Impacts of Forest Thinning and White-Tailed Deer Herbivory on Translocation of the Rare Terrestrial Orchid Platanthera integrilabia
Abstract
1. Introduction
2. Materials and Methods
2.1. Species Description
2.2. Study Location
2.3. Experimental Design
2.4. Data Collection
2.5. Data Analyses
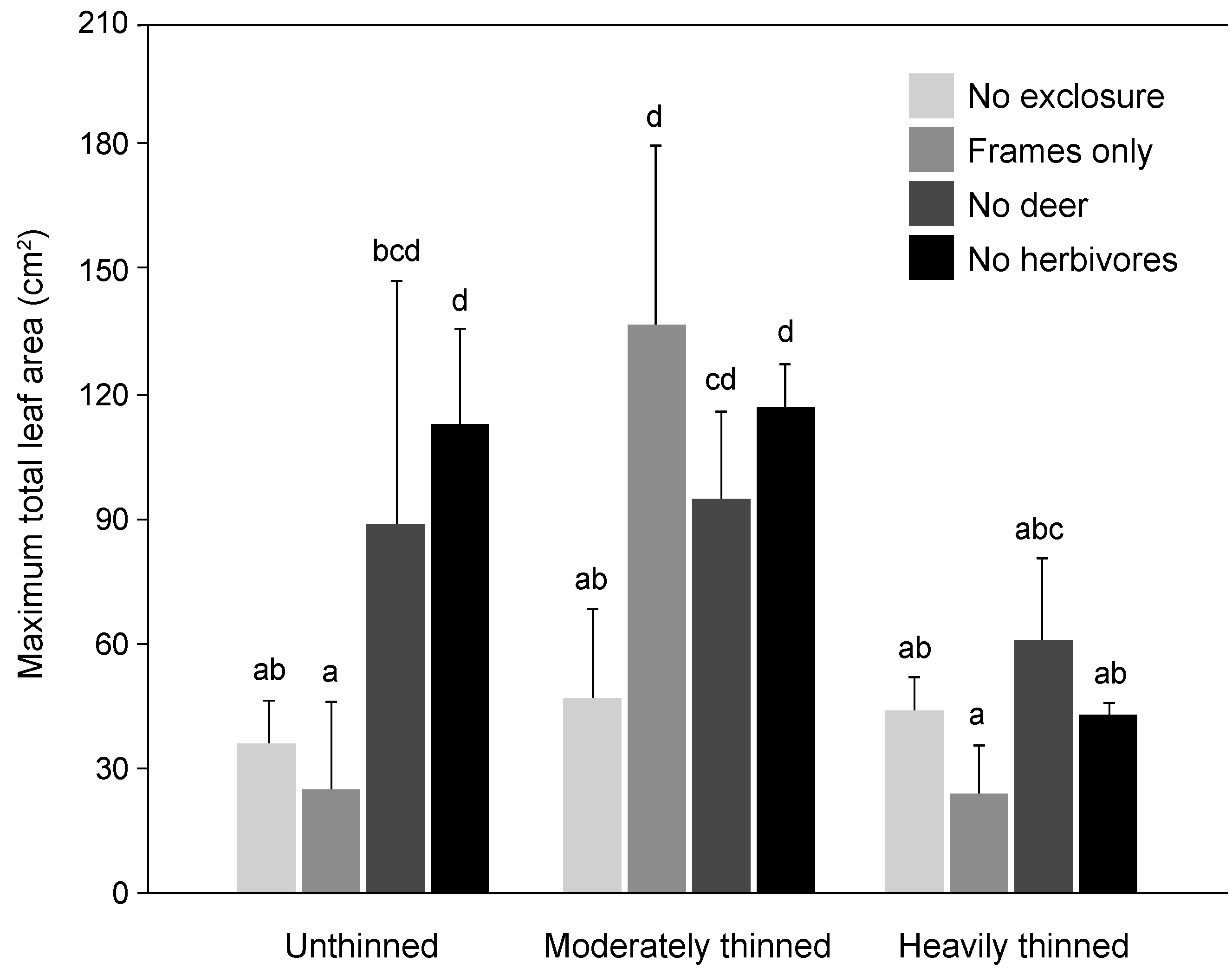
3. Results
4. Discussion
4.1. Overall Translocation Success
4.2. Influence of Light and Deer Herbivory on Platanthera integrilabia
4.3. Recommendations for Platanthera integrilabia Conservation and Management
4.4. Additional Considerations and Future Research
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Calster, H.; Vandenberghe, R.; Ruysen, M.; Verheyen, K.; Hermy, M.; Decocq, G. Unexpectedly high 20th century floristic losses in rural landscape in northern France. J. Ecol. 2008, 96, 927–936. [Google Scholar] [CrossRef]
- Mouillet, D.; Bellwood, D.R.; Baraloto, C.; Chave, J.; Galzin, R.; Harmelin-Vivien, M.; Kulbicki, M.; Lavergne, S.; Lavorel, S.; Mouquet, N.; et al. Rare species support vulnerable functions in high-diversity ecosystems. PLoS Biol. 2013, 11, 1001569. [Google Scholar] [CrossRef] [PubMed]
- Westin, A.; Lennartsson, T.; Bjorklund, J.-O. The historical ecology approach in species conservation—Identifying suitable habitat management for the endangered clouded Apollo butterfly (Parnassius mnemosyne L.) in Sweden. Environm. Sci. 2018, 5, 244–272. [Google Scholar] [CrossRef]
- Farnsworth, E.J. Plant life history traits of rare versus frequent plant taxa of sandplains: Implications for research and management trials. Biol. Conserv. 2006, 136, 44–52. [Google Scholar]
- Greenwalt, L.A.; Gehringer, J.W. Endangered and threatened species: Notice on critical habitat areas. Fed. Regist. 1975, 40, 17764–17765. [Google Scholar]
- Prendergast, J.R.; Quinn, R.M.; Lawton, J.H. The gaps between theory and practice in selecting nature reserves. Conserv. Biol. 1999, 13, 484–492. [Google Scholar] [CrossRef]
- Sunil, K.; Stohlgren, T.J. Maxent modeling for predicting suitable habitat for threatened and endangered tree Canacomyrica monticola in New Caledonia. J. Ecol. Nat. Environm. 2009, 1, 94–98. [Google Scholar]
- Caperta, A.D.; Espírito-Santo, M.D.; Silva, V.; Ferreira, A.; Paes, A.P.; Róis, A.S.; Costa, J.C.; Arsénio, P. Habitat specificity of a threatened and endemic, cliff-dwelling halophyte. AoB Plants 2004, 6, plu032. [Google Scholar] [CrossRef]
- Godefroid, S.; Le Pajolec, S.L. Pre-translocation considerations in rare plant reintroductions: Implications for designing protocols. Plant Ecol. 2016, 217, 169–182. [Google Scholar] [CrossRef]
- Armstrong, D.P.; Seddon, P.J. Directions in reintroduction biology. Trends Ecol. Evol. 2008, 23, 20–25. [Google Scholar] [CrossRef]
- Godefroid, S.; Piazza, C.; Rossi, G.; Buord, S.; Stevens, A.-D.; Aguraiuja, R.; Cowell, C.; Weekley, C.W.; Vogg, G.; Iriondo, J.M.; et al. How successful are plant species reintroductions? Biol. Conserv. 2011, 144, 672–682. [Google Scholar] [CrossRef]
- Rasmussen, H.N. Terrestrial Orchids from Seed to Mycotrophic Plant; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Cribbs, P.J.; Kell, S.P.; Dixon, K.W.; Barrett, R.L. Orchid conservation: A global perspective. In Orchid Conservation; Dixon, K.L., Kell, S.P., Barrett, R.L., Cribb, P.J., Eds.; Natural History Publications: Kota Kinabalu, Borneo, 2003; pp. 1–24. [Google Scholar]
- Chase, M.W.; Cameron, K.M.; Freudenstein, J.V.; Pridgeon, A.M.; Salazar, G.; Berg, C.; Schuiteman, A. An updated classification of Orchidaceae. Bot. J. Linnean Soc. 2015, 177, 151–174. [Google Scholar] [CrossRef]
- Swarts, N.D.; Dixon, K. Terrestrial orchid conservation in the age of extinction. Ann Bot 2009, 104, 543–556. [Google Scholar] [CrossRef]
- Tremblay, R.L.; Zimmerman, J.K.; Lebrón, L.; Bayman, P.; Sastre, I.; Axelrod, F.; Alers-García, J. Host specificity and low reproductive success in the rare endemic Puerto Rican orchid Lepanthes caritensis. Biol Conserv. 1998, 85, 297–304. [Google Scholar] [CrossRef]
- Bergman, E.; Ackerman, J.D.; Thompson, J.; Zimmerman, J.K. Land-use history affects the distribution of the saprophytic orchid Wullschlaegelia calcarata in Puerto Rico’s Tabonuco forest. Biotropica 2006, 38, 492–499. [Google Scholar] [CrossRef]
- Kolanowska, M.; Kras, M.; Lipińska, M.; Mystkowska, K.; Szalchetko, D.L.; Naczk, A.M. Global warming not so harmful for all plants—response of holomycotrophic orchid species for the future climate change. Sci. Rep. 2017, 7, 12704. [Google Scholar] [CrossRef]
- Fay, M. Orchid conservation: How can we meet the challenges in the twenty-first century? Bot. Sci. 2018, 59, 16. [Google Scholar] [CrossRef]
- Kottawa-Arachchi, J.D.; Gunasekara, S.R. Research priorities and future directions in conservation of wild orchids in Sri Lanka: A review. Nat. Conserv. Res. 2020. [Google Scholar] [CrossRef]
- Arditti, J. Fundamentals of Orchid Biology; John Wiley & Sons: Hoboken, NJ, USA, 1992. [Google Scholar]
- Delcourt, P.A.; Delcourt, H.R. Paleoecological insights on conservation of biodiversity: A focus on species, ecosystems, and landscapes. Ecol. Appl. 1991, 8, 921–934. [Google Scholar] [CrossRef]
- Noss, R.F.; Platt, W.J.; Sorrie, B.A.; Weakley, A.S.; Means, D.B.; Constanza, J.; Peet, R.K. How global biodiversity hotspots may go unrecognized: Lessons from the North American coastal plain. Divers. Distrib. 2015, 21, 236–244. [Google Scholar] [CrossRef]
- Whigham, D. Conserving our native orchid heritage—The what, how and when behind the North American Orchid Conservation Center. Nativ. Orchid Conf. J. 2012, 9, 24–31. [Google Scholar]
- United States Fish and Wildlife Service (USFWS). Threatened species status for Platanthera integrilabia (white fringeless orchid). Final rule. Fed. Regist. 2016, 81, 62826–62833. [Google Scholar]
- Correll, D.S. Native Orchids of North America North of Mexico; Stanford University Press: Redwood City, CA, USA, 1978. [Google Scholar]
- Shea, M. Status Survey Report on Platanthera integrilabia; Technical report to the United States Fish and Wildlife Service: Asheville, NC, USA, 1992.
- United States Fish and Wildlife Service (USFWS). Endangered and threatened wildlife and plants; threatened species status for Platanthera integrilabia (white fringeless orchid). Fed. Regist. 2015, 80, 55304–55321. [Google Scholar]
- Bentley, S.L. Native Orchids of the Southern Appalachian Mountains; The University of North Carolina Press: Chapel Hill, NC, USA, 2000. [Google Scholar]
- NatureServe Explorer: Platanthera integrilabia. Available online: https://explorer.natureserve.org/Taxon/ELEMENT_GLOBAL.2.155927/Platanthera_integrilabia (accessed on 20 August 2020).
- Botanic Gardens Conservation International (BGCI). North American Botanic Garden Strategy for Plant Conservation, 2016–2020; Botanic Gardens Conservation International, U.S.: San Marino, CA, USA, 2016. [Google Scholar]
- Luer, C.A. The Native Orchids of the United States and Canada Excluding Florida; New York Botanical Garden: New York, NY, USA, 1975. [Google Scholar]
- Zettler, L.W.; Fairley, J.E. The status of Platanthera integrilabia, an endangered terrestrial orchid. Lindleyana 1990, 5, 212–217. [Google Scholar]
- Boyd, J.N.; Raymond, G.A.; Call, G.P.; Pistrang, M.J. Ecophysiological performance of the rare terrestrial orchid Platanthera integrilabia across contrasting habitats. Plant Ecol. 2016, 217, 1259–1272. [Google Scholar] [CrossRef]
- Zettler, L.W. Extinction in our own backyard. Amer. Orchid Soc. Bull. 1994, 63, 686–688. [Google Scholar]
- Zettler, L.W.; Ahuja, N.S.; McInnis, T.M. Insect pollination of the endangered monkey-face orchid (Platanthera integrilabia) in McMinn County, Tennessee—One last glimpse of a once common spectacle. Castanea 1996, 61, 14–24. [Google Scholar]
- Rasmussen, H.N.; Whigham, D.F. Seed ecology of dust seeds in situ: A new study technique and its application in terrestrial orchids. Am. J. Bot. 1993, 80, 1374–1378. [Google Scholar] [CrossRef]
- Currah, R.; Zettler, L.; McInnis, T. Epulorhiza inquilina sp. nov. from Platanthera (Orchidaceae) and a key to Epulorhiza species. Mycotaxon 1997, 31, 335–342. [Google Scholar]
- Zettler, L.W.; McInnis, T.M. Propagation of Platanthera integrilabia (Correll) Luer, an endangered terrestrial orchid, through symbiotic seed germination. Lindleyana 1992, 7, 154–161. [Google Scholar]
- Albrecht, M.A.; Long, Q.G. Habitat suitability and herbivores determine reintroduction success of an endangered legume. Plant Divers. 2019, 41, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Sheviak, C.J. Platanthera. In Flora of North America; Flora of North America Association: Point Arena, CA, USA, 2003; Volume 26, p. 551. [Google Scholar]
- Crabtree, T. (Tennessee Department of Wildlife and Conservation, Nashville, Tennessee, USA). Personal communication, 2018.
- Douglas, J. (Tennessee Wildlife Resources Agency, Nashville, Tennessee, USA). Personal communication, 2018.
- NatureServe Explorer: Acer rubrum var. trilobum—Nyssa sylvatica/Osmunda cinnamomea—Carex intumescens/Sphagnum lescurii Seep Forest. Available online: https://explorer.natureserve.org/Taxon/ELEMENT_GLOBAL.2.687810/Acer_rubrum_var_trilobum_-_Nyssa_sylvatica_-_Osmunda_cinnamomea_-_Carex_intumescens_-_Sphagnum_lescurii_Seep_Forest (accessed on 20 August 2020).
- NatureServe Explorer: Central Interior—Appalachian Seepage Swamp. Available online: https://explorer.natureserve.org/Taxon/ELEMENT_GLOBAL.2.833244/Acer_rubrum_-_Nyssa_sylvatica_-_Liquidambar_styraciflua_Seepage_Forest_Group (accessed on 20 August 2020).
- Benson, A.R.; Boyd, J.N. Individual- and population-level effects of Odocoileus virginianus herbivory on the rare forest herb Scutellaria montana. Global Ecol. Conserv. 2014, 1, 80–92. [Google Scholar] [CrossRef]
- Sikkema, J.J.; Boyd, J.N. Impacts of invasive nonnative plant species on the rare forest herb Scutellaria montana. Acta Oecol. 2015, 69, 182–191. [Google Scholar] [CrossRef]
- Frankland, F.; Nelson, T. Impacts of white-tailed deer on spring wildflowers in Illinois, USA. Nat. Area. J. 2003, 23, 341–348. [Google Scholar]
- Fletcher, J.D.; McShea, W.J.; Shipley, L.A.; Shumway, D. Use of common forest forbs to measure browsing pressure by white-tailed deer (Odocoileus virginianus Zimmerman) in Virginia, USA. Nat. Area. J. 2001, 21, 172–176. [Google Scholar]
- Batty, A.L.; Brundrett, M.C.; Dixon, K.W.; Sivasithamparam, K. In situ symbiotic seed germination and propagation of terrestrial orchid seedlings for establishment at field site. Aus. J. Bot. 2006, 54, 375–381. [Google Scholar] [CrossRef]
- International Union for Conservation of Nature (IUCN)/Species Survival Commission (SSC). Guidelines for Reintroductions and Other Conservation Translocations, Version 1.0; IUCN Species Survival Commission: Gland, Switzerland, 2013. [Google Scholar]
- National Oceanographic and Atmospheric Administration: National Centers for Environmental Information. Climate Data Online. Available online: https://www.ncdc.noaa.gov/cdo-web/ (accessed on 28 September 2020).
- Ye, Z.-P. A new model for relationship between irradiance and the rate of photosynthesis in Oryza sativa. Photosynthetica 2007, 45, 637–640. [Google Scholar] [CrossRef]
- Smith, Z.F.; James, E.A.; McDonnell, M.J.; McLean, C.B. Planting conditions improve translocation success of the endangered terrestrial orchid Diuris fragrantissima (Orchidaceae). Aus. J. Bot. 2009, 57, 200–209. [Google Scholar] [CrossRef]
- Wright, M.; Cross, R.; Dixon, K.; Huynh, T.; Lawrie, A.; Nesbit, L.; Pritchard, A.; Swarts, N.; Thomson, R. Propagation and reintroduction of Caladenia. Aus. J. Bot. 2009, 57, 373–387. [Google Scholar] [CrossRef]
- Brundrett, M.C. Scientific approaches to Australian temperate terrestrial orchid conservation. Aus. J. Bot. 2007, 55, 293–307. [Google Scholar] [CrossRef]
- Williams, M.; Tennessee Department of Environment and Conservation, Nashville, TN, USA. Unpublished technical report. 2000.
- Littlefield, T.; Kentucky State Nature Preserves Commission, Frankfort, KY, USA. Unpublished technical report. 2013.
- Richards, M. (Atlanta Botanical Garden, Atlanta, GA, USA). Personal communication, 2016.
- Brumback, W.E.; Cairns, S.; Sperduto, M.B.; Fyler, C.W. Response of an Isotria medeoloides population to canopy thinning. Northeast. Nat. 2011, 18, 185–196. [Google Scholar] [CrossRef]
- Willems, J.H. Population dynamics of Spiranthes spiralis in South Limburg, The Netherlands. Mém. Soc. Royale Bot. Belgium. 1989, 11, 115–121. [Google Scholar]
- Willems, J.H.; Dorland, E. Flowering frequency and plant performance and their relation to age in the perennial orchid Spiranthes spiralis (L.) Chevall. Plant Biol. 2000, 2, 344–349. [Google Scholar] [CrossRef]
- Maron, J.L.; Crone, E. Herbivory: Effects on plant abundance, distribution and population growth. Proc. R. Soc. B 2006, 273, 2575–2584. [Google Scholar] [CrossRef]
- Moreira, X.; Castagneyrol, B.; Abdala-Roberts, L.; Traveset, A. A meta-analysis of herbivory effects on plant attractiveness to pollinators. Ecology 2019, 100, e02707. [Google Scholar] [CrossRef]
- Knapp, W.M.; Wiegand, R. Orchid (Orchidaceae) decline in the Catoctin Mountains, Frederick County, Maryland as documented by a long-term dataset. Biodivers. Conserv. 2014, 23, 1965–1976. [Google Scholar] [CrossRef]
- McCoy, R. (Tennessee Division of Natural Areas, Nashville, TN, USA). Personal communication, 2019.
- Holsinger, K.E.; Wallace, L.E. Bayesian approaches for the analysis of population genetic structure: An example from Platanthera leucophaea (Orchidaceae). Mol. Ecol. 2004, 13, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Morrison, L.W.; Haack-Gaynor, J.L.; Young, C.C.; DeBacker, M.D. A 20-year record of the western prairie fringed orchid (Platanthera praeclara): Population dynamics and moderling of precipitation effects. Nat. Area. J. 2015, 35, 246–255. [Google Scholar] [CrossRef]
- Jasinge, N.U.; Huynh, T.; Lawrie, A.C. Changes in orchid populations and endophytic fungi with rainfall and prescribed burning in Pterostylis revoluta in Victoria, Australia. Ann. Bot. 2018, 121, 321–334. [Google Scholar] [CrossRef]
- McCormick, M.K.; Jacquemyn, H. What constrains the distribution of orchid populations? New Phytol. 2014, 202, 392–400. [Google Scholar] [CrossRef]
- Albrecht, M.A.; Maschinski, J. Influence of founder population size, propagule stages, and life history on the survival of reintroduced plant populations. In Plant Reintroduction in a Changing Climate: Promises and Perils; Maschinski, J., Haskins, K.E., Eds.; Island Press: Washington, DC, USA, 2012; pp. 171–188. [Google Scholar]
- Kery, M.; Gregg, K.B. Demographic analysis of dormancy and survival in the terrestrial orchid Cypripedium reginae. J. Ecol. 2004, 92, 686–695. [Google Scholar] [CrossRef]
- Palumbi, S.R. The Evolution Explosion: How Humans Cause Rapid Evolutionary Change; W.W. Norton & Company: New York, NY, USA, 2001. [Google Scholar]
- Silcock, J.L.; Simmons, C.L.; Monks, L.; Dillon, R.; Reiter, N.; Jusaitis, M.; Vesk, P.A.; Byrne, M.; Coates, D.J. Threatened plant translocation in Australia. Biol. Conserv. 2019, 236, 211–222. [Google Scholar] [CrossRef]



| 2018 | 2019 | ||||||
|---|---|---|---|---|---|---|---|
| Source of Variation | Dependent Variable | Chi-Square | df | p | Chi-Square | df | p |
| Vegetation thinning | Emerged | 0.838 | 2 | 0.658 | 6.844 | 2 | 0.033 |
| Survived | 0.425 | 2 | 0.809 | 5.034 | 2 | 0.081 | |
| Produced stems | 3.628 | 2 | 0.163 | 8.913 | 2 | 0.012 | |
| Produced buds/flowers | 0.776 | 2 | 0.679 | 3.657 | 2 | 0.161 | |
| Evidenced herbivory | 3.917 | 2 | 0.141 | 1.828 | 2 | 0.401 | |
| Herbivore access | Emerged | 4.697 | 3 | 0.195 | 20.179 | 3 | <0.001 |
| Survived | 0.186 | 3 | 0.980 | 4.541 | 3 | 0.209 | |
| Produced stems | 0.557 | 3 | 0.906 | 3.848 | 3 | 0.278 | |
| Produced buds/flowers | 1.015 | 3 | 0.798 | 1.664 | 3 | 0.645 | |
| Evidenced herbivory | 1.884 | 3 | 0.597 | 1.720 | 3 | 0.632 | |
| Dependent Variable | Year | Treatment | Sum of Squares | df | Mean Square | F | p |
|---|---|---|---|---|---|---|---|
| Amax | 2018 | Vegetation thinning | 0.304 | 2 | 0.152 | 0.338 | 0.724 |
| Herbivore access | 6.760 | 3 | 2.253 | 5.012 | 0.036 | ||
| Vegetation herbivore | 3.144 | 6 | 0.524 | 1.166 | 0.418 | ||
| 2019 | Vegetation thinning | 3.909 | 2 | 1.955 | 0.603 | 0.564 | |
| Herbivore access | 8.859 | 3 | 2.953 | 0.911 | 0.467 | ||
| Vegetation herbivore | 5.293 | 6 | 0.882 | 0.272 | 0.939 | ||
| Rd | 2018 | Vegetation thinning | 0.024 | 2 | 0.012 | 0.948 | 0.442 |
| Herbivore access | 0.018 | 3 | 0.006 | 0.457 | 0.721 | ||
| Vegetation herbivore | 0.023 | 6 | 0.004 | 0.304 | 0.916 | ||
| 2019 | Vegetation thinning | 0.322 | 2 | 0.161 | 1.928 | 0.192 | |
| Herbivore access | 0.588 | 3 | 0.196 | 2.348 | 0.129 | ||
| Vegetation herbivore | 0.688 | 6 | 0.115 | 1.375 | 0.306 | ||
| QY | 2018 | Vegetation thinning | 0.001 | 2 | <0.001 | 0.357 | 0.712 |
| Herbivore access | <0.001 | 3 | <0.001 | 0.071 | 0.973 | ||
| Vegetation herbivore | 0.001 | 6 | <0.001 | 0.101 | 0.994 | ||
| 2019 | Vegetation thinning | 0.002 | 2 | 0.001 | 1.361 | 0.296 | |
| Herbivore access | 0.004 | 3 | 0.001 | 2.314 | 0.132 | ||
| Vegetation herbivore | 0.005 | 6 | 0.001 | 1.548 | 0.251 | ||
| LCP | 2018 | Vegetation thinning | 35.206 | 2 | 17.603 | 0.457 | 0.651 |
| Herbivore access | 46.285 | 3 | 15.428 | 0.401 | 0.757 | ||
| Vegetation herbivore | 100.031 | 6 | 16.672 | 0.433 | 0.836 | ||
| 2019 | Vegetation thinning | 1877.121 | 2 | 938.561 | 1.827 | 0.206 | |
| Herbivore access | 1264.779 | 3 | 421.593 | 0.821 | 0.509 | ||
| Vegetation herbivore | 1935.189 | 6 | 322.531 | 0.628 | 0.706 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wooten, S.; Call, G.; Dattilo, A.; Cruse-Sanders, J.; Boyd, J.N. Impacts of Forest Thinning and White-Tailed Deer Herbivory on Translocation of the Rare Terrestrial Orchid Platanthera integrilabia. Diversity 2020, 12, 412. https://doi.org/10.3390/d12110412
Wooten S, Call G, Dattilo A, Cruse-Sanders J, Boyd JN. Impacts of Forest Thinning and White-Tailed Deer Herbivory on Translocation of the Rare Terrestrial Orchid Platanthera integrilabia. Diversity. 2020; 12(11):412. https://doi.org/10.3390/d12110412
Chicago/Turabian StyleWooten, Savanna, Geoff Call, Adam Dattilo, Jennifer Cruse-Sanders, and Jennifer Nagel Boyd. 2020. "Impacts of Forest Thinning and White-Tailed Deer Herbivory on Translocation of the Rare Terrestrial Orchid Platanthera integrilabia" Diversity 12, no. 11: 412. https://doi.org/10.3390/d12110412
APA StyleWooten, S., Call, G., Dattilo, A., Cruse-Sanders, J., & Boyd, J. N. (2020). Impacts of Forest Thinning and White-Tailed Deer Herbivory on Translocation of the Rare Terrestrial Orchid Platanthera integrilabia. Diversity, 12(11), 412. https://doi.org/10.3390/d12110412





