Intraspecific Trait Variation Dilutes Deterministic Processes in Community Assembly of Arid Shrubs across Multiple Scales
Abstract
1. Introduction
2. Methods
2.1. Study Area
2.2. Sampling Site Establishment
2.3. Soil Sample Characteristics Measurement
2.4. Plant Sampling and Trait Measurement
2.5. Functional Diversity
2.6. Functional Trait-Based Community Assembly Pattern
2.7. Effect of Environmental Conditions on Functional Diversity
3. Results
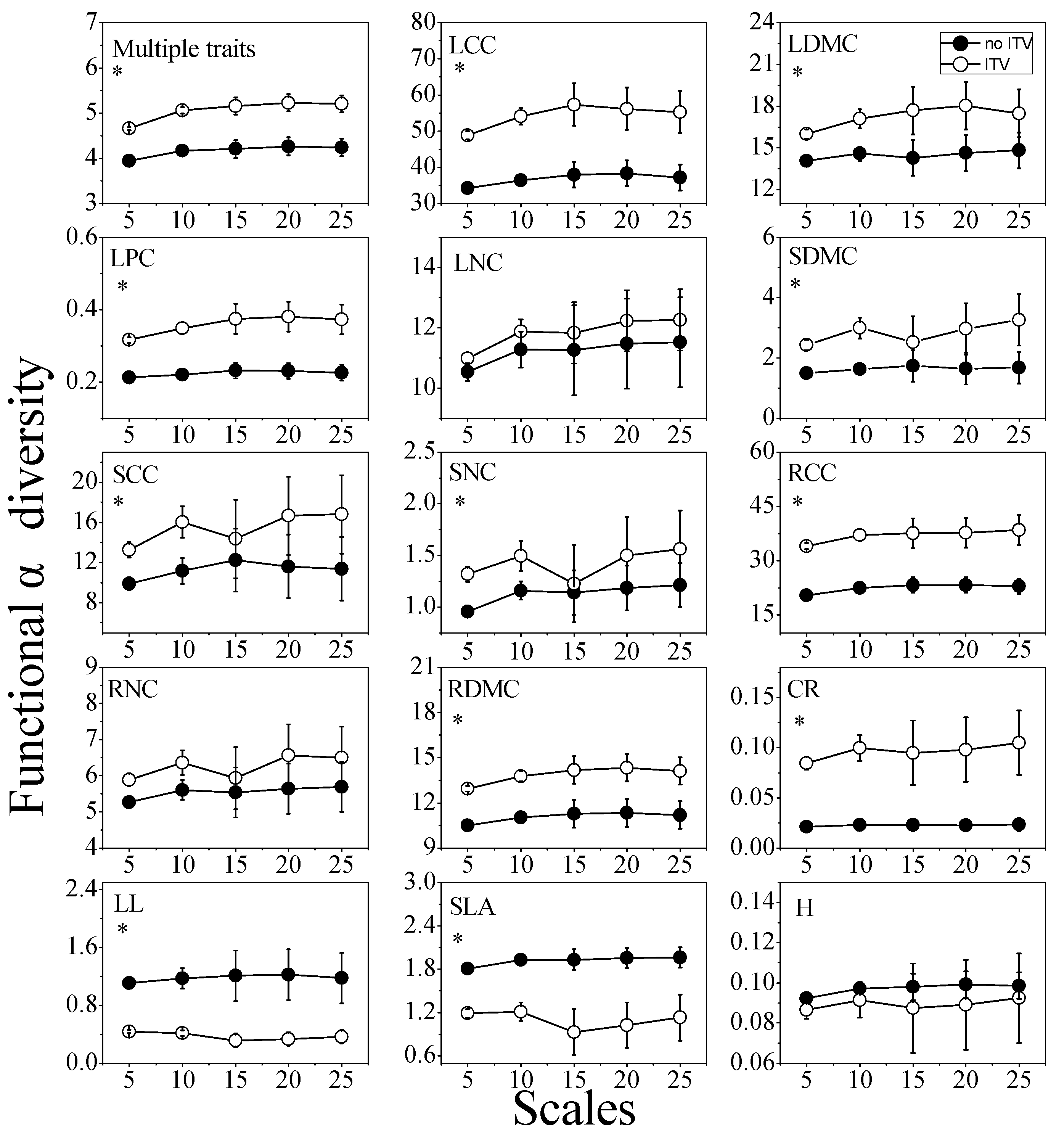
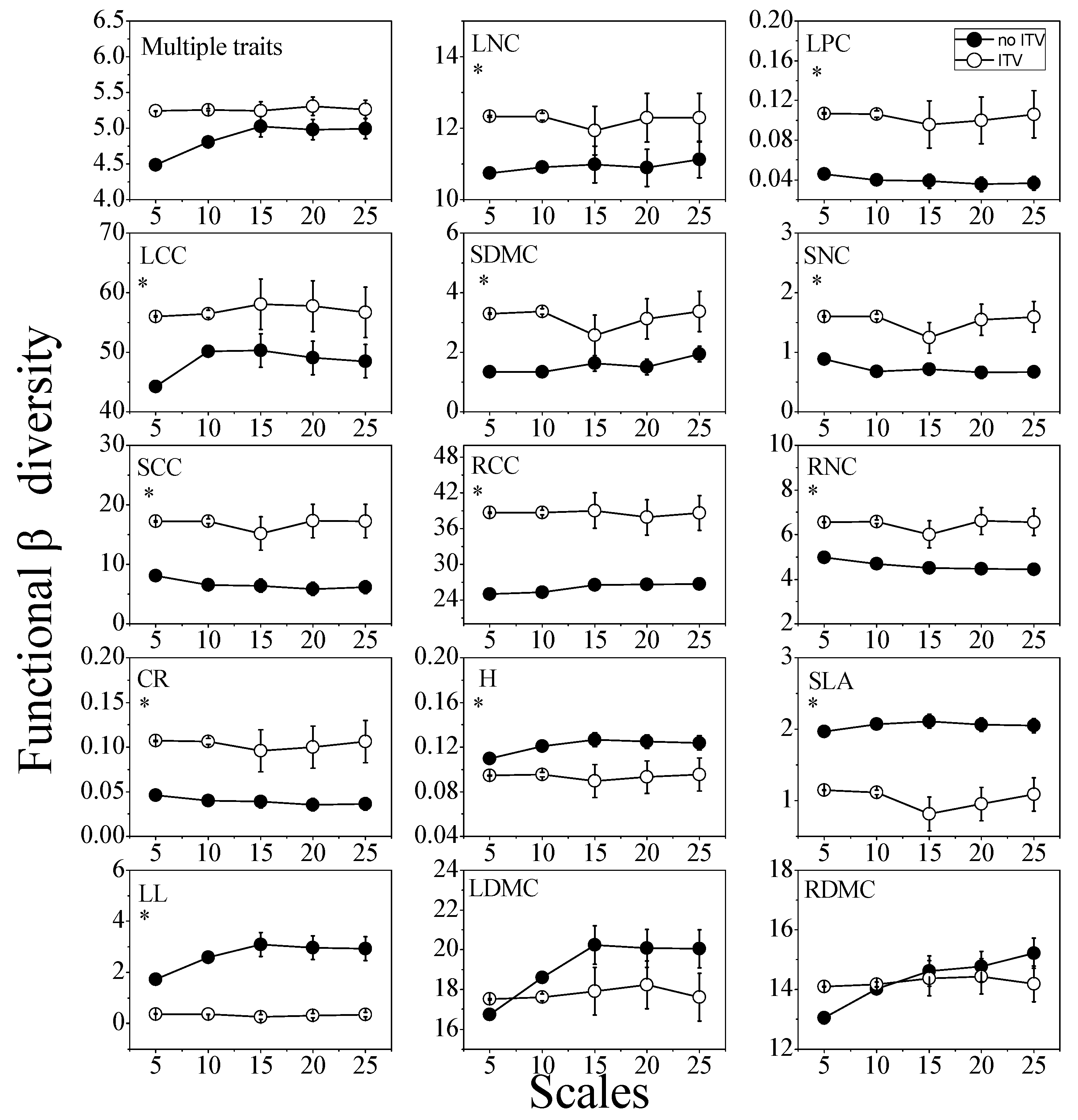
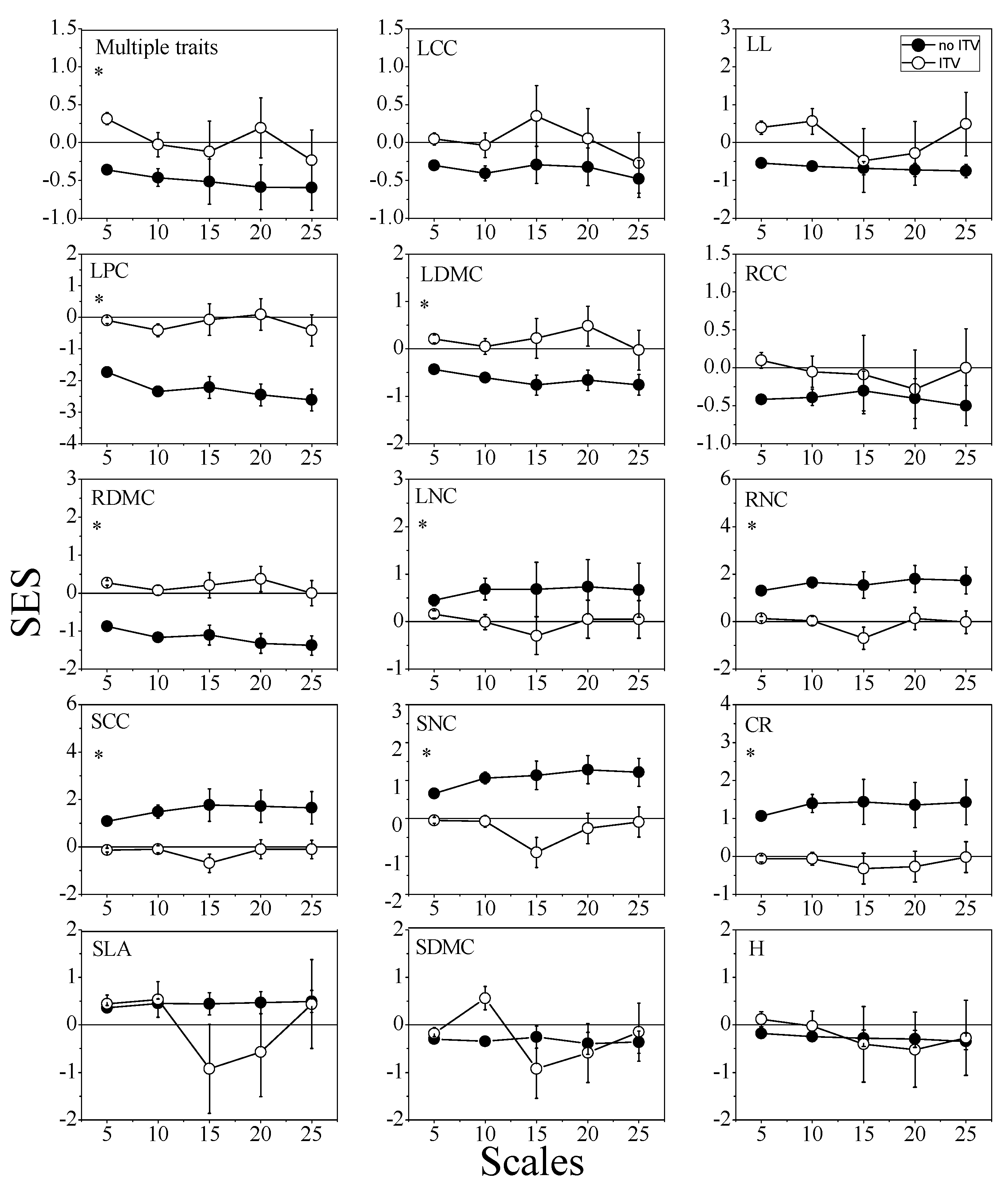
3.1. Functional Diversity and Community Assembly Patterns
3.2. The Effects of the Soil Environmental Factors on Functional Diversity
3.3. Functional Diversity and Community Assembly Patterns across Scales
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Data Accessibility Statement
References
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Evol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- McIntire, E.J.; Fajardo, A. Beyond description: The active and effective way to infer processes from spatial patterns. Ecology 2009, 90, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Bellwood, D.R.; Klanten, S.; Cowman, P.F.; Pratchett, M.S.; Konow, N.; van Herwerden, L. Evolutionary history of the butterflyfishes (f: Chaetodontidae) and the rise of coral feeding fishes. J. Evol. Biol. 2010, 23, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Ingram, T.; Shurin, J.B. Trait-based assembly and phylogenetic structure in northeast pacific rockfish assemblages. Ecology 2009, 90, 2444–2453. [Google Scholar] [CrossRef] [PubMed]
- Villéger, S.; Miranda, J.R.; Hernández, D.F.; Mouillot, D. Contrasting changes in taxonomic vs. functional diversity of tropical fish communities after habitat degradation. Ecol. Appl. 2010, 20, 1512–1522. [Google Scholar] [CrossRef]
- Paine, C.E.T.; Baraloto, C.; Chave, J.; Hérault, B. Functional traits of individual trees reveal ecological constraints on community assembly in tropical rain forests. Oikos 2011, 120, 720–727. [Google Scholar] [CrossRef]
- Laughlin, D.C.; Laughlin, D.E. Advances in modeling trait-based plant community assembly. Trends Plant Sci. 2013, 18, 584–593. [Google Scholar] [CrossRef]
- Rolhauser, A.G.; Pucheta, E. Annual plant functional traits explain shrub facilitation in a desert community. J. Veg. Sci. 2016, 27, 60–68. [Google Scholar] [CrossRef]
- McGill, B.J.; Enquist, B.J.; Weiher, E.; Westoby, M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Amarasekare, P.; Araújo, M.S.; Bürger, R.; Levine, J.M.; Novak, M.; Rudolf, V.H.W.; Schreiber, S.J.; Urban, M.C.; Vasseur, D.A. Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol. 2011, 26, 183–192. [Google Scholar] [CrossRef]
- Violle, C.; Enquist, B.J.; Mcgill, B.J.; Jiang, L.; Albert, C.H.; Hulshof, C.; Jung, V.; Messier, J. The return of the variance: Intraspecific variability in community ecology. Trends Ecol. Evol. 2012, 27, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, A.; Piper, F.I. Intraspecific trait variation and covariation in a widespread tree species (Nothofagus pumilio) in southern Chile. New Phytol. 2011, 189, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Fridley, J.D.; Grime, J.P.; Bilton, M. Genetic identity of interspecific neighbours mediates plant responses to competition and environmental variation in a species-rich grassland. J. Ecol. 2007, 95, 908–915. [Google Scholar] [CrossRef]
- Fridley, J.D.; Grime, J.P. Community and ecosystem effects of intraspecific genetic diversity in grassland microcosms of varying species diversity. Ecology 2010, 91, 2272–2283. [Google Scholar] [CrossRef] [PubMed]
- Jung, V.; Violle, C.; Mondy, C. Intraspecific variability and trait-based community assembly. J. Ecol. 2010, 98, 1134–1140. [Google Scholar] [CrossRef]
- Bergholz, K.; May, F.; Giladi, I. Environmental heterogeneity drives fine-scale species assembly and functional diversity of annual plants in a semi-arid environment. Perspect. Plant Ecol. Evol. Syst. 2017, 24, 138–146. [Google Scholar] [CrossRef]
- Shao, X.; Brown, C.; Worthy, S.J.; Liu, L.; Cao, M.; Li, Q.; Lin, L.; Swenson, N.G. Intra-specific relatedness, spatial clustering and reduced demographic performance in tropical rainforest trees. Ecol. Lett. 2018, 21, 1737–1751. [Google Scholar] [CrossRef]
- Chase, J.M.; Mcgill, B.J.; Thompson, P.L.; Antão Laura, H.; Bates, A.E.; Blowes, S.A. Species richness change across spatial scales. Oikos 2019, 128, 1079–1091. [Google Scholar] [CrossRef]
- Kraft, N.J.; Ackerly, D.D. Functional trait and phylogenetic tests of community assembly across spatial scales in an Amazonian forest. Ecol. Monogr. 2010, 80, 401–422. [Google Scholar] [CrossRef]
- Soliveres, S.; Smit, C.; Maestre, F.T. Moving forward on facilitation research: Response to changing environments and effects on the diversity, functioning and evolution of plant communities. Biol. Rev. 2015, 90, 297–313. [Google Scholar] [CrossRef]
- Koleff, P.; Gaston, K.J.; Lennon, J.J. Measuring beta diversity for presence–absence data. J. Anim. Ecol. 2003, 72, 367–382. [Google Scholar] [CrossRef]
- Swenson, N.G.; Enquist, B.J.; Thompson, J.; Zimmerman, J.K. The influence of spatial and size scale on phylogenetic relatedness in tropical forest communities. Ecology 2007, 88, 1770–1780. [Google Scholar] [CrossRef]
- Albert, C.H.; Grassein, F.; Schurr, F.M.; Vieilledent, G.; Violle, C. When and how should intraspecific variability be considered in trait-based plant ecology? Perspect. Plant Ecol. Evol. Syst. 2011, 13, 217–225. [Google Scholar] [CrossRef]
- Auger, S.; Shipley, B. Inter-specific and intra-specific trait variation along short environmental gradients in an old-growth temperate forest. J. Veg. Sci. 2013, 24, 419–428. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, G.; Ci, X.; Swenson, N.G.; Cao, M.; Sha, L.; Li, J.; Baskin, C.C.; Slik, J.W.F.; Lin, L.; et al. Functional and phylogenetic assembly in a Chinese tropical tree community across size classes, spatial scales and habitats. Funct. Ecol. 2014, 28, 520–529. [Google Scholar] [CrossRef]
- Siefert, A.; Violle, C.; Chalmandrier, L.; Albert, C.H.; Taudiere, A.; Fajardo, A.; Aarssen, L.W.; Baraloto, C.; Carlucci, M.B.; Cianciaruso, M.V.; et al. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecol. Lett. 2015, 18, 1406–1419. [Google Scholar] [CrossRef]
- Spasojevic, M.J.; Turner, B.L.; Myers, J.A. When does intraspecific trait variation contribute to functional beta-diversity? J. Ecol. 2016, 104, 487–496. [Google Scholar] [CrossRef]
- Siefert, A.; Fridley, J.D.; Ritchie, M.E. Community functional responses to soil and climate at multiple spatial scales: When does intraspecific variation matter? PLoS ONE 2014, 9, e111189. [Google Scholar] [CrossRef]
- Emerson, B.C.; Gillespie, R.G. Phylogenetic analysis of community assembly and structure over space and time. Trends Ecol. Evol. 2008, 23, 619–630. [Google Scholar] [CrossRef]
- Giladi, I.; Ziv, Y.; May, F.; Jeltsch, F. Scale-dependent determinants of plant species richness in a semi-arid fragmented agro-ecosystem. J. Veg. Sci. 2011, 22, 983–996. [Google Scholar] [CrossRef]
- Bernard-Verdier, M.; Garnier, E. Community assembly along a soil depth gradient: Contrasting patterns of plant trait convergence and divergence in a Mediterranean rangeland. J. Ecol. 2012, 100, 1422–1433. [Google Scholar] [CrossRef]
- de Bello, F.; Lavorel, S.; Lavergne, S.; Albert, C.H.; Boulangeat, J.; Mazel, F.; Thuiller, W. Hierarchical effects of environmental filters on the functional structure of plant communities: A case study in the French Alps. Ecography 2013, 36, 393–402. [Google Scholar] [CrossRef]
- Thompson, A.J.; Lester, H.A.; Lummis, S.C. The structural basis of function in Cys-loop receptors. Q. Rev. Biophys. 2010, 43, 449–499. [Google Scholar] [CrossRef] [PubMed]
- Weiher, E.; Freund, D.; Bunton, T.; Stefanski, A.; Lee, T.; Bentivenga, S. Advances, challenges and a developing synthesis of ecological community assembly theory. Philos. Trans. R. Soc. B-Biol. Sci. 2011, 366, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Götzenberger, L.; de Bello, F.; Bråthen, K.A.; Davison, J.; Dubuis, A.; Guisan, A.; Lepš, J.; Lindborg, R.; Moora, M.; Pärtel, M.; et al. Ecological assembly rules in plant communities-approaches, patterns and prospects. Biol. Rev. 2012, 87, 111–127. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Kozak, K.H.; Fine, P.V.; Kembel, S.W. The merging of community ecology and phylogenetic biology. Ecol. Lett. 2009, 12, 693–715. [Google Scholar] [CrossRef] [PubMed]
- Laliberté, E.; Norton, D.A.; Scott, D. Contrasting effects of productivity and disturbance on plant functional diversity at local and metacommunity scales. J. Veg. Sci. 2013, 24, 834–842. [Google Scholar] [CrossRef]
- Xu, J.S.; Chen, Y.; Zhang, L.X.; Chai, Y.F.; Wang, M.; Guo, Y.X.; Li, T.; Yue, M. Using phylogeny and functional traits for assessing community assembly along environmental gradients: A deterministic process driven by elevation. Ecol. Evol. 2017, 7, 5056–5069. [Google Scholar] [CrossRef]
- Aguiar, M.R.; Sala, O.E. Patch structure, dynamics and implications for the functioning of arid ecosystems. Trends Ecol. Evol. 1999, 14, 273–277. [Google Scholar] [CrossRef]
- Chen, S.; Xu, K.; Dong, A.; Xia, Q. Analysis on spatial distribution of cumulonimbus and its circulation characteristics over the Qilian Mountains. Arid Zone Res. 2010, 27, 114–120. [Google Scholar] [CrossRef]
- Wei, G.; Chen, S.; Zhang, Y. Sandstorms changing characteristics in the south edge of the Tengger Desert-Jingtai County, Gansu province as a case study. Arid Zone Res. 2015, 32, 1133–1139. [Google Scholar]
- Kong, L.; Shen, J. Analysis on species diversity of plant communities. J. Ningxia Agric. Coll. 2003, 24, 25–28. [Google Scholar]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; Steege, H.; Morgan, H.D.; Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Chacón-Labella, J.; Cruz, M.D.L.; Pescador, D.S.; Escudero, A. Individual species affect plant traits structure in their surroundings: Evidence of functional mechanisms of assembly. Oecologia 2016, 180, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.S.; Dang, H.; Wang, M.; Chai, Y.F.; Yue, M. Is phylogeny more useful than functional traits for assessing diversity patterns under community assembly processes? Forests 2019, 10, 1159. [Google Scholar] [CrossRef]
- Díaz, A.; Macnair, M.R. The effect of plant size on the expression of cleistogamy in Mimulus nasutus. Funct. Ecol. 1998, 12, 92–98. [Google Scholar] [CrossRef]
- Blonder, B.; Lamanna, C.; Violle, C.; Enquist, B.J. The n-dimensional hypervolume. Glob. Ecol. Biogeogr. 2014, 23, 595–609. [Google Scholar] [CrossRef]
- Mason, N.W.H.; MacGillivray, K.; Steel, J.B. An index of functional diversity. J. Veg. Sci. 2003, 14, 571–578. [Google Scholar] [CrossRef]
- Mason, N.W.H.; Mouillot, D.; Lee, W.G.; Wilson, J.B. Functional richness, functional evenness and functional divergence: The primary components of functional diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Mouchet, M.A.; Villéger, S.; Mason, N.W.; Mouillot, D. Functional diversity measures: An overview of their redundancy and their ability to discriminate community assembly rules. Funct. Ecol. 2010, 24, 867–876. [Google Scholar] [CrossRef]
- Swenson, N.G.; Stegen, J.C.; Davies, S.J.; Erickson, D.L.; Forero-Montaña, J.; Hurlbert, A.H.; Kress, W.J.; Thompson, J.; Uriarte, M.; Wright, S.J.; et al. Temporal turnover in the composition of tropical tree communities: Functional determinism and phylogenetic stochasticity. Ecology 2012, 93, 490–499. [Google Scholar] [CrossRef] [PubMed]
- de Bello, F.; Price, J.N.; Münkemüller, T.; Liira, J.; Zobel, M.; Thuiller, W.; Gerhold, P.; Götzenberger, L.; Lavergne, S.; Leps, J.; et al. Functional species pool framework to test for biotic effects on community assembly. Ecology 2012, 93, 2263–2273. [Google Scholar] [CrossRef] [PubMed]
- Chalmandrier, L.; Münkemüller, T.; Colace, M.P.; Renaud, J.; Aubert, S.; Carlson, B.Z.; Clément, J.C.; Legay, N.; Pellet, G.; Saillard, A.; et al. Spatial scale and intraspecific trait variability mediate assembly rules in alpine grasslands. J. Ecol. 2017, 105, 277–287. [Google Scholar] [CrossRef]
- Chalmandrier, L.; Münkemüller, T.; Gallien, L.; de Bello, F.; Mazel, F.; Lavergne, S.; Thuiller, W. A family of null models to distinguish between environmental filtering and biotic interactions in functional diversity patterns. J. Veg. Sci. 2013, 24, 853–864. [Google Scholar] [CrossRef] [PubMed]
- García-Cervigón, A.I.; Linares, J.C.; Aibar, P.; Olano, J.M. Facilitation promotes changes in leaf economics traits of a perennial forb. Oecologia 2015, 179, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Violle, C.; Castro, H.; Richarte, J.; Navas, M.L. Intraspecific seed trait variations and competition: Passive or adaptive response? Funct. Ecol. 2009, 23, 612–620. [Google Scholar] [CrossRef]
- Gubsch, M.; Buchmann, N.; Schmid, B.; Schulze, E.D.; Lipowsky, A.; Roscher, C. Differential effects of plant diversity on functional trait variation of grass species. Ann. Bot. 2011, 107, 157–169. [Google Scholar] [CrossRef]
- Münkemüller, T.; Gallien, L.; Lavergne, S.; Renaud, J.; Roquet, C.; Abdulhak, S.; Dullinger, S.; Garraud, L.; Guisan, A.; Lenoir, J.; et al. Scale decisions can reverse conclusions on community assembly processes. Glob. Ecol. Biogeogr. 2014, 23, 620–632. [Google Scholar] [CrossRef]
- Kazakou, E.; Violle, C.; Roumet, C.; Navas, M.L.; Vile, D.; Kattge, J.; Garnier, E. Are trait-based species rankings consistent across data sets and spatial scales? J. Veg. Sci. 2014, 25, 235–247. [Google Scholar] [CrossRef]
- Shiono, T.; Kusumoto, B.; Maeshiro, R.; Fujii, S.J.; Götzenberger, L.; de Bello, F.; Kubota, Y. Climatic drivers of trait assembly in woody plants in Japan. J. Biogeogr. 2015, 42, 1176–1186. [Google Scholar] [CrossRef]
- Kraft, N.J.; Valencia, R.; Ackerly, D.D. Functional traits and niche-based tree community assembly in an Amazonian forest. Science 2008, 322, 580–582. [Google Scholar] [CrossRef] [PubMed]
- Baraloto, C.; Hérault, B.; Cet, P.; Zimmerman, B.L. Contrasting taxonomic and functional responses of a tropical tree community to selective logging. J. Appl. Ecol. 2012, 49, 861–870. [Google Scholar] [CrossRef]
- Tirado, R.I.; Pugnaire, F. Community structure and positive interactions in constraining environments. Oikos 2005, 111, 437–444. [Google Scholar] [CrossRef]
- Mayfield, M.M.; Levine, J.M. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol. Lett. 2010, 13, 1085–1093. [Google Scholar] [CrossRef]
- Dante, S.K.; Schamp, B.S.; Aarssen, L.W. Evidence of deterministic assembly according to flowering time in an old-field plant community. Funct. Ecol. 2013, 27, 555–564. [Google Scholar] [CrossRef]
- Laliberté, E.; Zemunik, G.; Turner, B.L. Environmental filtering explains variation in plant diversity along resource gradients. Science 2014, 345, 1602–1605. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Dang, H.; Tian, T.; Chai, Y.; Quan, J.; Lei, M.; Liu, X.; Guo, Y.; Yue, M. Intraspecific Trait Variation Dilutes Deterministic Processes in Community Assembly of Arid Shrubs across Multiple Scales. Diversity 2020, 12, 447. https://doi.org/10.3390/d12120447
Xu J, Dang H, Tian T, Chai Y, Quan J, Lei M, Liu X, Guo Y, Yue M. Intraspecific Trait Variation Dilutes Deterministic Processes in Community Assembly of Arid Shrubs across Multiple Scales. Diversity. 2020; 12(12):447. https://doi.org/10.3390/d12120447
Chicago/Turabian StyleXu, Jinshi, Han Dang, Tingting Tian, Yongfu Chai, Jiaxin Quan, Maolin Lei, Xiao Liu, Yaoxin Guo, and Ming Yue. 2020. "Intraspecific Trait Variation Dilutes Deterministic Processes in Community Assembly of Arid Shrubs across Multiple Scales" Diversity 12, no. 12: 447. https://doi.org/10.3390/d12120447
APA StyleXu, J., Dang, H., Tian, T., Chai, Y., Quan, J., Lei, M., Liu, X., Guo, Y., & Yue, M. (2020). Intraspecific Trait Variation Dilutes Deterministic Processes in Community Assembly of Arid Shrubs across Multiple Scales. Diversity, 12(12), 447. https://doi.org/10.3390/d12120447




