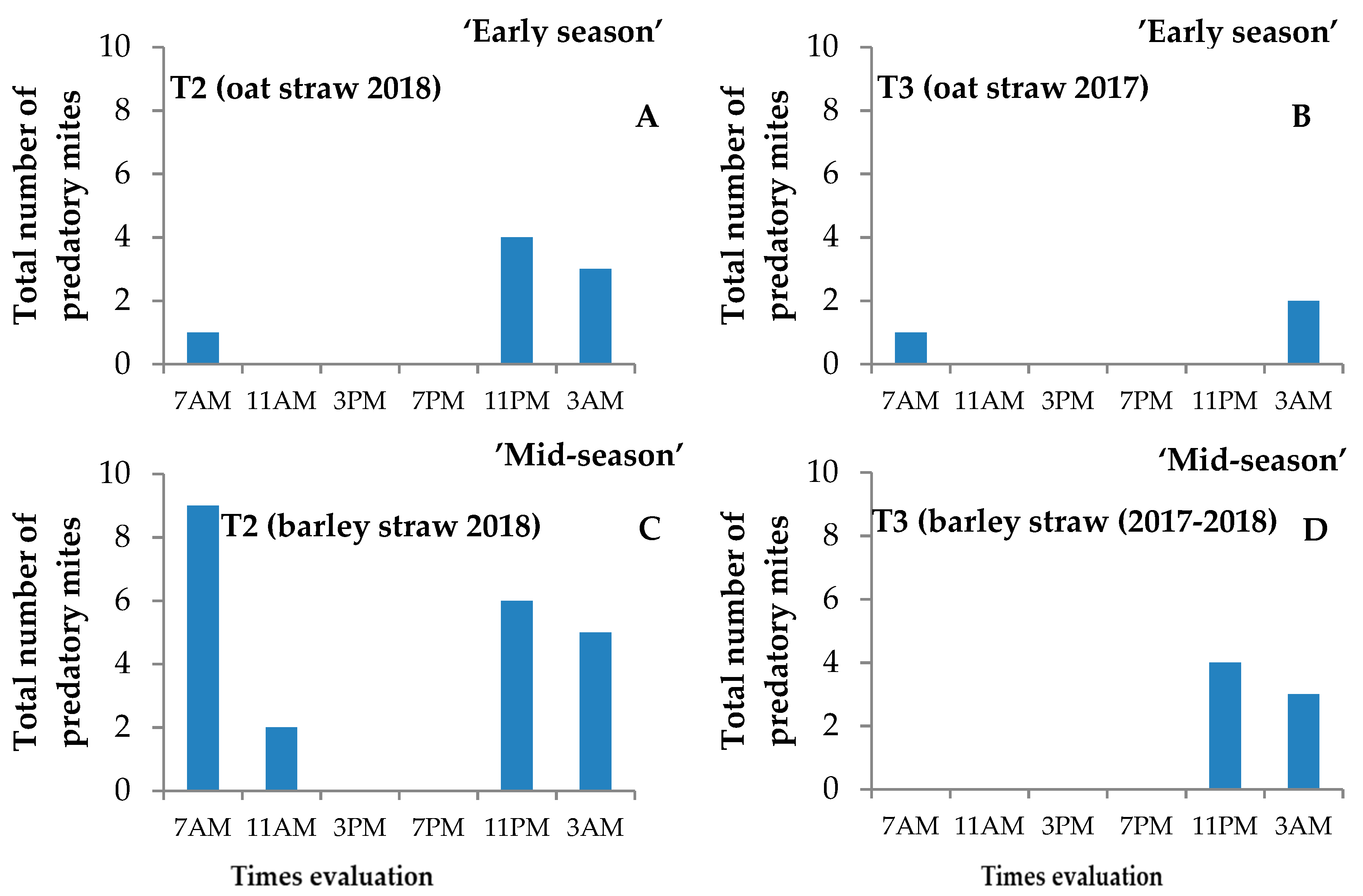
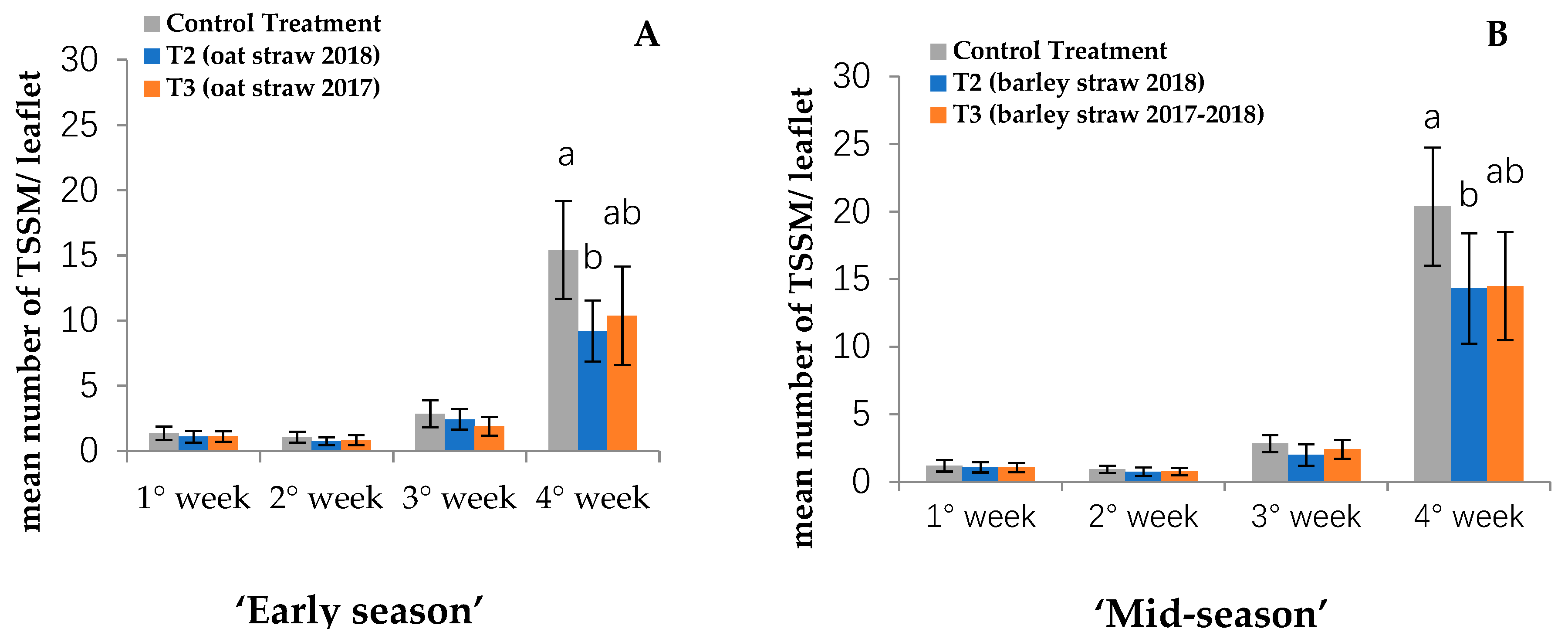
3. Results
3.1. Mites Extracted from Commercial Potting Substrate, Soil and Mulching Straw
3.2. Mites on Marked Leaflets
3.3. Mites Extracted from Whole Plants at the End of Experiments
4. Discussion
4.1. Mites from the Straw, Underlying Soil, and Commercial Potting Substrate
4.2. Mites on Strawberry Plants
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chakraborty, D.; Nagarajan, S.; Aggarwal, P.; Gupta, V.K.; Tomar, R.K.; Garg, R.N.; Sahoo, R.N.; Sarkar, A.; Chopra, U.K.; Sarma, K.S.S.; et al. Effect of mulching on soil and plant water status: And the growth and yield of wheat (Triticum aestivum L.) in a semi-arid environment. Agric. Water Manag. 2008, 95, 1323–1334. [Google Scholar] [CrossRef]
- Haslestad, J.; (Norsk Landbruksrådgivning, Ribadu, Hedmark, Norway). Personal communication, 2020.
- Vi Spiser Mer Utenlandske Enn Norske Jordbær. Available online: https://www.ssb.no/jord-skog-jakt-og-fiskeri/artikler-og-publikasjoner/vi-spiser-mer-utenlandske-enn-norske-jordbaer (accessed on 22 January 2020).
- Statistics Norway. Available online: https://www.ssb.no/en (accessed on 24 May 2020).
- Castilho, R.C.; Duarte, V.S.; Moraes, G.J.; Westrum, K.; Trandem, N.; Rocha, L.C.D.; Delalibera, I., Jr.; Klingen, I. Two-spotted spider mite and its natural enemies on strawberry grown as protected and unprotected crops in Norway and Brazil. Exp. Appl. Acarol. 2015, 66, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Trandem, N. Greenhouse production of strawberries and blackberries in Norway–arthropod pests and biological control. In Proceedings of the IOBC/ WPRS Working Group “Integrated Plant Protection in Orchards” Subgroup “Soft Fruits”, Dundee, Scotland, 18–21 September 2001; Gordon, S.C., Cross, J.V., Eds.; Scottish Crop Research Institute: Dundee, Scotland, 2003; Volume 26, pp. 45–50. [Google Scholar]
- Klingen, I.; Westrum, K.; Meyling, N.V. Effect of Norwegian entomopathogenic fungal isolates against Otiorhynchus sulcatus larvae at low temperatures and persistence in strawberry rhizospheres. Biol. Control. 2015, 81, 1–7. [Google Scholar] [CrossRef]
- Aasen, S.S.; Trandem, N. Strawberry blossom weevil Anthonomus rubi Herbst (Col.: Curculionidae): Relationships between bud damage, weevil density, insecticide use, and yield. J. Pest. Sci. 2006, 79, 169–174. [Google Scholar] [CrossRef]
- Kader, M.A.; Senge, M.; Mojid, M.A.; Ito, K. Recent advances in mulching materials and methods for modifying soil environment. Soil Till. Res. 2017, 168, 155–166. [Google Scholar] [CrossRef]
- Johnson, J.M.; Hough-Goldstein, J.A.; Vangessel, M.J. Effects of straw mulch on pest insects, predators, and weeds in watermelons and potatoes. Environ. Entomol. 2004, 33, 1632–1643. [Google Scholar] [CrossRef]
- Larentzaki, E.; Plate, J.; Nault, B.; Shelton, A. Impact of straw mulch on populations of onion thrips (Thysanoptera: Thripidae) in onion. J. Econ. Entomol. 2008, 101, 1317–1324. [Google Scholar] [CrossRef]
- Hoddle, M.S.; Morse, J.G.; Phillips, P.A.; Faber, B.A.; Jetter, K.M. Avocado thrips: A new challenge for growers. Cali Agric. 2002, 56, 103–107. [Google Scholar] [CrossRef]
- Jensen, L.; Simko, B.; Shock, C.; Saunders, L. Alternative Methods for Controlling Onion Thrips (Thrips tabaci) in Spanish Onions; Malheur Experiment Station Annual Report 2001; Oregon State University Agricultural Experiment Station: Ontario, OR, Canada, 2002; Volume 1038, pp. 104–111. [Google Scholar]
- Jamieson, L.E.; Stevens, P.S. The effect of mulching on adult emergence of Kelly’s citrus thrips (Pezothrips kellyanus). N. Z. Plant. Prot. 2006, 59, 42–46. [Google Scholar]
- Sánchez-Moreno, S.; Ferris, H. Suppressive service of the soil food web: Effects of environmental management. Agric. Ecosyst. Environ. 2007, 119, 75–87. [Google Scholar] [CrossRef]
- Carrillo, D.; Moraes, G.J.; Peña, J.E. Prospects for Biological Control. of Plant. Feeding Mites and Other Harmful Organisms; Springer International Publishing: Cham, Switzerland, 2015; p. 337. [Google Scholar]
- Walter, D.E.; Proctor, H.C. Mites: Ecology, Evolution and Behaviour Life at a Microscale, 2nd ed.; Springer Science and Business Media: Dordrecht, The Netherlands, 2013; p. 509. [Google Scholar]
- Bottinelli, N.; Jouquet, P.; Capowiez, Y.; Podwojewski, P.; Grimaldi, M.; Peng, X. Why is the influence of soil macrofauna on soil structure only considered by soil ecologists? Soil Till. Res. 2015, 146, 118–124. [Google Scholar] [CrossRef]
- Raven, P.H.; Evert, R.F.; Eichorn, S.E. Biologia Vegetal; Guanabara-Koogan: Rio de Janeiro, Brasil, 2001; p. 906. [Google Scholar]
- Esteca, F.C.N.; Rodrigues, L.R.; Moraes, G.J.; Júnior, I.D.; Klingen, I. Mulching with coffee husk and pulp in strawberry affects edaphic predatory mite and spider mite densities. Exp. Appl. Acarol. 2018, 76, 161–183. [Google Scholar] [CrossRef] [PubMed]
- Karg, W. Syno¨kologische Untersuchungen von Bodenmilben aus forstwirtschaftlich und landwirtschaftlich genutzten Böden. Pedobiologia 1967, 7, 198–214. [Google Scholar]
- van de Bund, C.F. Gamasides as predators of phytophagous nematodes. In Problems of Acarology, Symposium; Bozek, J., Suski, Z.W., Jakubowska, J., Eds.; Publisher: Polska Akademia Nauk, Polska, 1970; p. 280. [Google Scholar]
- Walter, D.E.; Behan-Pelletier, V. Mites in forest canopies: Filling the size distribution shortfall? Annu. Rev. Entomol. 1999, 44, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Onzo, A.; Hanna, R.; Zannou, I.; Sabelis, M.W.; Yaninek, J.S. Dynamics of refuge use: Diurnal, vertical migration by predatory and herbivorous mites within cassava plants. Oikos 2003, 101, 59–69. [Google Scholar] [CrossRef]
- Parecis-Silva, P.V.; Nuvoloni, F.M.; Feres, R.J. Day vs. night: The importance of the circadian cycle over metacommunities and predator–prey densities. Int. J. Acarol. 2016, 42, 141–148. [Google Scholar] [CrossRef]
- Fagan, L.L.; Didham, R.K.; Winchester, N.N.; Behan-Pelletier, V.; Clayton, M.; Lindquist, E.E.; Ring, R.A. An experimental assessment of biodiversity and species turnover in terrestrial versus canopy leaf litter. Oecologia 2006, 147, 335–347. [Google Scholar] [CrossRef]
- Britto, E.P.J.; Gago, E.; Moraes, G.J. How promising is Lasioseius floridensis as a control agent of Polyphagotarsonemus latus? Exp. Appl. Acarol. 2012, 56, 221–231. [Google Scholar] [CrossRef]
- Meier, U.; Graf, H.; Hess, M.; Kennel, W.; Klose, R.; Mappes, D.; Seipp, D.; Stauss, R.; Streif, J.; van den Boom, T. Phänologische Entwick-lungsstadien des Kernobstes (Malusdomestica Borkh. Und Pyrus communis L.), des Steinobstes (Prunus-Arten), der Johannisbeere (Ribes-Arten) und der Erdbeere (Fragaria x ananassa Duch.). Nachrichtenbl. Deut. Pflanzenschutzd 1994, 46, 141–153. [Google Scholar]
- Oliveira, A.R.; Moraes, G.J.; Demétrio, C.G.E.; Nardo, E.A.E. Efeito do vírus de poliedrose nuclear de Anticarsia gemmatalis sobre Oribatida edáficos (Arachnida: Acari) em um campo de soja. Embrapa Meio Ambiente 2001, 13, 5–31. [Google Scholar]
- Krantz, G.W.; Walter, D.E. A Manual of Acarology, 3rd ed.; Texas Tech University Press: Lubbock, TX, USA, 2009; p. 411. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing, v.3.0.1; R foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Pinzón, J.; Spence, J.R. Bark-dwelling spider assemblages (Araneae) in the boreal forest: Dominance, diversity, composition and life-histories. J. Insect. Conserv. 2010, 14, 439–458. [Google Scholar] [CrossRef]
- Sjursen, H.; Michelsen, A.; Jonasson, S. Effects of long term soil warming and fertilization on microarthropod abundances in three sub-artic ecosystems. Appl. Soil Ecol. 2005, 30, 148–161. [Google Scholar] [CrossRef]
- Blankinship, J.C.; Niklaus, P.A.; Hungate, B.A. A meta-analysis of responses of soil biota to global change. Oecologia 2011, 165, 553–565. [Google Scholar] [CrossRef]
- Bedano, J.C.; Ruf, A. Sensitivity of different taxonomic levels of soil Gamasina to land use and anthropogenic disturbances. Agric. Forest Entomol. 2010, 12, 203–212. [Google Scholar] [CrossRef]
- Hernandes, F.A.; Castro, T.M.M.; Venancio, R. Prostigmata (Acari: Trombidiformes) as biological control agents. In Prospects for Biological Control of Plant Feeding Mites and Other Harmful Organisms; Carillo, D., Moraes, G.J., Peña, J.E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 151–184. [Google Scholar]
- McMurtry, J.A.; Sourassou, N.F.; Demite, P.R. The Phytoseiidae (Acari: Mesostigmata) as biological control agents. In Prospects for Biological Control of Plant Feeding Mites and Other Harmful Organisms; Carillo, D., Moraes, G.J., Peña, J.E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 133–149. [Google Scholar]
- Knapp, M.; van Houten, Y.; van Baal, E.; Groot, T. Use of predatory mites in commercial biocontrol: Current status and future prospects. Acarologia 2018, 58, 72–82. [Google Scholar]
- Szlendak, E.; Lewandowski, M. Development and reproductive capacity of the predatory mite Parasitus consanguineus (Acari: Parasitidae) reared on the larval stages of Megaselia halterata and Lycoriella ingenua. Exp. Appl. Acarol. 2009, 47, 285–292. [Google Scholar] [CrossRef]
- Castilho, R.C.; Venancio, R.; Narita, J.P.Z. Mesostigmata as biological control agents, with emphasis on Rhodacaroidea and Parasitoidea. In Prospects for Biological Control of Plant Feeding Mites and Other Harmful Organisms; Carillo, D., Moraes, G.J., Peña, J.E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–31. [Google Scholar]
- Rueda-Ramírez, D.; Rios-Malaver, D.; Varela-Ramírez, A.; Moraes, G.J. Biology and predation capacity of Parasitus bituberosus (Acari: Mesostigmata: Parasitidae) on Frankliniella occidentalis (Thysanoptera: Thripidae), and free-living nematodes as its complementary prey. Pest. Manag. Sci. 2019, 75, 1819–1830. [Google Scholar] [CrossRef]
- Bolger, T.; Devlin, M.; Seniczak, A. First records of ten species of Mesostigmata (Acari, Mesostigmata) added to the published Norwegian species list. Nor. J. Entomol. 2018, 65, 94–100. [Google Scholar]
- Moraes, G.J.; Venancio, R.; dos Santos, V.L.V.; Paschoal, A.D. Potential of Ascidae, Blattisociidae and Melicharidae (Acari: Mesostigmata) as biological control agents of pest organisms. In Prospects for Biological Control of Plant Feeding Mites and Other Harmful Organisms; Carillo, D., Moraes, G.J., Peña, J.E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 33–75. [Google Scholar]
- Slomian, S.; Gulvik, M.E.; Madej, G.; Austad, I. Gamasina and Microgyniina (Acari, Gamasida) from soil and tree hollows at two traditional farms in Sogn og Fjordane, Norway. Norw. J. Entomol. 2005, 52, 39–48. [Google Scholar]
- Lindquist, E.E.; Krantz, G.W.; Walter, D.E. Order Mesostigmata. In A Manual of Acarology, 3rd ed.; Krantz, G.W., Walter, D.E., Eds.; Texas Tech University Press: Lubbock, TX, USA, 2009; pp. 124–232. [Google Scholar]
- Halliday, R.B. Alliphis siculus (Oudemans 1905) is not a synonym of Alliphis halleri (G. & R. Canestrini 1881) (Acari: Eviphididae). Syst. Appl. Acarol. 2008, 13, 51–64. [Google Scholar]
- Karg, W.; Grosse, E. Raubmilben als Antagonisten von Nematoden. Nachr. Pflanzenschutz DDR 1983, 37, 208–212. [Google Scholar]
- Mathys, G.; Tencalla, Y. Note préliminaire sur la biologie et la valeur prédatice de Proctolaelaps hypudaei Oudms (Acarien: Mesostigmata: Aceosejidae). Stn. Fédérales Essais Agric. 1959, 600, 645–654. [Google Scholar]
- Moraes, J.; Franklin, E.; Morais, J.W.; Souza, J.L.P. Species of edaphic mites (Acari: Oribatida) and effects of topography, soil properties and litter gradients on their qualitative and quantitative composition in 64 km2 of forest in Amazonia. Exp. Appl. Acarol. 2011, 55, 39–63. [Google Scholar] [CrossRef] [PubMed]
- Overmeer, W.P.J. Diapause. Spider Mites: Their Biology, Natural Enemies and Control; Elsevier: Amsterdam, The Netherlands, 1985; Volume 2, pp. 95–102. [Google Scholar]
- Veerman, A. Diapause. Spider Mites: Their Biology, Natural Enemies and Control; Elsevier: Amsterdam, The Netherlands, 1985; Volume 1, pp. 279–316. [Google Scholar]
- Laing, J.E.; Knop, N.F. Potential use of predaceous mites other than Phytoseiidae for biological control of orchard pests. In Biological Control of Pests by Mites, Special Publications; Hoy, M.A., Cunningham, G.L., Knutson, L., Eds.; University of California: Berkeley, CA, USA, 1983; pp. 12–20. [Google Scholar]
- Karg, W.; Edland, T. Neue Raubmilbenarten der Phytoseiidae Berlese, 1916. Dtsch. Entomol. Z. 1987, 34, 387–395. [Google Scholar] [CrossRef]
- Trandem, N.; Klingen, I.; Haukeland, S.; Moraes, G.J. The occurrence of two pest mites and three groups of biocontrol agents in organic and conventional strawberry fields (abstract only). IOBC Bull. 2011, 70, 96. [Google Scholar]
- Denmark, H.A.; Edland, T. The subfamily Amblyseiinae Muma (Acari: Phytoseiidae) in Norway. Int. J. Acarol. 2002, 28, 195–220. [Google Scholar] [CrossRef]
- Meshkov, Y.I. Phytoseiid Mites (Parasitiformes, Phytoseiidae) on Main Berry Plantings. Ph.D. Thesis, Bolshie Vjazemi, Moscow, Russia, 1996. [Google Scholar]
- Novotny, V.; Basset, Y.; Auga, J.; Boen, W.; Dal, C.; Drozd, P.; Kasbal, M.; Isua, B.; Kutil, R.; Manumbor, M. Predation risk for herbivorous insects on tropical vegetation: A search for enemy-free space and time. Aust. J. Ecol. 1999, 24, 477–483. [Google Scholar] [CrossRef]
- Saigusa, M.; Oishi, K.; Ikumoto, A.; Iwasaki, H.; Terajima, M. Emergence patterns of small subtidal arthropods in relation to day/night, tidal, and surface/bottom factors: Investigations in the Boreal Sea, Japan (Akkeshi, Hokkaido). J. Oceanogr. 2000, 56, 295–310. [Google Scholar] [CrossRef]
- Zaitsev, A.S.; Chauvat, M.; Pflug, A.; Wolters, V. Oribatid mite diversity and community dynamics in a spruce chronosequence. Soil Biol. Biochem. 2002, 34, 1919–1927. [Google Scholar] [CrossRef]
- Walter, D.E.; Hunt, H.W.; Elliott, E.T. Guilds or functional groups? An analysis of predatory arthropods from a shortgrass steppe soil. Pedobiologia 1988, 31, 247–260. [Google Scholar]
| Experiment Treatment (T) * | Date Collected | Temperature, RH, Precipitation ** | Location | Type of Straw | Length of Exposure in the Field |
|---|---|---|---|---|---|
| ‘Early season’ T2 | May 6, 2019 | 3.3–4.0 °C; 77–82% RH; 6 mm | 59°39′38″ N; 10°40′37″ E, Altitude 90 masl, Loam soil | Oat | Since autumn 2018 |
| ‘Early season’ T3 | May 6, 2019 | 3.3–4.0 °C; 77–82% RH; 6 mm | 59°39′30″ N; 10°41′13″ E, Altitude 100 masl, Silty loam soil | Oat | Since autumn 2017 |
| ‘Mid-season’ T2 | June 23, 2019 | 11.4–13.0 °C; 75–83% RH; 29 mm | 59°39′51″ N; 10°41′4″ E, Altitude 100 masl, Silty loam soil | Barley | Since autumn 2018 |
| ‘Mid-season’ T3 | June 23, 2019 | 11.4–13.0 °C; 75–83% RH; 29 mm | 59°39′51″ N; 10°41′4″ E, Altitude 100 masl, Silty loam soil | Barley | Mixture of autumn 2017 (lower layer) and 2018 (upper layer) |
| Experiments | ‘Early Season’ Experiment | ‘Mid-Season’ Experiment | ||||||
|---|---|---|---|---|---|---|---|---|
| Treatments | T2 (Oat Straw 2018) | T3 (Oat Straw 2017) | T2 (Barley Straw 2018) | T3 (Barley Straw 2017–2018) | ||||
| Taxa/Collection | B | E | B | E | B | E | B | E |
| Sarcoptiformes, Oribatida, Astigmatina | ||||||||
| Acaridae | ||||||||
| Tyrophagus putrescentiae | 48 | 92 | 130 | 35 | 17 | 22 | 15 | 7 |
| Winterschimidtiidae | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 0 |
| Sarcoptiformes, other Oribatida | ||||||||
| - | 119 | 11 | 109 | 7 | 23 | 5 | 149 | 16 |
| Trombidiformes, Prostigmata | ||||||||
| Anystidae | ||||||||
| Anystis sp. | 0 | 0 | 0 | 0 | 32 | 27 | 2 | 0 |
| Cunaxidae | ||||||||
| Cunaxoides croceus | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 |
| Ereynetidae | ||||||||
| Ereynetes spp. | 135 | 0 | 17 | 0 | 1 | 2 | 0 | 6 |
| Eupodidae | ||||||||
| Eupodes spp. | 239 | 0 | 46 | 0 | 64 | 50 | 31 | 66 |
| Pygmephoridae | ||||||||
| Siteroptes sp. | 1 | 0 | 2 | 0 | 0 | 0 | 1 | 0 |
| Tydeidae | ||||||||
| Lorryia oregonensis | 167 | 6 | 129 | 46 | 22 | 20 | 33 | 21 |
| Parasitiformes, Mesostigmata, Gamasina | ||||||||
| Ascidae | ||||||||
| Gamasellodes bicolor | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Neojordensia sinuata ♂ | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Blattisociidae | ||||||||
| Lasioseius sp. | 14 | 12 | 3 | 1 | 5 | 15 * | 1 | 14 * |
| Eviphididae | ||||||||
| Alliphis halleri | 9 * | 5 | 5 | 0 | 0 | 0 | 7 | 3 |
| Alliphis sp. immature | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Melicharidae | ||||||||
| Proctolaelaps sp. | 14 * | 102 * | 0 | 0 | 0 | 0 | 0 | 0 |
| Parasitidae | ||||||||
| Amblygamasus sp. | 0 | 0 | 0 | 0 | 4 | 3 | 20 | 0 |
| Amblygamasus sp. (immature) | 0 | 0 | 0 | 0 | 0 | 1 | 16 | 0 |
| Parasitus consanguineus | 0 | 0 | 1 | 7 | 0 | 0 | 0 | 0 |
| Parasitus sp. (deutonymph) | 1 | 0 | 7 | 0 | 0 | 0 | 0 | 0 |
| Pergamasus longicornis | 0 | 0 | 3 | 2 | 14 * | 4 | 42 * | 2 |
| Pergamasus septentrionalis | 0 | 0 | 10 | 0 | 0 | 0 | 5 | 2 |
| Pergamasus sp. (deutonymph) | 0 | 0 | 26 * | 2 | 1 | 4 | 17 | 0 |
| Pergamasus sp. ♂ | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Porrhostaspis lunulata | 0 | 0 | 0 | 0 | 0 | 8 | 4 | 7 * |
| Porrhostaspis sp. (deutonymph) | 0 | 0 | 1 | 2 | 0 | 0 | 1 | 0 |
| Immature | 1 | 0 | 2 | 1 | 2 | 1 | 3 | 0 |
| Phytoseiidae | ||||||||
| Neoseiulus alpinus | 1 | 2 | 3 | 1 | 0 | 0 | 0 | 0 |
| Neoseiulus cucumeris | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 1 |
| Neoseiulus sp. immature | 0 | 1 | 3 | 1 | 0 | 0 | 0 | 0 |
| Proprioseiopsis okanagensis | 0 | 0 | 3 | 0 | 1 | 2 | 0 | 0 |
| Typhlodromips masseei | 1 | 0 | 13 | 0 | 0 | 0 | 5 | 2 |
| Immature | 0 | 0 | 17 | 0 | 0 | 0 | 0 | 0 |
| Rhodacaridae | ||||||||
| Rhodacarellus epigynalis | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 3 |
| Rhodacarellus kreuzi | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Rhodacarellus sp. immature | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Veigaiidae | ||||||||
| Veigaia nemorensis | 1 | 0 | 0 | 0 | 0 | 0 | 22 * | 2 |
| Veigaia sp. immature | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| Total | 754 | 231 | 545 | 105 | 189 | 166 | 376 | 152 |
| Experiments | ‘Early Season’ Experiment | ‘Mid-Season’ Experiment | ||
|---|---|---|---|---|
| Taxa/Treatments | T2 (Oat Straw 2018) | T3 (Oat Straw 2017) | T2 (Barley Straw 2018) | T3 (Barley Straw 2017–2018) |
| Trombidiformes, Prostigmata | ||||
| Anystidae | ||||
| Anystis sp. | 0 | 0 | 22 | 0 |
| Parasitiformes, Mesostigmata, Gamasina | ||||
| Ascidae | ||||
| Neojordensia sinuata | 1 | 0 | 0 | 0 |
| Blattisociidae | ||||
| Lasioseius sp. | 0 | 3 | 1 | 11 * |
| Melicharidae | ||||
| Proctolaelaps sp. | 6 * | 0 | 0 | 0 |
| Parasitidae | ||||
| Parasitus sp. (deutonymph) | 0 | 0 | 1 | 0 |
| Pergamasus sp. (deutonymph) | 0 | 0 | 0 | 1 |
| Phytoseiidae | ||||
| Neoseiulus cucumeris | 0 | 0 | 1 | 0 |
| Typhlodromips masseei | 1 | 0 | 3 * | 0 |
| Proprioseiopsis okanagensis | 0 | 0 | 1 | 1 |
| Total | 8 | 3 | 29 | 13 |
| Treatments | T2 | T3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Experiments | ‘Early Season’ | ‘Mid-Season’ | ‘Early Season’ | ‘Mid-Season’ | ||||
| Taxa/Time | 11 a.m. | 11 p.m. | 11 a.m. | 11 p.m. | 11 a.m. | 11 p.m. | 11 a.m. | 11 p.m. |
| Trombidiformes, Prostigmata | ||||||||
| Anystidae | ||||||||
| Anystis sp. | 0 | 0 | 11 | 0 | 0 | 0 | 0 | 0 |
| Sarcoptiformes, Oribatida, Astigmatina | ||||||||
| Acaridae | ||||||||
| Tyrophagus putrescentiae | 0 | 14 | 0 | 11 | 0 | 16 | 0 | 24 |
| Parasitiformes, Mesostigmata, Gamasina | ||||||||
| Blattisociidae | ||||||||
| Lasioseius sp. | 0 | 5 | 0 | 11 * | 0 | 2 | 0 | 0 |
| Lasioseius sp. immature | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eviphididae | ||||||||
| Alliphis halleri | 0 | 11 | 0 | 0 | 0 | 0 | 0 | 1 |
| Melicharidae | ||||||||
| Proctolaelaps sp. | 0 | 33 * | 0 | 0 | 0 | 0 | 0 | 0 |
| Parasitidae | ||||||||
| Amblygamasus sp. | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Amblygamasus (deutonymph) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Pergamasus sp. (deutonymph) | 0 | 0 | 0 | 0 | 0 | 4 | 1 | 0 |
| Porrhostaspis lunulata | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 |
| Parasitus consanguineus | 0 | 0 | 0 | 0 | 0 | 16 * | 0 | 0 |
| Pergamasus longicornis | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 4 |
| Pergamasus septentrionalis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Immature | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 |
| Phytoseiidae | ||||||||
| Neoseiulus cucumeris | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 |
| Proprioseiopsis okanagensis | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Typhlodromips masseei | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| Rhodacaridae | ||||||||
| Rhodacarellus sp. (immature) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Total | 0 | 67 | 15 | 30 | 0 | 40 | 1 | 30 |
| Treatments | T2 | T3 | ||
|---|---|---|---|---|
| Experiments/Time | 11 a.m. | 11 p.m. | 11 a.m. | 11 p.m. |
| ‘Early season’ experiment | 0.0 ± 0.0 a | 4.1 ± 0.5 b | 0.0 ± 0.0 a | 1.8 ± 0.3 b |
| ‘Mid-season’ experiment | 0.3 ± 0.1 a | 1.5 ± 0.4 b | 0.2 ± 0.1 a | 0.5 ± 0.2 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neves Esteca, F.d.C.; Trandem, N.; Klingen, I.; Cruz Santos, J.; Delalibera Júnior, I.; de Moraes, G.J. Cereal Straw Mulching in Strawberry—A Facilitator of Plant Visits by Edaphic Predatory Mites at Night? Diversity 2020, 12, 242. https://doi.org/10.3390/d12060242
Neves Esteca FdC, Trandem N, Klingen I, Cruz Santos J, Delalibera Júnior I, de Moraes GJ. Cereal Straw Mulching in Strawberry—A Facilitator of Plant Visits by Edaphic Predatory Mites at Night? Diversity. 2020; 12(6):242. https://doi.org/10.3390/d12060242
Chicago/Turabian StyleNeves Esteca, Fernanda de Cássia, Nina Trandem, Ingeborg Klingen, Jandir Cruz Santos, Italo Delalibera Júnior, and Gilberto José de Moraes. 2020. "Cereal Straw Mulching in Strawberry—A Facilitator of Plant Visits by Edaphic Predatory Mites at Night?" Diversity 12, no. 6: 242. https://doi.org/10.3390/d12060242
APA StyleNeves Esteca, F. d. C., Trandem, N., Klingen, I., Cruz Santos, J., Delalibera Júnior, I., & de Moraes, G. J. (2020). Cereal Straw Mulching in Strawberry—A Facilitator of Plant Visits by Edaphic Predatory Mites at Night? Diversity, 12(6), 242. https://doi.org/10.3390/d12060242