Phylogenetic Affinity of Genolopa (Digenea: Monorchiidae) with Descriptions of Two New Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection and Morphological Analysis
2.2. Molecular Sequencing
2.3. Phylogenetic Analysis
3. Results—Morphological
3.1. Genolopa ampullacea Linton, 1910
3.1.1. Taxonomic Summary
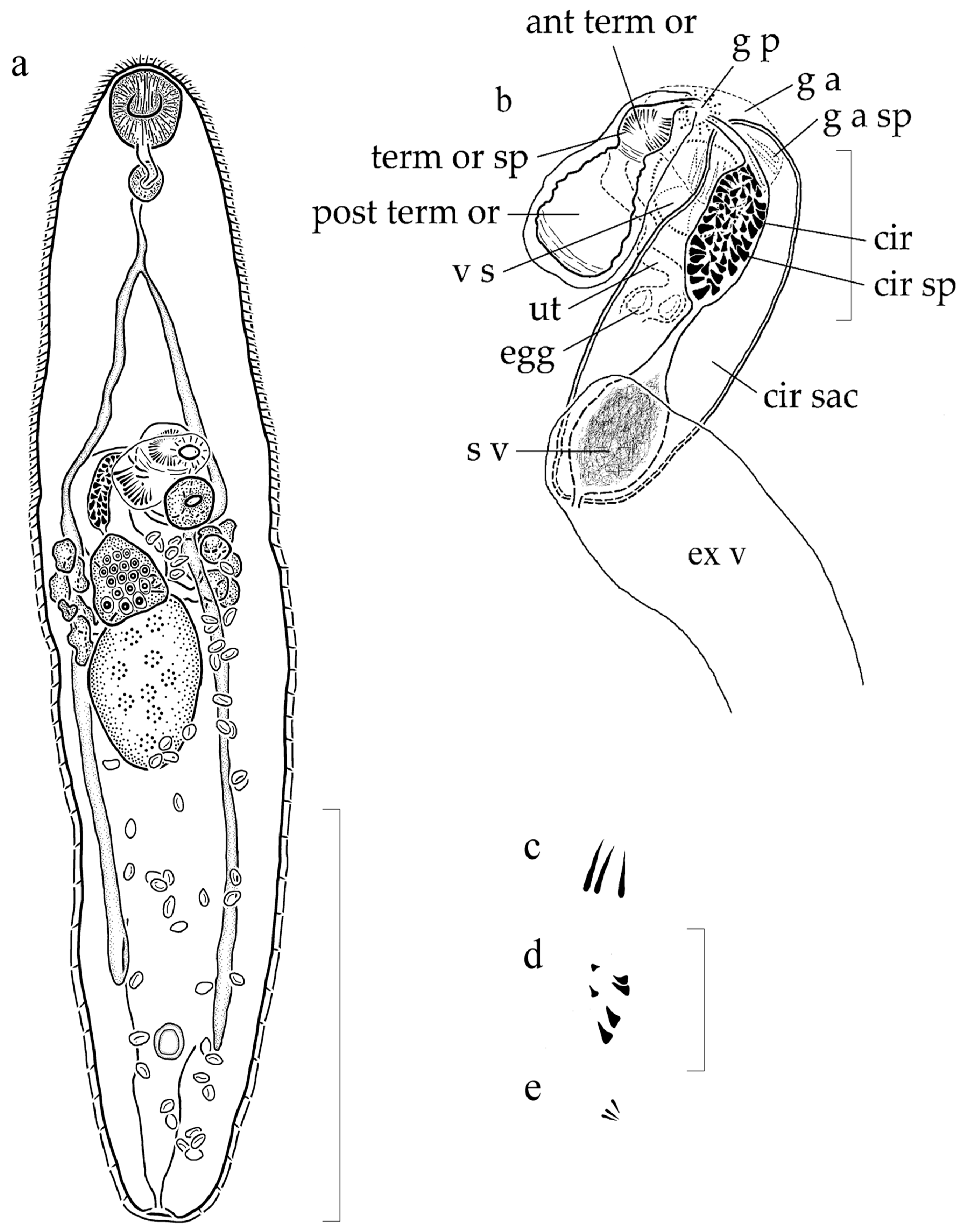
3.1.2. Supplemental Data (Figure 1) (Based on 5 Gravid, Adult Specimens from H. flavolineatum, Mounted without Pressure)
3.1.3. Remarks
3.2. Genolopa vesca n. sp.
3.2.1. Taxonomic Summary
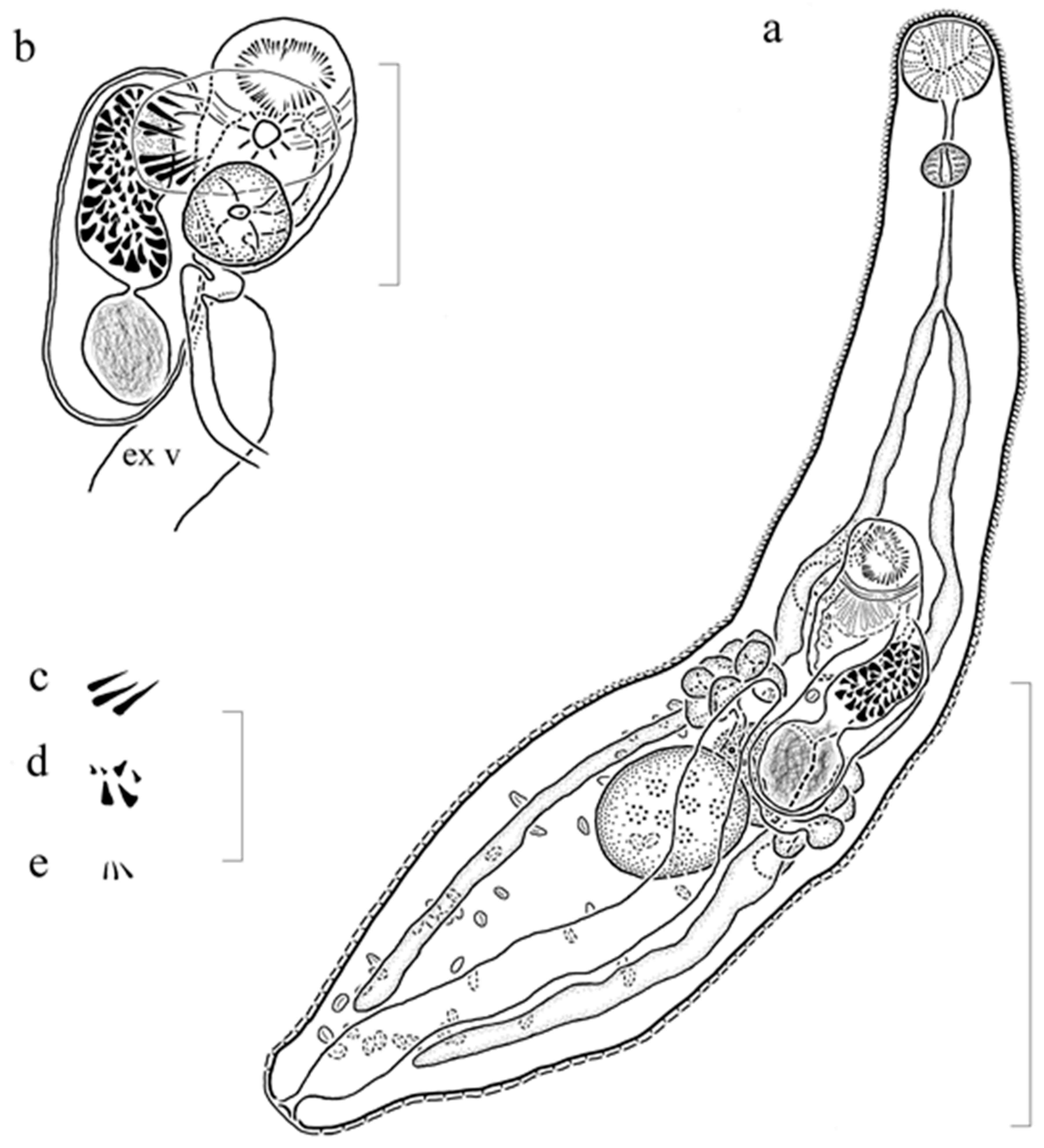
3.2.2. Description (Figure 2) (Based on 6 Gravid, Adult Specimens and 1 Non-Gravid Specimen, All Mounted without Pressure)
3.2.3. Remarks
3.3. Genolopa minuscula n. sp.
3.3.1. Taxonomic Summary
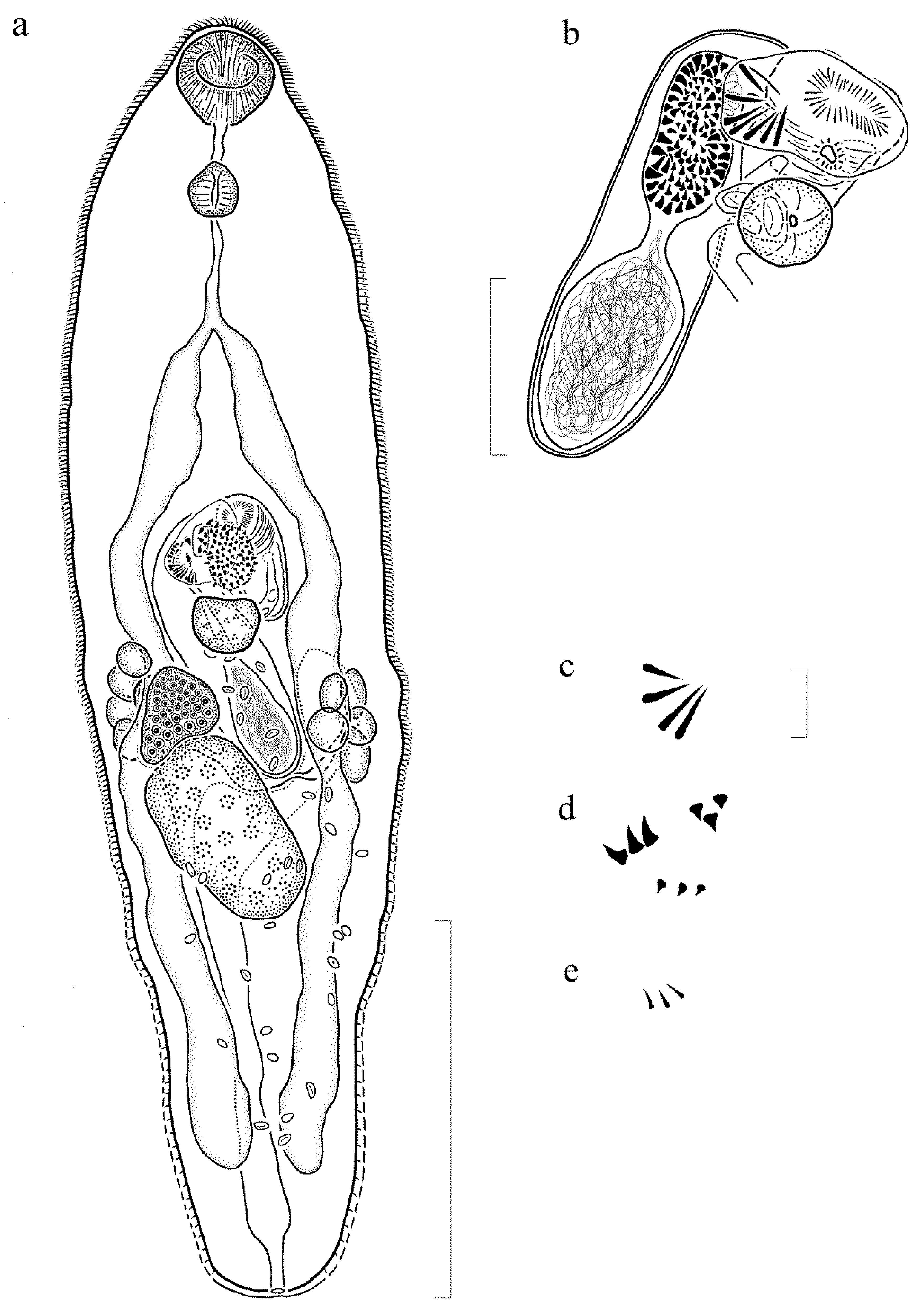
3.3.2. Description (Figure 3) (Based on 7 Gravid, Adult Specimens and 1 Non-Gravid Specimen, All Mounted without Pressure)
3.3.3. Remarks
3.4. Molecular Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Madhavi, R. Family Monorchiidae Odhner, 1911. In Keys to the Trematoda; Bray, R.A., Gibson, D.I., Jones, A., Eds.; CAB International and Natural History Museum: Cambridge, MA, USA, 2008; pp. 145–175. ISBN 978. [Google Scholar]
- Yamaguti, S. A synoptical review of the life histories of digenetic trematodes of vertebrates, 1st ed.; Yugaku-Sha, Ltd.: Tokyo, Japan, 1975. [Google Scholar]
- Gilardoni, C.; Carballo, M.C.; Cremonte, F. The life cycle and geographical distribution of the monorchiid Proctotrema bartolii (Digenea) in the clam Darina solenoides from the Patagonian coast, Argentina. J. Helminthol. 2013, 87, 392–399. [Google Scholar] [CrossRef]
- Bartoli, P.; Jousson, O.; Russell-Pinto, F. The life cycle of Monorchis parvus (Digenea: Monorchiidae) demonstrated by developmental and molecular data. J. Parasitol. 2000, 86, 479–489. [Google Scholar] [CrossRef]
- Young, R.T. Postmonorchis donacis, a new species of monorchid trematode from the Pacific coast, and its life history. J. Wash. Acad. Sci. 1953, 43, 88–93. [Google Scholar]
- Bagnato, E.; Gilardoni, C.; Pina, S.; Rodrigues, P.; Cremonte, F. Redescription and life cycle of the monorchiid Postmonorcheides maclovini Szidat, 1950 (Digenea) from the Southwestern Atlantic Ocean: morphological and molecular data. Parasitol. Int. 2016, 65, 44–49. [Google Scholar] [CrossRef]
- Manter, H.W. Some digenetic trematodes of marine fishes of Beaufort, North Carolina. Parasitology 1931, 23, 396–411. [Google Scholar] [CrossRef]
- Manter, H.W. Monorchiidae (Trematoda) from fishes of Tortugas, Florida. Trans. Am. Microsc. Soc. 1942, 61, 349–360. [Google Scholar] [CrossRef]
- Manter, H.W.; Pritchard, M.H. Studies on the digenetic trematodes of Hawaiian fishes: families Monorchiidae and Haploporidae. J. Parasitol. 1961, 47, 483–492. [Google Scholar] [CrossRef]
- Thomas, J.D. Trematodes of Ghanian sub-littoral Fishes. I. The Family Monorchiidae. J. Parasitol. 1959, 45, 95–113. [Google Scholar] [CrossRef]
- Hopkins, S.H. New genera and species of the family Monorchiidae (Trematoda), with a discussion of the excretory system. J. Parasitol. 1941, 27, 395–407. [Google Scholar] [CrossRef]
- Yamaguti, S. Synopsis of Digenetic Trematodes of Vertebrates; Keigaku: Tokyo, Japan, 1971. [Google Scholar]
- Machida, M. Monorchiidae (Trematoda, Digenea) from fishes of Japanese and adjacent Waters. Bull. Natl. Sci. Mus. Tokyo Ser. A Zool. 2005, 31, 123–136. [Google Scholar]
- Wee, N.Q.X.; Cutmore, S.C.; Cribb, T.H. Two monorchiid species from the freckled goatfish, Upeneus tragula Richardson (Perciformes: Mullidae), in Moreton Bay, Australia, including a proposal of a new genus. Syst. Parasitol. 2018, 95, 353–365. [Google Scholar] [CrossRef]
- Searle, E.L.; Cutmore, S.C.; Cribb, T.H. Monorchiid trematodes of the painted sweetlips, Diagramma labiosum (Perciformes: Haemulidae), from the southern Great Barrier Reef, including a new genus and three new species. Syst. Parasitol. 2014, 88, 195–211. [Google Scholar] [CrossRef]
- Atopkin, D.M.; Besprozvannykh, V.V.; Ngo, H.D.; Van Ha, N.; Van Tang, N.; Ermolenko, A.V.; Beloded, A.Y. Morphometric and molecular data of the two digenean species Lasiotocus lizae Liu, 2002 (Monorchiidae) and Paucivitellosus vietnamensis sp. n. (Bivesiculidae) from mullet fish in Tonkin Bay, Vietnam. J. Helminthol. 2017, 91, 346–355. [Google Scholar] [CrossRef]
- Olson, P.D.; Cribb, T.H.; Tkach, V.V.; Bray, R.A.; Littlewood, D.T.J. Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). Int. J. Parasitol. 2003, 33, 733–755. [Google Scholar] [CrossRef]
- McNamara, M.K.A.; Miller, T.L.; Cribb, T.H. Evidence for extensive cryptic speciation in trematodes of butterflyfishes (Chaetodontidae) of the tropical Indo-West Pacific. Int. J. Parasitol. 2014, 44, 37–48. [Google Scholar] [CrossRef]
- Tkach, V.V.; Pawlowski, J.; Mariaux, J.; Swiderski, Z. Molecular phylogeny of the suborder Plagiorchiata and its position in the system of Digenea. In Interrelationships of the Platyhelminthes; Littlewood, D.T.J., Bray, R.A., Eds.; Taylor & Francis: London, UK, 2001; pp. 186–193. [Google Scholar]
- Jousson, O.; Bartoli, P.; Pawlowski, J. Cryptic speciation among intestinal parasites (Trematoda: Digenea) infecting sympatric host fishes (Sparidae). J. Evol. Biol. 2000, 13, 778–785. [Google Scholar] [CrossRef]
- Cribb, T.H.; Wee, N.Q.X.; Bray, R.A.; Cutmore, S.C. Monorchis lewisi n. sp. (Trematoda: Monorchiidae) from the surf bream, Acanthopagrus australis (Sparidae), in Moreton Bay, Australia. J. Hazard. Mater. 2018, 92, 100–108. [Google Scholar] [CrossRef]
- Bray, R.A.; Palm, H.W.; Cutmore, S.C.; Cribb, T.H. Three members of Opisthomonorcheides Parukhin, 1966 (Digenea: Monorchiidae) from carangid fishes (Perciformes) from Indonesia, with a review of the genus. Syst. Parasitol. 2017, 94, 443–462. [Google Scholar] [CrossRef]
- Bagnato, E.; Gilardoni, C.; Di Giorgio, G.; Cremonte, F. A checklist of marine larval trematodes (Digenea) in molluscs from Argentina, Southwestern Atlantic coast. J. Biodivers. Data 2015, 11, 1–10. [Google Scholar] [CrossRef]
- Andres, M.J.; Pulis, E.E.; Curran, S.S.; Overstreet, R.M. On the systematics of some marine haploporids (Trematoda) with the description of a new species of Megasolena Linton, 1910. Parasitol. Int. 2018, 67, 805–815. [Google Scholar] [CrossRef]
- Cribb, T.H.; Bray, R.A. Gut wash, body soak, blender and heat-fixation: approaches to the effective collection, fixation and preservation of trematodes of fishes. Syst. Parasitol. 2010, 76, 1–7. [Google Scholar] [CrossRef]
- Curran, S.S.; Overstreet, R.M.; Tkach, V.V. Phylogenetic affinities of Plagiocirrus Van Cleave and Mueller, 1932 with the description of a new species from the Pascagoula River, Mississippi. J. Parasitol. 2007, 93, 1452–1458. [Google Scholar] [CrossRef]
- Overstreet, R.M. Some adult digenetic trematodes in striped mullet from the Northern Gulf of Mexico. J. Parasitol. 1971, 57, 967–974. [Google Scholar] [CrossRef]
- Pleijel, F.; Jondelius, U.; Norlinder, E.; Nygren, A.; Oxelman, B.; Schander, C.; Sundberg, P.; Thollesson, M. Phylogenies without roots? A plea for the use of vouchers in molecular phylogenetic studies. Mol. Phylogenet. Evol. 2008, 48, 369–371. [Google Scholar] [CrossRef]
- Tkach, V.V.; Snyder, S.D. Aptorchis megacetabulus n. sp. (Platyhelminthes: Digenea) from the northern long-necked turtle, Chelodina rugosa (Pleurodira: Chelidae), in Australia. J. Parasitol. 2007, 93, 404–408. [Google Scholar] [CrossRef]
- Curran, S.S.; Tkach, V.V.; Overstreet, R.M. A review of Polylekithum Arnold, 1934 and its familial affinities using morphological and molecular data, with description of Polylekithum catahoulensis sp. nov. Acta Parasitol. 2006, 51, 238–248. [Google Scholar] [CrossRef]
- Littlewood, D.T.J. Molecular phylogenetics of cupped oysters based on Partial 28S rRNA Gene Sequences. Mol. Phylogenet. Evol. 1994, 3, 221–229. [Google Scholar] [CrossRef]
- Keller, A.; Schleicher, T.; Schultz, J.; Müller, T.; Dandekar, T.; Wolf, M. 5.8S-28S rRNA interaction and HMM-based ITS2 annotation. Gene 2009, 430, 50–57. [Google Scholar] [CrossRef]
- Ankenbrand, M.J.; Keller, A.; Wolf, M.; Schultz, J.; Förster, F. ITS2 database V: Twice as much. Mol. Biol. Evol. 2015, 32, 3030–3032. [Google Scholar] [CrossRef]
- Sela, I.; Ashkenazy, H.; Katoh, K.; Pupko, T. Guidance 2: Accurate detection of unreliable alignment regions accounting for the uncertainty of multiple parameters. Nucleic Acids Res. 2015, 43, W7–W14. [Google Scholar] [CrossRef]
- Landan, G.; Graur, D. Local reliability measures from sets of co-optimal multiple sequence alignments. In Proceedings of the Pacific Symposium on Biocomputing, Kohala Coast, HI, USA, 4–8 January 2008; pp. 15–24. [Google Scholar]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinform. Appl. Note 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. Mrbayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Wee, N.Q.X.; Cutmore, S.C.; Cribb, T.H. Four new monorchiids from the golden trevally, Gnathanodon speciosus (Forsskål) (Perciformes: Carangidae), in Moreton Bay, Australia. Syst. Parasitol. 2019, 96, 265–278. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J. FigTree Version 1.4. 2012. [Google Scholar]
- Bray, R.A.; Cribb, T.H.; Cutmore, S.C. Lepocreadiidae Odhner, 1905 and Aephnidiogenidae Yamaguti, 1934 (Digenea: Lepocreadioidea) of fishes from Moreton Bay, Queensland, Australia, with the erection of a new family and genus. Syst. Parasitol. 2018, 95, 479–498. [Google Scholar] [CrossRef]
- Bray, R.A.; Cutmore, S.C.; Cribb, T.H. Lepotrema Ozaki, 1932 (Lepocreadiidae: Digenea) from Indo-Pacific fishes, with the description of eight new species, characterised by morphometric and molecular features. Syst. Parasitol. 2018, 95, 693–741. [Google Scholar] [CrossRef]
- Manter, H.W. The digenetic trematodes of marine fishes of Tortugas, Florida. Am. Midl. Nat. 1947, 38, 257. [Google Scholar] [CrossRef]
- Sparks, A.K. Some digenetic trematodes of marine fishes of the Bahama Islands. Bull. Mar. Sci. 1957, 7, 255–265. [Google Scholar]
- Sogandares-Bernal, F. Digenetic trematodes of marine fishes from the Gulf of Panama and Bimini, British West Indies. Tulane Stud. Zool. 1959, 7, 71–117. [Google Scholar]
- Nahhas, F.M.; Cable, R.M. Digenetic and aspidogastrid trematodes from marine fishes of Curaçao and Jamaica. Tulane Stud. Zool. 1964, 11, 169–228. [Google Scholar] [CrossRef]
- Rees, G. Some helminth parasites of fishes of Bermuda and an account of the attachment organ of Alcicornis carangis Maccallum, 1917 (Digenea: Bucephalidae). Parasitology 1970, 60, 195–221. [Google Scholar] [CrossRef] [PubMed]
- Nagaty, H.F.; Abdel-Aal, T.M. Trematodes of fishes from the Red Sea, Part 20. On four monorchiids, including a new genus and three new species. J. Egypt. Veternary Med. Assoc. 1972, 32, 207–213. [Google Scholar]
- Fischthal, J.H. Some sigenetic trematodes of marine fishes from the barrier reef and reef lagoon of Belize. Zool. Scr. 1977, 6, 81–88. [Google Scholar] [CrossRef]
- Kohn, A.; Macedo, B.; Fernandes, B.M.M. About some trematodes parasites of Haemulon sciurus (Shaw, 1803). Mem. Inst. Oswaldo Cruz 1982, 77, 153–157. [Google Scholar] [CrossRef]
- Centeno, L.; Bashirullah, A. Comunidades de parásitos metazoos en ocho especies de peces del género Haemulon (FAM: Haemulidae) del Golfo de Cariaco, Venezuela. Ciencia 2003, 11, 119–124. [Google Scholar]
- Bashirullah, A.K.; Díaz, M.T. Helminth infracommunity of Haemulon aurolineatum Cuvier, 1830 (Haemulidae) from the Gulf of Cariaco, Venezuela. Rev. Cient. la Fac. Ciencias Vet. la Univ. del Zulia 2015, 25, 167–172. [Google Scholar]
- Dyer, W.; Williams, E.; Bunkley-Williams, L. Homalometron dowgialloi sp. n. (Homalometridae) from Haemulon flavolineatum and additional records of digenetic trematodes of marine fishes in the West Indies. J. Helminthol. Soc. Wash. 1992, 59, 182–189. [Google Scholar]
- Curran, S.S.; Overstreet, R.M.; Tat The, D.; Thi Le, N. Singhiatrema vietnamensis sp. n. (Digenea, Ommatobrephidae) and Szidatia taiwanensis (Fischthal and Kuntz, 1975) comb. n. (Digenea: Cyathocotylidae) from colubrid snakes in Vietnam. Comp. Parasitol. 2001, 68, 219–227. [Google Scholar]
- Curran, S.S.; Tkach, V.V.; Overstreet, R.M. A new species of Homalometron (Digenea: Apocreadiidae) from fishes in the Northern Gulf of Mexico. J. Parasitol. 2013, 99, 93–101. [Google Scholar] [CrossRef]
- Linton, E. Helminth Fauna of the Dry Tortugas. Pap. Tortugas Lab. Carnegie Inst. Wash. 1910, 1, 157. [Google Scholar]
- Parasitology Laboratory. Investigation of parasites in sea eel from Fujian. J. Fujian Norm. Univ. Nat. Sci. Ed. 1976, 1, 108–112. [Google Scholar]
- Carella, F.; Culurgioni, J.; Aceto, S.; Fichi, G.; Pretto, T.; Luise, D.; Gustinelli, A.; De Vico, G. Postmonorchis sp. inq. (Digenea: Monorchiidae) metacercariae infecting natural beds of wedge clam Donax trunculus in Italy. Dis. Aquat. Org. 2013, 106, 163–172. [Google Scholar] [CrossRef]
- Mancini, E.; Furfaro, G.; Cervelli, M.; Di Giulio, A.; Oliverio, M.; Salvi, D.; Mariottini, P. Molecular detection of parasites (Trematoda, Digenea: Bucephalidae and Monorchiidae) in the European flat oyster Ostrea edulis (Mollusca: Bivalvia). Eur. Zool. J. 2018, 85, 8–16. [Google Scholar] [CrossRef]
- Ferrer-Maza, D.; Muñoz, M.; Lloret, J.; Faliex, E.; Vila, S.; Sasal, P. Health and reproduction of red mullet, Mullus barbatus, in the western Mediterranean Sea. Hydrobiologia 2015, 753, 189–204. [Google Scholar] [CrossRef]
- Knoff, M.; Amato, J.F. Nova especie do genero Genolopa Linton, 1910 (Monorchiidae, Lasiotocinae) parasite de tainha, Mugil platanus Gunther, 1880 da costa de estado do Rio de Janeiro, Brasil *. Rev. Bras. Biol. 1991, 51, 801–804. [Google Scholar]
- Siddiqi, A.H.; Cable, R.M. Digenetic trematodes of marine fishes of Puerto Rico. Scientific Survey of Porto Rico and the Virgin Islands 1960, 17, 256–369. [Google Scholar]
- Overstreet, R.M. Digenetic trematodes of marine teleost fishes from Biscayne Bay, Florida. Tulane Stud. Zool. Bot. 1969, 15, 119–176. [Google Scholar]
- Lozano, C.; Úbeda Ontiveros, J.; Rojas Álvarez, M.; Ariza Astolfi, C.; Guevara Benítez, D. Estudio de digénidos de peces marinos del sur de la Península Ibérica. Rev. Iber. Parasitol. 2001, 61, 103–116. [Google Scholar]
- Mosquera, O.; Mago, Y.; Chinchilla, O. Finding of Genolopa ampullacea Linton, 1910 (Digenea: Monorchiidae, Monorchiinae) in Haemulon bonariense Cuvier, 1830 from Mochima Bay, Sucre State, Venezuela. Saber 2014, 26, 121–126. [Google Scholar]
- Overstreet, R.M.; Brown, C.E. Lasiotocus trachinoti sp. n. (Digenea: Monorchiidae) from the pompano, Trachinotus carolinus (Linnaeus), along the East Coast of Florida. J. Parasitol. 1970, 56, 941–943. [Google Scholar] [CrossRef]

| Species | Host Species | GenBank Accession Number | Reference |
|---|---|---|---|
| Monorchiidae Odhner, 1911 | |||
| Cableia pudica | Cantherines pardalis | AY222251 | [17] |
| Diplomonorchis leiostomi * | Leiostomus xanthurus | AY222252 | [17] |
| Genolopa ampullacea * | Haemulon flavolineatum | MN984474 | present study |
| Helicometroides longicollis * | Diagramma labiosum | KJ658287 | [15] |
| Hurleytrematoides chaetodoni * | Chaetodon striatus | MH244116 | [24] |
| Hurleytrematoides galzini | Gnathanodon speciosus | MK501988 | [39] |
| Hurleytrematoides loi | Gnathanodon speciosus | MK501989 | [39] |
| Lasiotocus arrhichostoma | Diagramma labiosum | KJ658289 | [15] |
| Lasiotocus glebulentus | Mugil curema | MN984476 | present study |
| Lasiotocus lizae | Liza longimanus | LN831723 | [16] |
| Lasiotocus sp. | Menidia menidia | MN984477 | present study |
| Lasiotocus trachinoti | Trachinotus carolinus | MN984478 | present study |
| Lasiotocus typicum | Trachurus trachurus | AY222254 | [17] |
| Madhavia fellaminuta | Upeneus tragula | MG920219 | [14] |
| Monorchis lewisi | Acanthopagrus australis | MF503309 | [21] |
| Monorchis monorchis * | Diplodus vulgaris | AF184257 | [19] |
| Ovipusillus mayu * | Gnathanodon speciosus | MF503310 | [21] |
| Parachrisomon delicatus | Upeneus tragula | MG920218 | [14] |
| Postmonorchis orthopristis * | Haemulon flavolineatum | MN984475 | present study |
| Proctotrema addisoni | Diagramma labiosum | KJ658291 | [15] |
| Provitellus chaometra | Gnathanodon speciosus | MK501984 | [39] |
| Provitellus infrequens | Gnathanodon speciosus | MK501985 | [39] |
| Provitellus turrum * | Pseudocaranx dentex | AY222253 | [17] |
| Lissorchiidae Magath, 1917 | |||
| Lissorchis kritskyi | Minytrema melanops | EF032689 | [30] |
| Lepocreadiidae Odhner, 1905 | |||
| Bianium arabicum | Lagocephalus lunaris | MH157076 | [41] |
| Lepotrema adlardi | Abudefduf bengalensis | MH730015 | [42] |
| Reference | Linton [56] | Manter [8] | Manter [8] | Kohn et al. [50] | Present Study | Present Study |
|---|---|---|---|---|---|---|
| Material examined | syntypes | syntypes | new material | new material | syntypes | new material |
| Under pressure? | yes | yes | no | yes | yes | no |
| Fixed with an acid? | yes | yes | unknown | yes | yes | no |
| Host | H. macrostomum | H. macrostomum | H. album, H. carbonarium, H. flavolineatum, H. plumierii, H. sciurus, S. foetens | H. sciurus | H. macrostomum | H. flavolineatum |
| Locality | Dry Tortugas, FL | Dry Tortugas, FL | Tortugas, FL | Rio de Janeiro, Brazil | Dry Tortugas, FL | Florida Keys |
| Genital atrium spines | - | 34 to 36 | 34 to 36 | 30 to 36 | 30 to 38 | |
| Cirrus spines | - | 12 | 12 | 5 to 10 X 7 to 12 | 10 to 12 X 4 | 5 to 8, 8 to 12 X 2 to 3, 3 to 7 |
| Terminal organ spines | - | 17 | 17 | - | - | 8 to 14 X 1 to 3 |
| Body length | 1150 to 1420 | - | 425 to 1275 | 740 to 1580 | 1227 | 829 to 1265 |
| Body width | 630 | - | 187 to 365 | 310 to 590 | 602 | 202 to 253 |
| Oral sucker | 140 * | - | 50 to 96 | 120 to 210 X 150 to 170 | 131 x 174 | 70 to 82 X 66 to 82 |
| Ventral sucker | 120 * | - | 34 to 62 | 49 to 82 X 56 to 94 | 102 x 58 | 50 to 57 X 50 to 57 |
| Sucker ratio | - | 3:2 | 1:0.37 to 1:0.45 | 1:0.33 | 1:0.66 to 1:0.77 | |
| Pharynx | 40 * | - | 17 to 40 X 17 to 42 | 37 to 56 X 34 to 70 | 63 x 42 | 36 to 40 X 29 to 37 |
| Cirrus sac | - | - | 225 x 99 | - | 351 x 135 | 178 to 240 X 53 to 79 |
| Cirrus | - | - | - | - | 168 x 45 | 73 to 80 X 19 to 34 |
| Terminal organ | - | - | 150 x 85 | - | 242 x 106 | 122 to 136 X 49 to 52 |
| Testis | - | - | - | 150 to 300 X 100 to 180 | 186 x 155 | 154 to 170 X 109 to 126 |
| Percentage of post-testicular space to body length | - | - | 33% | - | 37% | 35 to 39% |
| Ovary | - | - | - | - | 113 x 118 | 67 to 85 X 61 to 81 |
| Eggs | 17 x 10 | - | 18 to 22 X 9 to 11 | 21 to 28 X 9 to 12 | 14 to 16 X 7 to 10 | 15 to 20 X 8 to 11 |
| Excretory vesicle | - | - | I - to ventral sucker | - | - | I – to posterior of cirrus sac |
| Species | G. minuscula | G. ampullacea | G. vesca | P. orthopristis |
|---|---|---|---|---|
| P. orthopristis | 45 (3.9) | 47 (4.0) | 24 (2.1) | — |
| G. minuscula | — | 19 (1.6) | 57 (4.9) | — |
| G. ampullacea | — | 57 (4.9) | — | |
| G. vesca | — | — |
| Species | Authority |
|---|---|
| Genolopa ampullacea * | Linton, 1910 |
| Genolopa anisotremi ** | (Nahhas and Cable, 1964) Yamaguti, 1971 |
| Genolopa brevicaecum ** | (Manter, 1942) Manter and Pritchard, 1961 |
| Genolopa bychowskii ** | Zhukov, 1977 |
| Genolopa cheilini ** | Nagaty and Abdel-Aal, 1972 |
| Genolopa loborchis ** | Wang, 1977 |
| Genolopa lunulata ** | Nagaty and Abdel-Aal, 1972 |
| Genolopa magnacirrus ** | Thatcher, 1996 |
| Genolopa microsoma ** | Lebedev, 1968 |
| Genolopa mintungensis ** | Wang, 1975 |
| Genolopa mugilis | Knoff and Amato, 1992 |
| Genolopa plectorhynchi | (Yamaguti, 1934) Hopkins, 1941 |
| Genolopa pritchardae ** | (Nahhas and Cable, 1964) Yamaguti, 1971 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panyi, A.J.; Curran, S.S.; Overstreet, R.M. Phylogenetic Affinity of Genolopa (Digenea: Monorchiidae) with Descriptions of Two New Species. Diversity 2020, 12, 51. https://doi.org/10.3390/d12020051
Panyi AJ, Curran SS, Overstreet RM. Phylogenetic Affinity of Genolopa (Digenea: Monorchiidae) with Descriptions of Two New Species. Diversity. 2020; 12(2):51. https://doi.org/10.3390/d12020051
Chicago/Turabian StylePanyi, Apryle J., Stephen S. Curran, and Robin M. Overstreet. 2020. "Phylogenetic Affinity of Genolopa (Digenea: Monorchiidae) with Descriptions of Two New Species" Diversity 12, no. 2: 51. https://doi.org/10.3390/d12020051
APA StylePanyi, A. J., Curran, S. S., & Overstreet, R. M. (2020). Phylogenetic Affinity of Genolopa (Digenea: Monorchiidae) with Descriptions of Two New Species. Diversity, 12(2), 51. https://doi.org/10.3390/d12020051




