Local and Landscape Compositions Influence Stingless Bee Communities and Pollination Networks in Tropical Mixed Fruit Orchards, Thailand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Region and Crop System
2.2. Study Sites
2.3. Environmental Multilevel Quantification
2.3.1. Local Conditions
2.3.2. Landscape Structure at Proximal and Broad Levels
2.4. Bee Sampling
2.5. Constructing the Pollination Networks
2.6. Statistical Analyses
3. Results
3.1. Plant Diversity
3.2. Stingless Bee Species
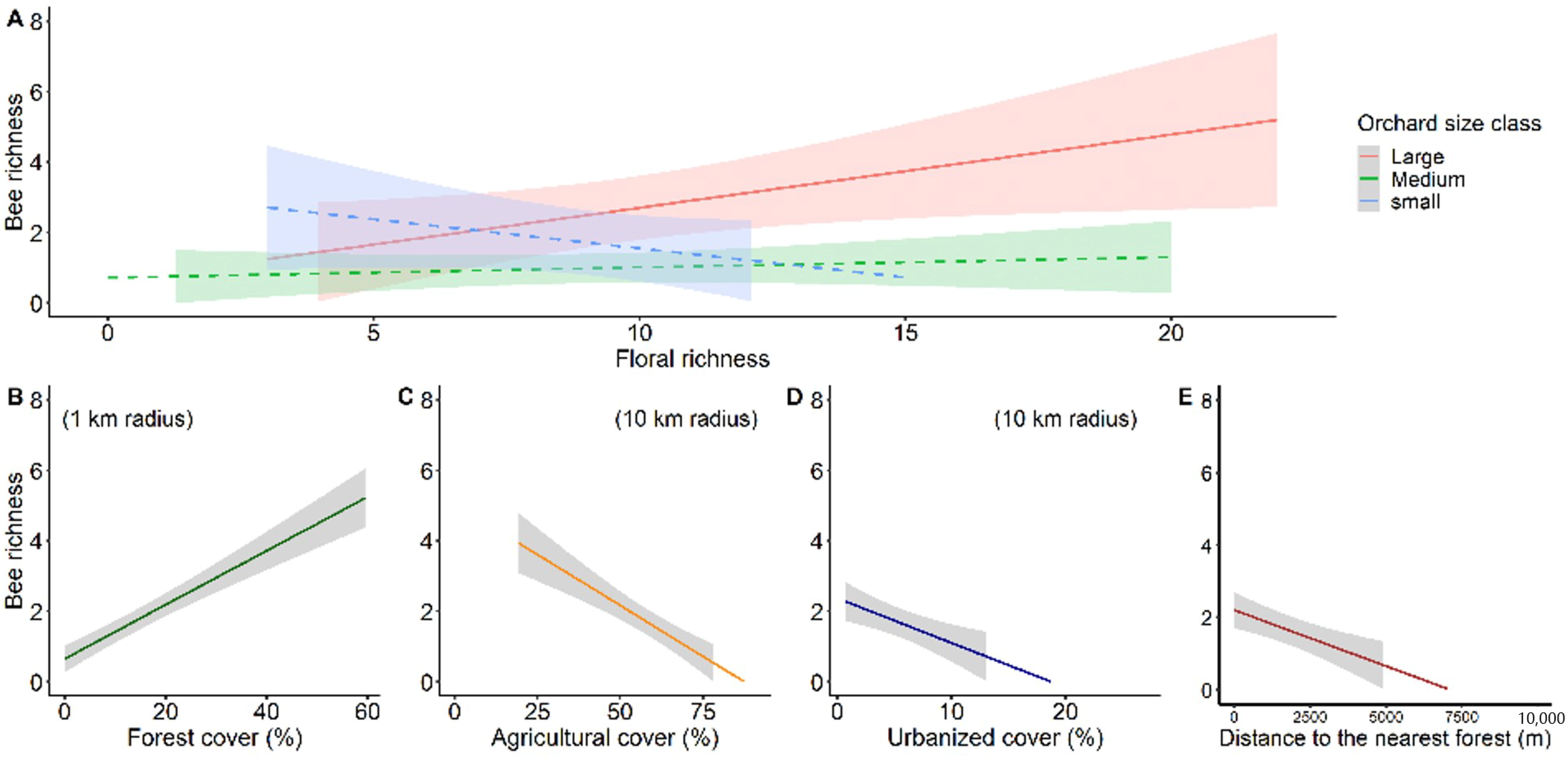
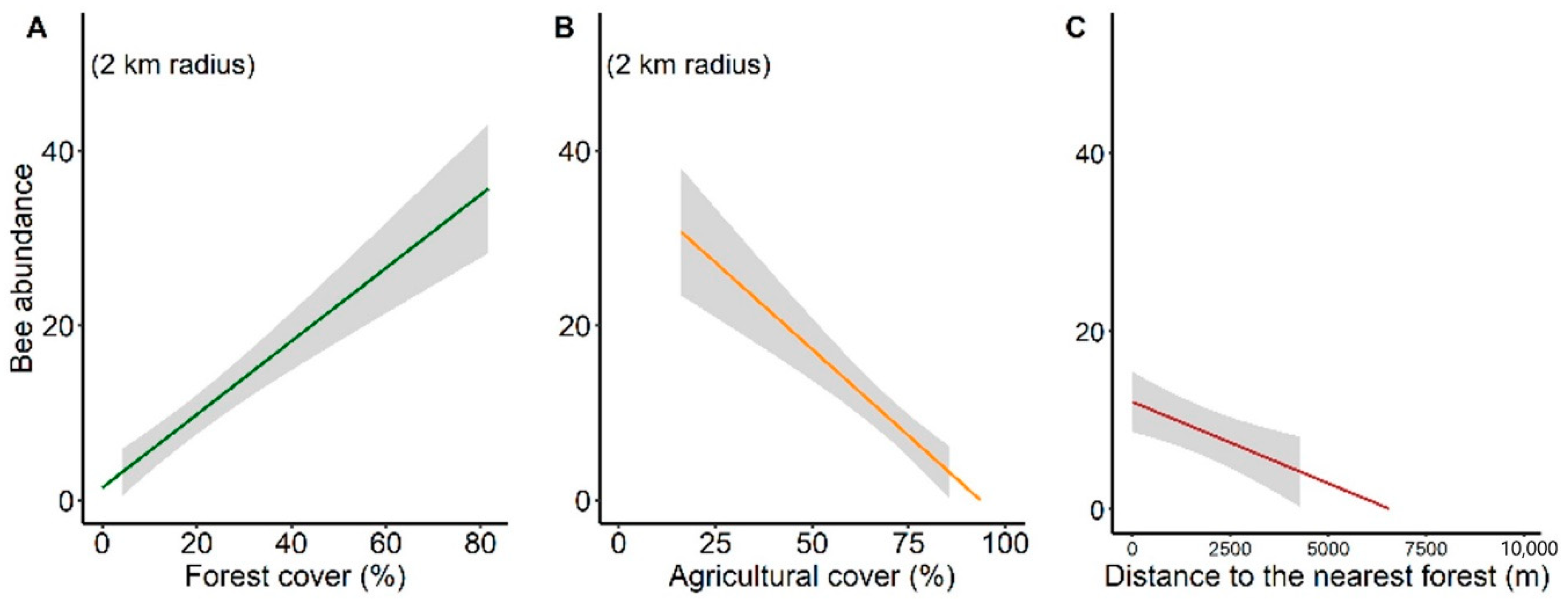
3.3. Environmental Multilevel Effects on Stingless Bee Richness and Abundance
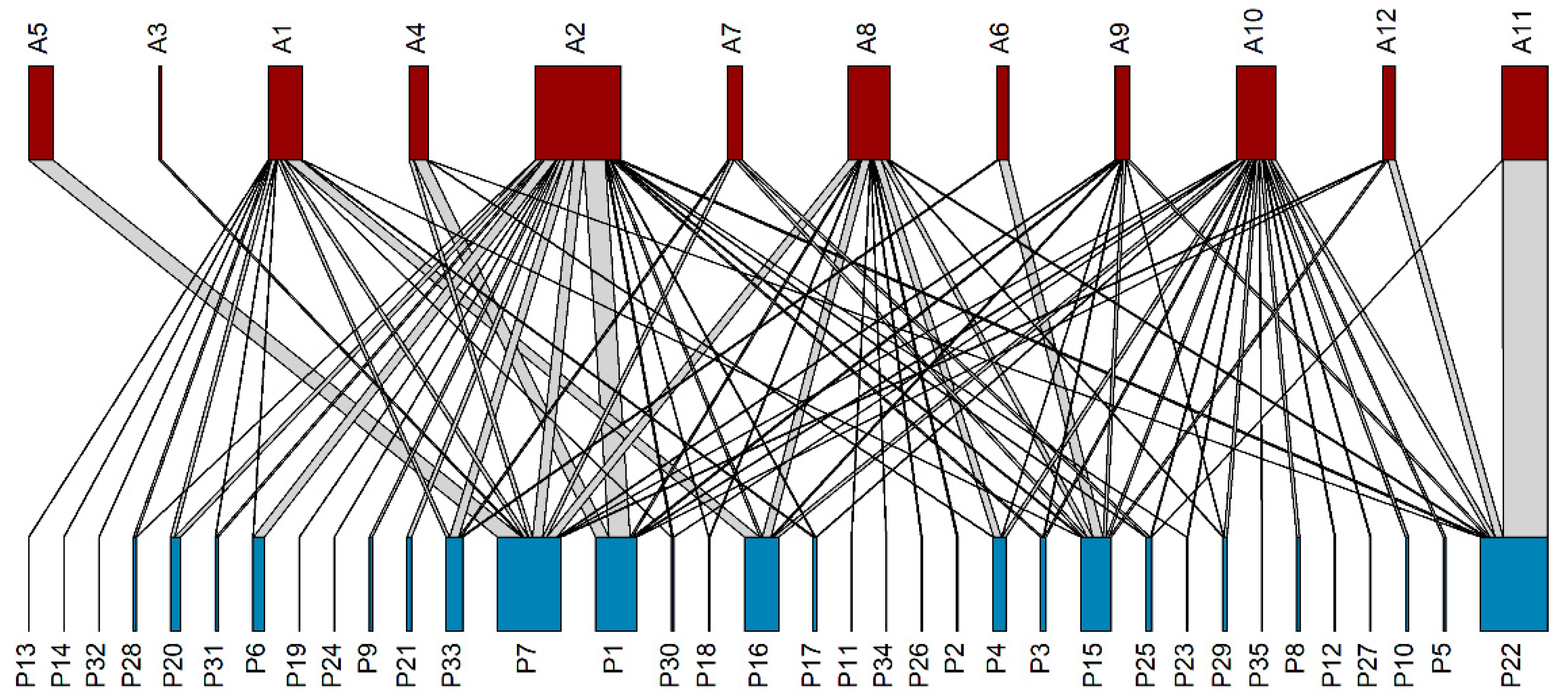
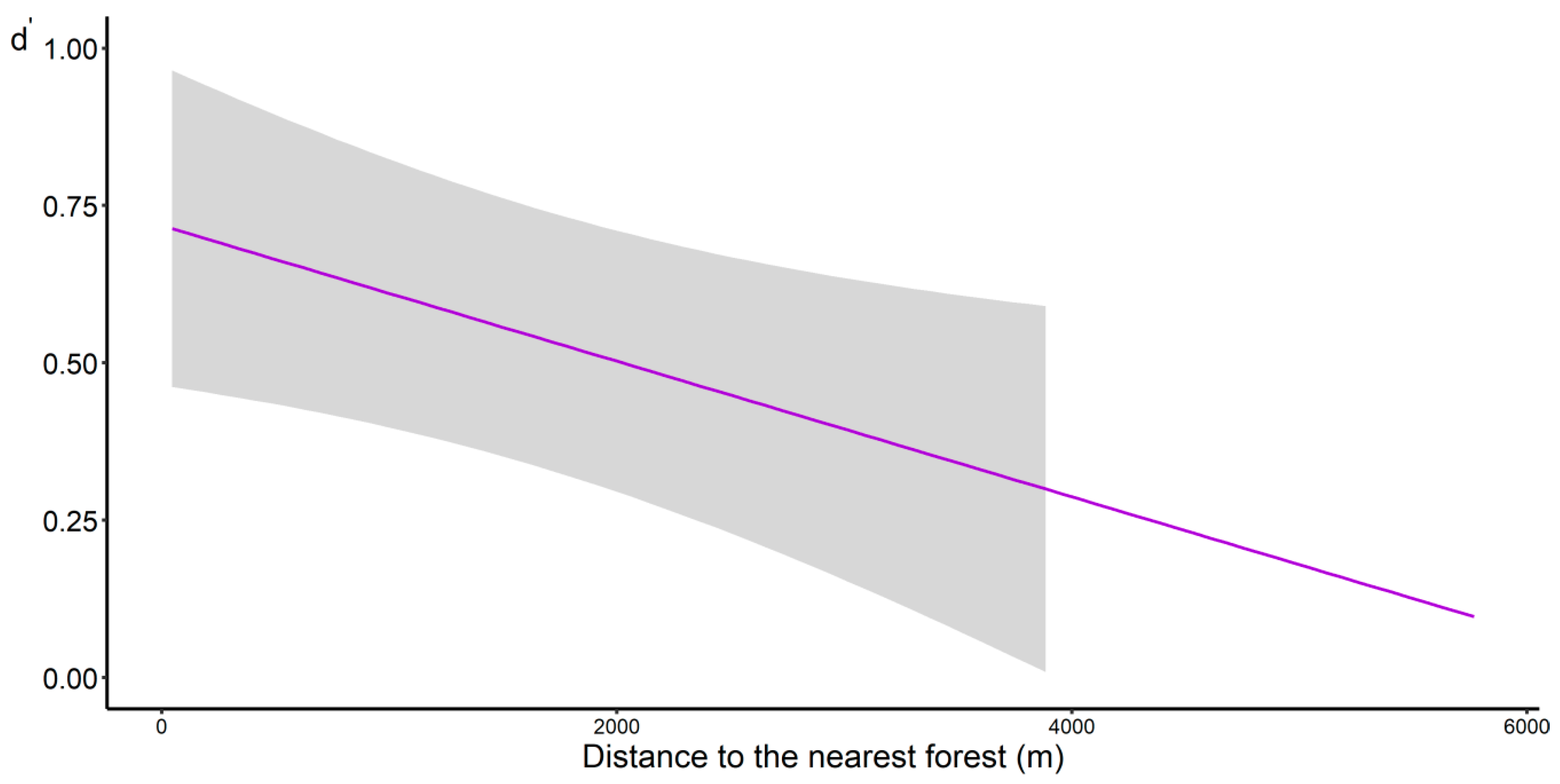
3.4. Pollination Network
4. Discussion
4.1. Plant and Stingless Bee Communities
4.2. Response of Stingless Bee Communities to Environmental Effect at Habitat-Level
4.3. Response of Stingless Bee Communities to Environmental Effect at Landscape-Level
4.4. Pollination Network
5. Implications for Conservation
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Animal Ethics
Appendix A
| No. | Meliponine Species | Abundance | Percent | |
|---|---|---|---|---|
| 1 | Heterotrigona | itama | 189 | 25.34 |
| 2 | Tetragonula | pagdeni | 94 | 12.60 |
| 3 | Tetrigona | apicalis | 87 | 11.66 |
| 4 | Geniotrigona | thoracica | 85 | 11.39 |
| 5 | Tetragonula | fuscobalteata | 81 | 10.86 |
| 6 | Lophotrigona | canifrons | 48 | 6.43 |
| 7 | Lepidotrigona | terminata | 37 | 4.96 |
| 8 | Tetragonula | laeviceps | 37 | 4.96 |
| 9 | Tetragonilla | collina | 31 | 4.16 |
| 10 | Tetrigona | melanoleuca | 27 | 3.62 |
| 11 | Tetragonilla | atripes | 24 | 3.22 |
| 12 | Lepidotrigona | satun | 4 | 0.54 |
| 13 | Lisotrigona | cacciae | 2 | 0.27 |
| Label | Plant Species | Label | Meliponine Species |
|---|---|---|---|
| P1 | Kyllinga brevifolia Rottb. | A1 | Geniotrigona thoracica |
| P2 | Scoparia dulcis L. | A2 | Heterotrigona itama |
| P3 | Musa spp. | A3 | Lepidotrigona satun |
| P4 | Ocimum tenuiflorum L. | A4 | Lepidotrigona terminata |
| P5 | Artocarpus heterophyllus Lam. | A5 | Lophotrigona canifrons |
| P6 | Melastoma malabathricum L. | A6 | Tetragonilla atripes |
| P7 | Nephelium lappaceum L. | A7 | Tetragonilla collina |
| P8 | Artocarpus integer (Thunb.) Merr. | A8 | Tetragonula fuscobalteata |
| P9 | Bidens pilosa L. | A9 | Tetragonula laeviceps |
| P10 | Tagetes erecta L. | A10 | Tetragonula pagdeni |
| P11 | Etlingera elatior (Jack) R.M. Sm. | A11 | Tetrigona apicalis |
| P12 | Ruellia tuberosa L. | A12 | Tetrigona melanoleuca |
| P13 | Averrhoa bilimbi L. | ||
| P14 | Calopogonium mucunoides Desv. | ||
| P15 | Durio zibethinus L. | ||
| P16 | Asystasia gangetica (L.) T. Anderson | ||
| P17 | Elaeis guineensis Jacq. | ||
| P18 | Cleome rutidosperma DC. | ||
| P19 | Leucas aspera (Willd.) Link | ||
| P20 | Benincasa hispida (Thunb.) Cogn. | ||
| P21 | Solanum virginianum L. | ||
| P22 | Cocos nucifera L. | ||
| P23 | Mangifera foetida Lour. | ||
| P24 | Solanum ferox L. | ||
| P25 | Mimosa pudica L. | ||
| P26 | Xanthostemon chrysanthus (F. Müll.) Benth. | ||
| P27 | Hippeastrum johnsonii (Gowen) Herb. | ||
| P28 | Oxalis barrelieri L. | ||
| P29 | Garcinia atroviridis Griff. ex T. Anderson | ||
| P30 | Salacca magnifica Mogea | ||
| P31 | Ageratum conyzoides (L.) L. | ||
| P32 | Chromolaena odorata (L.) R.M. King & H. Rob. | ||
| P33 | Urena lobata L. | ||
| P34 | Eulalia sp. | ||
| P35 | Ocimum basilicum L. |

References
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Dalsgaard, B. Land-use and climate impacts on plant–pollinator interactions and pollination services. Diversity 2020, 12, 168. [Google Scholar] [CrossRef]
- Rech, A.R.; Dalsgaard, B.; Sandel, B.; Sonne, J.; Svenning, J.C.; Holmes, N.; Ollerton, J. The macroecology of animal versus wind pollination: Ecological factors are more important than historical climate stability. Plant Ecol. Divers. 2016, 9, 253–262. [Google Scholar] [CrossRef]
- Klein, A.M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Winfree, R.; Aguilar, R.; Vazquez, D.P.; Lebuhn, G.; Aizen, M.A. A meta-analysis of bees’ responses to anthropogenic disturbance. Ecology 2009, 90, 2068–2076. [Google Scholar] [CrossRef]
- Winfree, R.; Bartomeus, I.; Cariveau, D.P. Native pollinators in anthropogenic habitats. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Ollerton, J. Pollinator Diversity: Distribution, Ecological Function, and Conservation. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 353–376. [Google Scholar] [CrossRef] [Green Version]
- Roulston, T.H.; Goodell, K. The role of resources and risks in regulating wild bee populations. Annu. Rev. Entomol. 2011, 56, 293–312. [Google Scholar] [CrossRef] [Green Version]
- Vaudo, A.D.; Tooker, J.F.; Grozinger, C.M.; Patch, H.M. Bee nutrition and floral resource restoration. Curr. Opin. Insect Sci. 2015, 10, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Brosi, B.J.; Daily, G.C.; Ehrlich, P.R. Bee community shifts with landscape context in a tropical countryside. Ecol. Appl. 2007, 17, 418–430. [Google Scholar] [CrossRef]
- Brosi, B.J.; Daily, G.C.; Shih, T.M.; Oviedo, F.; Durán, G. The effects of forest fragmentation on bee communities in tropical countryside. J. Appl. Ecol. 2008, 45, 773–783. [Google Scholar] [CrossRef]
- Brosi, B.J. The complex responses of social stingless bees (Apidae: Meliponini) to tropical deforestation. For. Ecol. Manag. 2009, 258, 1830–1837. [Google Scholar] [CrossRef]
- Lichtenberg, E.M.; Mendenhall, C.D.; Brosi, B. Foraging traits modulate stingless bee community disassembly under forest loss. J. Anim. Ecol. 2017, 86, 1404–1416. [Google Scholar] [CrossRef] [Green Version]
- Miljanic, A.S.; Loy, X.; Gruenewald, D.L.; Dobbs, E.K.; Gottlieb, I.G.W.; Fletcher, R.J.; Brosi, B.J. Bee communities in forestry production landscapes: Interactive effects of local-level management and landscape context. Landsc. Ecol. 2018, 1–18. [Google Scholar] [CrossRef]
- Liow, L.H.; Sodhi, N.S.; Elmqvist, T. Bee diversity along a disturbance gradient in tropical lowland forests of south-east Asia. J. Appl. Ecol. 2001, 38, 180–192. [Google Scholar] [CrossRef] [Green Version]
- Samejima, H.; Marzuki, M.; Nagamitsu, T.; Nakasizuka, T. The effects of human disturbance on a stingless bee community in a tropical rainforest. Biol. Conserv. 2004, 120, 577–587. [Google Scholar] [CrossRef]
- Stewart, A.B.; Sritongchuay, T.; Teartisup, P.; Kaewsomboon, S.; Bumrungsri, S. Habitat and landscape factors influence pollinators in a tropical megacity, Bangkok, Thailand. PeerJ 2018, 6, e5335. [Google Scholar] [CrossRef]
- Tangtorwongsakul, P.; Warrit, N.; Gale, G.A. Effects of landscape cover and local habitat characteristics on visiting bees in tropical orchards. Agric. For. Entomol. 2018, 20, 28–40. [Google Scholar] [CrossRef]
- Ricketts, T.H. Tropical forest fragments enhance pollinator activity in nearby coffee crops. Conserv. Biol. 2004, 18, 1262–1271. [Google Scholar] [CrossRef]
- Boonithee, A.; Juntawong, N.; Pechhacker, H.; Hüttinger, E. Floral visits to select crops by four Apis species and Trigona sp. in Thailand. Acta Hortic. 1991, 288, 74–80. [Google Scholar] [CrossRef]
- Kremen, C.; Williams, N.M.; Thorp, R.W. Crop pollination from native bees at risk from agricultural intensification. Proc. Natl. Acad. Sci. USA 2002, 99, 16812–16816. [Google Scholar] [CrossRef] [Green Version]
- Ricketts, T.H.; Daily, G.C.; Ehrlich, P.R.; Michener, C.D. Economic value of tropical forest to coffee production. Proc. Natl. Acad. Sci. USA 2004, 101, 12579–12582. [Google Scholar] [CrossRef] [Green Version]
- Kremen, C.; Williams, N.M.; Bugg, R.L.; Fay, J.P.; Thorp, R.W. The area requirements of an ecosystem service: Crop pollination by native bee communities in California. Ecol. Lett. 2004, 7, 1109–1119. [Google Scholar] [CrossRef]
- Hülsmann, M.; von Wehrden, H.; Klein, A.M.; Leonhardt, S.D. Plant diversity and composition compensate for negative effects of urbanization on foraging bumble bees. Apidologie 2015, 46, 760–770. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Steffan-Dewenter, I.; Kremen, C.; Morales, J.M.; Bommarco, R.; Cunningham, S.A.; Carvalheiro, L.G.; Chacoff, N.P.; Dudenhöffer, J.H.; Greenleaf, S.S.; et al. Stability of pollination services decreases with isolation from natural areas despite honey bee visits. Ecol. Lett. 2011, 14, 1062–1072. [Google Scholar] [CrossRef]
- Momose, K.; Yumoto, T.; Nagamitsu, T.; Kato, M.; Nagamasu, H.; Sakai, S.; Harrison, R.D.; Itioka, T.; Hamid, A.A.; Inoue, T. Pollination biology in a lowland dipterocarp forest in Sarawak, Malaysia. I. Characteristics of the plant-pollinator community in a lowland dipterocarp forest. Am. J. Bot. 1998, 85, 1477–1501. [Google Scholar] [CrossRef]
- Heard, T.A. The role of stingless bees in crop pollination. Annu. Rev. Entomol. 1999, 44, 183–206. [Google Scholar] [CrossRef]
- Eltz, T.; Bruhl, C.A.; Imiyabir, Z.; Linsenmair, K.E. Nesting and nest trees of stingless bees (Apidae: Meliponini) in lowland dipterocarp forests in Sabah, Malaysia, with implications for forest management. For. Ecol. Manag. 2003, 172, 301–313. [Google Scholar] [CrossRef]
- Hubbell, S.P.; Johnson, L. Competition and nest spacing in a tropical stingless bee community. Ecology 1997, 58, 949–963. [Google Scholar] [CrossRef]
- Inoue, T.; Nakamura, K.; Salmah, S.; Abbas, I. Population dynamics of animals in unpredictably-changing tropical environments. J. Biosci. 1993, 18, 425–455. [Google Scholar] [CrossRef]
- Eltz, T.; Brühl, C.A.; Van der Kaars, S.; Eduard Linsenmair, K. Determinants of stingless bee nest density in lowland dipterocarp forests of Sabah, Malaysia. Oecologia 2002, 131, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Chuttong, B.; Chanbang, Y.; Burgett, M. Meliponiculture—Stingless bee beekeeping in Thailand. Bee World 2014, 91, 41–45. [Google Scholar] [CrossRef]
- Brown, J.C.; Albrecht, C. The effect of tropical deforestation on stingless bees of the genus Melipona (Insecta: Hymenoptera: Apidae: Meliponini) in central Rondonia, Brazil. J. Biogeogr. 2001, 28, 623–634. [Google Scholar] [CrossRef]
- Round, P.D.; Gale, G.A.; Brockelman, W.Y. A comparison of bird communities in mixed fruit orchards and natural forest at Khao Luang, southern Thailand. Biodivers. Conserv. 2006, 15, 2873–2891. [Google Scholar] [CrossRef]
- Bumrungsri, S.; Sripaoraya, E.; Chongsiri, T.; Sridith, K.; Racey, P.A. The pollination ecology of durian (Durio zibethinus, Bombacaceae) in southern Thailand. J. Trop. Ecol. 2009, 25, 85–92. [Google Scholar] [CrossRef]
- Sritongchuay, T.; Kremen, C.; Bumrungsri, S. Effects of forest and cave proximity on fruit set of tree crops in tropical orchards in Southern Thailand. J. Trop. Ecol. 2016, 32, 269–279. [Google Scholar] [CrossRef] [Green Version]
- Montoya, J.M.; Pimm, S.L.; Solé, R.V. Ecological networks and their fragility. Nature 2006, 442, 259–264. [Google Scholar] [CrossRef]
- Kaiser-Bunbury, C.N.; Blüthgen, N. Integrating network ecology with applied conservation: A synthesis and guide to implementation. AoB Plants 2015, 7, 1–15. [Google Scholar] [CrossRef]
- Weiner, C.N.; Werner, M.; Linsenmair, K.E.; Blüthgen, N. Land-use impacts on plant-pollinator networks: Interaction strength and specialization predict pollinator declines. Ecology 2014, 95, 466–474. [Google Scholar] [CrossRef]
- Blüthgen, N.; Klein, A.M. Functional complementarity and specialisation: The role of biodiversity in plant-pollinator interactions. Basic Appl. Ecol. 2011, 12, 282–291. [Google Scholar] [CrossRef]
- Kaluza, B.F.; Wallace, H.; Heard, T.A.; Klein, A.M.; Leonhardt, S.D. Urban gardens promote bee foraging over natural habitats and plantations. Ecol. Evol. 2016, 6, 1304–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaluza, B.F.; Wallace, H.M.; Heard, T.A.; Minden, V.; Klein, A.; Leonhardt, S.D. Social bees are fitter in more biodiverse environments. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Land Development Department. GIS Data on Land Use Resources; Land Development Department: Bangkok, Thailand, 2019. [Google Scholar]
- Smith, J.P.; Heard, T.A.; Beekman, M.; Gloag, R. Flight range of the Australian stingless bee Tetragonula carbonaria (Hymenoptera: Apidae). Austral Entomol. 2017, 56, 50–53. [Google Scholar] [CrossRef]
- Wahala, S.; Huang, P. Foraging distance in the stingless bee Trigona thoracica. In Proceedings of the Tenth Annual Forestry & Environmental Symposium; Department of Forestry & Environmental Science, University of Sri Jayewardenepura: Nugegoda, Sri Lanka, 2005; pp. 62–63. [Google Scholar]
- Environmental Systems Research Institute (ESRI) ArcGIS Desktop Help 10.3 Geostatistical Analyst. 2014. Available online: https://desktop.arcgis.com/en/arcmap/10.3/get-started/quick-start-guides/arcgis-desktop-quick-start-guide.htm (accessed on 30 August 2020).
- Schwarz, H.F. The Indo-Malayan species of Trigona. Bull. Am. Museum Nat. Hist. 1939, 76, 83–141. [Google Scholar]
- Sakagami, S.F. Tetragonula Stingless Bees of the Continental Asia and Sri Lanka (Hymenoptera, Apidae). J. Fac. Sci. Hokkaido Univ. Ser. VI Zool. 1978, 21, 165–247. [Google Scholar]
- Attasopa, K.; Bänziger, H.; Disayathanoowat, T.; Packer, L. A new species of Lepidotrigona (hymenoptera: Apidae) from Thailand with the description of males of L. flavibasis and L. doipaensis and comments on asymmetrical genitalia in bees. Zootaxa 2018, 4442, 63–82. [Google Scholar] [CrossRef]
- Engel, M.S. A review of the Indo-Malayan meliponine genus Lisotrigona, with two new species (Hymenoptera: Apidae). Orient. Insects 2000, 34, 229–237. [Google Scholar] [CrossRef]
- Dormann, C.F.; Fruend, J.; Gruber, B.; Beckett, S.; Devoto, M.; Felix, G.; Iriondo, J.; Opsahl, T.; Pinheiro, R.; Strauss, R.; et al. Package “Bipartite”: Visualizing Bipartite Networks and Calculating Some (Ecological) Indices. Available online: https://cran.r-project.org/web/packages/bipartite/bipartite.pdf (accessed on 30 August 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Blüthgen, N.; Menzel, F.; Blüthgen, N. Measuring specialization in species interaction networks. BMC Ecol. 2006, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ripley, B.D.; Venables, W.N.; Bates, D.M.; Hornik, K.; Gebhardt, A.; Firth, D. Package ‘MASS’. Available online: http://www.stats.ox.ac.uk/pub/MASS4 (accessed on 30 August 2020).
- Bates, D.; Maechler, M. Matrix: Sparse and Dense Matrix Classes and Methods. Available online: https://cran.r-project.org/package=Matrix (accessed on 30 August 2020).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Linear Mixed-Effects Models using “Eigen” and S4. Available online: https://github.com/lme4/lme4/ (accessed on 30 August 2020).
- Fox, J.; Weisberg, S.; Price, B.; Adler, D.; Bates, D.; Baud-Bovy, G.; Bolker, B.; Ellison, S.; Firth, D.; Friendly, M.; et al. Package ‘Car’. Available online: https://cran.r-project.org/package=car (accessed on 30 August 2020).
- Sarkar, D.; Andrews, F.; Wright, K.; Klepeis, N.; Murrell, P. Package ‘Lattice’. Available online: http://lmdvr.r-forge.r-project.org (accessed on 30 August 2020).
- O’Hara, R.B.; Kotze, D.J. Do not log-transform count data. Methods Ecol. Evol. 2010, 1, 118–122. [Google Scholar] [CrossRef] [Green Version]
- Gittleman, J.L.; Kot, M. Adaptation: Statistics and a null model for estimating phylogenetic effects. Syst. Zool. 1990, 39, 227–241. [Google Scholar] [CrossRef]
- Paradis, E.; Blomberg, S.; Bolker, B.; Brown, J.; Claramunt, S.; Claude, J.; Cuong, H.S.; Desper, R.; Didier, G.; Durand, B.; et al. Package ‘Ape’. Available online: http://ape-package.ird.fr/ (accessed on 10 December 2020).
- Kuhn, M.; Jackson, S.; Cimentada, J. Corrr: Correlations in R. Available online: https://cran.r-project.org/package=corrr (accessed on 30 August 2020).
- Betts, M.G.; Forbes, G.J.; Diamond, A.W.; Taylor, P.D. Independent effects of fragmentation on forest songbirds: An organism-based approach. Ecol. Appl. 2006, 16, 1076–1089. [Google Scholar] [CrossRef]
- Barton, K. Package ‘MuMIn’. Available online: https://cran.r-project.org/package=MuMIn (accessed on 30 August 2020).
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D. Package ‘Ggplot2’. Available online: https://github.com/tidyverse/ggplot2 (accessed on 30 August 2020).
- Wayo, K.; Phankaew, C.; Stewart, A.B.; Bumrungsri, S. Bees are supplementary pollinators of self-compatible chiropterophilous durian. J. Trop. Ecol. 2018, 34, 41–52. [Google Scholar] [CrossRef]
- Blanche, K.R.; Ludwig, J.A.; Cunningham, S.A. Proximity to rainforest enhances pollination and fruit set in orchards. J. Appl. Ecol. 2006, 43, 1182–1187. [Google Scholar] [CrossRef]
- Chacoff, N.P.; Aizen, M.A. Edge effects on flower-visiting insects in grapefruit plantations bordering premontane subtropical forest. J. Appl. Ecol. 2006, 43, 18–27. [Google Scholar] [CrossRef]
- Klein, A.M.; Steffan-Dewenter, I.; Tscharntke, T. Fruit set of highland coffee increases with the diversity of pollinating bees. Proc. R. Soc. B Biol. Sci. 2003, 270, 955–961. [Google Scholar] [CrossRef] [Green Version]
- Klein, A.M.; Steffan-Dewenter, I.; Tscharntke, T. Pollination of Coffea canephora in relation to local and regional agroforestry management. J. Appl. Ecol. 2003, 40, 837–845. [Google Scholar] [CrossRef] [Green Version]
- Frias, B.E.D.; Barbosa, C.D.; Lourenço, A.P. Pollen nutrition in honey bees (Apis mellifera): Impact on adult health. Apidologie 2016, 47, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Hansen, K.; Sritongchuay, T.; Bumrungsri, S.; Simmons, B.I.; Strange, N.; Dalsgaard, B. Landscape-level effects of forest on pollinators and fruit set of guava (Psidium guajava L.) in orchards across southern Thailand. Diversity 2020, 12, 259. [Google Scholar] [CrossRef]
- Ricketts, T.H.; Regetz, J.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Bogdanski, A.; Gemmill-Herren, B.; Greenleaf, S.S.; Klein, A.M.; Mayfield, M.M.; et al. Landscape effects on crop pollination services: Are there general patterns? Ecol. Lett. 2008, 11, 499–515. [Google Scholar] [CrossRef]
- Greenleaf, S.S.; Williams, N.M.; Winfree, R.; Kremen, C. Bee foraging ranges and their relationship to body size. Oecologia 2007, 153, 589–596. [Google Scholar] [CrossRef]
- Winfree, R.; Griswold, T.; Kremen, C. Effect of human disturbance on bee communities in a forested ecosystem. Conserv. Biol. 2007, 21, 213–223. [Google Scholar] [CrossRef]
- Gutiérrez-Chacón, C.; Dormann, C.F.; Klein, A.M. Forest-edge associated bees benefit from the proportion of tropical forest regardless of its edge length. Biol. Conserv. 2018, 220, 149–160. [Google Scholar] [CrossRef]
- Cariveau, D.P.; Winfree, R. Causes of variation in wild bee responses to anthropogenic drivers. Curr. Opin. Insect Sci. 2015, 10, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Geslin, B.; Le Féon, V.; Folschweiller, M.; Flacher, F.; Carmignac, D.; Motard, E.; Perret, S.; Dajoz, I. The proportion of impervious surfaces at the landscape scale structures wild bee assemblages in a densely populated region. Ecol. Evol. 2016, 6, 6599–6615. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, A.; Grass, I.; Belavadi, V.V.; Tscharntke, T. How urbanization is driving pollinator diversity and pollination—A systematic review. Biol. Conserv. 2020, 241, 108321. [Google Scholar] [CrossRef]
- Ferreira, P.A.; Boscolo, D.; Lopes, L.E.; Carvalheiro, L.G.; Biesmeijer, J.C.; da Rocha, P.L.B.; Viana, B.F. Forest and connectivity loss simplify tropical pollination networks. Oecologia 2020, 192, 577–590. [Google Scholar] [CrossRef]
- Sritongchuay, T.; Hughes, A.C.; Memmott, J.; Bumrungsri, S. Forest proximity and lowland mosaic increase robustness of tropical pollination networks in mixed fruit orchards. Landsc. Urban Plan. 2019, 192, 103646. [Google Scholar] [CrossRef]
- Sritongchuay, T.; Hughes, A.C.; Bumrungsri, S. The role of bats in pollination networks is influenced by landscape structure. Glob. Ecol. Conserv. 2019, 20, 1–13. [Google Scholar] [CrossRef]
- Aizen, M.A.; Sabatino, M.; Tylianakis, J.M. Specialization and rarity predict nonrandom loss of interactions from mutualist networks. Science 2012, 335, 1486–1489. [Google Scholar] [CrossRef] [Green Version]
- Ashman, T.; Knight, T.M.; Steets, J.A.; Amarasekare, P.; Burd, M.; Campbell, D.R.; Dudash, M.R.; Johnston, M.O.; Mazer, S.J.; Mitchell, R.J.; et al. Pollen limitation of plant reproduction: Ecological and evolutionary causes and consequences. Ecology 2004, 85, 2408–2421. [Google Scholar] [CrossRef] [Green Version]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Antonini, Y.; Martins, R.P.; Aguiar, L.M.; Loyola, R.D. Richness, composition and trophic niche of stingless bee assemblages in urban forest remnants. Urban Ecosyst. 2013, 16, 527–541. [Google Scholar] [CrossRef]
| Indice | Explanatory Fixed Variable | Estimate | SE | z-Value | p-Value |
|---|---|---|---|---|---|
| Richness (AIC = 249.1) | Intercept | 0.059 | 0.003 | 18.56 | <0.001 *** |
| Orchard size | −1.929 | 0.003 | −607.92 | <0.001 *** | |
| Floral richness | −0.087 | 0.003 | −27.77 | <0.001 *** | |
| Forest cover (1 km) | 0.039 | 0.003 | 14.41 | <0.001 *** | |
| Orchard size * Floral richness | 0.322 | 0.003 | 101.58 | <0.001 *** | |
| (AIC = 252.1) | Intercept | 2.351 | 0.625 | 3.759 | <0.001 *** |
| Agricultural cover (10 km) | −0.044 | 0.011 | −3.943 | <0.001 *** | |
| (AIC = 261.5) | Intercept | 0.379 | 0.367 | 1.032 | 0.302 |
| Urbanized cover (10 km) | −0.106 | 0.046 | −2.322 | 0.020 * | |
| (AIC = 258.5) | Intercept | 0.354 | 0.315 | 1.122 | 0.262 |
| Distance to forest edge | −0.0003 | 0.0001 | −2.857 | 0.004 ** | |
| Abundance (AIC = 458.1) | Intercept | −0.323 | 0.465 | −0.695 | 0.487 |
| Forest cover (2 km) | 0.062 | 0.013 | 4.768 | <0.001 *** | |
| (AIC = 460.8) | Intercept | 5.132 | 1.004 | 5.115 | <0.001 *** |
| Agricultural cover (2 km) | −0.062 | 0.014 | −4.343 | <0.001 *** | |
| (AIC = 468) | Intercept | −0.497 | 1.175 | −0.423 | 0.673 |
| H′ | 0.936 | 0.503 | 1.859 | 0.063 | |
| Distance to forest edge | −5.477 | 2.058 | −2.662 | 0.008 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wayo, K.; Sritongchuay, T.; Chuttong, B.; Attasopa, K.; Bumrungsri, S. Local and Landscape Compositions Influence Stingless Bee Communities and Pollination Networks in Tropical Mixed Fruit Orchards, Thailand. Diversity 2020, 12, 482. https://doi.org/10.3390/d12120482
Wayo K, Sritongchuay T, Chuttong B, Attasopa K, Bumrungsri S. Local and Landscape Compositions Influence Stingless Bee Communities and Pollination Networks in Tropical Mixed Fruit Orchards, Thailand. Diversity. 2020; 12(12):482. https://doi.org/10.3390/d12120482
Chicago/Turabian StyleWayo, Kanuengnit, Tuanjit Sritongchuay, Bajaree Chuttong, Korrawat Attasopa, and Sara Bumrungsri. 2020. "Local and Landscape Compositions Influence Stingless Bee Communities and Pollination Networks in Tropical Mixed Fruit Orchards, Thailand" Diversity 12, no. 12: 482. https://doi.org/10.3390/d12120482
APA StyleWayo, K., Sritongchuay, T., Chuttong, B., Attasopa, K., & Bumrungsri, S. (2020). Local and Landscape Compositions Influence Stingless Bee Communities and Pollination Networks in Tropical Mixed Fruit Orchards, Thailand. Diversity, 12(12), 482. https://doi.org/10.3390/d12120482






