Plant Community Assembly in Invaded Recipient Californian Grasslands and Putative Donor Grasslands in Spain
Abstract
1. Introduction
2. Materials and Methods
2.1. Grassland Surveys
2.2. Statistical Analyses
3. Results
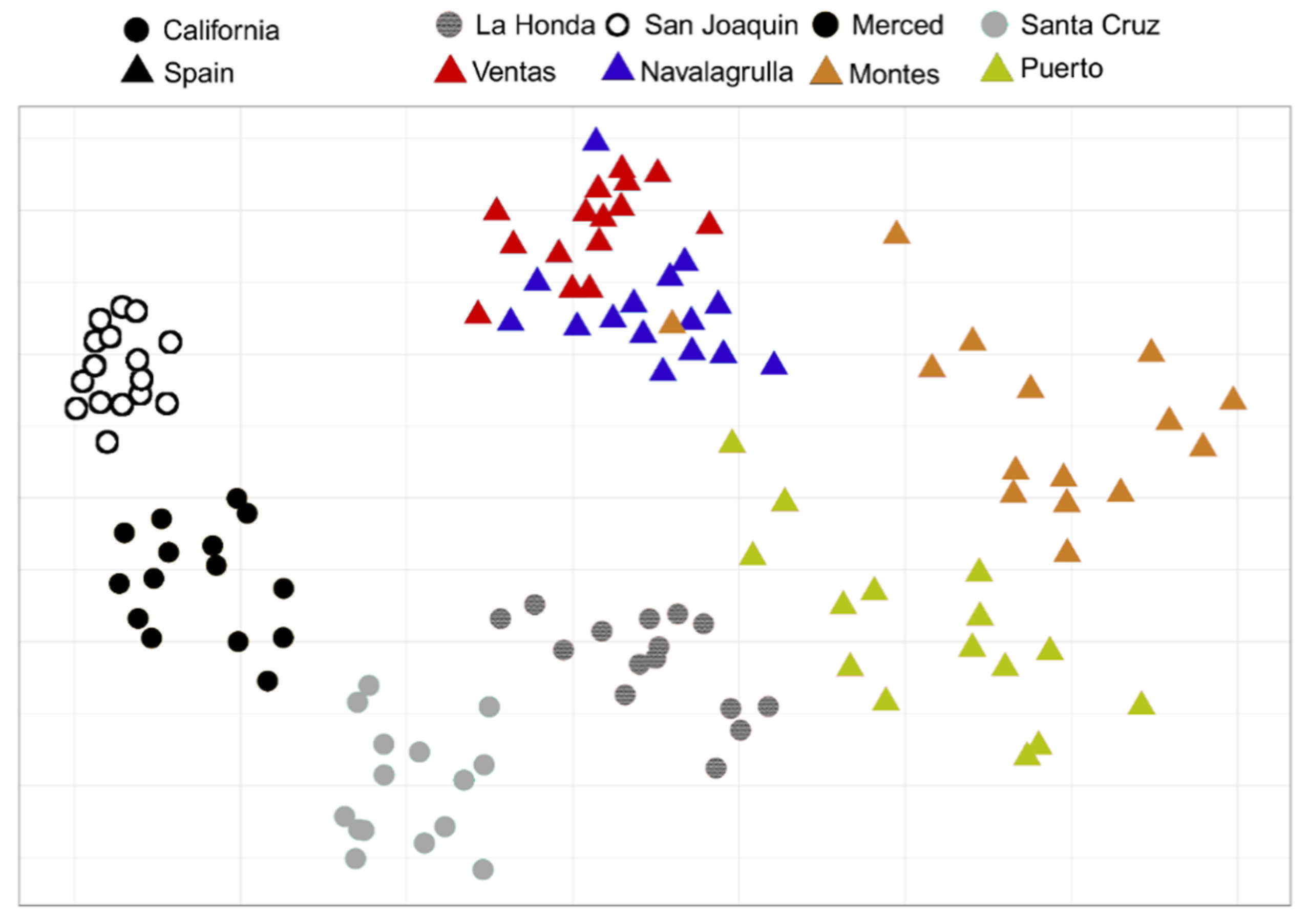
3.1. Similarities of Californian and Spanish Grassland Communities
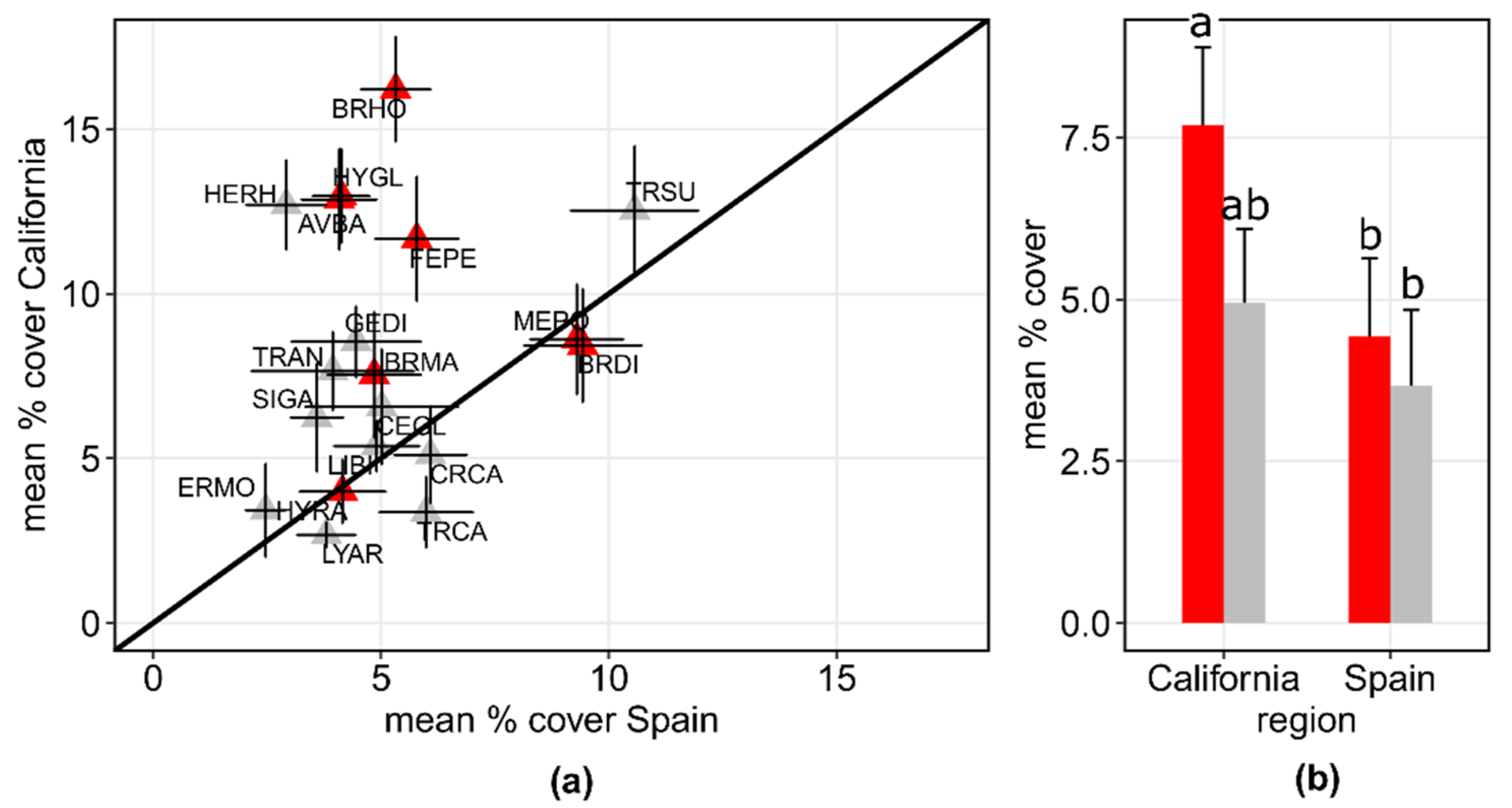
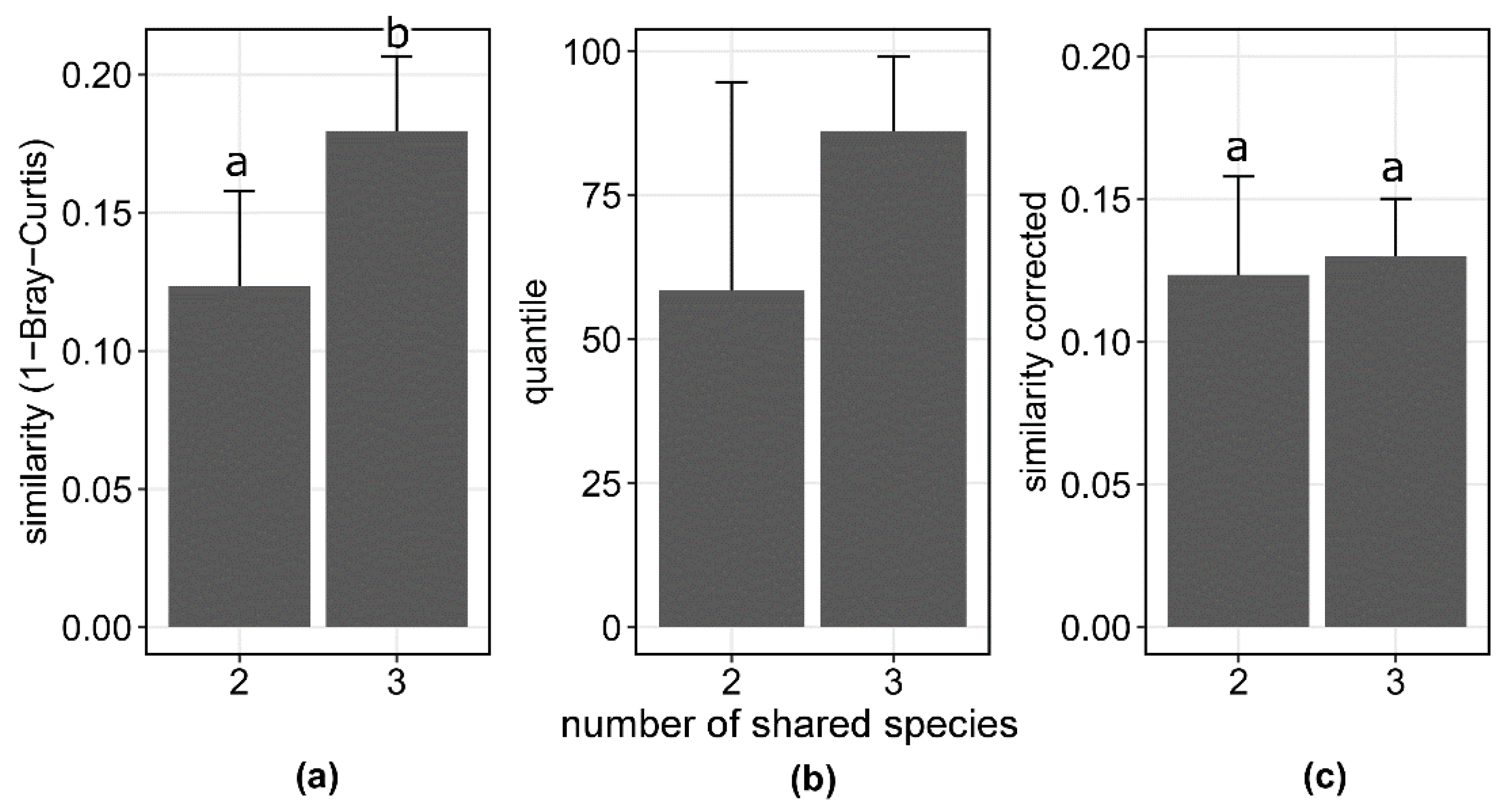
3.2. Assembly of Shared Species in Californian and Spanish Grasslands
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McKinney, M.L. Measuring floristic homogenization by non-native plants in North America. Glob. Ecol. Biogeogr. 2004, 13, 47–53. [Google Scholar] [CrossRef]
- Sax, D.F.; Gaines, S.D. Species diversity: From global decreases to local increases. Trends Ecol. Evol. 2003, 18, 561–566. [Google Scholar] [CrossRef]
- Winter, M.; Schweiger, O.; Klotz, S.; Nentwig, W.; Andriopoulos, P.; Arianoutsou, M.; Basnou, C.; Delipetrou, P.; Didziulis, V.; Hejda, M.; et al. Plant extinctions and introductions lead to phylogenetic and taxonomic homogenization of the European flora. Proc. Natl. Acad. Sci. USA 2009, 106, 21721–21725. [Google Scholar] [CrossRef] [PubMed]
- Bernardo-Madrid, R.; Calatayud, J.; González-Suárez, M.; Rosvall, M.; Lucas, P.M.; Rueda, M.; Antonelli, A.; Revilla, E. Human activity is altering the world’s zoogeographical regions. Ecol. Lett. 2019, 22, 1297–1305. [Google Scholar] [CrossRef]
- Stotz, G.C.; Cahill, J.F.; Bennett, J.A.; Carlyle, C.N.; Bork, E.W.; Askarizadeh, D.; Bartha, S.; Beierkuhnlein, C.; Boldgiv, B.; Brown, L.; et al. Not a melting pot: Plant species aggregate in their non-native range. Glob. Ecol. Biogeogr. 2020, 29, 482–490. [Google Scholar] [CrossRef]
- Van Kleunen, M.; Dawson, W.; Essl, F.; Pergl, J.; Winter, M.; Weber, E.; Kreft, H.; Weigelt, P.; Kartesz, J.; Nishino, M.; et al. Global exchange and accumulation of non-native plants. Nature 2015, 525, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Firn, J.; Moore, J.L.; MacDougall, A.S.; Borer, E.T.; Seabloom, E.W.; HilleRisLambers, J.; Harpole, W.S.; Cleland, E.E.; Brown, C.S.; Knops, J.M.H.H.; et al. Abundance of introduced species at home predicts abundance away in herbaceous communities. Ecol. Lett. 2011, 14, 274–281. [Google Scholar] [CrossRef]
- Pearson, D.E.; Eren, Ö.; Ortega, Y.K.; Villarreal, D.; Şentürk, M.; Miguel, M.F.; Weinzettel, C.M.; Prina, A.; Hierro, J.L. Are exotic plants more abundant in the introduced versus native range? J. Ecol. 2018, 106, 727–736. [Google Scholar] [CrossRef]
- Hierro, J.L.; Maron, J.L.; Callaway, R.M. A biogeographical approach to plant invasions: The importance of studying exotics in their introduced and native range. J. Ecol. 2005, 93, 5–15. [Google Scholar] [CrossRef]
- Hejda, M.; Štajerová, K.; Pyšek, P. Dominance has a biogeographical component: Do plants tend to exert stronger impacts in their invaded rather than native range? J. Biogeogr. 2017, 44, 18–27. [Google Scholar] [CrossRef]
- Hejda, M.; Štajerová, K.; Pergl, J.; Pyšek, P. Impacts of dominant plant species on trait composition of communities: Comparison between the native and invaded ranges. Ecosphere 2019, 10. [Google Scholar] [CrossRef]
- Sounding, E.; Sax, D.F.; Brown, J.H. The paradox of invasion. Glob. Ecol. Biogeogr. 2000, 9, 363–371. [Google Scholar] [CrossRef]
- Guerin, G.R.; Martín-Forés, I.; Sparrow, B.; Lowe, A.J. The biodiversity impacts of non-native species should not be extrapolated from biased single-species studies. Biodivers. Conserv. 2018, 27, 785–790. [Google Scholar] [CrossRef]
- Parker, J.D.; Torchin, M.E.; Hufbauer, R.A.; Bossdorf, O.; Byers, J.E.; Dunn, A.M.; Heckman, R.W.; Hejda, M.; Jarosik, V.; Kanarek, A.; et al. Do invasive species perform better in their new ranges? Ecology 2013, 94, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.; Hodgson, J.G.; Rich, T.C.G.G. Native and alien invasive plants: More of the same? Ecography 1995, 18, 390–402. [Google Scholar] [CrossRef]
- Colautti, R.; Parker, J.D.; Cadotte, M.W.; Pyšek, P.; Brown, C.S.; Sax, D.; Richardson, D. Quantifying the invasiveness of species. NeoBiota 2014, 21, 7–27. [Google Scholar] [CrossRef]
- Arianoutsou, M.; Delipetrou, P.; Vilà, M.; Dimitrakopoulos, P.G.; Celesti-Grapow, L.; Wardell-Johnson, G.; Henderson, L.; Fuentes, N.; Ugarte-Mendes, E.; Rundel, P.W. Comparative Patterns of Plant Invasions in the Mediterranean Biome. PLoS ONE 2013, 8, e79174. [Google Scholar] [CrossRef]
- De Miguel, J.M.; Martín-Forés, I.; Acosta-Gallo, B.; Del Pozo, A.; Ovalle, C.; Sánchez-Jardón, L.; Castro, I.; Casado, M.A. Non-random co-occurrence of native and exotic plant species in Mediterranean grasslands. Acta Oecologica 2016, 77, 18–26. [Google Scholar] [CrossRef]
- MacDougall, A.S.; McCune, J.L.; Eriksson, O.; Cousins, S.; Pärtel, M.; Firn, J.; Hierro, J.L. The Neolithic Plant Invasion Hypothesis: The role of preadaptation and disturbance in grassland invasion. New Phytol. 2018. [Google Scholar] [CrossRef]
- Casado, M.A.; Martín-Forés, I.; Castro, I.; De Miguel, J.M.; Acosta-Gallo, B. Asymmetric flows and drivers of herbaceous plant invasion success among Mediterranean-climate regions. Sci. Rep. 2018, 8, 16834. [Google Scholar] [CrossRef]
- Martín-Forés, I. Exotic Plant Species in the Mediterranean Biome: A Reflection of Cultural and Historical Relationships. Mediterr. Identities Environ. Soc. Cult. 2017. [Google Scholar] [CrossRef]
- Rejmánek, M.; Randall, J. Invasive alien plants in California: 1993 summary and comparison with other areas in North America. Madroño 1994, 41, 161–177. [Google Scholar]
- California Invasive Plant Inventory (Cal-IPC). Available online: www.cal-ipc.org (accessed on 25 September 2019).
- D’Antonio, C.M.; Vitousek, P.M. Biological Invasions by Exotic Grasses, the Grass/Fire Cycle, and Global Change. Annu. Rev. Ecol. Syst. 1992, 23, 63–87. [Google Scholar] [CrossRef]
- Minnich, R.A. California’s Fading Wildflowers: Lost Legacy and Biological Invasions; University of California Press: Berkeley, CA, USA, 2008; ISBN 9780520253537. [Google Scholar]
- Hamilton, J.G. Changing perceptions of pre-European grasslands in California. Madroñoo 1997, 44, 311–333. [Google Scholar]
- Stromberg, M.R.; Griffin, J.R. Long-Term Patterns in Coastal California Grasslands in Relation to Cultivation, Gophers, and Grazing. Ecol. Appl. 1996, 6, 1189–1211. [Google Scholar] [CrossRef]
- Corbin, J.D.; D’Antonio, C.M. Competition between native perennial and exotic annual grasses: Implications for an historical invasion. Ecology 2004, 85, 1273–1283. [Google Scholar] [CrossRef]
- HilleRisLambers, J.; Yelenik, S.G.; Colman, B.P.; Levine, J.M. California annual grass invaders: The drivers or passengers of change? J. Ecol. 2010, 98, 1147–1156. [Google Scholar] [CrossRef]
- Reilly, M.J.; McCord, M.G.; Brandt, S.M.; Linowksi, K.P.; Butz, R.J.; Jules, E.S. Repeated, high-severity wildfire catalyzes invasion of non-native plant species in forests of the Klamath Mountains, northern California, USA. Biol. Invasions 2020, 22, 1821–1828. [Google Scholar] [CrossRef]
- Stuble, K.L.; Young, T.P. Priority Treatment Leaves Grassland Restoration Vulnerable to Invasion. Diversity 2020, 12, 71. [Google Scholar] [CrossRef]
- Martín-Forés, I.; Guerin, G.R.; Lowe, A.J. Weed abundance is positively correlated with native plant diversity in grasslands of southern Australia. PLoS ONE 2017, 12, 1–13. [Google Scholar] [CrossRef]
- Valdés, B.; Talavera, S.; Galiano, E.F. Flora Vascular de Andalucía Occidental; Ketres Editores, S.A.: Barcelona, Spain, 1987; ISBN 84-85256-63-8. [Google Scholar]
- Calflora: Information on California Plants for Education, Research and Conservation. Available online: https://www.calflora.org/ (accessed on 25 September 2019).
- Fick, S.E.; Hijmans, R.J. Worldclim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Clarke, K.R.; Green, R.H. Statistical design and analysis for a “biological effects” study. Mar. Ecol. Prog. Ser. 1988, 46, 213–226. [Google Scholar] [CrossRef]
- Calatayud, J.; Andivia, E.; Escudero, A.; Melián, C.J.; Bernardo-Madrid, R.; Stoffel, M.; Aponte, C.; Medina, N.G.; Molina-Venegas, R.; Arnan, X.; et al. Positive associations among rare species and their persistence in ecological assemblages. Nat. Ecol. Evol. 2020, 4, 40–45. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing (R-3.6.1); R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Leiva, M.J.; Chapin, F.S.; Fernandez Ales, R. Differences in species composition and diversity among Mediterranean grasslands with different history—The case of California and Spain. Ecography 1997, 20, 97–106. [Google Scholar] [CrossRef]
- Eriksson, O. Species pools in cultural landscapes—Niche construction, ecological opportunity and niche shifts. Ecography 2013, 36, 403–413. [Google Scholar] [CrossRef]
- La Sorte, F.A.; Pyšek, P. Extra-regional residence time as a correlate of plant invasiveness: European archaeophytes in north America. Ecology 2009, 90, 2589–2597. [Google Scholar] [CrossRef]
- Martín-Forés, I.; Sánchez-Jardón, L.; Acosta-Gallo, B.; Del Pozo, A.; Castro, I.; De Miguel, J.M.; Ovalle, C.; Casado, M.A. From Spain to Chile: Environmental filters and success of herbaceous species in Mediterranean-climate regions. Biol. Invasions 2015, 17, 1425–1438. [Google Scholar] [CrossRef]
- Dark, S.J. The biogeography of invasive alien plants in California: An application of GIS and spatial regression analysis. Divers. Distrib. 2004, 10, 1–9. [Google Scholar] [CrossRef]
- Chytrý, M.; Jarošík, V.; Pyšek, P.; Hájek, O.; Knollová, I.; Tichý, L.; Danihelka, J.; Maskell, L.C.; Pino, J.; Pyšek, P.; et al. Separating Habitat Invasibility By Alien Plants. Ecology 2008, 89, 1541–1553. [Google Scholar] [CrossRef]
- Sutherland, S. What makes a weed a weed: Life history traits of native and exotic plants in the USA. Oecologia 2004, 141, 24–39. [Google Scholar] [CrossRef]
- Van Kleunen, M.; Weber, E.; Fischer, M. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol. Lett. 2010, 13, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Molinari, N.A.; D’Antonio, C.M. Structural, compositional and trait differences between native- and non-native-dominated grassland patches. Funct. Ecol. 2014, 28, 745–754. [Google Scholar] [CrossRef]
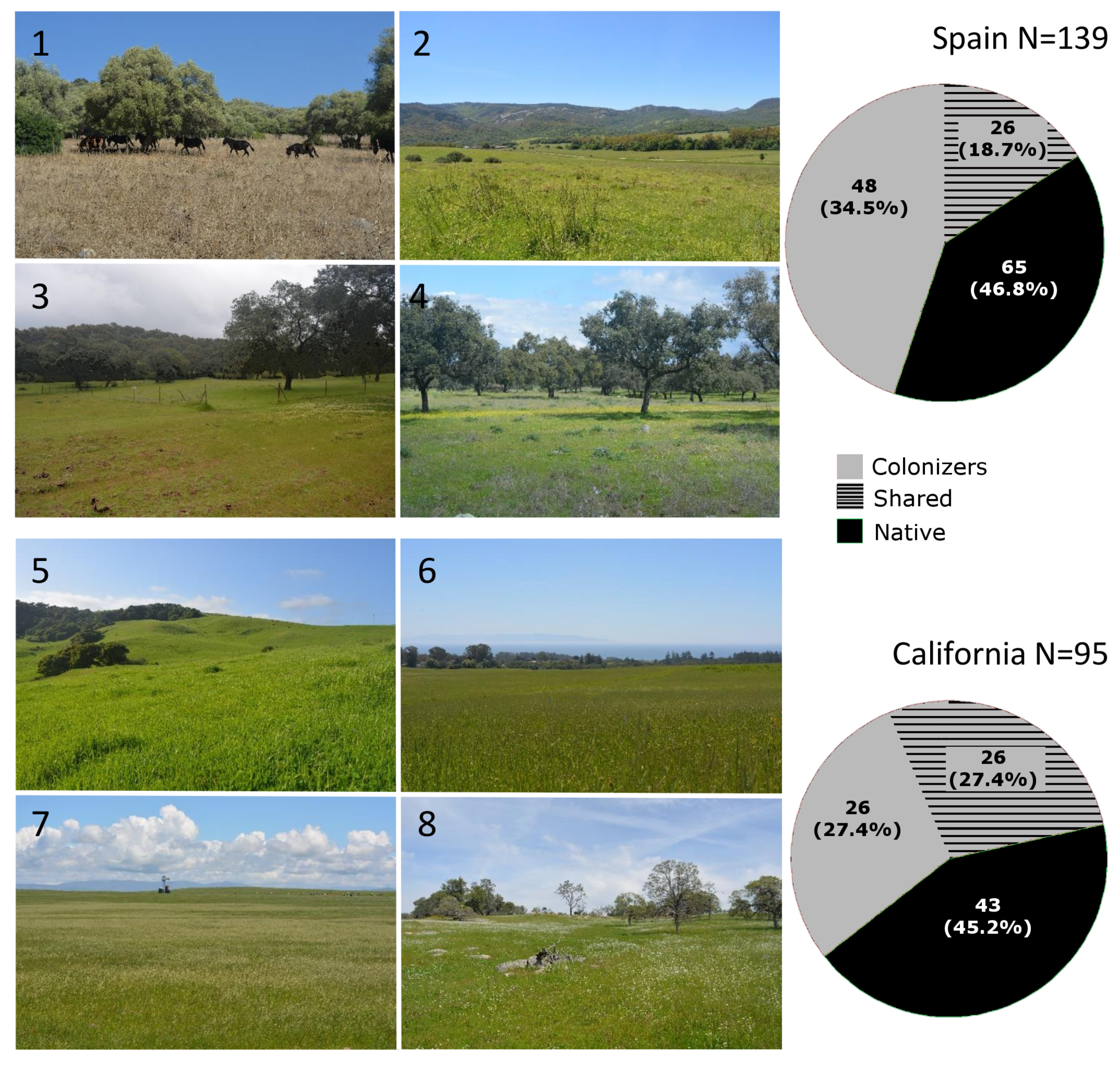
| Region | Grassland | Altitude (m) | MAR (mm) | MAT (°C) | MCM (°C) | N (%) | P (mg/kg) | C:N | OM (%) | pH | Richness | Natives | Colonizers | Shared |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spain | Navalagrulla | 300 | 596 | 17 | 4 | 0.13 ± 0.01 | 10.70 ± 1.46 | 12.41 ± 0.07 | 2.88 ± 0.16 | 6–7 | 63 | 18 | 45 | 19 |
| Ventas | 280 | 617 | 17 | 4 | 0.13 ± 0.02 | 7.26 ± 0.95 | 12.31 ± 0.52 | 2.78 ± 0.47 | 6–7 | 60 | 23 | 37 | 17 | |
| Montes | 180 | 753 | 17 | 7 | 0.23 ± 0.03 | 13.72 ± 3.92 | 12.18 ± 0.35 | 4.80 ± 0.77 | 6–7 | 53 | 23 | 30 | 11 | |
| Puerto | 245 | 796 | 16 | 5 | 0.17 ± 0.02 | 8.10 ± 0.79 | 12.32 ± 0.87 | 3.64 ± 0.49 | 6–7 | 61 | 28 | 33 | 14 | |
| California | San Joaquin | 310 | 519 | 16 | 2 | 0.08 ± 0.01 | 8.86 ± 3.30 | 13.01 ± 0.94 | 1.80 ± 0.21 | 6–7 | 31 | 17 | 14 | 8 |
| Merced | 100 | 375 | 16 | 2 | 0.23 ± 0.05 | 4.54 ± 1.00 | 10.70 ± 0.49 | 4.26 ± 1.05 | 6–7 | 32 | 19 | 13 | 10 | |
| Santa Cruz | 115 | 769 | 14 | 4 | 0.19 ± 0.02 | 4.44 ± 1.37 | 12.18 ± 0.63 | 3.99 ± 0.35 | 6–7 | 31 | 8 | 23 | 14 | |
| La Honda | 405 | 794 | 13 | 4 | 0.28 ± 0.03 | 18.66 ± 4.16 | 12.40 ± 0.68 | 5.82 ± 0.62 | 6–7 | 31 | 7 | 24 | 16 |
| Variable (per Plot) | California | Spain | Df | F | p |
|---|---|---|---|---|---|
| Total Shannon diversity | 2.09 ± 0.07 | 2.51 ± 0.11 | 1,6 | 15.38 | 0.007 |
| Total richness | 11.05 ± 1.05 | 16.65 ± 1.07 | 1,6 | 32.11 | <0.001 |
| Richness natives | 2.62 ± 1.25 | 5.69 ± 1.36 | 1,6 | 6.44 | 0.01 |
| Richness colonizers | 7.53 ± 1.13 | 10.71 ± 1.20 | 1,6 | 3.17 | 0.08 |
| Richness shared species | 5.39 ± 1.19 | 4.89 ± 1.28 | 1,6 | 0.16 | 0.69 |
| Cover natives | 17.64 ± 15.54 | 33.47 ± 23.39 | 1,6 | 7.49 | 0.03 |
| Cover colonizers | 81.11 ± 8.03 | 61.69 ± 11.43 | 1,6 | 5.75 | 0.05 |
| Cover shared species | 59.20 ± 11.15 | 26.57 ± 11.67 | 1,6 | 26.89 | 0.002 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galán Díaz, J.; de la Riva, E.G.; Parker, I.M.; Leiva, M.J.; Bernardo-Madrid, R.; Vilà, M. Plant Community Assembly in Invaded Recipient Californian Grasslands and Putative Donor Grasslands in Spain. Diversity 2020, 12, 193. https://doi.org/10.3390/d12050193
Galán Díaz J, de la Riva EG, Parker IM, Leiva MJ, Bernardo-Madrid R, Vilà M. Plant Community Assembly in Invaded Recipient Californian Grasslands and Putative Donor Grasslands in Spain. Diversity. 2020; 12(5):193. https://doi.org/10.3390/d12050193
Chicago/Turabian StyleGalán Díaz, Javier, Enrique G. de la Riva, Ingrid M. Parker, María José Leiva, Rubén Bernardo-Madrid, and Montserrat Vilà. 2020. "Plant Community Assembly in Invaded Recipient Californian Grasslands and Putative Donor Grasslands in Spain" Diversity 12, no. 5: 193. https://doi.org/10.3390/d12050193
APA StyleGalán Díaz, J., de la Riva, E. G., Parker, I. M., Leiva, M. J., Bernardo-Madrid, R., & Vilà, M. (2020). Plant Community Assembly in Invaded Recipient Californian Grasslands and Putative Donor Grasslands in Spain. Diversity, 12(5), 193. https://doi.org/10.3390/d12050193






