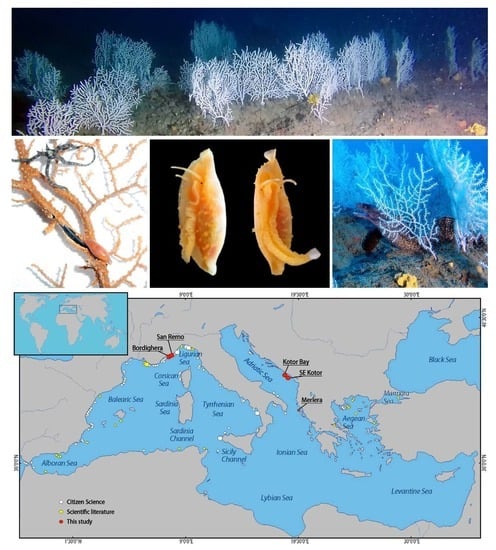
Vulnerable Forests of the Pink Sea Fan Eunicella verrucosa in the Mediterranean Sea
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. The Forest of Eunicella verrucosa off Sanremo
3.2. The Coral Forest off Bordighera
3.3. Additional Records of Eunicella verrucosa in the Adriatic and Ionian Seas
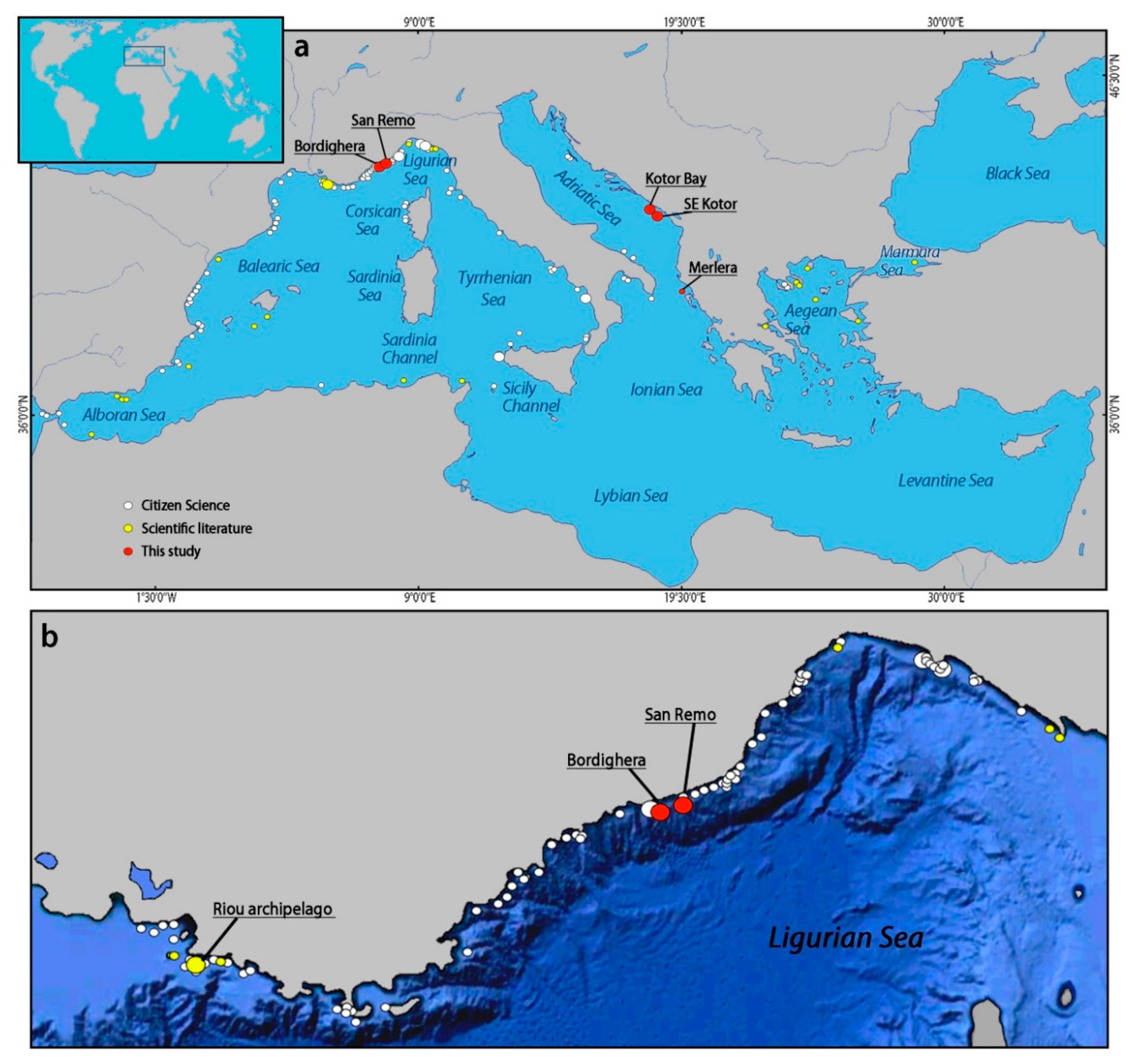
3.4. Distribution of Eunicella verrucosa in the Mediterranean Sea
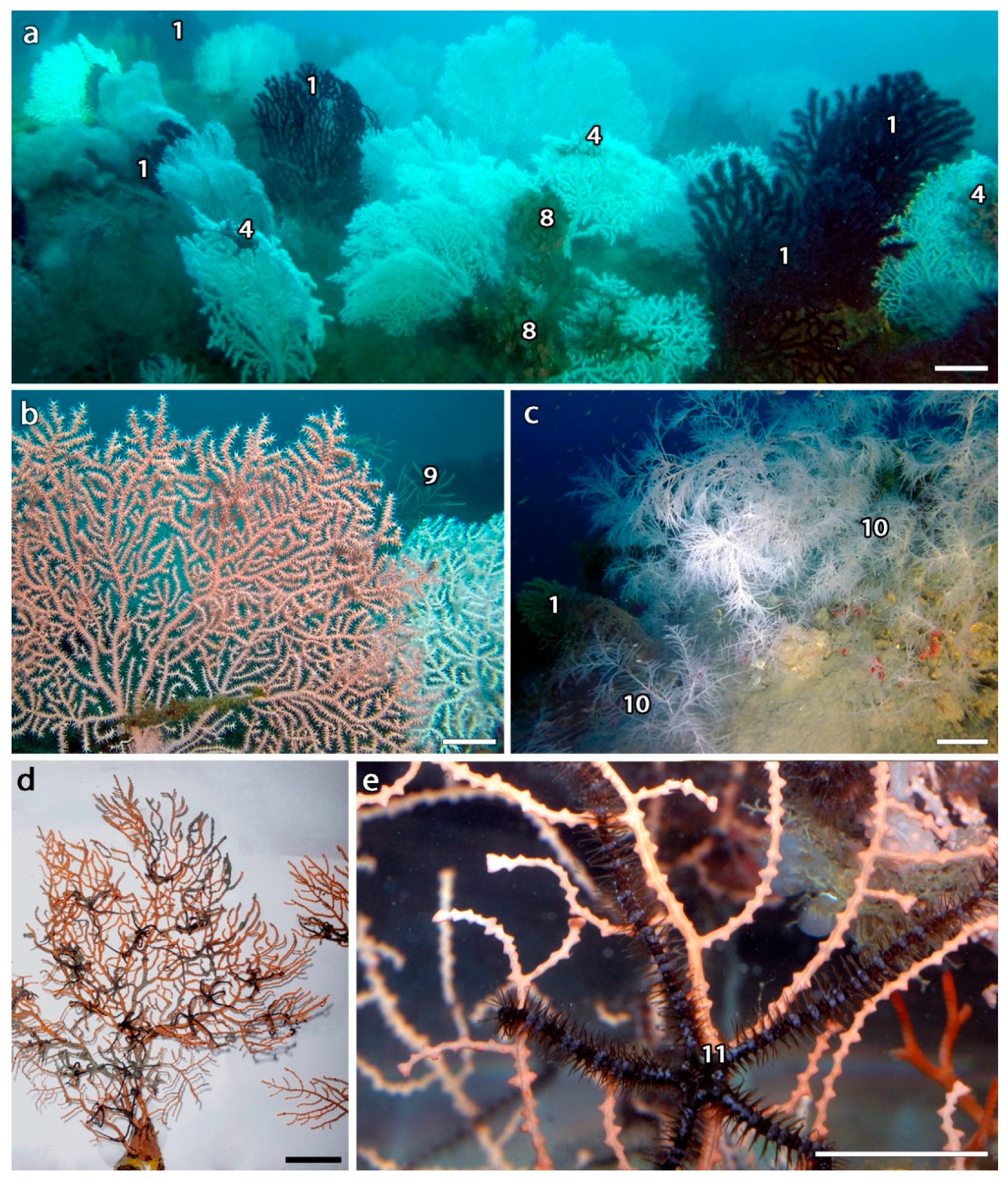
3.5. Biodiversity Associated with Eunicella verrucosa Forests
4. Discussion
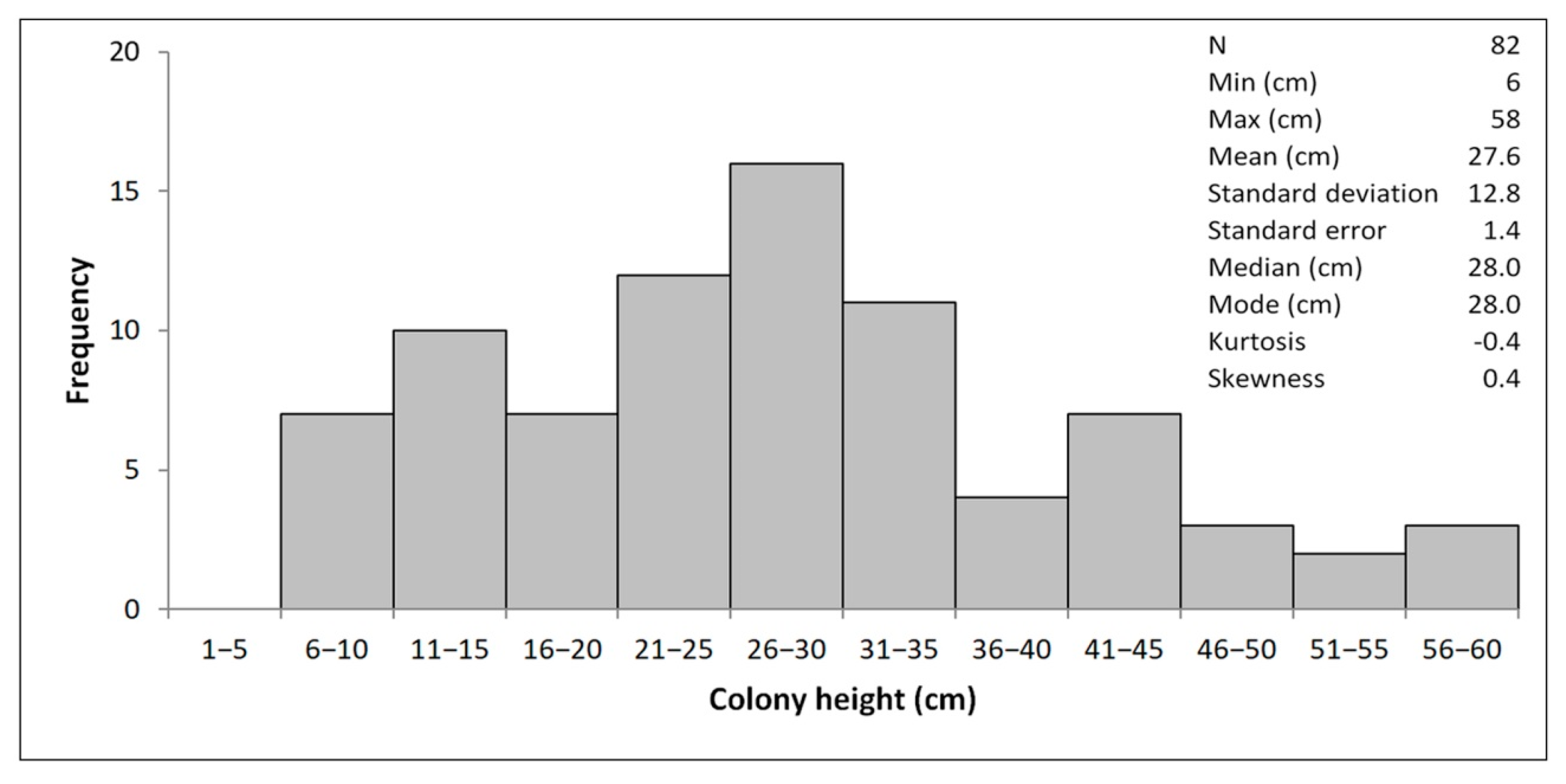
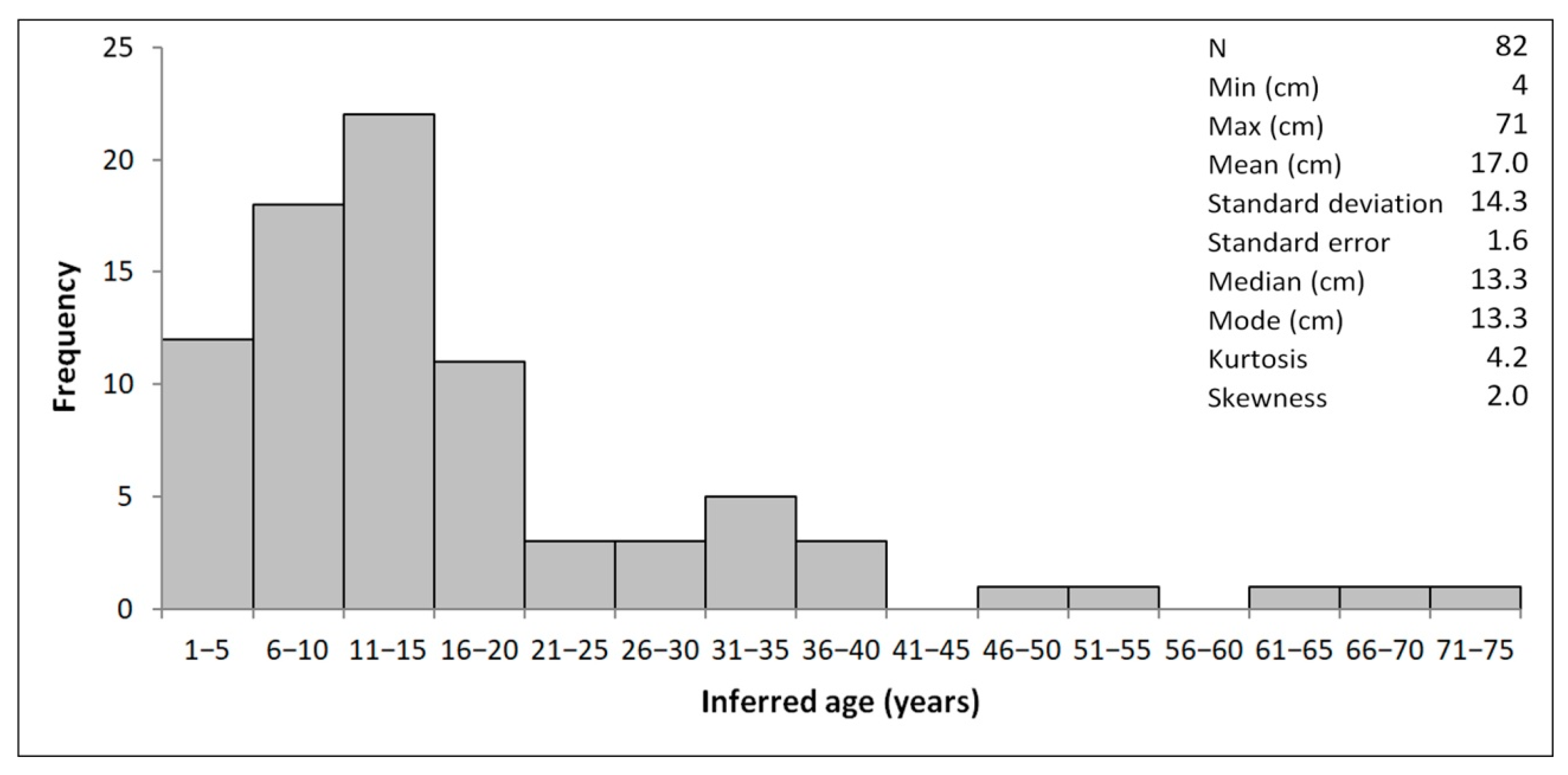
4.1. Morphometry and Age Inference
4.2. Eunicella verrucosa in the Mediterranean Sea
4.3. Impacts and Threats
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization (FAO). International Guidelines for the Management of Deep-Sea Fisheries in the High Seas; FAO: Rome, Italy, 2009; pp. 1–21. [Google Scholar]
- Lesser, M.P.; Slattery, M.; Leichter, J.J. Ecology of mesophotic coral reefs. J. Exp. Mar. Biol. Ecol. 2009, 375, 1–8. [Google Scholar] [CrossRef]
- Rossi, S.; Bramanti, L.; Gori, A.; Orejas, C. An overview of the animal forests of the world. In Marine Animal Forests; Rossi, S., Ed.; Springer International Publishing: Berlin, Germany, 2017; pp. 1–26. [Google Scholar]
- Chimienti, G.; Mastrototaro, F.; D’Onghia, G. Mesophotic and Deep-Sea Vulnerable Coral Habitats of the Mediterranean Sea: Overview and Conservation Perspectives. In The Benthos Zone; Soto, L., Ed.; IntechOpen: London, UK, 2019; pp. 1–20. [Google Scholar] [CrossRef] [Green Version]
- Carpine, C.; Grasshoff, M. Les Gorgonaires de la Mediterranean. Bull. Inst. Océanogr. Monaco 1975, 71, 1–140. [Google Scholar]
- Gili, J.M.; Coma, R. Benthic suspension feeders: Their paramount role in littoral marine food webs. Trends Ecol. Evol. 1998, 13, 316–321. [Google Scholar] [CrossRef]
- Linares, C.; Coma, R.; Garrabou, J.; Díaz, D.; Zabala, M. Size distribution, density and disturbance in two Mediterranean gorgonians: Paramuricea clavata and Eunicella singularis. J. Appl. Ecol. 2008, 45, 688–699. [Google Scholar] [CrossRef]
- Cerrano, C.; Danovaro, R.; Gambi, C.; Pusceddu, A.; Riva, A.; Schiaparelli, S. Gold coral (Savalia savaglia) and gorgonian forests enhance benthic biodiversity and ecosystem functioning in the mesophotic zone. Biodivers. Conserv. 2010, 19, 153–167. [Google Scholar] [CrossRef] [Green Version]
- Angeletti, L.; Taviani, M.; Canese, S.; Foglini, F.; Mastrototaro, F.; Argnani, A.; Trincardi, F.; Bakran–Petricioli, T.; Ceregato, A.; Chimienti, G.; et al. New deep-water cnidarian sites in the southern Adriatic Sea. Mediterr. Mar. Sci. 2014, 15, 225–238. [Google Scholar]
- Ponti, M.; Perlini, R.A.; Ventra, V.; Grech, D.; Abbiati, M.; Cerrano, C. Ecological shifts in Mediterranean coralligenous assemblages related to gorgonian forest loss. PLoS ONE 2014, 9, e102782. [Google Scholar] [CrossRef]
- Ponti, M.; Turicchia, E.; Ferro, F.; Cerrano, C.; Abbiati, M. The understorey of gorgonian forests in mesophotic temperate reefs. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 1153–1166. [Google Scholar] [CrossRef]
- Grinyó, J.; Gori, A.; Ambroso, S.; Purroy, A.; Calatayud, C.; Dominguez-Carrió, C.; Coppari, M.; Iacono, C.L.; López-González, P.J.; Gili, J.M. Diversity, distribution and population size structure of deep Mediterranean gorgonian assemblages (Menorca Channel, Western Mediterranean Sea). Prog. Oceanogr. 2016, 145, 42–56. [Google Scholar] [CrossRef]
- Chimienti, G.; Stithou, M.; Dalle Mura, I.; Mastrototaro, F.; D’Onghia, G.; Tursi, A.; Izzi, C.; Fraschetti, S. An explorative assessment of the importance of mediterranean coralligenous habitat to local economy: The case of recreational diving. J. Environ. Account. Manag. 2017, 5, 315–325. [Google Scholar] [CrossRef] [Green Version]
- Mastrototaro, F.; Chimienti, G.; Acosta, J.; Blanco, J.; Garcia, S.; Rivera, J.; Aguilar, R. Isidella elongata (Cnidaria: Alcyonacea) facies in the western Mediterranean Sea: Visual surveys and descriptions of its ecological role. Eur. Zool. J. 2017, 84, 209–225. [Google Scholar] [CrossRef] [Green Version]
- Carpine, C. Contribution à la connaissance des Gorgones Holaxonia de la Mediterranean occidentale. Bull. Inst. océanogr. Monaco 1963, 60, 1–52. [Google Scholar]
- Weinberg, S. Revision of the common Octocorallia of the Mediterranean circalittoral. I. Gorgonacea. Beaufortia 1976, 24, 63–104. [Google Scholar]
- Grasshoff, M. Die Flachwasser-Gorgonarien von Europa und Westafrika (Cnidaria, Anthozoa). Cour. Forsch. Inst. Senckenberg 1992, 149, 1–135. [Google Scholar]
- Lafargue, F. Peuplements sessiles de l’archipel des Glénans. Vie Milieu 1969, 20, 415–436. [Google Scholar]
- Hiscock, K. Where have all the corals gone? Mar. Conserv. 2003, 6, 8–9. [Google Scholar]
- Pikesley, S.K.; Godley, B.J.; Latham, H.; Richardson, P.B.; Robson, L.M.; Solandt, J.L.; Trundle, C.; Wood, C.; Witt, M.J. Pink sea fans (Eunicella verrucosa) as indicators of the spatial efficacy of Marine Protected Areas in southwest UK coastal waters. Mar. Policy 2016, 64, 38–45. [Google Scholar] [CrossRef]
- Otero, M.M.; Numa, C.; Bo, M.; Orejas, C.; Garrabou, J.; Cerrano, C.; Kružić, P.; Antoniadou, C.; Aguilar, R.; Kipson, S.; et al. Overview of the Conservation Status of Mediterranean Anthozoans; IUCN: Málaga, Spain, 2017; pp. 1–73. [Google Scholar]
- Sartoretto, S.; Francour, P. Bathymetric distribution and growth rates of Eunicella verrucosa (Cnidaria: Gorgoniidae) populations along the Marseilles coast (France). Sci. Mar. 2012, 76, 349–355. [Google Scholar] [CrossRef] [Green Version]
- Di Camillo, C.G.; Ponti, M.; Bavestrello, G.; Krzelj, M.; Cerrano, C. Building a baseline for habitat-forming corals by a multi-source approach, including Web Ecological Knowledge. Biodivers. Conserv. 2018, 27, 1257–1276. [Google Scholar] [CrossRef]
- Pérès, J.M.; Picard, J. Nouveau manuel de bionomie benthique de la mer Méditerranée. Rec. Trav. Stat. Mar. Endoume 1964, 31, 1–137. [Google Scholar]
- Ballesteros, E. Mediterranean coralligenous assemblages: A synthesis of present knowledge. Oceanogr. Mar. Biol. Ann. Rev. 2006, 44, 123–195. [Google Scholar]
- Ingrosso, G.; Abbiati, M.; Badalamenti, F.; Bavestrello, G.; Belmonte, G.; Cannas, R.; Benedetti-Cecchi, L.; Bertolino, M.; Bevilacqua, S.; Bianchi, C.N.; et al. Mediterranean bioconstructions along the Italian coast. Adv. Mar. Biol. 2018, 79, 61–136. [Google Scholar] [PubMed]
- Cocito, S.; Bedulli, D.; Sgorbini, S. Distribution patterns of the sublittoral epibenthic assemblages on a rocky shoal in the Ligurian Sea (NW Mediterranean). Sci. Mar. 2002, 66, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Rossi, L. Le specie di Eunicella (Gorgonaria) del golfo di Genova. Ann. Mus. Civ. Stor. Nat. Genova 1959, 71, 203–225. [Google Scholar]
- Vafidis, D.; Koukouras, A.; Voultsiadou-Koukoura, E. Octocoral fauna of the Aegean Sea with a check list of the Mediterranean species: New information, faunal comparisons. Ann. Inst. Oceanogr. Paris 1994, 70, 217–229. [Google Scholar]
- Cebrián, E.; Ballesteros, E. Zonation patterns of benthic communities in an upwelling area from the western Mediterranean (La Herradura, Alboran Sea). Sci. Mar. 2004, 68, 69–84. [Google Scholar] [CrossRef] [Green Version]
- Cerrano, C.; Bavestrello, G.; Palma, M.; Previati, M.; Schiapparelli, S. Una popolazione di Gerardia savaglia (Bertoloni, 1819) nell’area marina protetta di Portofino. Biol. Mar. Mediterr. 2007, 14, 156–157. [Google Scholar]
- Kružić, P. Anthozoan fauna of Telašćica Nature Park (Adriatic Sea, Croatia). Nat. Croat. 2007, 16, 233–266. [Google Scholar]
- Coppo, S.; Diviacco, G.; Tunesi, L. Environmental and conservation relevance of the Punta Manara coralligenous beds (Eastern Ligurian Sea). In Proceedings of the 1st Symposium on the Coralligenous and Other Calcareous Bioconcretions of the Mediterranean Sea, Tabarka, Tunisia, 15–16 January 2009; pp. 75–81. [Google Scholar]
- Bo, M.; Bava, S.; Canese, S.; Angiolillo, M.; Cattaneo-Vietti, R.; Bavestrello, G. Fishing impact on deep Mediterranean rocky habitats as revealed by ROV investigation. Biol. Conserv. 2014, 171, 167–176. [Google Scholar] [CrossRef]
- Gori, A.; Bavestrello, G.; Grinyó, J.; Dominguez-Carrió, C.; Ambroso, S.; Bo, M. Animal forests in deep coastal bottoms and continental shelf of the Mediterranean Sea. In Marine Animal Forests; Rossi, S., Ed.; Springer International Publishing: Berlin, Germany, 2017; pp. 207–233. [Google Scholar]
- de la Torriente, A.; González-Irusta, J.M.; Aguilar, R.; Fernández-Salas, L.M.; Punzón, A.; Serrano, A. Benthic habitat modelling and mapping as a conservation tool for marine protected areas: A seamount in the western Mediterranean. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 732–750. [Google Scholar] [CrossRef]
- Ghanem, R.; Kechaou, E.S.; Ben Souissi, J.; Garrabou, J. Overview on the distribution of gorgonian species in Tunisian marine coastal waters (central Mediterranean). Sci. Mar. 2018, 82, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Weinberg, S. The minimal area problem in invertebrate communities of Mediterranean rocky substrata. Mar. Biol. 1978, 49, 33–40. [Google Scholar] [CrossRef]
- Ambroso, S.; Gori, A.; Dominguez-Carrió, C.; Gili, J.M.; Berganzo, E.; Teixidó, N.; Greenacre, M.; Rossi, S. Spatial distribution patterns of the soft corals Alcyonium acaule and Alcyonium palmatum in coastal bottoms (Cap de Creus, northwestern Mediterranean Sea). Mar. Biol. 2014, 160, 3059–3070. [Google Scholar] [CrossRef]
- Chimienti, G.; Angeletti, L.; Rizzo, L.; Tursi, A.; Mastrototaro, F. ROV vs. trawling approaches in the study of benthic communities: The case of Pennatula rubra (Cnidaria: Cennatulacea). J. Mar. Biol. Assoc. UK 2018, 98, 1859–1869. [Google Scholar] [CrossRef]
- Komsta, L.; Novomestky, F. Moments, Cumulants, Skewness, Kurtosis and Related Tests. R Package Version 0.13. 2012. Available online: http://CRAN.R-project.org/package=moments (accessed on 13 April 2020).
- Anscombe, F.J.; Gynn, W.J. Distribution of the kurtosis statistic b2 for normal samples. Biometrika 1983, 70, 227–234. [Google Scholar] [CrossRef]
- Demir, M. Boğazlar ve adalar sahillerinin omurgasız dip hayvanları. Ist. Univ. Rac. Sci. Hydrobiol. Res. Ins. Publ. 1952, 3, 1–615. [Google Scholar]
- Gori, A.; Bramanti, L.; López-González, P.; Thoma, J.N.; Gili, J.M.; Grinyó, J.; Uceira, V.; Rossi, S. Characterization of the zooxanthellate and azooxanthellate morphotypes of the Mediterranean gorgonian Eunicella singularis. Mar. Biol. 2012, 159, 1485–1496. [Google Scholar] [CrossRef]
- Pax, F.; Müller, I. Die Anthozoenfauna der Adria; Institut für Ozeanographie und Fischerei: Split, Croatia, 1962; pp. 1–343. [Google Scholar]
- Cerrano, C.; Milanese, M.; Ponti, M. Diving for science-science for diving: Volunteer scuba divers support science and conservation in the Mediterranean Sea. Aquat. Conserv. 2017, 7, 303–323. [Google Scholar] [CrossRef]
- Furfaro, G.; Trainito, E.; De Lorenzi, F.; Fantin, M.; Doneddu, M. Tritonia nilsodhneri Marcus Ev., 1983 (Gastropoda, Heterobranchia, Tritoniidae): First records for the Adriatic Sea and new data on ecology and distribution of Mediterranean populations. Acta Adriat. 2017, 58, 261–270. [Google Scholar] [CrossRef]
- Fabri, M.C.; Pedel, L.; Beuck, L.; Galgani, F.; Hebbeln, D.; Freiwald, A. Megafauna of vulnerable marine ecosystems in French mediterranean submarine canyons: Spatial distribution and anthropogenic impacts. Deep Sea Res. Part II Top. Stud. Oceanogr. 2014, 104, 184–207. [Google Scholar] [CrossRef] [Green Version]
- Bo, M.; Bavestrello, G.; Angiolillo, M.; Calcagnile, L.; Canese, S.; Cannas, R.; Cau, A.; D’Elia, M.; D’Oriano, F.; Follesa, M.C.; et al. Persistence of pristine deep-sea coral gardens in the Mediterranean Sea (SW Sardinia). PLoS ONE 2015, 10, e0119393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chimienti, G.; Bo, M.; Taviani, M.; Mastrototaro, F. Occurrence and Biogeography of Mediterranean Cold-Water Corals. In Mediterranean Cold-Water Corals: Past, Present and Future; Orejas, C., Jimennez, C., Eds.; Springer International Publishing: Berlin, Germany, 2019; Volume 9, pp. 213–243. [Google Scholar]
- Ocaña, A.; Sánchez Tocino, L.; López-González, P.J. Consideraciones faunísticas y biogeográficas de los antozoos (Cnidaria: Anthozoa) de la costa de Granada (Mar de Alborán). Zool. Baetica 2000, 11, 51–65. [Google Scholar]
- Chimienti, G.; Bo, M.; Mastrototaro, F. Know the distribution to assess the changes: Mediterranean cold-water coral bioconstructions. Rend. Lincei Sci. Fis. Nat. 2018, 29, 583–588. [Google Scholar] [CrossRef]
- Coz, R.; Ouisse, V.; Artero, C.; Carpentier, A.; Crave, A.; Feunteun, E.; Olivier, J.M.; Perrin, B.; Ysnel, F. Development of a new standardised method for sustainable monitoring of the vulnerable pink sea fan Eunicella verrucosa. Mar. Biol. 2012, 159, 1375–1388. [Google Scholar] [CrossRef]
- Murillo, F.J.; MacDonald, B.W.; Kenchington, E.; Campana, S.E.; Sainte-Marie, B.; Sacau, M. Morphometry and growth of sea pen species from dense habitats in the Gulf of St. Lawrence, eastern Canada. Mar. Biol. Res. 2018, 14, 366–382. [Google Scholar] [CrossRef]
- Chimienti, G.; Di Nisio, A.; Lanzolla, A.M.L.; Andria, G.; Tursi, A.; Mastrototaro, F. Towards non-invasive methods to assess population structure and biomass in vulnerable sea pen fields. Sensors 2019, 19, 2255. [Google Scholar] [CrossRef] [Green Version]
- Mistri, M.; Ceccherelli, V.U. Growth of the Mediterranean gorgonian Lophogorgia ceratophyta (L., 1758). Mar. Ecol. 1993, 14, 329–340. [Google Scholar] [CrossRef]
- Coma, R.; Ribes, M.; Zabala, M.; Gili, J.M. Growth in a modular colonial marine invertebrates. Estuar. Coast. Shlef Sci. 1998, 47, 459–470. [Google Scholar] [CrossRef] [Green Version]
- Andrews, A.H.; Cordes, E.E.; Mahoney, M.M.; Munk, K.; Coale, K.H.; Caillet, G.M.; Heifetz, J. Age, growth and radiometric age validation of a deep-sea, habitat-forming gorgonian (Primnoa resedaeformis) from the Gulf of Alaska. Hydrobiologia 2002, 471, 101–110. [Google Scholar] [CrossRef]
- Company, J.B.; Ramirez-Llodra, E.; Sardà, F. Submarine canyons in the Catalan Sea (NW Mediterranean): Megafaunal biodiversity patterns and anthropogenic threats. In Mediterranean Submarine Canyons: Ecology and Governance; Würtz, M., Ed.; IUCN: Gland, Switzerland; Málaga, Spain, 2012; pp. 133–144. [Google Scholar]
- Hinz, H. Impact of bottom fishing on animal forests: Science, conservation, and fisheries management. In Marine Animal Forests; Rossi, S., Ed.; Springer International Publishing: Berlin, Germany, 2017; pp. 1041–1059. [Google Scholar]
- Mytilineou, C.; Smith, C.J.; Anastasopoulou, A.; Papadopoulou, K.N.; Christidis, G.; Bekas, P.; Kavadas, S.; Dokos, J. New cold-water coral occurrences in the eastern Ionian Sea: Results from experimental long line fishing. Deep Sea Res. Part II Top. Stud. Oceanogr. 2014, 99, 146–157. [Google Scholar] [CrossRef]
- Sampaio, I.; Braga-Henriques, A.; Pham, C.; Ocaña, O.; De Matos, V.; Morato, T.; Porteiro, F.M. Cold-water corals landed by bottom longline fisheries in the Azores (north-eastern Atlantic). J. Mar. Biol. Assoc. UK 2012, 92, 1547–1555. [Google Scholar] [CrossRef]
- D’Onghia, G. Cold-water corals as shelter, feeding and life-history critical habitats for fish species: Ecological interactions and fishing impact. In Mediterranean Cold-Water Corals: Past, Present and Future; Orejas, C., Jimennez, C., Eds.; Springer International Publishing: Berlin, Germany, 2019; Volume 9, pp. 335–356. [Google Scholar]
- Enrichetti, F.; Dominguez-Carrió, C.; Toma, M.; Bavestrello, G.; Betti, F.; Canese, S.; Bo, M. Megabenthic communities of the Ligurian deep continental shelf and shelf break (NW Mediterranean Sea). PLoS ONE 2019, 14, e0223949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO (Food and Agriculture Organization). Abandoned, lost or otherwise discarded gillnets and trammel nets: Methods to estimate ghost fishing mortality, and the status of regional monitoring and management. In FAO Fisheries and Aquaculture Technical Paper No. 600; Gilman, E., Chopin, F., Suuronen, P., Kuemlangan, B., Eds.; FAO: Italy, Rome, 2016; pp. 1–79. [Google Scholar]
- Garrabou, J.; Coma, R.; Bensoussan, N.; Bally, M.; Chevaldonné, P.; Cigliano, M.; Diaz, D.; Harmelin, J.G.; Gambi, M.C.; Kersting, D.K.; et al. Mass mortality in NW Mediterranean rocky benthic communities: Effects of the 2003 heat wave. Glob. Chang. Biol. 2009, 15, 1090–1103. [Google Scholar] [CrossRef]
- Hall-Spencer, J.M.; Pike, J.; Munn, C.B. Diseases affect cold-water corals too: Eunicella verrucosa (Cnidaria: Gorgonacea) necrosis in SW England. Dis. Aquat. Org. 2007, 76, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Morato, T.; González-Irusta, J.M.; Dominguez-Carrió, C.; Wei, C.L.; Davies, A.; Sweetman, A.K.; Taranto, G.H.; Beazley, L.; García-Alegre, A.; Grehan, A.; et al. Climate-induced changes in the suitable habitat of cold-water corals and commercially important deep-sea fishes in the North Atlantic. Glob. Chang. Biol. 2020, 26, 2181–2202. [Google Scholar] [CrossRef]


| Basin | Country | Locality | Lat N | Lon E | N | Depth | Year | Source |
|---|---|---|---|---|---|---|---|---|
| Balearic Sea | Fr | Maire Island, Riou Archipelago | 43.2076 | 5.3402 | - | 20–40 | 2007 | [22] |
| Ligurian Sea | It | I tuvi Bordighera | 43.7691 | 7.6878 | >50 | 30–33 | 2012 | RCMed |
| Ligurian Sea | It | Bordighera | 43.768 | 7.694 | >50 | 47–50 | 2020 | This study |
| Ligurian Sea | It | Sanremo, Scoglio dell’Astice | 43.800 | 7.838 | >150 | 65–70 | 2020 | This study |
| Ligurian Sea | It | Pilone | 43.9158 | 8.1404 | >50 | 33–35 | 2014 | RCMed |
| Ligurian Sea | It | Portofino, Punta Chiappa | 44.3230 | 9.1456 | >50 | 45–55 | 2006 | RCMed |
| Ligurian Sea | It | Portofino, Punta del Faro | 44.2976 | 9.2181 | >50 | 40–70 | 2015 | RCMed |
| Ligurian Sea | It | Portofino, Punta del Faro | 44.2980 | 9.2183 | >50 | 42–45 | 2013 | RCMed |
| Ligurian Sea | It | Portofino, Punta del Faro | 44.2980 | 9.2196 | >50 | 45–50 | 2013 | RCMed |
| Ligurian Sea | It | Punta Manara | 44.2427 | 9.4027 | >50 | 40–45 | 2006 | RCMed |
| Tyrrhenian Sea | It | Scalea | 39.8177 | 15.7731 | >100 | 44–55 | 2010 | RCMed |
| Sicily Channel | It | Favignana | 37.9048 | 12.3059 | >50 | 25–30 | 2010 | RCMed |
| Sicily Channel | It | Favignana, Trigone | 37.9068 | 12.3037 | >50 | 25–30 | 2010 | RCMed |
| Sicily Channel | It | Favignana, Galeotta | 37.9114 | 12.2999 | >50 | 18–30 | 2010 | RCMed |
| Sicily Channel | It | Favignana, Toro Canyon | 37.8771 | 12.3095 | > 100 | 16–32 | 2010 | RCMed |
| Sicily Channel | It | Favignana, Secca del Toro | 37.8782 | 12.3097 | >100 | 20–30 | 2010 | RCMed |
| Sicily Channel | It | Favignana, Secca Fondale | 37.8744 | 12.3091 | >100 | 20–30 | 2010 | RCMed |
| Adriatic Sea | Mo | Kotor Bay | 42.3015 | 18.5127 | - | 108 | 2014 | This study |
| Adriatic Sea | Mo | SE Kotor | 42.2312 | 18.5919 | - | 110 | 2014 | This study |
| Phylum, Class | Taxon | Location | Protection | ||
|---|---|---|---|---|---|
| B | S | I | |||
| Foraminifera | |||||
| Globothalamea | Miniacina miniacea (Pallas, 1766) | SE Kotor | |||
| Porifera | |||||
| Demospongiae | Agelas oroides (Schmidt, 1864) | Sanremo | |||
| Aplysina aerophoba (Nardo, 1833) | Sanremo | II | |||
| Axinella brondstedi Bergquist, 1970 | Bordighera, Sanremo | ||||
| Axinella damicornis (Esper, 1794) | Sanremo | ||||
| Axinella polypoides Schmidt, 1862 | Bordighera, Sanremo | II | II | ||
| Chondrosia reniformis Nardo, 1847 | Sanremo | ||||
| Cliona sp. | Riou [22], Bordighera | ||||
| Dysidea avara (Schmidt, 1862) | Bordighera | ||||
| Geodia sp. | Sanremo | ||||
| Hemimycale columella (Bowerbank, 1874) | Sanremo | ||||
| Ircinia sp. | Sanremo | ||||
| Pachastrella monilifera Schmidt, 1868 | Sanremo | ||||
| Petrosia sp. | Bordighera, Sanremo | ||||
| Phorbas sp. | Sanremo | ||||
| Pleraplysilla spinifera (Schulze, 1879) | Sanremo | ||||
| Raspaciona aculeata (Johnston, 1842) | Bordighera | ||||
| Sarcotragus cf. foetidus Schmidt, 1862 | Sanremo | II | |||
| Spongia sp. | Sanremo | ||||
| Suberitidae | Sanremo | ||||
| Homoscleromorpha | Oscarella lobularis (Schmidt, 1862) | Sanremo | |||
| Cnidaria | |||||
| Hydrozoa | Aglaophenia sp. | Sanremo | |||
| Halecium sp. | SE Kotor | ||||
| Anthozoa | Alcyonium acaule Marion, 1878 | Sanremo | LC | ||
| Caryophyllia sp. | Kotor Bay, SE Kotor, Bordighera, Sanremo | ||||
| Eunicella cavolini (Koch, 1887) | Riou [22], Bordighera, Sanremo | NT | |||
| Leptogorgia sarmentosa (Esper, 1789) | Riou [22], Bordighera | LC | |||
| Paramuricea clavata (Risso, 1826) | Riou [22], Bordighera, Sanremo | VU | |||
| Parazoanthus axinellae (Schmidt, 1862) | Bordighera, Sanremo | LC | |||
| Savalia savaglia (Bertoloni, 1819) | Sanremo | II | II | NT | |
| Mollusca | |||||
| Gastropoda | Felimare picta (Philippi, 1836) | Sanremo | |||
| Simnia spelta (Linnaeus, 1758) 1 | Riou [22], SE Kotor | ||||
| Tritonia nilsodhneri Marcus Ev., 1983 1 | Grananda [47], Riou [22], Portofino [31] | ||||
| Bivalvia | Pteria hirundo (Linnaeus, 1758) 1 | SE Kotor | |||
| Annelida | |||||
| Polychaeta | Bonellia viridis Rolando, 1822 | Bordighera, Sanremo | |||
| Filograna-Salmacina complex 1 | Bordighera, Sanremo | ||||
| Sabella pavonina Savigny, 1822 | Sanremo | ||||
| Serpula vermicularis Linnaeus, 1767 | Sanremo | ||||
| Serpulidae | Sanremo, SE Kotor | ||||
| Arthropoda | |||||
| Malacostraca | Balssia gasti (Balss, 1921) 1 | Riou [22] | |||
| Homarus gammarus (Linnaeus, 1758) | Sanremo | III | III | LC | |
| Maja squinado (Herbst, 1788) | Sanremo | III | III | ||
| Palinurus elephas (Fabricius, 1787) | Sanremo | III | III | VU | |
| Bryozoa | |||||
| Gymnolaemata | Adeonella calveti Canu & Bassler, 1930 | Sanremo | |||
| Myriapora truncata (Pallas, 1766) | Sanremo | ||||
| Pentapora fascialis (Pallas, 1766) | Sanremo | ||||
| Reteporella cf. grimaldii (Jullien, 1903) 1 | Bordighera, Sanremo | ||||
| Schizomavella (Schizomavella) mamillata (Hincks, 1880) | Sanremo, Kotor Bay | ||||
| Schizomavella sp. | Sanremo | ||||
| Smittina cervicornis (Pallas, 1766) | Sanremo | ||||
| Echinodermata | |||||
| Crinoidea | Antedon mediterranea (Lamarck, 1816) 1 | Kotor Bay, SE Kotor | |||
| Holothuroidea | Holothuria (Holothuria) tubulosa Gmelin, 1791 | Bordighera, Sanremo | LC | ||
| Holothuria (Panningothuria) forskali Delle Chiaje, 1823 | Bordighera, Sanremo | LC | |||
| Holothuria (Roweothuria) poli Delle Chiaje, 1824 | Bordighera, Sanremo | LC | |||
| Asteroidea | Anseropoda placenta (Pennant, 1777) | SE Kotor | |||
| Echinaster (Echinaster) sepositus (Retzius, 1783) | Sanremo | ||||
| Hacelia attenuata Gray, 1840 | Sanremo | ||||
| Marthasterias glacialis (Linnaeus, 1758) | Sanremo | ||||
| Peltaster placenta (Müller & Troschel, 1842) | Sanremo | ||||
| Ophiuroidea | Astrospartus mediterraneus (Risso, 1826) 1 | Bordighera, Sanremo | |||
| Ophiacantha setosa (Bruzelius, 1805) 1 | Corsica [5], Kotor Bay, SE Kotor | ||||
| Echinoidea | Echinus melo Lamarck, 1816 | Sanremo | |||
| Chordata | |||||
| Ascidiacea | Didemnidae | Sanremo, Kotor Bay, SE Kotor | |||
| Diplosoma spongiforme (Giard, 1872) | Sanremo | ||||
| Halocynthia papillosa (Linnaeus, 1767) | Sanremo | ||||
| Actinopterygii | Anthias anthias (Linnaeus, 1758) | Sanremo | LC | ||
| Callanthias ruber (Rafinesque, 1810) | Sanremo | LC | |||
| Conger conger (Linnaeus, 1758) | Sanremo | LC | |||
| Coris julis (Linnaeus, 1758) | Sanremo | LC | |||
| Epinephelus marginatus (Lowe, 1834) | Bordighera | III | III | VU | |
| Labrus mixtus Linnaeus, 1758 | Sanremo | LC | |||
| Mullus barbatus barbatus Linnaeus, 1758 | Sanremo | LC | |||
| Muraena helena Linnaeus, 1758 | Sanremo | LC | |||
| Phycis phycis (Linnaeus, 1766) | Sanremo | LC | |||
| Polyprion americanus (Bloch & Schneider, 1801) | Sanremo | NT | |||
| Scorpaena notata Rafinesque, 1810 | Sanremo | LC | |||
| Scorpaena scrofa Linnaeus, 1758 | Bordighera, Sanremo | LC | |||
| Serranus cabrilla (Linnaeus, 1758) | Sanremo | LC | |||
| Spicara sp. | Sanremo | LC | |||
| Trachurus sp. | Sanremo | LC | |||
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chimienti, G. Vulnerable Forests of the Pink Sea Fan Eunicella verrucosa in the Mediterranean Sea. Diversity 2020, 12, 176. https://doi.org/10.3390/d12050176
Chimienti G. Vulnerable Forests of the Pink Sea Fan Eunicella verrucosa in the Mediterranean Sea. Diversity. 2020; 12(5):176. https://doi.org/10.3390/d12050176
Chicago/Turabian StyleChimienti, Giovanni. 2020. "Vulnerable Forests of the Pink Sea Fan Eunicella verrucosa in the Mediterranean Sea" Diversity 12, no. 5: 176. https://doi.org/10.3390/d12050176
APA StyleChimienti, G. (2020). Vulnerable Forests of the Pink Sea Fan Eunicella verrucosa in the Mediterranean Sea. Diversity, 12(5), 176. https://doi.org/10.3390/d12050176