Abstract
Bats are killed at wind energy facilities worldwide and we must improve our understanding of why this is happening and implement effective strategies to minimize impacts. To this end, we need accurate assessments of which individuals from which bat species are being killed at individual wind projects and at regional and range-wide scales. Traditional fatality searches have relied on physical characteristics to ascertain species and sex of bat carcasses collected at wind turbines; however, the resulting data can be incomplete and inaccurate. In contrast, the use of readily available and low-cost molecular methods improves both the quality and quantity of available data. We applied such methods to a bat fatality dataset (n = 439 bats) from far-south Texas, USA. Using DNA barcoding, we increased accurate species identification from 83% to 97%, and discovered the presence of 2 bat species outside of their known geographic ranges. Using a PCR-based approach to determine sex, the number of carcasses with correct sex assignment increased from 35% to 94%, and we documented a female-biased sex ratio for all species combined and for Dasypterus ega. We recommend that molecular methods be used during future survey efforts to accurately assess the impacts of wind energy on bats.
Keywords:
bats; conservation genetics; Dasypterus; DNA barcoding; Lasiurus; mortality; wind energy; wind turbine; yellow bats 1. Introduction
Bats currently face a multitude of threats worldwide, making their study and conservation essential for the preservation of species and the important ecosystem services that they provide [1,2,3,4]. Many aspects of bat biology are poorly understood, and fear of bats has led to the extermination of entire colonies and roost destruction, which are major drivers of species decline [3,4,5]. Changes in land use, driven by increased agriculture and urbanization, have eliminated valuable bat habitats, while also accelerating the effects of climate change, which together threaten both bats and their ecosystem services [6]. Efforts being made to reduce the effects of climate change have altered the way we produce energy in favor of renewable processes, like wind energy. Mitigating the impacts of climate change by reducing greenhouse gas emissions through an increase in electricity generation from renewable sources like wind power has the potential to benefit wildlife conservation [7,8]. Nonetheless, bat mortality at wind energy facilities is an unanticipated consequence that unfortunately may simultaneously threaten the persistence of bat populations [4,9,10].
Several studies have identified the potential for wind energy development to cause population declines in bat species from the northern hemisphere, yet few studies have provided quantitative estimates of bat-wind fatality and population viability [11,12]. In order to minimize the detrimental effects wind development could have on bats, there is a pressing need to establish conservation strategies protecting bats from this source of mortality. Operational minimization, smart curtailment, and acoustic deterrents have reduced bat fatalities where implemented [13,14,15,16], but the application of these strategies is not yet widespread, and operational minimization incurs a loss in power generation. The U.S. Department of Energy, technology vendors, and wind project owners have therefore invested considerable financial resources in the further development and testing of impact reduction technologies that minimize power generation loss. However, to fully assess the effectiveness of these strategies, stakeholders must know which individuals of which bat species are being impacted, both before and after the implementation of a specific mitigation method. Accurate species identification of bat carcasses collected during turbine searches is not always possible and worsens with increased estimated time since death [17]. Furthermore, sex assignment based on external morphology can generate inaccurate sex ratio estimates, that could in turn provide a misleading representation of which individuals are being killed by wind-turbines [17,18]. Therefore, DNA barcoding and molecular sexing are viable means, by which species identification and sex determination can be obtained from bat carcasses collected at wind energy facilities [17,19].
Recent wind energy expansion into the Lower Rio Grande Valley of Texas has led to two additional bat species, the northern yellow bat (Dasypterus intermedius) and the southern yellow bat (Dasypterus ega), as being identified as collision fatalities at wind turbines [20]. Dasypterus species are morphologically similar and poorly studied, but the potential for impacts from wind-related mortality is high, given the level of annual mortality in other closely related tree bats, Lasiurus borealis and Aeorestes cinereus [21,22]. (Note: Recent phylogenetic analyses of the lasiurine bats [23,24] suggest several taxonomic revisions, including the recognition of three separate genera from what was formerly the genus Lasiurus: Aeorestes for hoary bats, Lasiurus for red bats, and Dasypterus for yellow bats. We have elected to use the new nomenclature here.) Thus, wind energy is a new source of mortality for these yellow bats, and as both species have a limit to their respective geographic ranges in Texas [25,26], these fatalities provide a unique opportunity to understand how populations may be impacted at the edge of their geographic range due to wind energy development. Therefore, the objective of our study was to demonstrate the effectiveness of mitochondrial DNA sequencing (DNA barcoding) and molecular sex methods when applied to carcasses collected from a region with closely related and morphologically similar bat species. By comparing both the quality and quantity of data obtained from field-based and molecular methods, we provide a quantitative estimate of the gains possible from applying these techniques to post-construction fatality datasets from wind energy development in: 1) new regions where species impacts may be unknown; 2) at sites with diverse, yet morphologically similar, bat species; and 3) at sites with bat species of conservation concern where correctly identifying protected bat species is a higher priority.
2. Materials and Methods
We obtained wing tissue samples from bat carcasses collected during post-construction fatality surveys at wind energy facilities in Starr and Hidalgo Counties (TX, USA) in the Lower Rio Grande Valley region near the USA-Mexico border, from March through November of 2017 and 2018 (n = 439 carcasses). These facilities are in the Texas-Tamaulipan Thornscrub Level IV ecoregion [27], and the habitat includes scrub and shrub woodlands, cultivated crops, pasture and hay fields, and some developed lands. Bat carcasses were collected in accordance with the Texas State University Institutional Animal Care and Use Committee (IACUC: permit number 20171185494, to S. Weaver) and Texas Parks and Wildlife Department (TPWD: permit number SPR-0213-023, to S. Weaver). The climate, habitats, and bat community at this study site are similar to other regions in the southwestern USA and northwestern Mexico [25,28], for which data on the impacts of wind energy development on bats are largely unknown. Time since death (< 1 day, 2–3 days, ≥ 4 days) was estimated based on the state of decomposition and decay as well as the body tissues present [29]. We assigned species identifications (D. ega or D. intermedius) based on pelage color, presence of fur on anterior half of tail membrane, and/or forearm length [25], combined with epiphyseal-diaphyseal fusion status [30] when these structures were available to observe. Sex was assigned to only those carcasses with an estimated time since death < 1 day (the remaining carcasses were assigned sex unknown, due to uncertainty associated with rapid decomposition using external morphology) [29]. Wing tissue samples were taken from carcasses and stored in vials containing 95% ethanol. In addition to yellow bats, Brazilian free-tailed bat (Tadarida brasiliensis) fatalities have been documented in high numbers at this site [16,28], but we do not include them here, because they are morphologically distinct and better studied than the Dasypterus species [31,32,33].
We extracted DNA from the preserved Dasypterus tissue samples following the methods detailed in Korstian et al. [19] We sequenced the DNA samples at a 550 bp section of the mitochondrial cytochrome c oxidase I (COI) gene. To amplify the COI gene using a polymerase chain reaction (PCR), we used an M13-tailed primer cocktail [34], cocktail 2 in [35]. PCR reactions (10 µL) contained 10–50 ng DNA, 0.2 µM of the primer cocktail, 1X BSA, and 1X AccuStart™ II PCR SuperMix. PCR reactions were completed using an ABI 2720 thermal cycler with parameters: one cycle at 94 °C for 15 min, followed by 30 cycles of 30 s at 94 °C, 90 s at 57 °C, 90 s at 72 °C, and then a final extension of 5 min at 72 °C. Negative PCR controls were included in each set of amplifications. Products were sequenced in both directions using ABI Big Dye Terminator Cycle Sequencing v3.1 Chemistry (Applied Biosystems, USA), with the M13 primers. DNA sequences were analyzed on an ABI 3130XL Genetic Analyzer (Applied Biosystems, USA); trimmed, edited, and assembled into contigs using Sequencher v5.1 (Gene Codes, USA); and then aligned in MEGA v10 [36]. Aligned sequences were translated to verify the absence of stop codons, after which, they were compared to GenBank voucher sequences compiled by Korstian et al. [17], to generate a species ID. Only sequences > 400 bp in length were used and our criterion to accept a molecular species identification required an identity value > 98% in BLAST. Unique sequence haplotypes were detected using GenAlEx v6.5 [37]. We used Chi-square contingency tests to determine if correct species identification was independent of estimated time since death (< 1 day, 2–3 days, ≥ 4 days; = 0.05). All unique sequences have been deposited in GenBank (accession numbers: MT510982–MT510998).
We compiled sequences from this study, Korstian et al. [17], and GenBank, including sequences for D. i. floridanus (MN326026) and D. i. intermedius (MF990089). We constructed an unrooted maximum-likelihood tree in MEGA X, to visualize relationships among haplotypes [36]. The tree was inferred using the HKY + G model of base substitution with the highest value of the Bayesian information criterion (BIC) and the pairwise deletion option for missing data. Bootstrap values for the branching pattern were calculated using 1000 replicates.
Using PCR, we amplified the zfx and zfy introns found in the sex chromosomes using the primers in Korstian et al. [19]. PCR reactions deviated from the mitochondrial sequencing protocol, as 0.5 µM of X-primer and 0.35 µM of Y-primer were used in the 10 µL reaction. PCR cycling parameters were: one cycle at 95 °C for 15 min, followed by 30 cycles of 30 s at 94 °C, 15 s at 57 °C, 30 s at 72 °C. PCR products were fluorescently stained with Gel Red, electrophoresed at 200 volts in a 1% agarose gel for 20 min, and visualized using UV light. Sex was determined by the presence of one band in females corresponding to the X-chromosome intron (245 bp), whereas males had two bands, one for the X-chromosome intron and one for the Y-chromosome intron (80 bp). We used Fisher’s exact tests to compare the proportions of males to females, using both molecular and field sex identification (α = 0.05). We used Chi-square contingency tests to determine if correct sex determination was independent of estimated time since death (< 1 day, 2–3 days, ≥ 4 days; = 0.05).
3. Results
3.1. DNA Barcoding
Of the 439 tissue samples collected during surveys, 83% of carcasses (n = 366) were correctly identified to species based on DNA barcoding analysis (Figure 1). DNA barcoding improved the overall proportion of carcasses with accurate species identification to 97% (n = 425; Figure 1). Correct species identification in the field was independent of time since death, suggesting that decomposition and scavenging did not interfere with species identification (χ2 = 1.45, df = 2, p = 0.484). DNA barcoding revealed the presence of two additional species in the dataset: D. xanthinus (n = 36) and Lasiurus blossevillii (n = 3). Of the 295 carcasses that were field-identified as D. intermedius, 88% (n = 261) were correctly identified as D. intermedius, whereas 5% were D. ega (n = 14), and 4% were D. xanthinus (n = 11). Of the 144 carcasses that were field-identified as D. ega, 73% (n = 105) were correctly identified as D. ega, whereas 17% were D. xanthinus (n = 25), 4% were D. intermedius (n = 6), and 2% were D. blossevillii (n = 3; Figure 1). Due to DNA degradation, we were unable to obtain DNA barcodes for 3% (n = 9) of field-identified D. intermedius and 4% (n = 5) of field-identified D. ega. The maximum-likelihood tree using the 20 unique haplotypes we found in this study and from Korstian et al. [17], has clades corresponding to each of the three Dasypterus species (Figure 2). Bootstrap values separating species are in excess of 95. D. intermedius was split into two well supported monophyletic clades that corresponded to each putative subspecies, D. i. floridanus and D. i. intermedius.
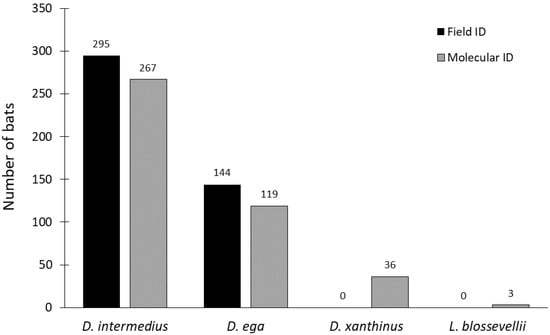
Figure 1.
Number of individual bats that were identified to each species, based on either external morphology in the field or DNA barcoding.
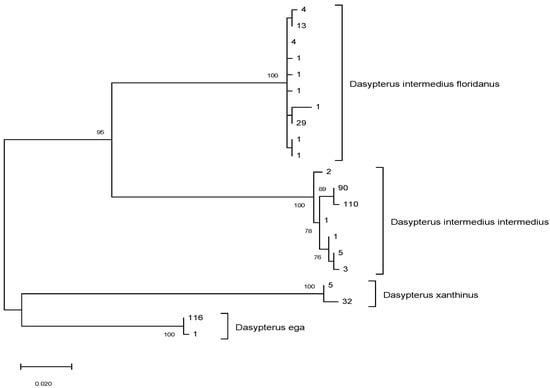
Figure 2.
Maximum likelihood tree of unique cytochrome c oxidase I (COI) haplotypes from Dasypterus samples in this study, as well as samples obtained from Korstian et al. [17] and from GenBank. Numbers at the nodes indicate bootstrap support following 1000 replicates, and branch length represents genetic distance. Numbers at branch tips represent the number of individuals with that unique haplotype.
3.2. Molecular Sex Determination
Survey efforts in the field assigned sex to just 35% (n = 153) of the total carcasses, of which 94% had a correct sex assignment that was confirmed using molecular methods (Figure 3). Of carcasses identified as either male or female based on external morphology, there was no significant deviation from a 50:50 male:female sex ratio (Fisher’s exact test: p = 0.87). Using molecular methods, 94% (n = 411) of the DNA samples amplified at the sex chromosome introns. With all species pooled together, 56% of bats were female (n = 230), with a significant deviation from a 50:50 sex ratio (Fisher’s exact test: p = 0.016; Figure 3) when using molecular sex determination. All three L. blossevillii bats were female. Of the 112 D. ega with successful sex chromosome intron amplification, 62% (n = 69) were female, representing a significant deviation from a 50:50 sex ratio (Fisher’s exact test: p = 0.018; Figure 4). Of the 254 D. intermedius with successful sex chromosome intron amplification, 53% (n = 134) were female, which was not significantly different from a 50:50 sex ratio (Fisher’s exact test: p = 0.415; Figure 4). Of the 34 D. xanthinus with successful sex chromosome intron amplification, 62% (n = 21) were female, which was also not significantly different from a 50:50 sex ratio (Fisher’s exact test: p = 0.229; Figure 4).
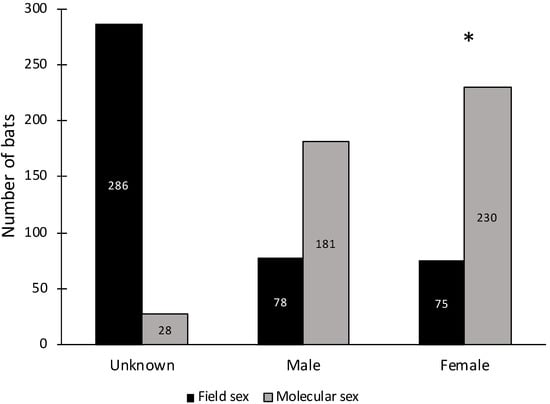
Figure 3.
Number of individual bats from all species combined that were assigned the sex of unknown, male, or female based on external morphology in the field or by using molecular methods (n = 439). The star above the female category indicates a significant deviation from a 50:50 sex ratio (Fisher’s exact test: p = 0.016).
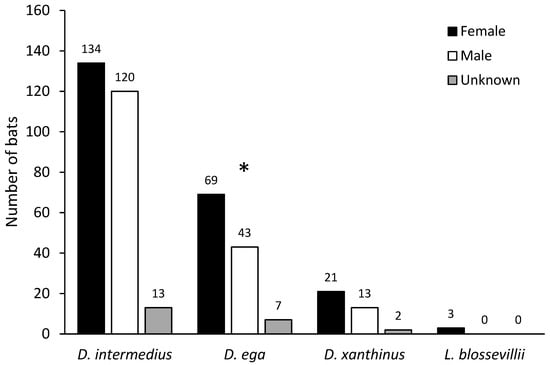
Figure 4.
Number of individual bats from each species identified as female, male, or sex unknown using molecular methods. The star above D. ega indicates a significant deviation from a 50:50 sex ratio (Fisher’s exact test: p = 0.018).
In contrast to species identification, correct sex determination based on external morphology was dependent upon time since death (χ2 = 162.1, df = 2, p < 0.001); fresh carcasses ( < 1 day) were significantly more likely to be correctly identified as male or female, compared to carcasses with longer estimated times since death.
4. Discussion
The error rate for field identification of Dasypterus species (14% misidentified overall) included in this study was higher than what was reported for L. borealis (2.6% misidentified) and A. cinereus (0.4% misidentified), killed at a north-central Texas wind energy facility [17]. Species misidentifications in this study likely occurred at a higher rate due to the morphological similarity between the two focal species and the presence of two additional bat species that were outside their known geographic ranges (L. blossevilli and D. xanthinus; [25]). Field personnel were unaware of the potential presence of these additional species. For the D. ega samples for which we obtained a DNA barcode, 24% of the bats had been misidentified in the field, perhaps due to the presence of D. xanthinus, which is morphologically very similar to D. ega [25] and comprised 74% of D. ega misidentifications.
A recent study by Decker et al. [26] provides updated geographic ranges, including range extensions at the county level, for all three Dasypterus species in Texas; but it does not, however, differentiate between the putative subspecies of D. intermedius (see Figure 5 for the study site location and range maps of D. ega, D. intermedius, D. xanthinus, and L. blossevillii within the continental USA [38,39,40,41]). Although only D. i. intermedius was suspected to occur in this region of south Texas [25], the DNA barcoding results of this study clearly indicate that both D. i. floridanus and D. i. intermedius are present at our study site. Additional support for the presence of both species is also provided by Decker [42]. This finding, in combination with the abundance of D. xanthinus samples in our dataset, recent evidence of D. xanthinus range expansion in New Mexico [43], and close proximity of our study site to a new county record for D. xanthinus in Texas [26], suggests that more research is needed to fully understand the distributions of Dasypterus species, especially in light of on-going land use modification and climate change. Similarly, recent studies of capture and specimen records for Lasiurus bats from the western USA and Canada have documented the range expansion for L. borealis in North America [44,45], and clarified the eastern and northern range limits of L. blossevilli in southwestern USA [45]; although, there is still much uncertainty regarding the extent of sympatry for these two Lasiurus species in far western and southern Texas [45]. In light of these recent findings, we will continue to underestimate the number of bat species and subspecies that may be impacted by wind energy, as development continues to expand into new areas if we rely solely on historic range maps and county-level records to make inferences regarding potential impacts. The presence of these previously undocumented species in the region also has implications for bat acoustic monitoring studies if biologists are not considering their potential for occurrence.
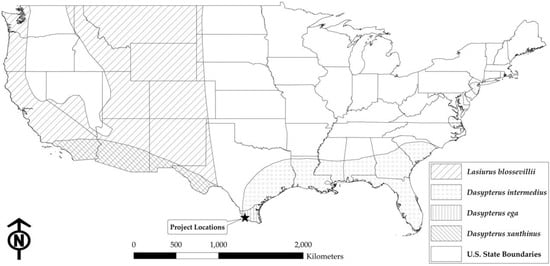
Figure 5.
IUCN (International Union for the Conservation of Nature) Red List range maps for L. blossevillii [38], D. intermedius [39], D. ega [40], and D. xanthinus [41] within the continental USA. The star indicates the location of the wind energy facilities included in this study.
In addition to errors with species identification, errors or missing data for sex assignment also introduce uncertainty regarding the impacts of wind energy development on bats. Variation in post-construction fatality monitoring protocols (e.g., search interval length, carcass persistence times), as well as how field technicians are trained, introduce variation in the quantity and quality of sex assignment data that can be obtained in the field. For example, in this study, personnel only assigned sex to 35% of the Dasypterus carcasses they collected in the field (i.e., those with estimated time since death < 1 day), meaning that any inferences related to a potential sex-bias or a lack of sex-bias in mortality would be limited to only a small subset of carcasses. By applying molecular methods, we were able to increase both the quantity and quality of the sex data for Dasypterus killed at these wind energy facilities: the percentage of bat carcasses with sex information was increased to 94% (n = 411 of 439 bats), and we improved accuracy by identifying errors in sex assignment of 6% of the bats that were assigned sex in the field. In a similar study from north-central Texas, Korstian et al. [19] showed that sex determination based on external morphology had a 10% error rate, and that by using molecular methods, they increased the number of bats with sex data from 554 to 867, representing a 56% increase in available data.
Some previous studies have reported male-biased sex ratios for migratory tree bats killed at wind energy facilities [46,47]; however, it is unknown if this observed bias is real or if it reflects the greater uncertainty of assigning sex to female bat carcasses, especially when they may have been partially scavenged and decomposing. Using molecular methods, sex ratios for L. borealis killed at wind energy facilities did not differ from 50:50 [18,19], whereas sex ratios for A. cinereus either did not differ from 50:50 [19] or were female-biased [18]. In this study, sex identification based on external morphology suggested a 50:50 sex ratio, whereas molecular methods revealed a female-biased sex ratio across all species combined and for D. ega analyzed separately. We do not know if the female-biased sex ratio we observed is due to an overall female bias in Dasypterus populations, or if the behavior of female bats increases their collision risk relative to males. Sex-specific conservation strategies for female Dasypterus may be warranted, as females are responsible for providing all offspring care and their mortality could lead to population declines over time [48]. In contrast to molecular species identification, which would be required only under certain conditions (i.e., in new regions exposed to wind energy development or in areas with morphologically similar species or species of conservation concern where correct species identification is paramount), we recommend molecular sex determination be more broadly utilized in bat-wind turbine fatality studies. Assigning sex based on morphology is simply inadequate to accurately characterize which sex of any given bat species is most susceptible to wind turbine mortality. Moving forward, there is a need to gather more accurate data on sex ratios of bat-wind turbine fatalities to determine if sex-biases are consistent across species and geographic areas before making range-wide management decisions.
Like previous studies, we found that reliance on morphology alone to identify species and determine sex of bats killed at wind energy facilities can be inaccurate and produce biased sex ratio estimates [17,18,19]. Accurate species identification and sex determination have a powerful influence on the ways in which we create, implement, and evaluate conservation strategies [49,50]. Uncertainty regarding species presence or probable presence makes it difficult to make informed siting decisions or habitat conservation plans to minimize the impacts of future development. Furthermore, minimization and mitigation strategies designed to reduce the number of bats killed at wind energy facilities require population-level knowledge of the impacted species to fully assess the effectiveness of these management practices [13,15]. The application of inexpensive and reliable molecular methods, such as those employed in this study, increases the number of individuals with sex and species identifications and reduces identification errors made during fatality surveys. Our in-house cost per sample (including DNA extraction, sequencing the barcode region, and determining sex) is USD $ 7.35. Sex determination alone, from DNA extraction to PCR, would be USD $ 1.00. The work described here could be conducted by a commercial research lab or alternatively at academic or governmental research labs with access to the necessary equipment, software (for analyzing the DNA barcoding results), and expertise. Numerous individuals and institutions already have the skills and expertise in place to generate these types of data. Enhancing our knowledge of which individual bats are being impacted adversely by wind energy development will allow for the implementation of effective sex- and species-specific management strategies.
Author Contributions
Conceptualization, A.S.C., A.M.H., S.P.W. and D.A.W.; methodology, A.S.C., S.P.W. and D.A.W.; statistical analyses, A.S.C., A.M.H. and D.A.W.; writing—original draft preparation, A.S.C., A.M.H. and D.A.W.; writing—review and editing, A.S.C., A.M.H., S.P.W. and D.A.W.; funding acquisition, A.S.C., A.M.H., S.P.W. and D.A.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a TCU College of Science & Engineering SERC Graduate Student Grant (G 190301) and a TCU Biology Department Adkins Fellowship to A.S.C. Supplies were funded by a Texas State University College of Science & Engineering Dorothy Coker Fellowship to S.P.W. and a Texas State University Graduate College Doctoral Research Support Fellowship to S.P.W.
Acknowledgments
We thank NextEra Energy Resources for their continued interest and support of our wind-wildlife research efforts. We thank Duke Energy and EDP Renewables for allowing access to their wind energy facilities, and the many field technicians who collected tissue samples.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Boyles, J.C.; Cryan, P.M.; McCracken, G.F.; Kunz, T.H. Economic importance of bats in agriculture. Science 2011, 332, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Kunz, T.H.; de Torrez, E.B.; Bauer, D.; Lobova, T.; Fleming, T.H. Ecosystem services provided by bats. Ann. N. Y. Acad. Sci. 2011, 1233, 1–38. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, T.J.; Cryan, P.M.; Hayman, D.T.S.; Plowright, R.K.; Steicker, D.G. Multiple mortality events in bats: A global review. Mamm. Rev. 2016, 46, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Frick, W.F.; Kingston, T.; Flanders, J. A review of the major threats and challenges to global bat conservation. Ann. N. Y. Acad. Sci. 2019, 1–21. [Google Scholar] [CrossRef]
- Tuttle, M.D. Fear of bats and its consequences. Bat Res. Cons. 2017, 10, 1. [Google Scholar] [CrossRef]
- Williams-Guillén, K.; Olimpi, E.; Maas, B.; Taylor, P.; Arlettaz, R. Bats in the anthropogenic matrix: Challenges and opportunities for the conservation of Chiroptera and their ecosystem services in agricultural landscapes. In Bats in the Anthropocene: Conservation of Bats in a Changing World; Voigt, C., Kingston, T., Eds.; Springer: New York, NY, USA, 2016; pp. 151–186. [Google Scholar]
- Kiesecker, J.M.; Evans, J.S.; Fargione, J.; Doherty, K.; Foresman, K.R.; Kunz, T.H.; Naugle, D.; Nibbelink, N.P.; Niemuth, N.D. Win-win for wind and wildlife: A vision to facilitate sustainable development. PLoS ONE 2011, 6, e17566. [Google Scholar] [CrossRef]
- Allison, T.D.; Diffendorfer, J.E.; Baerwald, E.F.; Beston, J.A.; Drake, D.; Hale, A.M.; Hein, C.D.; Huso, M.M.; Loss, S.R.; Lovich, J.E.; et al. Impacts to wildlife of wind energy siting and operation in the United States. Issues Ecol. 2019, 21, 1–24. [Google Scholar]
- Kunz, T.H.; Arnett, E.B.; Erickson, W.P.; Hoar, A.R.; Johnson, G.D.; Larkin, R.P.; Strickland, M.D.; Thresher, R.W.; Tuttle, M.D.M. Ecological impacts of wind energy development on bats: Questions, research needs, and hypotheses. Fron. Ecol. Environ. 2007, 5, 315–324. [Google Scholar] [CrossRef]
- Erickson, R.A.; Thogmartin, W.E.; Diffendorfer, J.E.; Russell, R.E.; Szymanski, J.A. Effects of wind energy generation and white-nose syndrome on the viability of the Indiana bat. PeerJ. 2016, 4, e2830. [Google Scholar] [CrossRef]
- Frick, W.F.; Baerwald, E.F.; Pollock, J.F.; Barclay, R.M.R.; Szymanski, J.A.; Weller, T.J.; Russell, A.L.; Loeb, S.C.; Medellin, R.A.; McGuire, L.P. Fatalities at wind turbines may threaten population viability of a migratory bat. Biol. Cons. 2017, 209, 172–177. [Google Scholar] [CrossRef]
- Rodhouse, T.J.; Rodriguez, R.M.; Banner, K.M.; Ormsbee, P.C.; Barnett, J.; Irvine, K.M. Evidence of region-wide bat population decline from long-term monitoring and Bayesian occupancy models with empirically informed priors. Ecol. Evo. 2019, 9, 11078–11088. [Google Scholar] [CrossRef] [PubMed]
- Arnett, E.B.; Huso, M.M.; Schirmacher, M.R.; Hayes, J.P. Altering turbine speed reduces bat mortality at wind-energy facilities. Fron. Ecol. Environ. 2011, 9, 209–214. [Google Scholar] [CrossRef]
- Martin, C.M.; Arnett, E.B.; Stevens, R.D.; Wallace, M.C. Reducing bat fatalities at wind facilities while improving the economic efficiency of operational mitigation. J. Mamm. 2017, 98, 378–385. [Google Scholar] [CrossRef]
- Hayes, M.A.; Hooton, L.A.; Gilland, K.L.; Grandgent, C.; Smith, R.L.; Lindsay, S.R.; Collins, J.D.; Schumacher, S.M.; Rabie, P.A.; Gruver, J.C.; et al. A smart curtailment approach for reducing bat fatalities and curtailment time at wind energy facilities. Ecol. Appl. 2019, 29, e01881. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.P.; Hein, C.D.; Simpson, T.R.; Evans, J.W.; Castro-Arellano, I. Ultrasonic acoustic deterrents significantly reduce bat fatalities at wind turbines. Global. Ecol. Cons. 2020, e01099. [Google Scholar] [CrossRef]
- Korstian, J.M.; Hale, A.M.; Bennett, V.J.; Williams, D.A. Using DNA barcoding to improve bat carcass identification at wind farms in the United States. Cons. Gen. 2016, 8, 27–34. [Google Scholar] [CrossRef]
- Nelson, D.M.; Nagel, J.; Trott, R.; Campbell, C.J.; Pruitt, L.; Good, R.E.; Iskali, G.; Gugger, P.F. Carcass age and searcher identity affect morphological assessment of sex of bats. J. Wild Man 2018, 82, 1582–1587. [Google Scholar] [CrossRef]
- Korstian, J.M.; Hale, A.M.; Bennett, V.J.; Williams, D.A. Advances in sex determination in bats and its utility in wind-wildlife studies. Mol. Ecol. Res. 2013, 13, 776–780. [Google Scholar] [CrossRef]
- American Wind Wildlife Institute (AWWI). AWWI Technical Report: A Summary of Bat Fatality Data in a Nationwide Database; AWWI: Washington, DC, USA, 2018; Available online: www.awwi.org (accessed on 12 November 2019).
- Arnett, E.; Baerwald, E.F. Impacts of wind energy development on bats: Implications for conservation. In Bat Evolution, Ecology, and Conservation; Adams, R., Pederson, C., Eds.; Springer: New York, NY, USA, 2013; pp. 435–456. [Google Scholar]
- Zimmerling, J.R.; Francis, C.M. Bat mortality due to wind turbines in Canada. J. Wild Man 2016, 80, 1360–1369. [Google Scholar] [CrossRef]
- Baird, A.B.; Braun, J.K.; Mares, M.A.; Morales, J.C.; Patton, J.C.; Tran, C.Q.; Bickham, J.W. Molecular systematic revision of tree bats (Lasiurini): Doubling the native mammals of the Hawaiian Islands. J. Mamm. 2015, 96, 1255–1274. [Google Scholar] [CrossRef]
- Baird, A.B.; Braun, J.K.; Engstrom, M.D.; Holbert, A.C.; Huerta, M.G.; Lim, B.K.; Mares, M.A.; Patton, J.C.; Bickham, J.W. Nuclear and mtDNA phylogenetic analyses clarify the evolutionary history of two species of native Hawaiian bats and the taxonomy of Lasiurini (Mammalia: Chiroptera). PLoS ONE 2017, 12, e0186085. [Google Scholar] [CrossRef] [PubMed]
- Ammerman, L.K.; Hice, C.L.; Schmidly, D.J. Bats of Texas; Texas A&M University Press: College Station, TX, USA, 2012. [Google Scholar]
- Decker, S.K.; Krejsa, D.M.; Lindsey, L.L.; Amoateng, R.P.; Ammerman, L.K. Updated distributions of three species of yellow bat (Dasypterus) in Texas based on specimen records. W Wildl. 2020, 7, 2–8. [Google Scholar]
- Griffith, G.; Bryce, S.; Omernik, J.; Rogers, A. Ecoregions of Texas; Texas Commission on Environmental Quality: Austin, TX, USA,, 2007. [Google Scholar]
- Ceballos, G. Mammals of Mexico; Johns Hopkins University Press: Baltimore, MD, USA, 2014. [Google Scholar]
- Weaver, S.P. Understanding wind energy impacts on bats and testing reduction strategies in south Texas. Ph.D. Thesis, Texas State University, San Marcos, TX, USA, 2019. [Google Scholar]
- Brunet-Rossinni, A.K.; Wilkinson, G.S. Methods for age estimation and the study of senescence in bats. In Ecological and Behavioral Methods for the Study of Bats; Kunz, T.J., Parsons, S., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2009; pp. 315–328. [Google Scholar]
- Kunz, T.H.; Whitaker, J.O.; Wadanoli, M.D. Dietary energetics of the insectivorous Mexican free-tailed bat (Tadarida brasiliensis) during pregnancy and lactation. Oecologia 1995, 101, 407–415. [Google Scholar] [CrossRef] [PubMed]
- McCracken, G.F.; Gillam, E.H.; Westbrook, J.K.; Lee, Y.F.; Jensen, M.L.; Balsley, B.B. Brazilian free-tailed bats (Tadarida brasiliensis: Molossidae, Chiroptera) at high altitude: Links to migratory insect populations. Integr. Comp. Biol. 2008, 48, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.L.; Cox, M.P.; Brown, V.A.; McCracken, G.F. Population growth of Mexican free-tailed bats (Tadarida brasiliensis mexicana) predates human agricultural activity. BMC Evol. Biol. 2011, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, N.V.; Zemlak, T.S.; Hanner, R.H.; Hebert, P.D.N. Universal primer cocktails for fish DNA barcoding. Mol. Ecol. Notes 2007, 7, 544–548. [Google Scholar] [CrossRef]
- Clare, E.L.; Lim, B.K.; Engstrom, M.D.; Eger, J.L.; Hebert, P.D.N. DNA barcoding of Neotropical bats: Species identification and discovery within Guyana. Mol. Ecol. Notes 2007, 7, 184–190. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Gonzalez, E.; Barquez, R.; Miller, B. Lasiurus blossevillii. The IUCN Red List of Threatened Species 2016: E.T88151055A22120040. Available online: https://dx.doi.org/10.2305/IUCN.UK.2016-1.RLTS.T88151055A22120040.en (accessed on 20 January 2020).
- Miller, B.; Rodriguez, B. Lasiurus intermedius (errata version published in 2017). The IUCN Red List of Threatened Species 2016: E.T11352A115101697. Available online: https://dx.doi.org/10.2305/IUCN.UK.2016-3.RLTS.T11352A22119630.en (accessed on 20 January 2020).
- Barquez, R.; Diaz, M. Lasiurus ega. The IUCN Red List of Threatened Species 2016: E.T11350A22119259. Available online: https://dx.doi.org/10.2305/IUCN.UK.2016-3.RLTS.T11350A22119259.en (accessed on 20 January 2020).
- Arroyo-Cabrales, J.; Álvarez-Castañeda, S.T. Lasiurus xanthinus. The IUCN Red List of Threatened Species 2017: E.T41532A22004260. Available online: https://dx.doi.org/10.2305/IUCN.UK.2017-2.RLTS.T41532A22004260.en. (accessed on 20 January 2020).
- Decker, S.K. Phylogeographic analysis of northern yellow bats, Dasypterus intermedius, by molecular analysis. Honors Thesis, Angelo State University, San Angelo, TX USA, 2019. Available online: https://hdl.handle.net/2346.1/30937.en (accessed on 21 May 2019).
- Zabriskie, J.E.; Cutler, P.L.; Stuart, J.N. Range extension of the western yellow bat (Dasypterus xanthinus) in New Mexico. W Wildl. 2019, 6, 1–4. [Google Scholar]
- Geluso, K.; Valdez, E.W. First records of the eastern red bat (Lasiurus borealis) in Arizona, Utah, and western New Mexico; Occasional Papers, Number 361; Museum of Texas Tech University: Lubbock, TX, USA, 2019. [Google Scholar]
- Solick, D.I.; Barclay, R.M.R.; Bishop-Boros, L.; Hays, Q.R.; Lausen, C.L. Distributions of eastern and western red bats in western North America. West. NA Nat. 2020, 80, 90–97. [Google Scholar] [CrossRef]
- Johnson, G.D.; Erickson, W.P.; Strickland, M.D.; Shepherd, M.F.; Shepherd, D.A.; Sarappo, S.A. Mortality of bats at a large-scale wind power development at Buffalo Ridge, Minnesota. Am. Mid. Nat. 2003, 150, 332–342. [Google Scholar] [CrossRef]
- Arnett, E.B.; Brown, W.K.; Erickson, W.P.; Fiedler, J.K.; Hamilton, B.L.; Henry, T.H.; Jain, A.; Johnson, G.D.; Kerns, J.; Koford, R.R.; et al. Patterns of bat fatalities at wind energy facilities in North America. J. Wild Man 2008, 72, 61–78. [Google Scholar] [CrossRef]
- Grüebler, M.U.; Schuler, H.; Müller, M.; Sparr, R.; Horch, P.; Naef-Daenzer, B. Female biased mortality caused by anthropogenic nest loss contributes to population decline and adult sex ratio of a meadow bird. Biol. Cons. 2008, 141, 3030–3049. [Google Scholar] [CrossRef]
- Cryan, P.M.; Jameson, J.W.; Baerwald, E.F.; Willis, C.K.R.; Barclay, R.M.R.; Snider, E.A.; Crichton, E.G. Evidence of late-summer mating readiness and early sexual maturation in migratory tree-roosting bats found dead at wind turbines. PLoS ONE 2012, 7, e47586. [Google Scholar] [CrossRef] [PubMed]
- Pickett, E.J.; Stockwell, M.P.; Pollard, C.J.; Garnham, J.I.; Clulow, J.; Mahony, M.J. Estimates of sex ratio require the incorporation of unequal catchability between sexes. Wild. Res. 2012, 39, 350–354. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).