Patterns of Rotifer Diversity in the Chihuahuan Desert
Abstract
1. Introduction
2. Materials and Methods
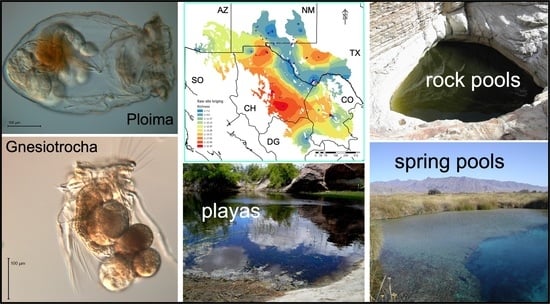
2.1. Collection Sites
2.2. Species Identification
2.3. Diversity Indices
2.4. Sampling Effort
2.5. Indicator Species Identification
2.6. Nestedness
2.7. Relationship between Species Richness and Geographic Distance
2.8. Prediction of Biodiversity Hotspots
3. Results
3.1. Species Composition
3.2. Diversity Indices
3.3. Sampling Effort
3.4. Indicator Species Identification
3.5. Nestedness
3.6. Relationship between Species Richness and Geographic Distance
3.7. Prediction of Biodiversity Hotspots
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Site Name/Location | Habitat Type | Latitude | Longitude | Species Richness | Sampling Effort |
|---|---|---|---|---|---|
| Arizona | |||||
| Triangle Pond, BANWR | spring | 31.55 | −111.533889 | 6 | 2 |
| Lake Arivaca, BANWR | lake | 31.531896 | −111.253136 | 6 | 1 |
| New Mexico | |||||
| Lazy Lagoon, BLSP | playa | 33.3541666 | −104.3417666 | 3 | 2 |
| Cottonwood Lake, BLSP | lake | 33.3388666 | −104.3340277 | 6 | 2 |
| Mirror Lake, BLSP | lake | 33.3363666 | −104.3327333 | 2 | 2 |
| Figure Eight Lake, BLSP | lake | 33.3339333 | −104.3324666 | 2 | 2 |
| Pasture Lake, BLSP | lake | 33.3310666 | −104.3295666 | 16 | 2 |
| Lea Lake, BLSP | lake | 33.3170833 | −104.3303666 | 8 | 2 |
| Elephant Butte Reservoir | lake | 33.1607361 | −107.1885194 | 2 | 2 |
| Rio Grande, Williamsburg | river | 33.10335 | −107.293983 | 12 | 2 |
| Caballo Reservoir | lake | 32.8977222 | −107.2985583 | 13 | 1 |
| Dune Pond 1, WHSA | playa | 32.7243 | −106.393367 | 1 | 1 |
| Dune Pond 3, WHSA | playa | 32.72365 | −106.394917 | 3 | 1 |
| Lost River, WHSA | stream | 32.8802 | −106.1708833 | 3 | 1 |
| Lower Lost River Pool, WHSA | stream | 32.8775333 | −106.1789333 | 1 | 1 |
| Lake Holloman | lake | 32.80745 | −106.1227833 | 6 | 1 |
| Backcountry Trailhead, WHSA | playa | 32.797 | −106.26965 | 2 | 1 |
| Garton Spring, WHSA | spring | 32.775067 | −106.145267 | 1 | 2 |
| Lake Lucero, WHSA | playa | 32.6976333 | −106.4511666 | 7 | 2 |
| Cattle Tank, WHSA | tank | 32.67485 | −106.44345 | 4 | 2 |
| Dripping Springs | spring | 32.3231888 | −106.5725138 | 6 | 2 |
| La Mancha Wetlands | river | 32.278092 | −106.828626 | 13 | 2 |
| Red Lake | lake | 32.8615027 | −104.1771791 | 2 | |
| Sitting Bull Falls, LNF | spring | 32.243666 | −104.696599 | 7 | 1 |
| Sitting Bull Falls, LNF | spring | 32.2434916 | −104.6962916 | 19 | 2 |
| Sitting Bull Falls Pool 1, LNF | spring | 32.2390333 | −104.7025333 | 19 | 1 |
| Sitting Bull Falls Pool 2, LNF | spring | 32.2385 | −104.702667 | 3 | 1 |
| Rattlesnake Spring, CAVE | spring | 32.1097 | −104.471625 | 33 | 2 |
| 404A Playa | playa | 32.0125844 | −106.523427 | 16 | |
| 404B Playa | playa | 32.022586 | −106.508957 | 17 | 1 |
| McKittrick Creek, GUMO | stream | 31.985783 | −104.769383 | 1 | 2 |
| Smith Spring, GUMO | spring | 31.9186111 | −104.806667 | 3 | 1 |
| Manzanita Spring, GUMO | spring | 31.9103194 | −104.79855 | 23 | 3 |
| Chosa Spring south side, GUMO | spring | 31.9065333 | −104.7821166 | 5 | 2 |
| Chosa Spring north side, GUMO | spring | 31.906397 | −104.782996 | 4 | 2 |
| Upper Pine Spring Pool #1, GUMO | spring | 31.9032666 | −104.81785 | 4 | 2 |
| Upper Pine Spring Pool #2, GUMO | spring | 31.9029666 | −104.81765 | 7 | 2 |
| Guadalupe Canyon Seepage 1, GUMO | spring | 31.869527 | −104.8380166 | 3 | 1 |
| Guadalupe Canyon Seepage 3, GUMO | spring | 31.8696 | −104.8377833 | 5 | 1 |
| Columbus Playa, NM | playa | 31.805433 | −107.103833 | 12 | 1 |
| NM Highway 180 | river | 32.508553 | −106.957176 | 10 | 2 |
| Rio Grande, Percha Dam | river | 32.868149 | −107.304454 | 5 | 2 |
| Rio Grande, Anthony | river | 32.005933 | −106.639733 | 9 | 3 |
| Texas | |||||
| BRH, HTSPHS | playa | 31.927081 | −106.041142 | 4 | 5 |
| Heart, HTSPHS | rock pool | 31.924848 | −106.042467 | 2 | 5 |
| Hex, HTSPHS | rock pool | 31.924734 | −106.04221 | 2 | 5 |
| Stacia, HTSPHS | rock pool | 31.924685 | −106.042592 | 1 | 5 |
| North Temp, HTSPHS | rock pool | 31.924682 | −106.042347 | 4 | 5 |
| Vero, HTSPHS | rock pool | 31.924675 | −106.042662 | 2 | 5 |
| Boo’s Pond, HTSPHS | playa | 31.9246611 | −106.045825 | 3 | 5 |
| South Temp, HTSPHS | rock pool | 31.924658 | −106.042285 | 6 | 5 |
| Cammie, HTSPHS | rock pool | 31.924642 | −106.042669 | 1 | 5 |
| Laguna Prieta, HTSPHS | playa | 31.9246388 | −106.046675 | 17 | 5 |
| Al, HTSPHS | rock pool | 31.924634 | −106.042674 | 1 | 5 |
| Walsh, HTSPHS | rock pool | 31.924628 | −106.042628 | 2 | 5 |
| Julie, HTSPHS | rock pool | 31.924622 | −106.042497 | 1 | 5 |
| Luisa, HTSPHS | rock pool | 31.924768 | −106.042617 | 1 | 5 |
| Jamie, HTSPHS | rock pool | 31.92456 | −106.042433 | 1 | 5 |
| Behind East, HTSPHS | playa | 31.919195 | −106.041106 | 13 | 5 |
| Mescalero Canyon, HTSPHS | playa | 31.9188166 | −106.040366 | 44 | 5 |
| Clammation, HTSPHS | rock pool | 31.922556 | −106.042508 | 1 | 4 |
| Shelby, HTSPHS | rock pool | 31.924622 | −106.042668 | 1 | 5 |
| Pia, HTSPHS | rock pool | 31.924544 | −106.042239 | 1 | 4 |
| Monica, HTSPHS | rock pool | 31.925051 | −106.045727 | 1 | 4 |
| Kettle 1, HTSPHS | rock pool | 31.918455 | −106.040106 | 2 | 4 |
| Kettle 2, HTSPHS | rock pool | 31.918455 | −106.040107 | 2 | 4 |
| Kettle 3, HTSPHS | rock pool | 31.918455 | −106.040101 | 2 | 4 |
| Kettle 4, HTSPHS | rock pool | 31.918446 | −106.040105 | 4 | 5 |
| Kettle 5, HTSPHS | rock pool | 31.918484 | −106.040087 | 2 | 4 |
| Behind Picnic, HTSPHS | rock pool | 31.924831 | −106.045855 | 2 | 3 |
| 1 of 4, HTSPHS | rock pool | 31.924826 | −106.045663 | 2 | 4 |
| 2 of 4, HTSPHS | rock pool | 31.92482 | −106.04567 | 1 | 4 |
| 3 of 4, HTSPHS | rock pool | 31.924813 | −106.045669 | 1 | 4 |
| 4 of 4, HTSPHS | rock pool | 31.924799 | −106.045673 | 1 | 4 |
| Abelex, HTSPHS | rock pool | 31.924624 | −106.042526 | 1 | 3 |
| Iceskating Pond, HTSHPS | playa | 31.924729 | −106.045909 | 4 | 3 |
| Rio Grande, Borderland | river | 31.8859527 | −106.5988777 | 12 | 1 |
| Crossroads Pond | lake | 31.836988 | −106.580518 | 4 | 2 |
| Keystone Heritage Park Wetland | spring | 31.8224694 | −106.5642444 | 5 | 2 |
| Rio Grande, American Dam | river | 31.786506 | −106.526992 | 15 | 3 |
| Ascarate Lake | lake | 31.7501777 | −106.4047527 | 33 | 4 |
| Ascarate Duck Pond | lake | 31.7473027 | −106.4035527 | 7 | 1 |
| Feather Lake | lake | 31.6890972 | −106.305 | 24 | 2 |
| Rio Bosque Wetland Cell 1 | tank | 31.64202 | −106.315503 | 2 | 1 |
| Rio Bosque Wetland Cell 2 | tank | 31.636467 | −106.310833 | 8 | 2 |
| Rio Grande, San Elizario | river | 31.669737 | −106.337114 | 18 | 3 |
| Rio Grande, Fort Quitman | river | 31.087533 | −105.60933 | 4 | 2 |
| Rio Grande, Presidio | river | 29.60365 | −104.45197 | 2 | 2 |
| Rio Grande, C 50 | river | 30.585217 | −104.892833 | 5 | 2 |
| Rio Grande, C 20 | river | 30.36695 | −104.8118 | 3 | 2 |
| Rio Grande, Candelaria | river | 30.133417 | −104.69 | 1 | 2 |
| Rio Grande, Guadalupe POE | river | 31.431854 | −106.148343 | 4 | 2 |
| Rio Grande, Montoya Drain | river | 31.799933 | −106.556490 | 11 | 3 |
| Montoya and Doniphan | river | 31.873037 | −106.592262 | 4 | 2 |
| Rio Grande Fabens | river | 31.430277 | −106.14222 | 18 | 2 |
| Album Park | playa | 31.783419 | −106.346349 | 5 | 3 |
| McNary Reservoir | lake | 31.2242138 | −105.7890083 | 12 | 1 |
| Diamond Y Roadside | spring | 31.0088 | −102.922533 | 13 | 2 |
| Diamond Y Spring | spring | 31.0010666 | −102.9242833 | 18 | 2 |
| East Sandia Flow | spring | 30.9910833 | −103.7286 | 10 | 2 |
| East Sandia Spring | spring | 30.9909666 | −103.7288666 | 22 | 2 |
| Balmorhea Lake | lake | 30.9663333 | −103.7134 | 5 | 2 |
| Balmorhea Main Pool | spring | 30.9445833 | −103.7876666 | 5 | 2 |
| Balmorhea Wetland 1 | spring | 30.9449166 | −103.7835 | 27 | 3 |
| Balmorhea Wetland 2 | spring | 30.945413 | −103.785982 | 5 | 2 |
| Balmorhea Canal | spring | 30.9444472 | −103.7851583 | 32 | 3 |
| Roadside Wetland | river | 30.8551333 | −105.3608833 | 17 | 1 |
| Soda Spring | spring | 30.8276388 | −105.3173055 | 10 | 1 |
| Beauty Spring B | spring | 30.8243333 | −105.3148611 | 2 | 2 |
| Stump Spring A | spring | 30.8225883 | −105.3151466 | 7 | 1 |
| Masims Spring | spring | 30.8219666 | −105.314733 | 2 | 1 |
| Dynamite Spring | spring | 30.8218833 | −105.31545 | 6 | 1 |
| Squaw Spring | spring | 30.7972166 | −105.0111833 | 2 | 2 |
| Corral Tank, IMRS | tank | 30.785263 | −104.984084 | 9 | 2 |
| Peccary Tank, IMRS | tank | 30.755556 | −105.004167 | 3 | 1 |
| Rattlesnake Tank, IMRS | tank | 30.743611 | −105.008333 | 1 | 1 |
| Red Tank, IMRS | tank | 30.7303083 | −104.9891083 | 2 | 2 |
| Miller Ranch 96 Well | spring | 30.6238533 | −104.6739988 | 9 | 2 |
| Miller Ranch 2 (Spring) | spring | 30.55025 | −104.66645 | 13 | 1 |
| Miller Ranch Glidewell | spring | 30.571483 | −104.657317 | 8 | 1 |
| Pinto Canyon Stream | stream | 30.0308666 | −104.468433 | 10 | 1 |
| Kimball Hole Miller Ranch | spring | 30.585278 | −104.626667 | 5 | 1 |
| Sanderson Canyon | rock pool | 29.8472 | −102.1837055 | 6 | 1 |
| La Mesa Canyon Tule 2 | rock pool | 29.829091 | −102.360993 | 26 | 1 |
| Rio Grande, Above Dryden | river | 29.8090277 | −102.1481138 | 1 | 1 |
| Lower Madison Falls Seep 1 | spring | 29.7967666 | −102.3779333 | 7 | 2 |
| Silber Hotspring 2 | spring | 29.76835 | −102.5635833 | 2 | 1 |
| Below Hotsprings Texas | spring | 29.7484 | −102.5406833 | 3 | 1 |
| Fuentes Ranch Shafter | stream | 29.7936833 | −104.27665 | 11 | 1 |
| Buttrill Springs, BIBE | spring | 29.54585 | −103.2738 | 6 | 2 |
| McKinney Spring 1, BIBE | spring | 29.4090166 | −103.08715 | 3 | 1 |
| Grapevine Spring, BIBE | spring | 29.4075666 | −103.19085 | 1 | 1 |
| McKinney Wall Spring, BIBE | spring | 29.407466 | −103.0885166 | 1 | 1 |
| McKinney Tinaja, BIBE | rock pool | 29.4073666 | −103.0886833 | 1 | 1 |
| Dripping Spring Cliff, BIBE | spring | 29.4066833 | −103.3103166 | 1 | 1 |
| Dripping Spring, BIBE | spring | 29.4049666 | −103.3078583 | 1 | 2 |
| Dripping Spring Upper, BIBE | spring | 29.4049491 | −103.3078470 | 1 | 1 |
| Onion Tinaja, BIBE | rock pool | 29.4014 | −103.32585 | 1 | 1 |
| Paint Gap Tank, BIBE | tank | 29.3878555 | −103.302675 | 10 | 3 |
| San Felipe Creek Del Rio | stream | 29.36985 | −100.8838166 | 1 | 1 |
| Croton Spring, BIBE | spring | 29.3446166 | −103.3471166 | 10 | 3 |
| Croton Stream, BIBE | spring | 29.3437833 | −103.3465 | 4 | 2 |
| Government Spring 2, BIBE | spring | 29.3406167 | −103.2559833 | 2 | 2 |
| Government Spring 1, BIBE | spring | 29.3405666 | −103.2560833 | 2 | 4 |
| Oak Creek, BIBE | spring | 29.2828666 | −103.3421833 | 6 | 3 |
| Window Trail Pool A, BIBE | rock pool | 29.28003 | −103.3299472 | 2 | 2 |
| Window Trail Pool B, BIBE | rock pool | 29.28003 | −103.33 | 4 | 2 |
| Window Trail Pool C, BIBE | rock pool | 29.28009 | −103.33018 | 1 | 2 |
| Window Trail Pool D, BIBE | rock pool | 29.2802 | −103.33038 | 2 | 2 |
| Window Trail Pool E, BIBE | rock pool | 29.28025 | −103.33043 | 6 | 3 |
| Window Trail Pool F, BIBE | rock pool | 29.28031 | −103.3305 | 6 | 3 |
| Window Trail Pool G, BIBE | rock pool | 29.28035 | −103.3305388 | 4 | 3 |
| Window Trail Pool H, BIBE | rock pool | 29.2804138 | −103.3305388 | 4 | 2 |
| Window Trail Pool I, BIBE | rock pool | 29.2804611 | −103.3305388 | 6 | 2 |
| Window Trail Pool Donut, BIBE | rock pool | 29.2802722 | −103.330475 | 5 | 2 |
| Carlota Tinaja, BIBE | rock pool | 29.2790833 | −103.0354166 | 1 | 1 |
| Cattail Spring A, BIBE | spring | 29.2731805 | −103.3355138 | 35 | 4 |
| Cattail Spring B, BIBE | spring | 29.2731833 | −103.33555 | 25 | 4 |
| Cattail Spring C, BIBE | spring | 29.2731833 | −103.3355861 | 17 | 4 |
| Cattail Spring C’, BIBE | spring | 29.2731833 | −103.3356305 | 9 | 3 |
| Cattail Spring C’’, BIBE | spring | 29.2731833 | −103.335675 | 8 | 3 |
| Cattail Spring C-D, BIBE | spring | 29.2731555 | −103.3357336 | 13 | 3 |
| Cattail Spring D, BIBE | spring | 29.2731527 | −103.3358277 | 17 | 4 |
| Cattail Spring E, BIBE | spring | 29.2731444 | −103.3359666 | 18 | 4 |
| Cattail Spring F, BIBE | spring | 29.2731333 | −103.3360833 | 21 | 4 |
| Cattail Spring G, BIBE | spring | 29.2731666 | −103.3361638 | 29 | 4 |
| Cattail Spring H, BIBE | spring | 29.2731694 | −103.3362388 | 23 | 4 |
| Ernst Tinaja 1, BIBE | rock pool | 29.2568666 | −103.0100833 | 6 | 3 |
| Ernst Tinaja 2, BIBE | rock pool | 29.2567416 | −103.0103583 | 5 | 3 |
| Ernst Tinaja 3, BIBE | rock pool | 29.2567415 | −103.0104 | 6 | 2 |
| Ernst Tinaja 4, BIBE | rock pool | 29.2562666 | −103.0112916 | 2 | 2 |
| Ernst Tinaja 4A, BIBE | rock pool | 29.2563611 | −103.0111083 | 6 | 2 |
| Ernst Tinaja 5, BIBE | rock pool | 29.2560416 | −103.0117361 | 8 | 3 |
| Ernst Tinaja 6, BIBE | rock pool | 29.2559972 | −103.0119166 | 6 | 3 |
| Ernst Tinaja 7, BIBE | rock pool | 29.2559944 | −103.01195 | 5 | 3 |
| Ernst Tinaja 8, BIBE | rock pool | 29.2559888 | −103.0119694 | 1 | 2 |
| Ernst Tinaja 9, BIBE | rock pool | 29.2559805 | −103.0119972 | 5 | 3 |
| Ernst Tinaja 10, BIBE | rock pool | 29.255975 | −103.0120138 | 3 | 2 |
| Ernst Tinaja Hueco, BIBE | rock pool | 29.2551 | −103.0148833 | 6 | 1 |
| Ward Spring 2, BIBE | spring | 29.24445 | −103.3505833 | 1 | 1 |
| Tule Cattle Tank, BIBE | tank | 29.2424333 | −103.4438305 | 21 | 3 |
| Tule Spring A, BIBE | spring | 29.2422833 | −103.4426666 | 6 | 3 |
| Tule Spring B, BIBE | spring | 29.24155 | −103.4428333 | 3 | 3 |
| Burro Spring, BIBE | spring | 29.2373 | −103.4259 | 14 | 3 |
| Rio Grande Village Cattail Pond, BIBE | tank | 29.189 | −102.9716166 | 28 | 3 |
| Rio Grande Village Canal, BIBE | river | 29.18615 | −102.97225 | 6 | 2 |
| Rio Grande Rio Grande Village, BIBE | river | 29.18555 | −102.979666 | 16 | 3 |
| Langford Hot Springs, BIBE | spring | 29.1794944 | −102.995466 | 3 | 2 |
| Rio Grande Village Pump House, BIBE | river | 29.17945 | −102.95325 | 16 | 2 |
| Rio Grande Village Upper Pond, BIBE | river | 29.1785472 | −102.9531833 | 30 | 4 |
| Rio Grande Village Lower Pond, BIBE | river | 29.1785166 | −102.95375 | 34 | 4 |
| Glenn Springs, BIBE | spring | 29.1744166 | −103.1575 | 21 | 3 |
| Trap Spring, BIBE | spring | 29.1636333 | −103.4194166 | 3 | 2 |
| Mule Ears Spring (Middle), BIBE | spring | 29.1624 | −103.4082666 | 2 | 1 |
| Mule Ears Spring (Lower), BIBE | spring | 29.16235 | −103.4082833 | 5 | 2 |
| Rio Grande, Santa Elena | river | 29.15415 | −103.598683 | 4 | 1 |
| Tuff Canyon Falls (wall), BIBE | rock pool | 29.15115 | −103.4855 | 2 | 1 |
| Tuff Canyon 1, BIBE | rock pool | 29.1507666 | −103.48605 | 1 | 2 |
| Tuff Canyon 3, BIBE | rock pool | 29.1507666 | −103.4859 | 2 | 2 |
| Tuff Canyon 4, BIBE | rock pool | 29.15077 | −103.4857666 | 3 | 2 |
| Tuff Canyon 5, BIBE | rock pool | 29.1509 | −103.48575 | 2 | 2 |
| Tuff Canyon 6, BIBE | rock pool | 29.15095 | −103.485389 | 1 | 1 |
| Mexico | |||||
| Presa Chihuahua | lake | 28.5762166 | −106.1711833 | 32 | 2 |
| Delicias Beisbol Field Pool | tank | 28.1648166 | −105.498500 | 6 | 1 |
| Presa Francisco Ignacio Madero | lake | 28.1626166 | −105.6321833 | 19 | 2 |
| Lago Colina | lake | 27.5724 | −105.4004666 | 43 | 2 |
| Presa de la Boquilla | lake | 27.5361333 | −105.4011333 | 23 | 2 |
| Laguna La Leche | playa | 27.2860833 | −102.9161666 | 7 | 1 |
| San Jose del Anteojo, APFFC | spring | 26.9693166 | −102.1208166 | 21 | 2 |
| Tio Julio, APFFC | spring | 26.9462833 | −102.0592 | 10 | 1 |
| Poza Tortugas, APFFC | spring | 26.93145 | −102.1247 | 27 | 3 |
| Poza Azul, APFFC | spring | 26.9226666 | −102.1226333 | 3 | 2 |
| Rio Mesquites, APFFC | river | 26.9222222 | −102.1083333 | 8 | 2 |
| Poza Marcelo, APFFC | spring | 26.9104 | −102.0363166 | 6 | 2 |
| Las Playitas, APFFC | spring | 26.9085166 | −102.01745 | 7 | 2 |
| Los Gatos, APFFC | spring | 26.88875 | −101.9980333 | 14 | 2 |
| Poza la Becerra, APFFC | spring | 26.8784166 | −102.1377666 | 13 | 2 |
| Los Hundidos Main pool, APFFC | spring | 26.8711666 | −102.0204166 | 13 | 2 |
| La Campana, APFFC | spring | 26.8683666 | −102.0278333 | 3 | 1 |
| Poza El Arco B, APFFC | spring | 26.8683333 | −102.0228 | 6 | 1 |
| Poza Churince, APFFC | spring | 26.8404166 | −102.1342333 | 15 | 3 |
| Ejido El Venado Entrance, APFFC | spring | 26.9146333 | −102.047 | 14 | 1 |
| Ejido El Venado Grande, APFFC | spring | 26.8199 | −101.904833 | 1 | 1 |
| Ejido El Venado A, APFFC | spring | 26.8194666 | −101.9053166 | 7 | 1 |
| Presa Francisco Zarco Durango | lake | 25.2693055 | −103.7727222 | 2 | 1 |
| Ojos Altos A | spring | 31.40685 | −107.6181833 | 1 | 3 |
| Ojos Altos B | spring | 31.4068 | −107.6179666 | 1 | 2 |
| Ojos Altos C | spring | 31.4035166 | −107.616 | 12 | 3 |
| Ojos Altos D | spring | 31.4032666 | −107.6163 | 9 | 3 |
| Ojo de la Punta, ANPMS | spring | 31.3859166 | −106.6022666 | 32 | 4 |
| Ojo de en Medio ANPMS | spring | 31.37885 | −106.5877833 | 26 | 3 |
| Ojo de la Casa ANPMS | spring | 31.3656166 | −106.5322333 | 21 | 3 |
| DunasCampestre ANPMS | spring | 31.335967 | −106.491333 | 8 | 3 |
| El Huerfano ANPMS | spring | 31.294817 | −106.511017 | 10 | 3 |
| Ojo de Santa Maria | spring | 31.1552777 | −107.3172222 | 22 | 2 |
| Upper Mexican Hotsprings | spring | 29.7460833 | −102.5455666 | 11 | 2 |
References
- Emerson, B.C.; Gillespie, R.G. Phylogenetic analysis of community assembly and structure over space and time. TREE 2008, 23, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Leibold, M.A.; Economo, E.P.; Peres-Neto, P.R. Metacommunity phylogenetics: Separating the roles of environmental filters and historical biogeography. Ecol. Lett. 2010, 13, 1290–1299. [Google Scholar] [CrossRef] [PubMed]
- Guisan, A.; Rahbek, C. SESAM –A new framework integrating macroecological and species distribution models for predicting spatio-temporal patterns of species assemblages. J. Biogeogr. 2011, 38, 1433–1444. [Google Scholar] [CrossRef]
- Fontaneto, D. Long-distance passive dispersal in microscopic aquatic animals. Mov. Ecol. 2019, 7, 10. [Google Scholar] [CrossRef]
- Fenchel, T.; Finlay, B.J. The ubiquity of small species: Patterns of local and global diversity. Bioscience 2004, 54, 777. [Google Scholar] [CrossRef]
- Velasco-Castrillón, A.; Page, T.J.; Gibson, J.A.E.; Stevens, M.I. Surprisingly high levels of biodiversity and endemism amongst Antarctic rotifers uncovered with mitochondrial DNA. Biodiversity 2014, 15, 130–142. [Google Scholar] [CrossRef]
- Segers, H.; Shiel, R.J. Microfaunal diversity in a biodiversity hotspot: New rotifers from southwestern Australia. Zool. Stud. 2003, 42, 516–521. [Google Scholar]
- Dumont, H.J. Biogeography of rotifers. Hydrobiologia 1983, 104, 19–30. [Google Scholar] [CrossRef]
- Segers, H. A biogeographical analysis of rotifers of the genus Trichocerca Lamarck, 1801 (Trichocercidae, Monogononta, Rotifera), with notes on taxonomy. Hydrobiologia 2003, 500, 103–114. [Google Scholar] [CrossRef]
- Ning, N.; Gawne, B.; Cook, R.A.; Ielsen, D.L.N. Zooplankton dynamics in response to the transition from drought to flooding in four Murray–Darling Basin rivers affected by differing levels of flow regulation. Hydrobiologia 2012, 702, 45–62. [Google Scholar] [CrossRef]
- Vanschoenwinkel, B.; Waterkeyn, A.; Nhiwatiwa, T.; Pinceel, T.; Spooren, E.; Geerts, A.; Clegg, B.; Brendonck, L. Passive external transport of freshwater invertebrates by elephant and other mud-wallowing mammals in an African savannah habitat. Freshw. Biol. 2011, 56, 1606–1619. [Google Scholar] [CrossRef]
- Frisch, D.; Green, A.J.; Figuerola, J.; Green, A.J. High dispersal capacity of a broad spectrum of aquatic invertebrates via waterbirds. Aquat. Sci. 2007, 69, 568–574. [Google Scholar] [CrossRef]
- Rivas, J.A.; Mohl, J.E.; Van Pelt, R.S.; Leung, M.-Y.; Wallace, R.L.; Gill, T.E.; Walsh, E.J. Evidence for regional aeolian transport of freshwater micrometazoans in arid regions. Limnol. Oceanogr. Lett. 2018, 3, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Rivas, J.A.; Schröder, T.; Gill, T.E.; Wallace, R.L.; Walsh, E.J. Anemochory of diapausing stages of microinvertebrates in North American drylands. Freshw. Biol. 2019, 64, 1303–1314. [Google Scholar] [CrossRef]
- Rousselet, C.F. On the geographic distribution of the Rotifera. J. Quekett Microsc. Cl. 1909, 2, 465–470. [Google Scholar]
- Fontaneto, D.; Barraclough, T.G.; Chen, K.; Ricci, C.; Herniou, E.A. Molecular evidence for broad-scale distributions in bdelloid rotifers: Everything is not everywhere but most things are very widespread. Mol. Ecol. 2008, 17, 3136–3146. [Google Scholar] [CrossRef]
- Segers, H. Global diversity of rotifers (Rotifera) in freshwater. Hydrobiologia 2008, 198, 49–59. [Google Scholar]
- Segers, H.; De Smet, W.H. Diversity and endemism in Rotifera: A review, and Keratella Bory de St Vincent. Biodiver. Conservation 2008, 17, 303–316. [Google Scholar] [CrossRef]
- Kuczyńska-Kippen, N.; Basińska, A. Habitat as the most important influencing factor for the rotifer community structure at landscape level. Int. Rev. Hydrobiol. 2014, 99, 58–64. [Google Scholar] [CrossRef]
- Walsh, E.J.; Smith, H.A.; Wallace, R.L. Rotifers of temporary waters. Int. Rev. Hydrobiol. 2014, 99, 3–19. [Google Scholar] [CrossRef]
- Murphy, A.L.; Pavlova, A.; Thompson, R.; Davis, J.; Sunnucks, P. Swimming through sand: Connectivity of aquatic fauna in deserts. Ecol. Evol. 2015, 5, 5252–5264. [Google Scholar] [CrossRef] [PubMed]
- Sada, D.W.; Fleishman, E.; Murphy, D.D. Associations among spring-dependent aquatic assemblages and environmental and land use gradients in a Mojave Desert mountain range. Divers. Distrib. 2005, 11, 91–99. [Google Scholar] [CrossRef]
- Hendrickson, J.; Johnston, M.C. Vegetation and Community Types of the Chihuahuan Desert. In Second Symposium of Resources of the Chihuahuan Desert Region: U.S. and Mexico; Chihuahuan Desert Research Institute, Sul Ross State University: Alpine, TX, USA, 1986; Volume II, pp. 20–39. [Google Scholar]
- Olsen, D.M.; Dinerstein, E. The Global 200: A representation approach to conserving the Earth’s most biologically valuable ecoregions. Cons. Biol. 1998, 12, 502–515. [Google Scholar] [CrossRef]
- Dinerstein, E.; Olsen, D.; Atchley, J.; Loucks, C.; Contreras-Balderas, S.; Abell, R.; Inigo, E.; Enkerlin, E.; Williams, C.; Castilleja, G. Ecoregion-Based Conservation in the Chihuahuan Desert: A Biological Assessment; World Wildlife Fund: Washington, DC, USA, 2000; p. 318. [Google Scholar]
- Minckley, W.L. Environments of the Bolsón of Cuatro Ciénegas, Coahuila, Mexico, with Special Reference to the Aquatic Biota; Texas Western Press: El Paso, TX, USA, 1969; p. 65. [Google Scholar]
- Minckley, W.L. Endemic fishes of the Cuatro Ciénegas Basin, Northern Coahuila, Mexico. In Symposium on the Biological Resources of the Chihuahuan Desert Region, United States and Mexico; Wauer, R.H., Riskind, D.H., Eds.; U.S. National Park Service Transactions and Proceedings Series 3; Government Printing Office: Washington, DC, USA, 1978; pp. 383–404. [Google Scholar]
- Hershler, R. Systematic revision of the Hydrobiid snails (Gastropoda: Rissoacea) of the Cuatro Ciénegas Basin, Coahuila, Mexico. Malacologia 1985, 26, 31–123. [Google Scholar]
- Hershler, R.; Liu, H.-P.; Mulvey, M. Phylogenetic relationships within the aquatic snail genus tryonia: Implications for biogeography of the North American Southwest. Mol. Phylogenetics Evol. 1999, 13, 377–391. [Google Scholar] [CrossRef]
- Taylor, D.W. A remarkable snail fauna from Coahuila, México. The Veliger 1966, 9, 152–228. [Google Scholar]
- Stanislawczyk, K.; Walters, A.D.; Haan, T.J.; Sei, M.; Lang, B.K.; Berg, D.J. Variation among macroinvertebrate communities suggests the importance of conserving desert springs. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 944–953. [Google Scholar] [CrossRef]
- Hershler, R.; Liu, H.P.; Landye, J. New species and records of springsnails (Caenogastropods: Cochliopidae: Tryonia) from the Chihuahuan Desert (Mexico and United States), an imperiled biodiversity hotspot. Zootaxa 2011, 3001, 1–32. [Google Scholar] [CrossRef]
- Ripley, B.J.; Simovich, M.A. Species richness on islands in time: Variation in ephemeral pond crustacean communities in relation to habitat duration and size. Hydrobiologia 2008, 617, 181–196. [Google Scholar] [CrossRef]
- Kneitel, J.M. Inundation timing, more than duration, affects the community structure of California vernal pool mesocosms. Hydrobiology 2014, 732, 71–83. [Google Scholar] [CrossRef]
- Serrano, L.; Fahd, K. Zooplankton communities across a hydroperiod gradient of temporary ponds in the Doñana National Park (SW Spain). Wetlands 2005, 25, 101–111. [Google Scholar] [CrossRef]
- Aridland Springs in North America: Ecology and Conservation; Stevens, L.E., Meretsky, V.J., Eds.; The University of Arizona Press: Tucson, AZ, USA, 2008; p. 406. [Google Scholar]
- Hurt, C.; Hedrick, P.W. Conservation genetics in aquatic species: General approaches and case studies in fishes and springsnails of arid lands. Aquat. Sci. 2004, 66, 402–413. [Google Scholar] [CrossRef]
- Carson, E.W.; Dowling, T.E. Influence of hydrogeographic history and hybridization on the distribution of genetic variation in the pupfishes Cyprinodon atrorus and C. bifasciatus. Mol. Ecol. 2005, 15, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Kodric-Brown, A.; Brown, J.H. Native fishes, exotic mammals, and the conservation of desert springs. Front. Ecol. Environ. 2007, 5, 549–553. [Google Scholar] [CrossRef]
- Tobler, M.; Carson, E.W. Environmental variation, hybridization, and phenotypic diversification in Cuatro Ciénegas pupfishes. J. Evol. Biol. 2010, 23, 1475–1489. [Google Scholar] [CrossRef]
- Hershler, R.; Mulvey, M.; Liu, H.-P. Genetic variation in the Desert Springsnail (Tryonia porrecta): Implications for reproductive mode and dispersal. Mol. Ecol. 2005, 14, 1755–1765. [Google Scholar] [CrossRef]
- Hershler, R.; Liu, H.-P.; Lang, B.K. Genetic and morphologic variation of the Pecos assiminea, an endangered mollusk of the Rio Grande region, United States and Mexico (Caenogastropoda: Rissooidea: Assimineidae). Hydrobiologia 2007, 579, 317–335. [Google Scholar] [CrossRef][Green Version]
- Hershler, R.; Liu, H.-P.; Stockwell, E.A. A new genus and species of aquatic gastropods (Rissooidea: Hydrobiidae) from the North American Southwest: Phylogenetics relationships and biogeography. Proc. Biol. Soc. Wash. 2002, 115, 171–188. [Google Scholar]
- Moline, A.B.; Shuster, S.M.; Hendrickson, D.A.; Marks, J.C. Genetic variation in a desert aquatic snail (Nymphophilus minckleyi) from Cuatro Ciénegas, Coahuila, Mexico. Hydrobiology 2004, 522, 179–192. [Google Scholar] [CrossRef]
- Johnson, S.G. Age, phylogeography and population structure of the microendemic banded spring snail, Mexipyrgus churinceanus. Mol. Ecol. 2005, 14, 2299–2311. [Google Scholar] [CrossRef]
- Gervasio, V.; Berg, D.J.; Lang, B.K.; Allan, N.L.; Guttman, S.I. Genetic diversity in the Gammarus pecos species complex: Implications for conservation and regional biogeography in the Chihuahuan Desert. Limnol. Oceanogr. 2004, 49, 520–531. [Google Scholar] [CrossRef]
- Adams, N.E.; Inoue, K.; Seidel, R.A.; Lang, B.K.; Berg, D.J. Isolation drives increased diversification rates in freshwater amphipods. Mol. Phylogenetics Evol. 2018, 127, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Cottenie, K.; Michels, E.; Nuytten, N.; De Meester, L. Zooplankton Metacommunity structure: Regional vs. local processes in highly interconnected ponds. Ecology 2003, 84, 991–1000. [Google Scholar] [CrossRef]
- Mouquet, N.; Loreau, M. Community patterns in source-sink metacommunities. Am. Nat. 2003, 162, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Ricklefs, R.E. A comprehensive framework for global patterns in biodiversity. Ecol. Lett. 2004, 7, 1–15. [Google Scholar] [CrossRef]
- Jocque, M.; Riddoch, B.J.; Brendonck, L. Successional phases and species replacements in freshwater rock pools: Towards a biological definition of ephemeral systems. Freshw. Biol. 2007, 52, 1734–1744. [Google Scholar] [CrossRef]
- Jocque, M.; Graham, T.; Brendonck, L. Local structuring factors of invertebrate communities in ephemeral freshwater rock pools and the influence of more permanent water bodies in the region. Hydrobiology 2007, 592, 271–280. [Google Scholar] [CrossRef]
- Wallace, R.L.; Walsh, E.J.; Arroyo, M.; Starkweather, P.L. Life on the edge: Rotifers from springs and ephemeral waters in the Chihuahuan Desert, Big Bend National Park (Texas, USA). Hydrobiology 2005, 546, 147–157. [Google Scholar] [CrossRef]
- Walsh, E.J.; Schroder, T.; Arroyo, M.L.; Wallace, R.L. How well do single samples reflect rotifer species diversity? A test based on interannual variation of rotifer communities in Big Bend National Park (Texas, USA). Hydrobiology 2007, 593, 39–47. [Google Scholar] [CrossRef]
- Walsh, E.J.; Schröder, T.; Wallace, R.L.; Ríos-Arana, J.V.; Rico-Martínez, R. Rotifers from selected inland saline waters in the Chihuahuan Desert of México. Saline Syst. 2008, 4, 7. [Google Scholar] [CrossRef]
- Walsh, E.J.; Schröder, T.; Wallace, R.; Rico-Martínez, R. Cryptic speciation in Lecane bulla (Monogononta: Rotifera) in Chihuahuan Desert waters. SIL Proc. 1922–2010 2009, 30, 1046–1050. [Google Scholar] [CrossRef]
- Wallace, R.L.; Walsh, E.J.; Schröder, T.; Rico-Martínez, R.; Ríos-Arana, J.V. Species composition and distribution of rotifers in Chihuahuan Desert waters of México: Is everything everywhere? SIL Proc. 1922–2010 2008, 30, 73–76. [Google Scholar] [CrossRef]
- Örstan, A. A new species of bdelloid rotifer from Sonora, Mexico. Southwest. Nat. 1995, 40, 255–258. [Google Scholar]
- Mackay, W.P.; Loring, S.J.; Frost, T.M.; Whitford, W.G. Population dynamics of a playa community in the Chihuahuan Desert. Southwest. Nat. 1990, 35, 393. [Google Scholar] [CrossRef]
- Kubly, D.M. Aquatic invertebrates in desert mountain rock pools: The White Tank Mountains, Maricopa County, Arizona. Limnology Aquat. Biol. Southwest 1992, 2, 55–69. [Google Scholar]
- Rico-Martínez, R.; Silva-Briano, M. Contribution to the knowledge of the rotifera of Mexico. Hydrobiologia 1993, 255/256, 467–474. [Google Scholar] [CrossRef]
- Sarma, S.S.S. Checklist of rotifers (Rotifera) from Mexico. Environ. Ecol. 1999, 17, 978–983. [Google Scholar]
- Sarma, S.S.S.; Elías-Gutiérrez, M. Rotifers from Mexico: New records in high altitude ponds. Southwest Nat. 2000, 45, 366. [Google Scholar] [CrossRef]
- Hart, C.M.; González, M.R.; Simpson, E.P.; Hurlbert, S.H. Salinity and fish effects on Salton Sea microecosystems: Zooplankton and nekton. Hydrobiology 1998, 381, 129–152. [Google Scholar] [CrossRef]
- Kuperman, B.I.; Matey, V.E.; Dexter, D.M.; Tiffany, M.A. Invertebrates of the Salton Sea: A scanning electron microscopy portfolio. Hydrobiology 2002, 473, 203–216. [Google Scholar] [CrossRef]
- Riedel, R.; Costa-Pierce, B.A. Feeding ecology of Salton Sea Tilapia (Oreochromis spp.). Bull. South Calif. Acad. Sci. 2005, 104, 26–36. [Google Scholar] [CrossRef]
- Tiffany, M.A.; Swan, B.K.; Watts, J.M.; Hurlbert, S.H. Metazooplankton dynamics in the Salton Sea, California, 1997–1999. Salton Sea 2002, 473, 103–120. [Google Scholar] [CrossRef]
- Walker, B.W. (Ed.) The Ecology of the Salton Sea, California, in Relation to the Sportfishery; Fish Bulletin 1961, No. 113: 199–204; The Resources Agency of California, Department of Fish and Game: Sacramento, CA, USA, 1961.
- De Ridder, M. Rotifers from Algeria. J. Afr. Zool. 1991, 105, 473–483. [Google Scholar]
- Furst, D.; Aldridge, K.; Shiel, R.; Ganf, G.; Mills, S.; Brookes, J. Floodplain connectivity facilitates significant export of zooplankton to the main River Murray channel during a flood event. Inland Waters 2014, 4, 413–424. [Google Scholar] [CrossRef]
- Segers, H.; Shiel, R.J. Diversity of cryptic Metazoa in Australian freshwaters: A new genus and two new species of sessile rotifer (Rotifera, Monogononta, Gnesiotrocha, Flosculariidae). Zootaxa 2008, 1750, 19–31. [Google Scholar] [CrossRef]
- Koste, W.; Shiel, R.J.; Brock, M.A. Rotifera from Western Australian wetlands with descriptions of two new species. Hydrobiologia 1983, 104, 9–17. [Google Scholar] [CrossRef]
- Shiel, R.J.; Koste, W. Rotifera from Australian inland waters. VIII. Trichocercidae (Monogononta). Trans. R. Soc. S. Aust. 1992, 116, 1–27. [Google Scholar]
- Koste, W.; Shiel, R.J. Rotifera from Australian inland waters. VII. Notommatidae (Rotifera: Monogononta). Trans. R. Soc. S. Aust. 1991, 115, 111–159. [Google Scholar]
- Koste, W.; Shiel, R.J. Rotifera from Australian inland waters. VI. Proalidae, Lindiidae (Rotifera: Monogononta). Trans. R. Soc. S. Aust. 1990, 114, 129–143. [Google Scholar]
- Koste, W.; Shiel, R.J. Rotifera from Australian inland waters. V. Lecanidae (Rotifera: Monogononta). Trans. R. Soc. S. Aust. 1990, 114, 1–36. [Google Scholar]
- Shiel, R.J.; Koste, W. Rotifera from Australian inland waters. IX. Gastropodiae, Synchaetidae, Asplanchnidae (Rotifera: Monogononta). Trans. R. Soc. S. Aust. 1993, 117, 111–139. [Google Scholar]
- Koste, W.; Shiel, R.J. Rotifera from Australian inland waters. IV. Colurellidae (Rotifera: Monogononta). Trans. R. Soc. S. Aust. 1989, 113, 119–143. [Google Scholar]
- Koste, W.; Shiel, R.J. Rotifera from Australian inland waters. III. Euchlanidae, Mytilinidae and Trichotriidae (Rotifera: Monogononta). Trans. R. Soc. S. Aust. 1989, 113, 85–114. [Google Scholar]
- Koste, W.; Shiel, R. Rotifera from Australian inland waters. II. Epiphanidae and Brachionidae (Rotifera: Monogononta). Invertebr. Syst. 1987, 1, 949–1021. [Google Scholar] [CrossRef]
- Koste, W.; Shiel, R. Rotifera from Australian inland waters. I. Bdelloidea (Rotifera: Digononta). Mar. Freshw. Res. 1986, 37, 765–792. [Google Scholar] [CrossRef]
- Brain, C.K.; Shiel, R.J. Rotifers of the Kalahari Gemsbok National Park, South Africa. Hydrobiology 1995, 313, 319–324. [Google Scholar] [CrossRef]
- Brain, C.K.; Koste, W. Rotifers of the genus Proales from saline springs in the Namib desert, with the description of a new species. Hydrobiologia 1993, 255/256, 449–454. [Google Scholar] [CrossRef]
- Segers, H.; Dumont, H.J. Rotifera from Arabia, with descriptions of two new species. Fauna Saudi Arab. 1993, 13, 3–26. [Google Scholar]
- Mazuelos, N.; Toja, J.; Guisande, C. Rotifers in ephemeral ponds of Doñana National Park. Hydrobilogia 1993, 255/256, 429–434. [Google Scholar] [CrossRef]
- Dumont, H.J.; Coussement, M. Rotifers from Rio de Oro (North-Western Sahara). Hydrobiologia 1976, 51, 109–112. [Google Scholar] [CrossRef]
- Jersabek, C.D.; Bolortsetseg, E. Mongolian rotifers (Rotifera, Monogononta)–Checklist with annotations on global distribution and autecology. Proc. Acad. Nat. Sci. Phila 2010, 159, 119–168. [Google Scholar] [CrossRef]
- Walsh, E.J.; Arroyo, M.L.; Schröder, T.; Wallace, R.L. Species richness and species turnover (complementarity) of Rotifera in selected aquatic systems of Big Bend National Park, Texas. In Proceedings of the Sixth Symposium on the Natural Resources of the Chihuahuan Desert Region, Fort Davis, TX, USA, 14–17 October 2004; pp. 185–204. [Google Scholar]
- Schröder, T.; Howard, S.; Arroyo, M.L.; Walsh, E.J. Sexual reproduction and diapause of Hexarthra sp. (Rotifera) in short-lived ponds in the Chihuahuan Desert. Freshw. Biol. 2007, 52, 1033–1042. [Google Scholar] [CrossRef]
- Ríos-Arana, J.V.; Agüero-Reyes, L.D.C.; Wallace, R.L.; Walsh, E.J. Limnological characteristics and rotifer community composition of Northern Mexico Chihuahuan Desert Springs. J. Arid. Environ. 2019, 160, 32–41. [Google Scholar] [CrossRef]
- Donner, J. Ordnung Bdelloidea (Rotatoria, Rädertiere); Akademie-Verlag: Berlin, Germany, 1965; p. 297. [Google Scholar]
- Ricci, C.; Melone, G. Key to the identification of the genera of bdelloid rotifers. Hydrobiologia 2000, 418, 73–80. [Google Scholar] [CrossRef]
- Edmondson, W.T. Rotifera. In Freshwater Biology, 2nd ed.; Edmondson, W.T., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1959; pp. 420–494. [Google Scholar]
- Edmondson, W.T. A Formula Key to the Rotatorian Genus Ptygura. Trans. Am. Microsc. Soc. 1949, 68, 127. [Google Scholar] [CrossRef]
- Bērziņš, B. On the collothecacean Rotatoria with special reference to the species found in the Aneboda district, Sweden. Arkiv Zoologi 1951, 1, 565–592. [Google Scholar]
- Elliott, J.M.; Ruttner-Kolisko, A. Plankton Rotifers: Biology and taxonomy. J. Anim. Ecol. 1976, 45, 617. [Google Scholar] [CrossRef]
- Koste, W. Rotatoria. In Die Rädertiere Mitteleuropas; 2 volumes; Gebrüder Borntraeger: Stuttgart, Germany, 1978. [Google Scholar]
- Stemberger, R.S. A Guide to Rotifers of the Laurentian Great Lakes; US Environmental Protection Agency: Cincinnati, OH, USA; National Technical Information Service (PB80-101280): Springfield, VA, USA, 1979.
- Wallace, R.L.; Snell, T.W.; Ricci, C.; Nogrady, T. Rotifera. Biology, Ecology and Systematics, 2nd ed.; Backhuys Publishers: Leiden, The Netherlands, 2006; Volume 1, p. 299. [Google Scholar]
- Rotifera: Asplanchnidae, Gastropodidae, Lindiidae, Microcodidae, Synchaetidae, Trochosphaeridae and Filinia; Nogrady, T., Segers, H., Eds.; SPB Academic Publishers BV: The Hague, The Netherlands, 2002; Volume 6, p. 264. [Google Scholar]
- De Smet, W.H.; Pourriot, R. Rotifera: The Dicranophoridae (Monogononta) and: The Ituridae (Monogononta); SPB Academic Publishing: Amsterdam, The Netherlands, 1997; Volume 5, p. 344. [Google Scholar]
- De Smet, W.H. Rotifera: The Proalidae (Monogononta); SPB Academic Publishing BV: Amsterdam, The Netherlands, 1996; Volume 4, p. 102. [Google Scholar]
- Nogrady, T.; Pourriot, R.; Segers, H. Rotifera: The Notommatidae and: The Scaridiidae; SPB Academic Publishing: The Hague, The Netherlands, 1995; Volume 3, p. 248. [Google Scholar]
- Segers, H. Rotifera: The Lecanidae (Monogononta); SPB Academic Publishing BV: Amsterdam, The Netherlands, 1995; Volume 2, p. 226. [Google Scholar]
- Segers, H.H. A reappraisal of the Scaridiidae (Rotifera, Monogononta). Zoologica Scripta 1995, 24, 91–100. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monographs 1997, 67, 345–366. [Google Scholar] [CrossRef]
- De Caceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Atmar, W.; Patterson, B.D. The measure of order and disorder in the distribution of species in fragmented habitat. Oecologia 1993, 96, 373–382. [Google Scholar] [CrossRef]
- Almeida-Neto, M.; Guimarães, P.; Guimarães, P.R., Jr.; Loyola, R.D.; Ulrich, W. A consistent metric for nestedness analysis in ecological systems: Reconciling concept and measurement. Oikos 2008, 117, 1227–1239. [Google Scholar] [CrossRef]
- Guimarães, P.R., Jr.; Guimarães, P. Improving the analyses of nestedness for large sets of matrices. Environ. Model. Softw. 2006, 21, 1512–1513. [Google Scholar] [CrossRef]
- Ulrich, W.; Almeida-Neto, M.; Gotelli, N.J. A consumer’s guide to nestedness analysis. Oikos 2009, 118, 3–17. [Google Scholar] [CrossRef]
- Hijmans, R.J. Introduction to the ”Geosphere” Package (Version 1.5-10). 2019. Available online: https://rdrr.io/cran/geosphere/ (accessed on 1 July 2020).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: An Introduction to Ordination. Community Ecology Package. Available online: https://rdrr.io/cran/vegan/ (accessed on 1 July 2020).
- Krivoruchko, K. Empirical Bayesian Kriging. Esri: Redlands, CA. 2012. Available online: http://www.esri.com/news/arcuser/1012/empirical-byesian-kriging.html (accessed on 4 August 2020).
- Segers, H. Annotated checklist of the rotifers (Phylum Rotifera), with notes on nomenclature, taxonomy and distribution. Zootaxa 2007, 1564, 1–104. [Google Scholar] [CrossRef]
- Shiel, R.J.; Koste, W. Rotifer communities of billabongs in northern and south-eastern Australia. Hydrobiologia 1983, 104, 41–47. [Google Scholar] [CrossRef]
- Kuczyńska-Kippen, N.; Ejsmont-Karabin, J. Rotifera of various aquatic environments of Costa Rica in reference to Central American rotifer fauna. Turk. J. Zool. 2020, 44. [Google Scholar] [CrossRef]
- Sharma, B.K.; Dudani, V.K. Rotifers from some tropical ponds in Bihar: Species composition, similarities and trophic indicators. J. Indian Inst. Sci. 1992, 72, 121–130. [Google Scholar]
- Duggan, I.C.; Green, J.D.; Shiel, R.J. Distribution of rotifer assemblages in North Island, New Zealand, lakes: Relationships to environmental and historical factors. Freshw. Biol. 2002, 47, 195–206. [Google Scholar] [CrossRef]
- Kaya, M.; Fontaneto, D.; Segers, H.; Altindağ, A. Temperature and salinity as interacting drivers of species richness of planktonic rotifers in Turkish continental waters. J. Limnol. 2010, 69, 297–304. [Google Scholar] [CrossRef]
- Hansson, L.-A.; Hylander, S.; Dartnall, H.J.G.; Lidström, S.; Svensson, J.-E. High zooplankton diversity in the extreme environments of the McMurdo Dry Valley lakes, Antarctica. Antarct. Sci. 2011, 24, 131–138. [Google Scholar] [CrossRef]
- De Smet, W.H.; Beyens, L. Rotifers from the Canadian High Arctic (Devon Island, Northwest Territories). Hydrobiologia 1995, 313/314, 29–34. [Google Scholar] [CrossRef]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’Amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial ecoregions of the world: A new map of life on Earth. Bioscience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- Paskevich, V. U.S. STATE_BOUNDS.SHP: Internal US State Boundaries. Open-File Report 2005-1071; Woods Hole Science Center: Woods Hole, MA, USA, 2020. [Google Scholar]
- Špoljar, M.; Dražina, T.; Lajtner, J.; Duić Sertić, M.; Radanović, I.; Wallace, R.L.; Matulić, D.; Tomljanović, T. Zooplankton assemblage in four temperate shallow waterbodies in association with habitat heterogeneity and alternative states. Limnologica 2018, 71, 51–61. [Google Scholar] [CrossRef]
- Vasseur, D.A.; Fox, J.W.; Gonzalez, A.; Adrian, R.; Beisner, B.E.; Helmus, M.R.; Johnson, C.; Kratina, P.; Kremer, C.; de Mazancourt, C.; et al. Synchronous dynamics of zooplankton competitors prevail in temperate lake ecosystems. Proc. R. Soc. B. 2014, 281, 20140633. [Google Scholar] [CrossRef]
- Beach, N.W. A study of the planktonic rotifers of the Ocqueoc River system, Presque Isle County, Michigan. Ecol. Monogr. 1960, 30, 339–358. [Google Scholar] [CrossRef]
- May, L.; Wallace, R.L. An examination of long-term ecological studies of rotifers: Comparability of methods and results, insights into drivers of change and future research challenges. Hydrobiologia 2019, 844, 129–147. [Google Scholar] [CrossRef]
- Matthews, D.; Effler, S.; Prestigiacomo, A.; O’Donnell, S. Trophic state responses of Onondaga Lake, New York to reductions in phosphorus loading from advanced wastewater treatment. Inland Waters 2015, 5, 125–138. [Google Scholar] [CrossRef]
- Hampton, S.E.; Scheuerell, M.D.; Schindler, D.E. Coalescence in the Lake Washington story: Interaction strengths in a planktonic food web. Limnol. Oceanogr. 2006, 51, 2042–2051. [Google Scholar] [CrossRef]
- Hampton, S.E. Increased niche differentiation between two Conochilus species over 33 years of climate change and food web alteration. Limnol. Oceanogr. 2005, 50, 421–426. [Google Scholar] [CrossRef]
- Hampton, S.E.; Izmest’Eva, L.R.; Moore, M.V.; Katz, S.L.; Dennis, B.; Silow, E.A. Sixty years of environmental change in the world’s largest freshwater lake - Lake Baikal, Siberia. Glob. Chang. Biol. 2008, 14, 1947–1958. [Google Scholar] [CrossRef]
- Herzig, A. The analysis of planktonic rotifer populations: A plea for long-term investigations. Hydrobiologia 1987, 147, 163–180. [Google Scholar] [CrossRef]
- Molinero, J.C.; Anneville, O.; Souissi, S.; Balvay, G.; Gerdeaux, D. Anthropogenic and climate forcing on the long-term changes of planktonic rotifers in Lake Geneva, Europe. J. Plankton Res. 2005, 28, 287–296. [Google Scholar] [CrossRef]
- Duggan, I.C.; Green, J.D.; Shiel, R.J. Distribution of rotifers in North Island, New Zealand, and their potential use as bioindicators of lake trophic state. Hydrobiologia 2001, 446/447, 155–164. [Google Scholar] [CrossRef]
- Duggan, I.C.; Barnes, G. Assessment of Trophic State Change in Selected Lakes of the Auckland Region Based on Rotifer Assemblages; Centre for Biodiversity and Ecology Research, University of Waikato: Hamilton, New Zealand, 2005; p. 31. [Google Scholar]
- Duggan, I.C. An Assessment of the Water Quality of Ten Waikato Lakes Based on Zooplankton Community Composition; Centre for Biodiversity and Ecology Research Contract Report 60; The University of Waikato: Hamilton, New Zealand, 2007. [Google Scholar]
- Muirhead, J.R.; Ejsmont-Karabin, J.; MacIsaac, H.J. Quantifying rotifer species richness in temperate lakes. Freshw. Biol. 2006, 51, 1696–1709. [Google Scholar] [CrossRef]
- Smith, H.A.; Ejsmont-Karabin, J.; Hess, T.M.; Wallace, R.L. Paradox of planktonic rotifers: Similar structure but unique trajectories in communities of the Great Masurian Lakes (Poland). Verh. Internat. Verein. Limnol. 2009, 30, 951–956. [Google Scholar] [CrossRef]
- Magnuson, J.; Carpenter, S.; Stanley, E. North Temperate Lakes LTER: Zooplankton–Trout Lake Area 1982—Current ver 34. Environmental Data Initiative. Available online: https://lter.limnology.wisc.edu/node/55119 (accessed on 28 July 2020).
- Mills, S. Investigations of the Brachionus plicatilis Species Complex, with Particular Reference to Southwest Western Australia. Ph.D. Thesis, The University of Western Australia, Crawley, Western Australia, 2006. [Google Scholar]
- Mills, S.; Lunt, D.H.; Gómez, A. Global isolation by distance despite strong regional phylogeography in a small metazoan. BMC Evol. Biol. 2007, 7, 225. [Google Scholar] [CrossRef]
- Meksuwan, P.; Jaturapruek, R.; Supiyanit Maiphae, S. Two new species of genus Limnias (Rotifera, Gnesiotrocha) from Thailand. ZooKeys 2018, 787, 1–15. [Google Scholar] [CrossRef]
- Meksuwan, P.; Pholpunthin, P.; Segers, H.H. Molecular phylogeny confirms Conochilidae as ingroup of Flosculariidae (Rotifera, Gnesiotrocha). Zoologica Scripta 2015, 44, 562–573. [Google Scholar] [CrossRef]
- Mills, S.; Alcántara-Rodríguez, J.A.; Ciros-Pérez, J.; Gómez, A.; Hagiwara, A.; Galindo, K.H.; Jersabek, C.D.; Malekzadeh-Viayeh, R.; Leasi, F.; Lee, J.-S.; et al. Fifteen species in one: Deciphering the Brachionus plicatilis species complex (Rotifera, Monogononta) through DNA taxonomy. Hydrobiologia 2017, 796, 39–58. [Google Scholar] [CrossRef]
- Wen, X.; Xi, Y.; Zhang, G.; Xue, Y.; Xiang, X. Coexistence of cryptic Brachionus calyciflorus (Rotifera) species: Roles of environmental variables. J. Plankton Res. 2016, 38, 478–489. [Google Scholar] [CrossRef]
- Kordbacheh, A.; Garbalena, G.; Walsh, E.J. Population structure and cryptic species in the cosmopolitan rotifer Euchlanis dilatata. Zool. J. Linn. Soc. 2017, 181, 757–777. [Google Scholar] [CrossRef]
- Zweerus, N.L.; Sommer, S.; Fontaneto, D.; Ozgul, A. Life-history responses to environmental change revealed by resurrected rotifers from a historically polluted lake. Hydrobiologia 2017, 796, 121–130. [Google Scholar] [CrossRef]
- Shiel, R.H. Zooplankton of the Murray-Darling system. In The Ecology of River Systems; Davies, B.R., Walker, K.F., Eds.; Dr. W. Junk Publishers: Dordrecht, The Netherlands, 1986. [Google Scholar]
- Shiel, R.J.; Costelloe, J.F.; Reid, J.R.W.; Hudson, P.J.; Powling, J. Zooplankton diversity and assembly in arid zone rivers of the Lake Eyre basin, Australia. Mar. Freshw. Res. 2006, 57, 49–60. [Google Scholar] [CrossRef]
- Koste, W.; Shiel, R. Tasmanian rotifer: Affinities with the Australian fauna. Hydrobiologia 1987, 147, 31–43. [Google Scholar] [CrossRef]
- Shiel, R.J.; Koste, W.; Tan, L.W. Tasmania revisited: Rotifer communities and habitat heterogeneity. Hydrobiologia 1989, 186/187, 239–245. [Google Scholar] [CrossRef]
- Shiel, R.J.; Green, J.D. Rotifera recorded from New Zealand, 1859–1995, with comments on zoogeography. N. Z. J. Zool. 1996, 23, 191–207. [Google Scholar] [CrossRef]
- Shiel, R.J.; Koste, W. Rotifera recorded from Australia. Trans. R. Soc. S. Aust. 1979, 103, 57–68. [Google Scholar]
- Sanoamuang, L. Rotifera of some freshwater habitats in the floodplain of the River Nan, northern Thailand. Hydrobiologia 1998, 387/388, 27–33. [Google Scholar] [CrossRef]
- Weithoff, G. On the ecology of the rotifer Cephalodella hoodi from an extremely acidic lake. Freshw. Biol. 2005, 50, 14641473. [Google Scholar] [CrossRef]
- Jersabek, C.D.; Weithoff, G.; Weisse, T. Cephalodella acidophila n. sp. (Monogononta: Notommatidae), a new rotifer species from highly acidic mining lakes. Zootaxa 2011, 2939, 50–58. [Google Scholar] [CrossRef]
- Brett, M.T. The rotifer communities of acid-stressed lakes of Maine. Hydrobiologia 1989, 186, 181–189. [Google Scholar] [CrossRef]
- Garcia-Morales, A.E.; Elias-Gutierrez, M. DNA barcoding of freshwater Rotifera in Mexico: Evidence of cryptic speciation in common rotifers. Mol. Ecol. Resour. 2013, 13, 1097–1107. [Google Scholar] [CrossRef]
- Zhang, W.; Lemmen, K.D.; Zhou, L.; Papakostas, S.; Declerck, S.A.J. Patterns of differentiation in the life history and demography of four recently described species of the Brachionus calyciflorus cryptic species complex. Freshw. Biol. 2019, 64, 1994–2005. [Google Scholar] [CrossRef]
- Meksuwan, P.; Pholpunthin, P.; Walsh, E.J.; Segers, H.; Wallace, R.L. Nestedness in sessile and periphytic rotifer communities: A meta-analysis. Int. Rev. Hydrobiol. 2014, 99, 48–57. [Google Scholar] [CrossRef]
- Dražina, T.; Špoljar, M.; Primc, B.; van Habdija, I. Distribution of rotifers and other meiofauna in the bryophytes and hyporheic zone of a karst hydrosystem – an example of a nested community. Mar. Freshw. Res. 2017, 68, 43–52. [Google Scholar] [CrossRef]
- Stendera, S.E.S.; Johnson, R.K. Additive partitioning of aquatic invertebrate species diversity across multiple spatial scales. Freshw. Biol. 2005, 50, 1360–1375. [Google Scholar] [CrossRef]
- Kimpel, D.; Gockel, J.; Gerlach, G.; Bininda-Emonds, O.R.P. Population structuring in the monogonont rotifer Synchaeta pectinata: High genetic divergence on a small geographical scale. Freshw. Biol. 2015, 60, 1364–1378. [Google Scholar] [CrossRef]
- Thielsch, A.; Brede, N.; Petrusek, A.; De Meester, L.; Schwenk, K. Contribution of cyclic parthenogenesis and colonization history to population structure in Daphnia. Mol. Ecol. 2009, 18, 1616–1628. [Google Scholar] [CrossRef]
- García-Morales, A.E.; Domínguez-Domínguez, O. Cryptic molecular diversity in the morphologically variable rotiferan Brachionus quadridentatus (Rotifera: Monogononta). Rev. Biol. Trop. 2019, 67, 1114–1130. [Google Scholar]
- Kusumoto, B.; Costello, M.J.; Kubota, Y.; Shiono, T.; Wei, C.L.; Yasuhara, M.; Chao, A. Global distribution of coral diversity: Biodiversity knowledge gradients related to spatial resolution. Ecol. Res. 2020, 35, 315–326. [Google Scholar] [CrossRef]
- de Wit, R.; Bouvier, T. ‘Everything is everywhere, but, the environment selects’; what did Baas Becking and Beijerinck really say? Environ. Microbiol. 2006, 8, 755–758. [Google Scholar] [CrossRef]
- Dumont, H.J. Workshop on taxonomy and biogeography. Hydrobiologia 1980, 73, 205–206. [Google Scholar] [CrossRef]
- Kellogg, C.A.; Griffin, D.W. Aerobiology and the global transport of desert dust. Trends Ecol. Evol. 2006, 21, 638–644. [Google Scholar] [CrossRef] [PubMed]
- De Meester, L.; Gómez, A.; Okamura, B.; Schwenk, K. The Monopolization Hypothesis and the dispersal–gene flow paradox in aquatic organisms. Acta Oecologia 2002, 23, 121–125. [Google Scholar] [CrossRef]
- De Meester, L.; Vanoverbeke, J.; Kilsdonk, L.J.; Urban, M.C. Evolving perspectives on monopolization and priority effects. TREE 2016, 31, 136–146. [Google Scholar] [CrossRef]
- Silberstorf, R.R. Structural Analysis of Ernst Tinaja Canyon Focusing on Strike-slip Faulting and Folding, Big Bend National Park, Texas; Stephen F. Austin State University: Nacogdoches, TX, USA, 2017. [Google Scholar]
- Barker, D.S. Down to earth at Tuff Canyon, Big Bend National Park, Texas; Bureau of Economic Geology, the University of Texas: Austin, TX, USA, 2000. [Google Scholar]
- Gabaldón, C.; Fontaneto, D.; Carmona, M.J.; Montero-Pau, J.; Serra, M. Ecological differentiation in cryptic rotifer species: What we can learn from the Brachionus plicatilis complex. Hydrobiologia 2017, 796, 7–18. [Google Scholar] [CrossRef]
- Fontaneto, D. Molecular phylogenies as a tool to understand diversity in rotifers. Int. Rev. Hydrobiol. 2014, 99, 178–187. [Google Scholar] [CrossRef]
- Hamdan, L.K. Ecology and Genetics of Philodina megalotrocha (Rotifera, Bdelloidea) from Chihuahuan Desert Populations. Master’s Thesis, Department of Biological Sciences, University of Texas at El Paso, El Paso, TX, USA, 2010; 85p. [Google Scholar]
- Fontaneto, D.; Ficetola, G.F.; Ambrosini, R.; Ricci, C. Patterns of diversity in microscopic animals: Are they comparable to those in protists or in larger animals? Glob. Ecol. Biogeogr. 2006, 15, 153–162. [Google Scholar] [CrossRef]
- Fontaneto, D.; Barbosa, A.M.; Segers, H.; Pautasso, M. The ‘rotiferologist’ effect and other global correlates of species richness in monogonont rotifers. Ecography 2012, 35, 174–182. [Google Scholar] [CrossRef]
- Clavel, J.; Julliard, R.; Devictor, V. Worldwide decline of specialist species: Toward a global functional homogenization? Front. Ecol. Environ. 2011, 9, 222–228. [Google Scholar] [CrossRef]
- Michaloudi, E.; Mills, S.; Papakostas, S.; Stelzer, C.-P.; Triantafyllidis, A.; Kappas, I.; Vasileiadou, K.; Proios, K.; Abatzopoulos, T.J. Morphological and taxonomic demarcation of Brachionus asplanchnoidis Charin within the Brachionus plicatilis cryptic species complex (Rotifera, Monogononta). Hydrobiologia 2017, 796, 19–37. [Google Scholar] [CrossRef]
- Obertegger, U.; Flaim, G.; Fontaneto, D. Cryptic diversity within the rotifer Polyarthra dolichoptera along an altitudinal gradient. Freshw. Biol. 2014, 59, 2413–2427. [Google Scholar] [CrossRef]
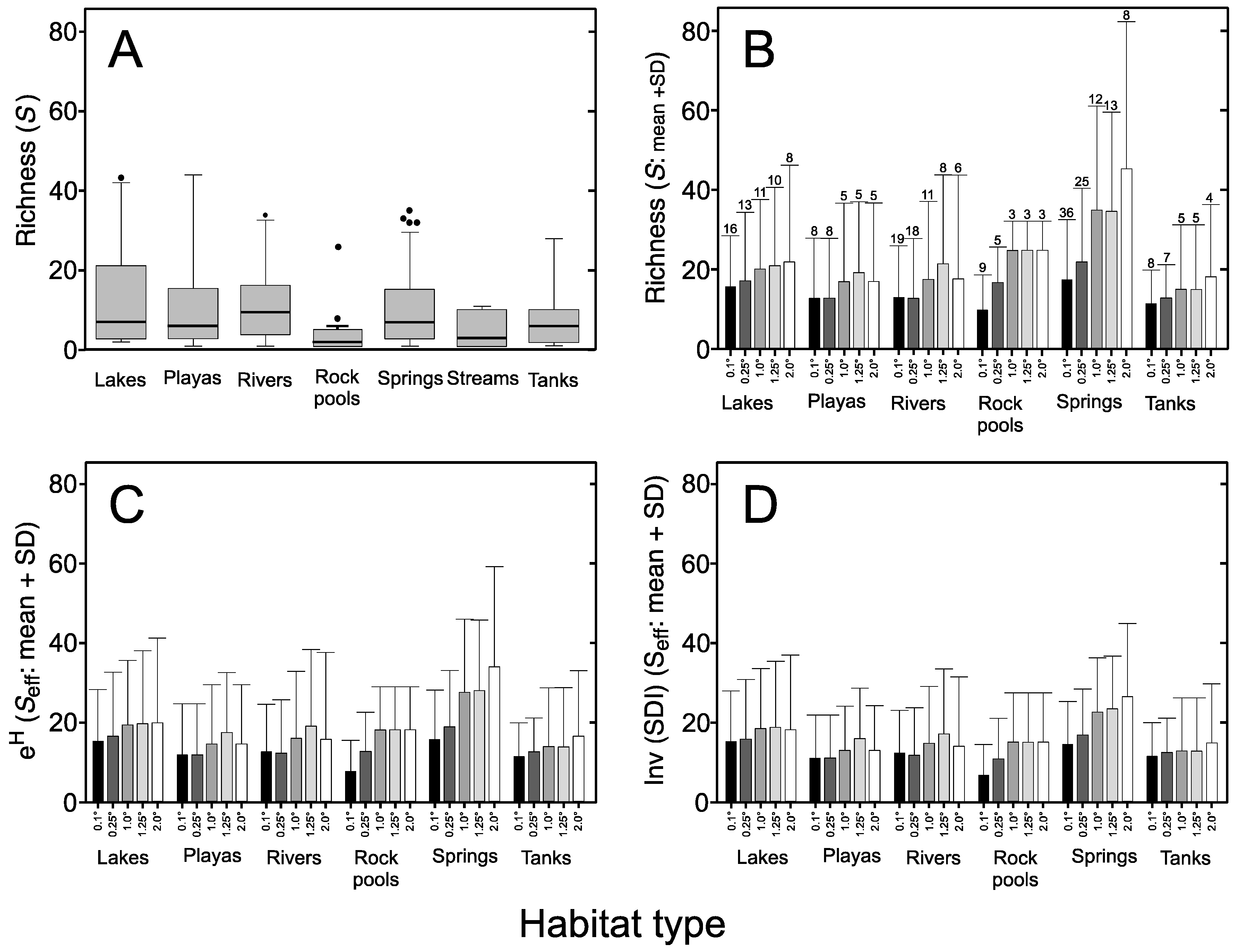
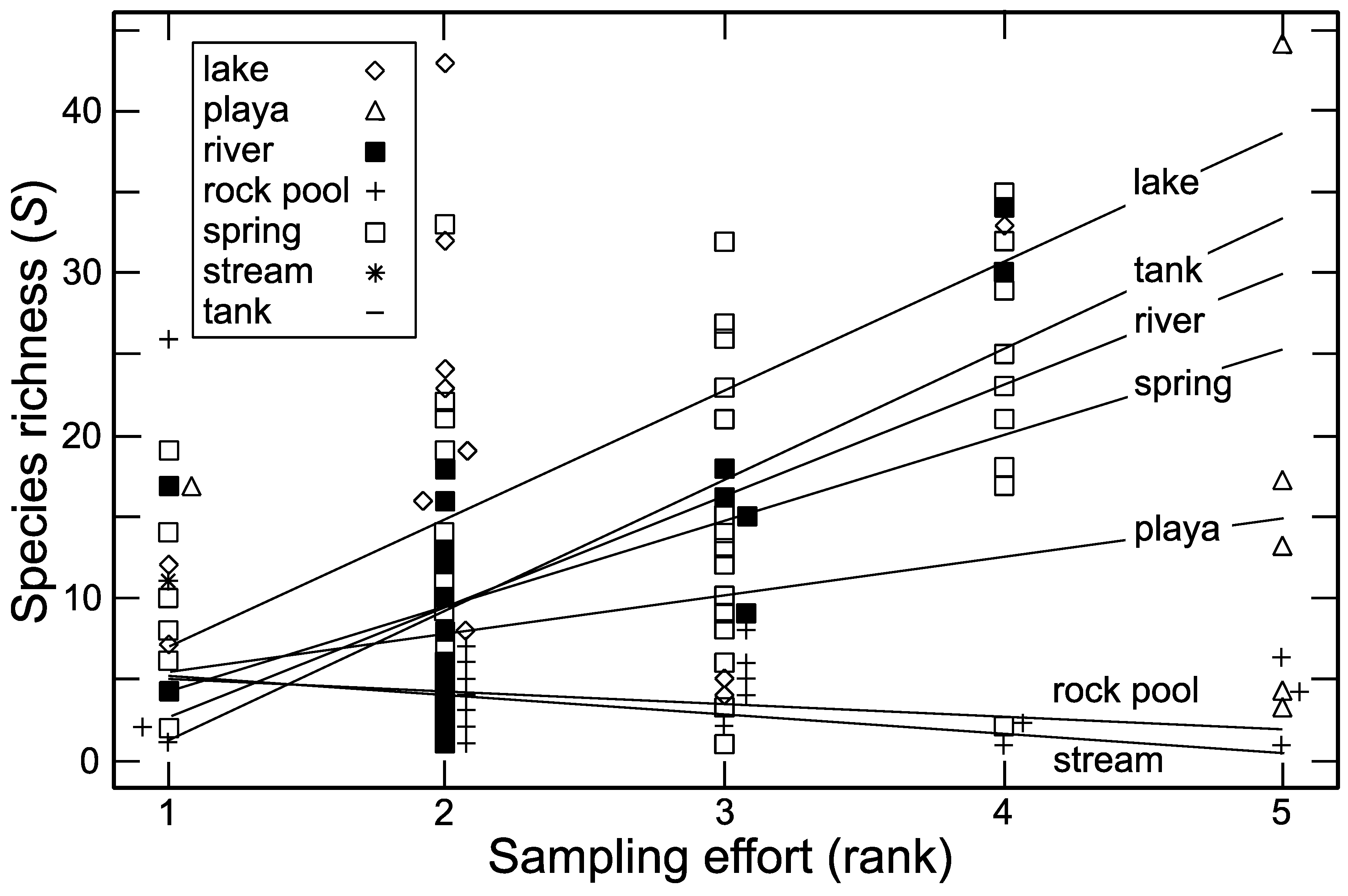
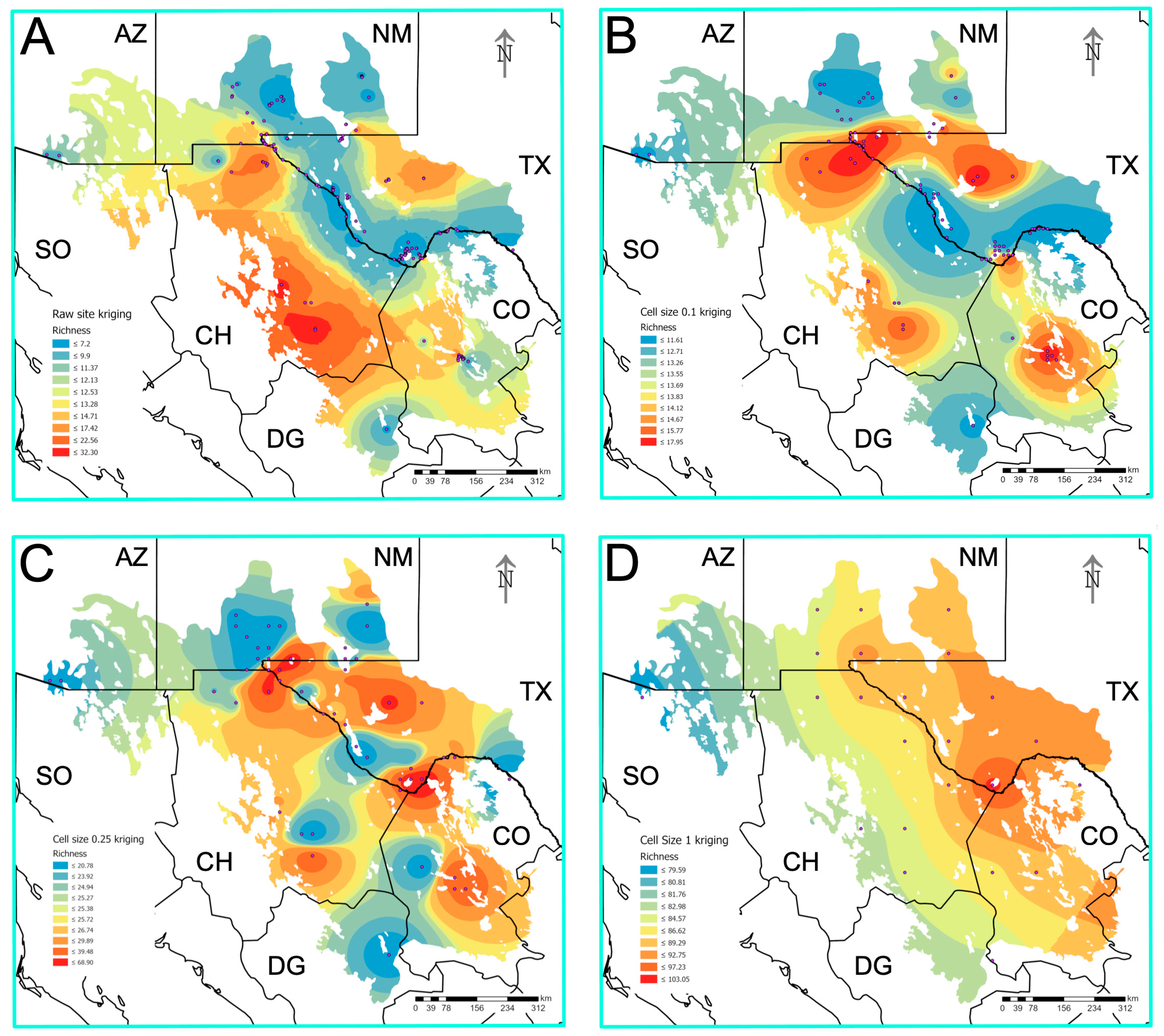
| Habitat Type | Species Richness (S) | Unique Species * | Versus Lake | Versus Playa | Versus Rock Pool | Versus Spring | Versus Tank |
|---|---|---|---|---|---|---|---|
| Lake | 114 | 12 (10.5) | — | 42 | 36 | 77 | 33 |
| Playa | 66 | 6 (9.1) | 0.47 | — | 24 | 45 | 26 |
| Rock pool | 53 | 3 (5.7) | 0.44 | 0.40 | — | 44 | 20 |
| Spring | 175 | 60 (34.3) | 0.54 | 0.38 | 0.39 | — | 39 |
| Tank | 57 | 6 (10.5) | 0.39 | 0.42 | 0.36 | 0.34 | — |
| Habitat Type | Number of Associated Species | Indicator Species | IndVal | p Value |
|---|---|---|---|---|
| Lake | 30 | Trichocerca pusilla | 0.483 | 0.003 |
| Asplanchna priodonta | 0.378 | 0.008 | ||
| Playa | 16 | Epiphanes brachionus | 0.538 | 0.001 |
| Rhinoglena ovigera | 0.458 | 0.011 | ||
| Filinia cornuta | 0.433 | 0.002 | ||
| Asplanchna sieboldii | 0.387 | 0.012 | ||
| Lacinularia flosculosa | 0.354 | 0.048 | ||
| Rock Pool | 6 | Hexarthra n. sp. | 0.632 | 0.001 |
| Stream | 3 | Dicranophorus grandis | 0.378 | 0.027 |
| Wulfertia ornata | 0.378 | 0.027 | ||
| Tank | 13 | Filinia cf. pejleri | 0.481 | 0.005 |
| Brachionus dimidiatus | 0.360 | 0.018 | ||
| Lake + River | 5 | Keratella americana | 0.432 | 0.011 |
| Lake + Rock Pool | 1 | Trichocerca similis | 0.514 | 0.004 |
| Lake + Stream | 3 | Colurella adriatica | 0.423 | 0.014 |
| Lake + Tank | 6 | Asplanchna brightwellii | 0.433 | 0.013 |
| Brachionus caudatus | 0.354 | 0.031 | ||
| Brachionus havanaensis | 0.350 | 0.041 | ||
| Euchlanis calpidia | 0.345 | 0.042 | ||
| Mytilina ventralis | 0.332 | 0.042 | ||
| Playa + Stream | 1 | Trichocerca rattus | 0.445 | 0.004 |
| River + Spring | 2 | Dipleuchlanis propatula | 0.396 | 0.034 |
| River + Tank | 6 | Plationus patulus | 0.446 | 0.015 |
| Eosphora najas | 0.397 | 0.022 | ||
| Brachionus bidentatus | 0.364 | 0.026 | ||
| Lake + Playa + Spring | 3 | Lecane closterocerca | 0.439 | 0.049 |
| Lake + Playa + Stream | 2 | Brachionus plicatilis | 0.486 | 0.006 |
| Notommata glyphura | 0.356 | 0.039 | ||
| Lake + River + Spring | 7 | Colurella uncinata | 0.482 | 0.010 |
| Lake + River + Tank | 4 | Keratella cochlearis | 0.467 | 0.003 |
| Brachionus variabilis | 0.431 | 0.011 | ||
| Polyarthra dolichoptera | 0.431 | 0.028 | ||
| Testudinella patina | 0.403 | 0.040 | ||
| Playa + River + Tank | 3 | Brachionus quadridentatus | 0.674 | 0.001 |
| Brachionus angularis | 0.439 | 0.019 | ||
| Lake + Playa + River + Stream | 1 | Cephalodella catalina | 0.455 | 0.019 |
| Lake + Playa + River + Tank | 4 | Brachionus calyciflorus | 0.432 | 0.026 |
| Epiphanes chihuahuaensis | 0.368 | 0.036 | ||
| Playa + River + Stream + Tank | 2 | Euchlanis dilatata | 0.628 | 0.001 |
| Platyias quadricornis | 0.462 | 0.012 | ||
| Lake + Playa + River + Spring + Stream | 2 | Lecane bulla | 0.668 | 0.001 |
| Lake + River + Spring + Stream + Tank | 1 | Philodina megalotrocha | 0.598 | 0.007 |
| Lake + Playa + River + Spring + Stream + Tank | 4 | Lecane luna | 0.564 | 0.008 |
| Cephalodella gibba | 0.495 | 0.017 |
| Regions Analyzed 1 | Number of Taxa | Number of Genera | Number of Families | Packed Matrix T° | Null Support 2 | Idiosyncratic Species 3 | Idiosyncratic Habitats 4 |
|---|---|---|---|---|---|---|---|
| Chihuahuan Desert(this study) | |||||||
| All sites | 246 | 59 | 25 | 2.4 | 4 | Hexarthra n. sp.; Trichocerca similis | Caballo Reservoir, NM; Cattail Spring Pools C-D, BIBE, TX; Lake Lucero, WHSA, NM; Langford Hot Springs, BIBE, TX; Miller Ranch 2 (Spring), TX; Presa Chihuahua, MX; Rio Grande Village Cattail Pond, BIBE, TX; Rio Grande Village Upper Pond, BIBE, TX |
| By habitat type | |||||||
| 1. All lakes | 112 | 38 | 24 | 14.2 | 4 | Encentrum cf. algente; Lecane arcula; Lecane quadridentata; Polyarthra vulgaris; Synchaeta cf. oblonga | Presa Chihuahua, Chihuahua, MX |
| 2. All playas | 66 | 30 | 19 | 11.9 | 4 | Lecane hornemanni; Lecane thalera | None |
| 3. All tanks | 57 | 27 | 14 | 11.1 | 4 | Brachionus durgae; Epiphanes brachionus; Lepadella patella | Tule Cattle Tank, BIBE, TX |
| 4. All springs | 175 | 49 | 23 | 5.0 | 4 | Adineta vaga; Aspelta aper; Cephalodella catellina; Cephalodella tenuiseta; Colurella adriatica; Encentrum saundersiae; Filinia brachiata; Lepadella acuminata; Mytilina mucronata; Notommata cf. haueri | Balmorhea State Park Main Pool, TX; Balmorhea Wetland 2, TX; Miller Ranch 96 Well, TX; Oak Creek BIBE, TX; Ojo de la Punta, ANPMS, MX; Sitting Bull Falls LNF, NM |
| Selected springs in Mexico | 57 | 24 | 15 | 21.9 | 4 | Cephalodella cf. graciosa; Cephalodella megalocephala; Pleurotrocha petromyzon; Pleurotrocha sigmoidea | One small, impounded spring: Ojo de en Medio, ANPMS |
| 5. Cascading pools (BIBE) | |||||||
| A. All rock pools | 72 | 21 | 14 | 5.4 | 4 | Epiphanes daphnicola; Trichocerca similis | Second pool of the flowage – surrounded by lush vegetation |
| B. Cattail Springs | 65 | 19 | 11 | 23.7 | 4 | Colurella obtusa; Lecane pyriformis; Proales cryptopus; Tripleuchlanis plicata | Small pool isolated from the main flowage at this site. |
| C. Ernst canyon | 16 | 9 | 8 | 19.0 | 4 | None | None |
| D. Tuff canyon | 4 | 4 | 3 | 11.7 | 0 | None | Shallow rock pool (Tuff Canyon Site #4) |
| E. Window Trail canyon | 16 | 7 | 6 | 23.3 | 2 | Lecane pyriformis | Small tinaja nearly filled with small rocks and sediment, surrounded by plants |
| 6. Rock pools at HTSPHS | |||||||
| A. Isolated rock pools | 14 | 11 | 9 | 4.9 | 4 | None. However, Hexarthra n. sp. was found in all sites except for the two artificially enlarged, sheltered rock pools noted here | Two, artificially enlarged, rock pools sheltered by an overhanging shelf |
| B. Mesocosms: artificial rock pools | 9 | 6 | 5 | 22.9 | 1 | Lecane nana | None |
| By Geospatial scale (grid size) | |||||||
| 1. Grid 0.1° | 246 | 59 | 25 | 4.4 | 4 | Adineta vaga; Brachionus plicatilis; Brachionus variabilis; Cephalodella cf. misgurnus/pachyodon; Lecane hornemanni; Lecane inermis; Synchaeta cf. oblonga; Trichocerca similis | 20755: Northern BIBE (Cattail Springs, Window trail, Croton spring) 29355:Caballo reservoir and Percha dam 30345:BLSP |
| 2. Grid 0.25° | 246 | 59 | 25 | 6.0 | 4 | Brachionus caudatus; Brachionus variabilis; Cephalodella cf. misgurnus/pachyodon; Epiphanes chihuahuaensis; Paradicranophorus sordidus; Polyarthra vulgaris; Trichocerca similis; Wulfertia ornata | 3310:Northern BIBE 4842:BLSP |
| 3. Grid 1.0° | 246 | 59 | 25 | 11.6 | 4 | Brachionus bidentatus; Brachionus plicatilis; Cephalodella calosa; Euchlanis triquetra; Filinia brachiata; Keratella americana; Keratella cochlearis; Philodina acuticornis; Philodina megalotrocha; Proales cognita; Wolga spinifera; Wulfertia ornata | 177: Delicias Beisbol field pool and Presa Francisco Ignacio Madero (southern pond and reservoir respectively) 298: BLSP |
| 4. Grid 1.25° | 246 | 59 | 25 | 10.5 | 4 | Dicranophorus mesotis; Euchlanis calpidia; Hexarthra n.sp.; Lacinularia flosculosa; Lecane aeganea; Lecane undulata; Paradicranophorus sordidus; Polyarthra vulgaris; Proales cf. halophila; Squatinella lamellaris f. mutica; Testudinella patina; Trichocerca similis | El Paso area including HTSPHS |
| 5. Grid 2.0° | 246 | 59 | 25 | 9.5 | 4 | Encentrum cf. cruentum; Euchlanis calpidia; Paradicranophorus sordidus; Plationus patulus; Polyarthra vulgaris; Trichocerca similis | 64: El Paso/Juarez area including ANPMS, HTSPHS, IMRS 65: GUMO and Balmorhea SP |
| Other aridland biomes | |||||||
| 1. Billabongs (Australia) | 52 | 25 | 18 | 39.3 | 2 | Mytilina mucronata; Epiphanes daphnicola; Trichocerca rattus | None |
| 2. Various habitats (Oman) | 66 | 20 | 12 | 45.9 | 3 | Cephalodella gibba; Colurella obtusa; Trichocerca tenuior | Ravine (Wadi O7) |
| 3. Various habitats (Saudi Arabia) | 19 | 10 | 7 | 11.1 | 3 | Lecane ungulata | Brackish water lagoon (Sabkhat S7) |
| 4. Various habitats (Yemen) | 74 | 26 | 16 | 11.3 | 4 | Brachionus urceolaris; Cephalodella forficula; Colurella adriatica; Lophocharis salpina | Wet Wadi (Y30) with Phragmites |
| 5. Dune pools (Spain) | 34 | 18 | 12 | 16.5 | 4 | Lophocharis salpina; Trichocerca bidens; Trichocerca rattus | Two pools: (1) mobile dune region; (2) stable dune region and close to a salt marsh |
| Tropical biomes | |||||||
| 1. Costa Rican habitats | 105 | 33 | 17 | 10.1 | 4 | Ascomorpha klementi; Keratella americana; Lecane nana; Lepadella patella; Resticula melandoca; Trichocerca dixonnuttalli | Artificial Lake; Bromelia; Lake Turrialba |
| 2. Eutrophic tropical fish ponds | 57 | 22 | 15 | 61.8 | 0 | None | None |
| Temperate biomes | |||||||
| 1. North Island, NZ | 79 | 32 | 20 | 26.3 | 4 | Filinia cf. pejleri; Keratella australis; Keratella tropica; Lecane flexilis; Lepadella acuminata; Trichocerca longiseta | Lake Okaro; Lake Ototoa; Lake Tutira |
| 2. Develi Plain, Turkey | 84 | 33 | 17 | 31.6 | 3 | Lecane quadridentata; Lepadella biloba; Scaridium longicauda | None |
| Cold biomes | |||||||
| 1. Antarctica & sub-Antarctica | 24 | 6 | 3 | 22.7 | 2 | Brachionus quadridentatus; Notholca hollowdayi | None |
| 2. Canadian High Arctic | 70 | 26 | 16 | 29.5 | 4 | Collotheca sp. 2; Cephalodella catellina; Squatinella sp.; Trichocerca sp. | Small pool, 8 (P208) |
| Region | Mantel r Statistic | P-Value | n |
|---|---|---|---|
| All sites | |||
| sites | 0.12 | <0.001 | 236 |
| 0.1° | 0.12 | 0.01 | 84 |
| 0.25° | 0.14 | 0.02 | 55 |
| 1° | 0.03 | 0.22 | 24 |
| 1.25° | 0.20 | 0.08 | 21 |
| 2° | 0.20 | 0.10 | 14 |
| By habitat | |||
| Lakes | |||
| sites | 0.30 | 0.001 | 21 |
| 0.1° | 0.25 | 0.044 | 16 |
| 0.25° | 0.20 | 0.105 | 13 |
| 1° | 0.31 | 0.048 | 11 |
| 1.25° | 0.32 | 0.085 | 10 |
| 2° | 0.35 | 0.095 | 8 |
| Playas | |||
| sites | 0.55 | <0.001 | 16 |
| 0.1° | 0.60 | 0.009 | 8 |
| 0.25° | 0.62 | 0.002 | 7 |
| 1° | 0.74 | 0.008 | 5 |
| 1.25° | 0.58 | 0.083 | 5 |
| 2° | 0.80 | 0.008 | 5 |
| Rivers | |||
| sites | 0.27 | <0.001 | 26 |
| 0.1° | 0.41 | <0.001 | 19 |
| 0.25° | 0.48 | <0.001 | 18 |
| 1° | 0.42 | 0.012 | 11 |
| 1.25° | 0.13 | 0.271 | 8 |
| 2° | 0.13 | 0.350 | 6 |
| Rock pools | |||
| sites | 0.12 | <0.001 | 60 |
| 0.1° | −0.16 | 0.696 | 9 |
| 0.25° | 0.61 | 0.133 | 5 |
| Springs | |||
| sites | 0.02 | 0.334 | 95 |
| 0.1° | −0.06 | 0.752 | 36 |
| 0.25° | −0.05 | 0.663 | 25 |
| 1° | 0.06 | 0.321 | 12 |
| 1.25° | 0.02 | 0.406 | 13 |
| 2° | 0.16 | 0.253 | 8 |
| Tanks | |||
| sites | 0.41 | 0.012 | 11 |
| 0.1° | 0.35 | 0.063 | 8 |
| 0.25° | 0.28 | 0.147 | 7 |
| 1° | 0.60 | 0.017 | 5 |
| 1.25° | 0.67 | 0.008 | 5 |
| 2° | 0.77 | 0.083 | 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, P.D.; Schröder, T.; Ríos-Arana, J.V.; Rico-Martinez, R.; Silva-Briano, M.; Wallace, R.L.; Walsh, E.J. Patterns of Rotifer Diversity in the Chihuahuan Desert. Diversity 2020, 12, 393. https://doi.org/10.3390/d12100393
Brown PD, Schröder T, Ríos-Arana JV, Rico-Martinez R, Silva-Briano M, Wallace RL, Walsh EJ. Patterns of Rotifer Diversity in the Chihuahuan Desert. Diversity. 2020; 12(10):393. https://doi.org/10.3390/d12100393
Chicago/Turabian StyleBrown, Patrick D., Thomas Schröder, Judith V. Ríos-Arana, Roberto Rico-Martinez, Marcelo Silva-Briano, Robert L. Wallace, and Elizabeth J. Walsh. 2020. "Patterns of Rotifer Diversity in the Chihuahuan Desert" Diversity 12, no. 10: 393. https://doi.org/10.3390/d12100393
APA StyleBrown, P. D., Schröder, T., Ríos-Arana, J. V., Rico-Martinez, R., Silva-Briano, M., Wallace, R. L., & Walsh, E. J. (2020). Patterns of Rotifer Diversity in the Chihuahuan Desert. Diversity, 12(10), 393. https://doi.org/10.3390/d12100393