Richness of Primary Producers and Consumer Abundance Mediate Epiphyte Loads in a Tropical Seagrass System
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Selection and Experimental Design
2.2. Sample Analysis
2.3. Statistical Analysis
2.3.1. Model Selection
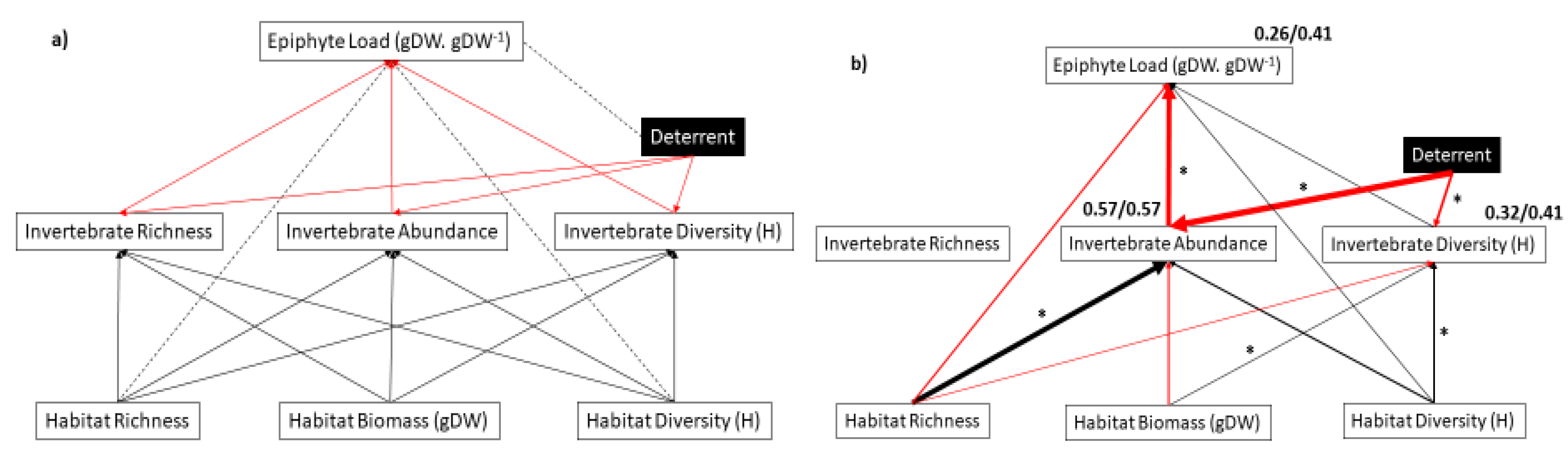
2.3.2. Path Analysis of Combined Interaction Web
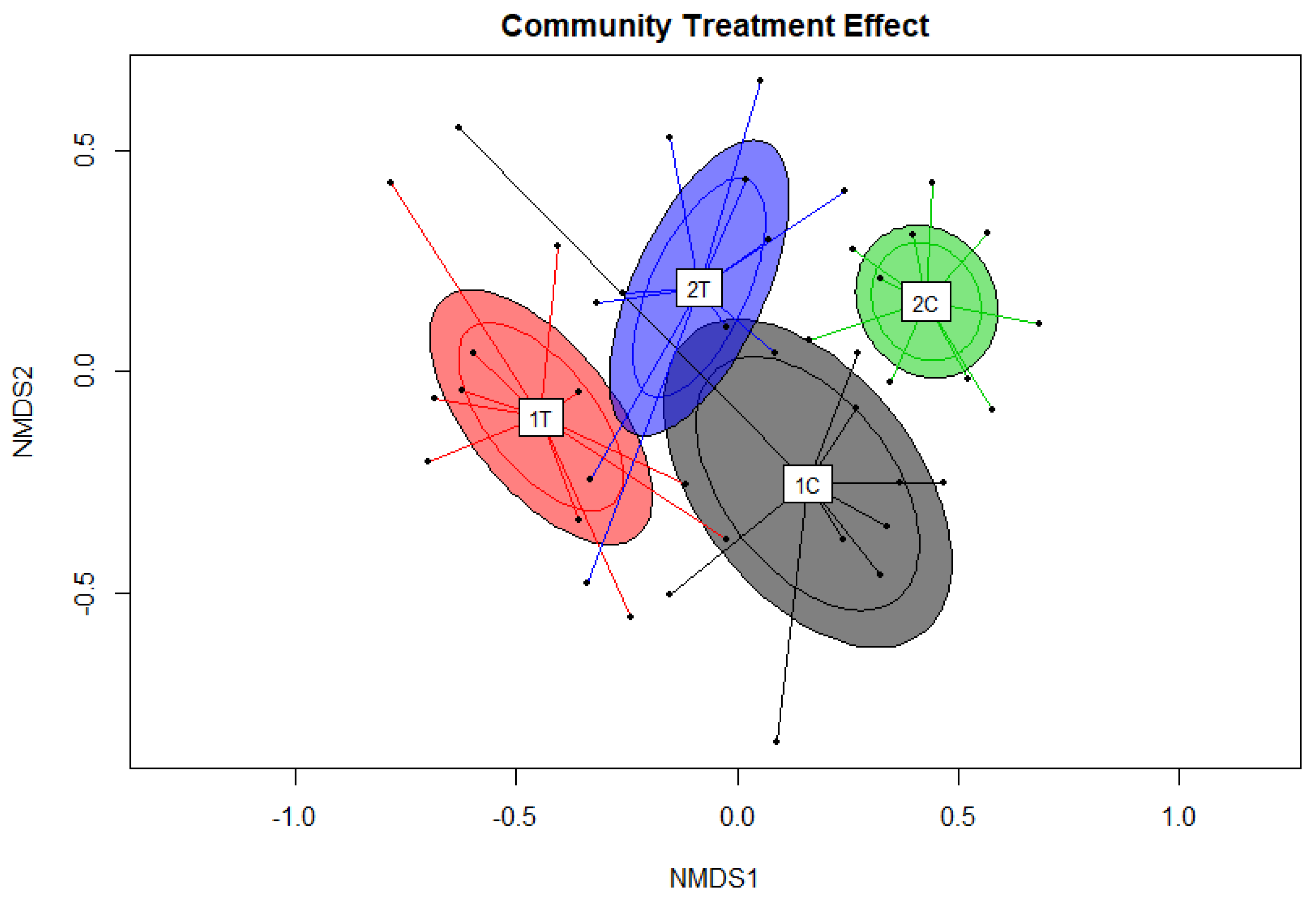
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
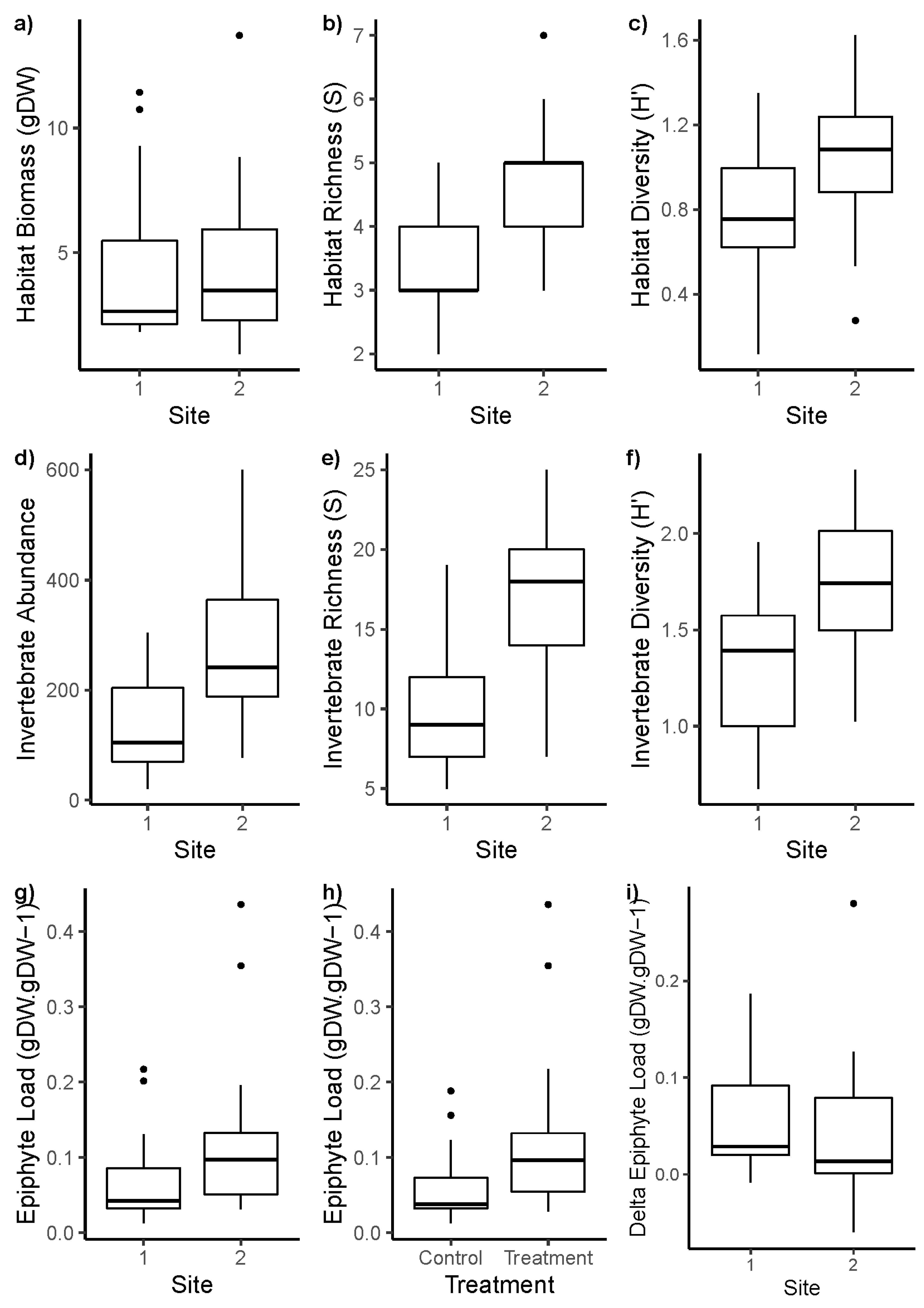
Appendix A
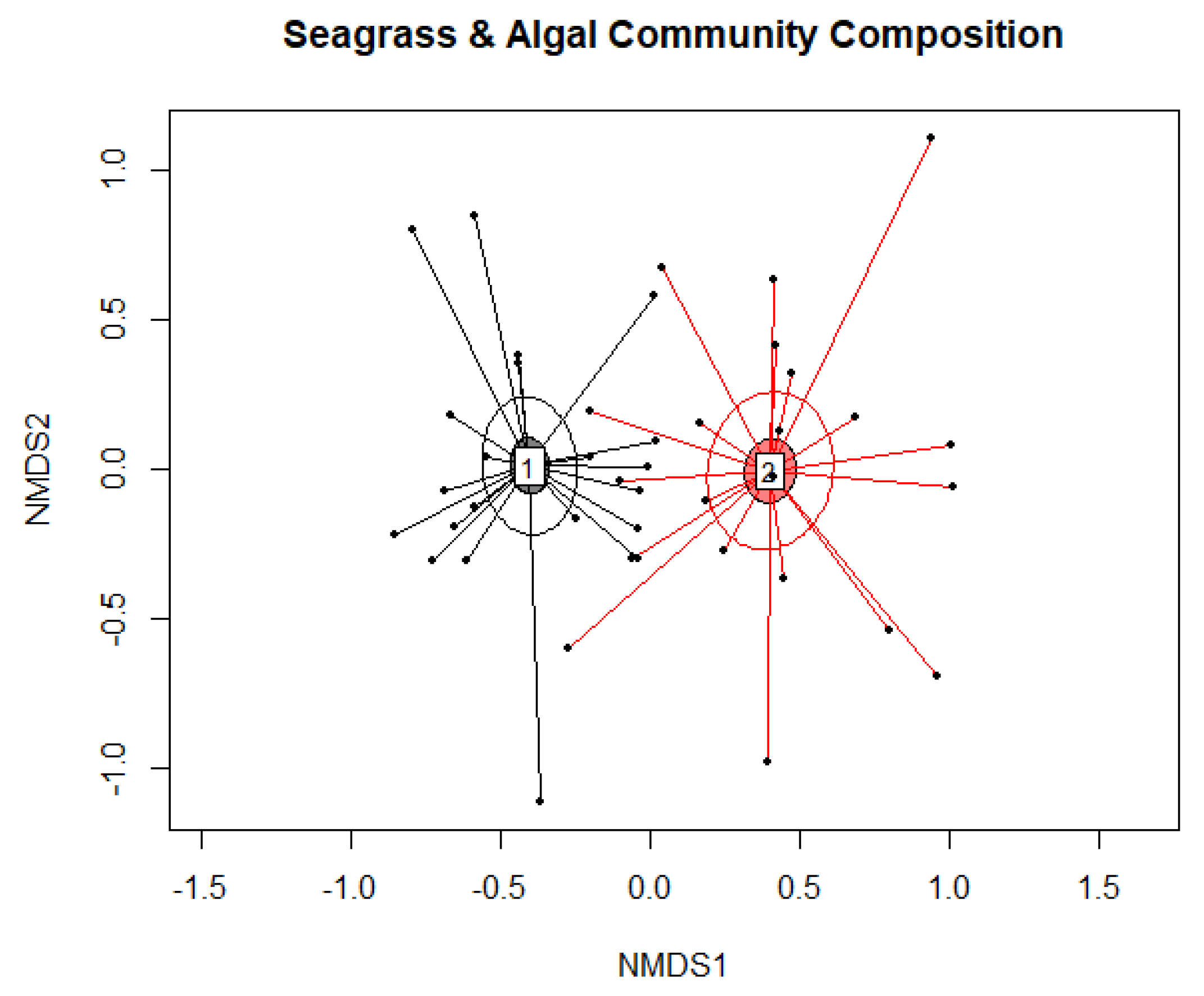
Appendix B
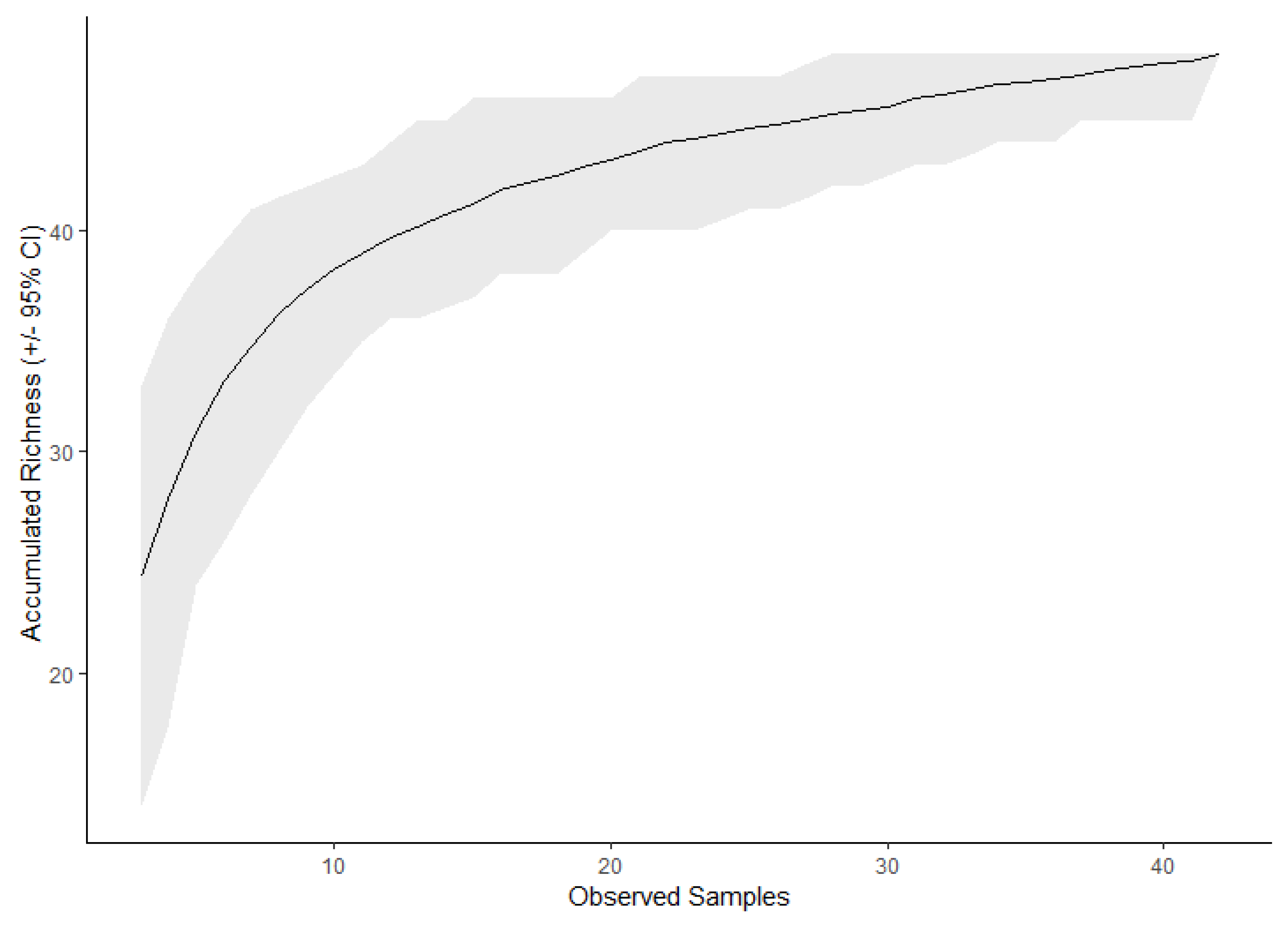
| Method | Expected # of Taxa | ±95% C.I. |
|---|---|---|
| Observed | 48 | - |
| Chao | 71.9 | ±59.6 |
| Jackknife 1 | 54.8 | ±6.9 |
| Jackknife 2 | 60.6 | ±6.9 |
| Bootstrap | 51.3 | ±3.7 |
Appendix C
References
- Proulx, M.; Mazumder, A. Reversal of Grazing Impact on Plant Species Richness in Nutrient-Poor vs. Nutrient-Rich. Ecology 1998, 79, 2581–2592. [Google Scholar] [CrossRef]
- Worm, B.; Lotze, H.K.; Hillebrand, H.; Sommer, U. Consumer versus resource control of species diversity and ecosystem functioning. Nature 2002, 417, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Gruner, D.S.; Smith, J.E.; Seabloom, E.W.; Sandin, S.A.; Ngai, J.T.; Hillebrand, H.; Harpole, S.W.; Elser, J.J.; Cleland, E.E.; Bracken, M.E.S.; et al. A cross-system synthesis of consumer and nutrient resource control on producer biomass. Ecol. Lett. 2008, 11, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Huntly, N. Herbivores and the dynamics or communities and ecosystems. Annu. Rev. Ecol. Syst. 1991, 22, 477–503. [Google Scholar] [CrossRef]
- Borer, E.T.; Seabloom, E.W.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Lind, E.M.; Adler, P.B.; Alberti, J.; Anderson, T.M.; Bakker, J.D.; et al. Herbivores and nutrients control grassland plant diversity via light limitation. Nature 2014, 508, 517–521. [Google Scholar] [CrossRef]
- Scott, A.L.; York, P.H.; Duncan, C.; Macreadie, P.I.; Connolly, R.M.; Ellis, M.T.; Jarvis, J.C.; Jinks, K.I.; Marsh, H.; Rasheed, M.A. The Role of Herbivory in Structuring Tropical Seagrass Ecosystem Service Delivery. Front. Plant Sci. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Hillebrand, H.; Gruner, D.S.; Borer, E.T.; Bracken, M.E.S.; Cleland, E.E.; Elser, J.J.; Harpole, W.S.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; et al. Consumer versus Resource Control of Producer Diversity Depends on Ecosystem Type and Producer Community Structure. Proc. Natl. Acad. Sci. USA 2007, 104, 10904–10909. [Google Scholar] [CrossRef]
- Grace, J.B.; Michael Anderson, T.; Seabloom, E.W.; Borer, E.T.; Adler, P.B.; Stanley Harpole, W.; Hautier, Y.; Hillebrand, H.; Lind, E.M.; Pärtel, M.; et al. Integrative modelling reveals mechanisms linking productivity and plant species richness. Nature 2016, 529, 390–395. [Google Scholar] [CrossRef]
- France, K.E.; Duffy, J.E. Diversity and dispersal interactively affect predictability of ecosystem function. Nature 2006, 441, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.E.; Altieri, A.H.; Johnston, L.N.; Kuempel, C.D.; Paperno, R.; Paul, V.J.; Duffy, J.E. Herbivore community determines the magnitude and mechanism of nutrient effects on subtropical and tropical seagrasses. J. Ecol. 2018, 106, 401–412. [Google Scholar] [CrossRef]
- Duffy, J.E.; Cardinale, B.J.; France, K.E.; McIntyre, P.B.; Thébault, E.; Loreau, M. The functional role of biodiversity in ecosystems: Incorporating trophic complexity. Ecol. Lett. 2007, 10, 522–538. [Google Scholar] [CrossRef] [PubMed]
- Alcoverro, T.; Mariani, S. Patterns of Fish and Sea Urchin Grazing on Tropical Indo-Pacific Seagrass Beds. Ecography 2004, 27, 361–365. [Google Scholar] [CrossRef]
- Christianen, M.J.A.; Govers, L.L.; Bouma, T.J.; Kiswara, W.; Roelofs, J.G.M.; Lamers, L.P.M.; van Katwijk, M.M. Marine megaherbivore grazing may increase seagrass tolerance to high nutrient loads. J. Ecol. 2011, 100, 546–560. [Google Scholar] [CrossRef]
- Christianen, M.J.A.; Herman, P.M.J.; Bouma, T.J.; Lamers, L.P.M.; van Katwijk, M.M.; van der Heide, T.; Mumby, P.J.; Silliman, B.R.; Engelhard, S.L.; van de Kerk, M.; et al. Habitat collapse due to overgrazing threatens turtle conservation in marine protected areas. Proc. R. Soc. B. 2014, 281. [Google Scholar] [CrossRef] [PubMed]
- Orth, R.J.; van Montfrans, J. Epiphyte-seagrass relationships with an emphasis on the role of micrograzing: A review. Aquat. Bot. 1984, 18, 43–69. [Google Scholar] [CrossRef]
- Heck, K.L.; Valentine, J.F. Plant-herbivore interactions in seagrass meadows. J. Exp. Mar. Biol. Ecol. 2006, 330, 420–436. [Google Scholar] [CrossRef]
- Myers, J.A.; Heck, K.L. Amphipod control of epiphyte load and its concomitant effects on shoalgrass Halodule wrightii biomass. Mar. Ecol. Prog. Ser. 2013, 483, 133–142. [Google Scholar] [CrossRef]
- Reynolds, P.L.; Richardson, J.P.; Duffy, J.E. Field experimental evidence that grazers mediate transition between microalgal and seagrass dominance. Limnol. Oceanogr. 2014, 59, 1053–1064. [Google Scholar] [CrossRef]
- Duffy, J.E.; Reynolds, P.L.; Boström, C.; Coyer, J.A.; Cusson, M.; Donadi, S.; Douglass, J.G.; Eklöf, J.S.; Engelen, A.H.; Eriksson, B.K.; et al. Biodiversity mediates top-down control in eelgrass ecosystems: A global comparative-experimental approach. Ecol. Lett. 2015, 18, 696–705. [Google Scholar] [CrossRef]
- Baden, S.; Emanuelsson, A.; Pihl, L.; Svensson, C.; Åberg, P. Shift in seagrass food web structure over decades is linked to overfishing. Mar. Ecol. Prog. Ser. 2012, 451, 61–73. [Google Scholar] [CrossRef]
- Duffy, J.E.; Macdonald, K.S.; Rhode, J.M.; Parker, J.D. Grazer diversity, functional redundancy, and productivity in seagrass beds. Ecology 2009, 82, 2417–2434. [Google Scholar] [CrossRef]
- Lefcheck, J.S.; Duffy, E.J. Multitrophic functional diversity predicts ecosystem functioning in experimental assemblages of estuarine consumers. Ecology 2015, 96, 2973–2983. [Google Scholar] [CrossRef] [PubMed]
- Duffy, E.; Richardson, P.; Canuel, E. Grazer diversity effects on ecosystem functioning in seagrass beds. Ecol. Lett. 2003, 6, 637–645. [Google Scholar] [CrossRef]
- Hughes, A.R.; Stachowicz, J.J. Genetic diversity enhances the resistance of a seagrass ecosystem to disturbance. Proc. Natl. Acad. Sci. USA 2004, 101, 8998–9002. [Google Scholar] [CrossRef] [PubMed]
- Reusch, T.B.H.; Ehlers, A.; Hä, A.; Worm, B. Ecosystem recovery after climatic extremes enhanced by genotypic diversity. Proc. Natl. Acad. Sci. USA 2005, 102, 2826–2831. [Google Scholar] [CrossRef] [PubMed]
- Whalen, M.A.; Duffy, J.E.; Grace, J.B. Temporal shifts in top-down vs. bottom-up control of epiphytic algae in a seagrass ecosystem. Ecology 2013, 94, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.B.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; et al. Accelerating Loss of Seagrasses across the Globe Threatens Coastal Ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef]
- Howarth, R.W.; Anderson, D.B.; Cloern, J.E.; Elfring, C.; Hopkinson, C.S.; Lapointe, B.; Maloney, T.J.; Marcus, N.; McGlathery, K.; Sharpley, A.N.; et al. Nutrient pollution of coastal rivers, bays, and seas. Issues Ecol. 2000, 7, 1–16. [Google Scholar]
- Jackson, J.B.C.; Kirby, M.X.; Berger, W.H.; Bjorndal, K.A.; Botsford, L.W.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.; Erlandson, J.; Estes, J.A.; et al. Historical Overfishing and the Recent Collapse of Coastal Ecosystems. Science 2001, 293, 629–638. [Google Scholar] [CrossRef]
- Hootsmans, M.J.M.; Vermaat, J.E. The Effect of Periphyton-Grazing by Three Epifaunal Species on the Growth of Zostera marina L. Under Experimental Conditions. Aquat. Bot. 1985, 22, 83–88. [Google Scholar] [CrossRef]
- Howard, R.K.; Short, F.T. Seagrass Growth and Survivorship Under the Influence of Epiphite Grazers. Aquat. Bot. 1986, 24, 287–302. [Google Scholar] [CrossRef]
- Neckles, H.A.; Wetzel, R.L.; Orth, R.J. Relative Effects of Nutrient Enrichment and Grazing on Epiphyte-Macrophyte (Zostera marina L.) Dynamics. Oecologia 1993, 93, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.L.; Ruckelshaus, M.H. Effects of Nitrogen Availability and Herbivory on Eelgrass (Zostera marina) and Epiphytes. Ecology 1993, 74, 904–918. [Google Scholar] [CrossRef]
- Orth, R.J.; Carruthers, T.J.B.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; Kendrick, G.A.; Kenworthy, W.J.; Olyarnik, S.; et al. A Global Crisis for Seagrass Ecosystems. Bioscience 2006, 56, 987–996. [Google Scholar] [CrossRef]
- Brown, J.H. Why are there so many species in the tropics? J. Biogeogr. 2014, 41, 8–22. [Google Scholar] [CrossRef]
- Leopardas, V.; Uy, W.; Nakaoka, M. Benthic macrofaunal assemblages in multispecific seagrass meadows of the southern Philippines: Variation among vegetation dominated by different seagrass species. J. Exp. Mar. Biol. Ecol. 2014, 457, 71–80. [Google Scholar] [CrossRef]
- Baxter, I.N. Green Island Information Review; Great Barrier Reef Marine Park Authority: Townsville, QLD, Australia, 1990. [Google Scholar]
- Rasheed, M.A. Recovery and succession in a multi-species tropical seagrass meadow following experimental disturbance: The role of sexual and asexual reproduction. J. Exp. Mar. Biol. Ecol. 2004, 310, 13–45. [Google Scholar] [CrossRef]
- Poore, A.G.B.; Campbell, A.H.; Steinberg, P.D. Natural densities of mesograzers fail to limit growth of macroalgae or their epiphytes in a temperate algal bed. J. Ecol. 2009, 97, 164–175. [Google Scholar] [CrossRef]
- Hoffmann, L. Richness of primary producers and consumer abundance mediate epiphyte loads in a tropical seagrass system (dataset). In Tropical Data Hub; James Cook University: Cairns, Australia, 2020. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Community Ecology Package ‘Vegan’, 2019, Version 2.5-6. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 7 October 2020).
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2018; Available online: https://www.R-project.org (accessed on 3 September 2019).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Bartoń, K. MuMIn: Multi-Model Inference. R Package 2020, Version 1.43.17. Available online: https://cran.r-project.org/web/packages/MuMIn/index.html (accessed on 7 October 2020).
- Burnham, K.P.; Anderson, D.R. Multimodel Inference Understanding AIC and BIC in Model Selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R.; Huyvaert, K.P. AIC model selection and multimodel inference in behavioral ecology: Some background, observations. Behav. Ecol. Sociobiol. 2011, 65, 23–35. [Google Scholar] [CrossRef]
- Shipley, B. The AIC model selection method applied to path analytic models compared using a d-separation test. Ecology 2013, 94, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Lefcheck, J.S. piecewiseSEM: Piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Grace, J.B.; Schoolmaster, D.R.; Guntenspergen, G.R.; Little, A.M.; Mitchell, B.R.; Miller, K.M.; Schweiger, E.W. Guidelines for a graph-theoretic implementation of structural equation modeling. Ecosphere 2012, 3, 73. [Google Scholar] [CrossRef]
- Shipley, B. Confirmatory path analysis in a generalized multilevel context. Ecology 2009, 90, 363–368. [Google Scholar] [CrossRef]
- Shipley, B. A New Inferential Test for Path Models Based on Directed Acyclic Graphs. Struct. Equ. Model. 2000, 7, 206–218. [Google Scholar] [CrossRef]
- Bostrom, C.; Bonsdorff, E. Zoobenthic community establishment and habitat complexity—The importance of seagrass shoot-density, morphology and physical disturbance for faunal recruitment. Mar. Ecol. Prog. Ser. 2000, 205, 123–138. [Google Scholar] [CrossRef]
- Macreadie, P.I.; Connolly, R.M.; Jenkins, G.P.; Hindell, J.S.; Keough, M.J. Edge patterns in aquatic invertebrates explained by predictive models. Mar. Freshw. Res. 2010, 61, 214. [Google Scholar] [CrossRef]
- Tomas, F.; Abbott, J.M.; Steinberg, C.; Balk, M.; Williams, S.L.; Stachowicz, J.J. Plant genotype and nitrogen loading influence seagrass productivity, biochemistry, and plant-herbivore interactions. Ecology 2011, 92, 1807–1817. [Google Scholar] [CrossRef]
- Lefcheck, J.S.; Marion, S.R.; Lombana, A.V.; Orth, R.J. Faunal Communities are Invariant to Fragmentation in Experimental Seagrass Landscapes. PLoS ONE 2016, 11, e0156550. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.C.; Fourqurean, J.W. Competition between the tropical alga, Halimeda incrassata, and the seagrass, Thalassia testudinum. Aquat. Bot. 2001, 71, 217–232. [Google Scholar] [CrossRef]
- Koch, M.; Bowes, G.; Ross, C.; Zhang, X.H. Climate change and ocean acidification effects on seagrasses and marine macroalgae. Glob. Chang. Biol. 2013, 19, 103–132. [Google Scholar] [CrossRef] [PubMed]
- Blake, R.E.; Duffy, J.E. Grazer diversity affects resistance to multiple stressors in an experimental seagrass ecosystem. Oikos 2010, 119, 1625–1635. [Google Scholar] [CrossRef]
- Jones, D.; Morgan, G. A Field Guide to Crustaceans of Australian Waters; Young, J., Ed.; Reed: Chatswood, NSW, Australia, 1994. [Google Scholar]
- Dauby, P.; Scailteur, Y.; de Broyer, C. Trophic diversity within the Eastern Weddell Sea amphipod community. Hydrobiologia 2001, 443, 69–86. [Google Scholar] [CrossRef]
- Farlin, J.; Lewis, L.; Anderson, T.; Lai, C. Functional diversity in amphipods revealed by stable isotopes in an eelgrass ecosystem. Mar. Ecol. Prog. Ser. 2010, 420, 277–281. [Google Scholar] [CrossRef]
- Jinks, K.I.; Brown, C.J.; Rasheed, M.A.; Scott, A.L.; Sheaves, M.; York, P.H.; Connolly, R.M. Habitat complexity influences the structure of food webs in Great Barrier Reef seagrass meadows. Ecosphere 2019, 10. [Google Scholar] [CrossRef]
- Kharlamenko, V.; Kiyashko, S.; Imbs, A.; Vyshkvartzev, D. Identification of food sources of invertebrates from the seagrass Zostera marina community using carbon and sulfur stable isotope ratio and fatty acid analyses. Mar. Ecol. Prog. Ser. 2001, 220, 103–117. [Google Scholar] [CrossRef]
- Jaschinski, S.; Brepohl, D.; Sommer, U. Carbon sources and trophic structure in an eelgrass Zostera marina bed, based on stable isotope and fatty acid analyses. Mar. Ecol. Prog. Ser. 2008, 358, 103–114. [Google Scholar] [CrossRef]
- York, P.H.; Smith, T.M.; Coles, R.G.; McKenna, S.A.; Connolly, R.M.; Irving, A.D.; Jackson, E.L.; McMahon, K.; Runcie, J.W.; Sherman, C.D.H.; et al. Identifying knowledge gaps in seagrass research and management: An Australian perspective. Mar. Environ. Res. 2017, 127, 163–172. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Altieri, A.H.; Angelini, C.; Bishop, M.J.; Gribben, P.E.; Lear, G.; He, Q.; Schiel, D.R.; Silliman, B.R.; South, P.M.; et al. Secondary foundation species enhance biodiversity. Nat. Ecol. Evol. 2018, 2, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Cadenasso, M.L.; Pickett, S.T.A.; Grove, J.M. Dimensions of ecosystem complexity: Heterogeneity, connectivity, and history. Ecol. Complex. 2006, 3, 1–12. [Google Scholar] [CrossRef]
- Gonzalez, A.; Loreau, M. The Causes and Consequences of Compensatory Dynamics in Ecological Communities. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 393–414. [Google Scholar] [CrossRef]
- Navarro-Barranco, C.; Guerra-García, J.M. Spatial distribution of crustaceans associated with shallow soft-bottom habitats in a coral reef lagoon. Mar. Ecol. 2016, 37, 77–87. [Google Scholar] [CrossRef]
- Spivak, A.; Canuel, E.; Duffy, E. Top-down and bottom-up controls on sediment organic matter composition in an experimental seagrass ecosystem. Limnol. Oceanogr. 2007, 52, 2595–2607. [Google Scholar] [CrossRef]
- Grech, A.; Chartrand-Miller, K.; Erftemeijer, P.; Fonseca, M.; McKenzie, L.; Rasheed, M.; Taylor, H.; Coles, R. A comparison of threats, vulnerabilities and management approaches in global seagrass bioregions. Environ. Res. Lett. 2012, 7, 024006. [Google Scholar] [CrossRef]

| Interaction Grouping | Variables Included within Models | ||
|---|---|---|---|
| Epiphytes | Epiphyte Load (gDW·gDW−1) | ||
| Invertebrate Assemblages | Invertebrate Abundance | Invertebrate Richness (S) | Invertebrate Diversity (H′) |
| Habitat Community | Habitat Biomass (gDW) | Habitat Richness (S) | Habitat Diversity (H′) |
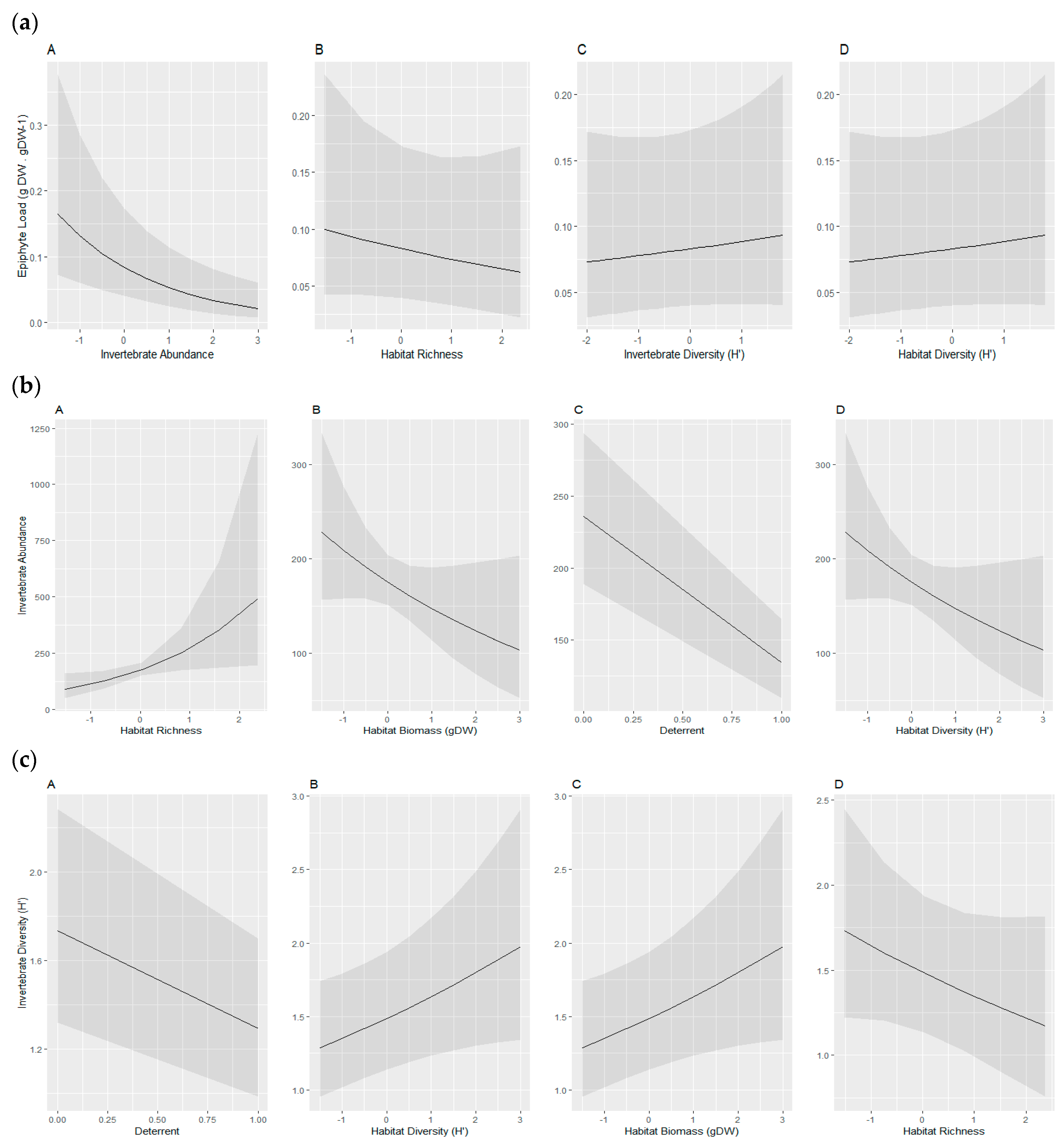
| (a) Epiphyte Load (Epiphyte gDW/Seagrass Biomass gDW−1) Model Coefficients | ||||||||
| Model # | Site (Intercept) | Habitat Diversity | Invertebrate Diversity | Invertebrate Abundance | Habitat Richness | df | AICc | Δ Weight |
| 1 | −2.49 | NA | NA | −0.46 | NA | 4 | −127.1 | 0 |
| 2 | −2.49 | NA | NA | −0.42 | −0.07 | 5 | −124.8 | 1.53 |
| 3 | −2.49 | NA | 0.06 | −0.47 | NA | 5 | −124.8 | 2.08 |
| 4 | −2.49 | 0.02 | NA | −0.47 | NA | 5 | −124.6 | 2.98 |
| Averaged Model | −2.49 | 0.02 | 0.06 | −0.46 | −0.07 | |||
| (b) Invertebrate Abundance Model Coefficients | ||||||||
| Model # | Site (Intercept) | Habitat Diversity | Habitat Richness (S) | Habitat Biomass (gDW) | Deterrent | df | AICc | Δ Weight |
| 1 | 5.46 | NA | 0.57 | −0.23 | −0.55 | 6 | 498.5 | 0 |
| 2 | 5.46 | 0.22 | 0.23 | NA | −0.56 | 6 | 499.9 | 1.36 |
| 3 | 5.46 | 0.13 | 0.44 | −0.18 | −0.56 | 7 | 500.4 | 1.86 |
| 4 | 5.45 | 0.37 | NA | NA | −0.53 | 5 | 500.8 | 2.22 |
| 5 | 5.45 | NA | 0.40 | NA | −0.52 | 5 | 500.9 | 2.43 |
| Averaged Model | 5.46 | 0.23 | 0.45 | −0.22 | −0.55 | |||
| (c) Invertebrate Diversity (H′) Model Coefficients | ||||||||
| Model # | Site (Intercept) | Habitat Diversity | Habitat Richness (S) | Habitat Biomass (gDW) | Deterrent | df | AICc | Δ Weight |
| 1 | 0.54 | NA | NA | NA | −0.27 | 4 | 44.3 | 0 |
| 2 | 0.55 | 0.06 | NA | NA | −0.28 | 5 | 44.5 | 0.17 |
| 3 | 0.54 | NA | NA | 0.05 | −0.28 | 5 | 44.8 | 0.47 |
| 4 | 0.55 | 0.06 | NA | 0.05 | −0.28 | 6 | 45.2 | 0.85 |
| 5 | 0.54 | NA | 0.05 | NA | −0.27 | 5 | 45.7 | 1.40 |
| 6 | 0.55 | 0.12 | −0.10 | 0.09 | −0.29 | 7 | 46.3 | 2.02 |
| 7 | 0.55 | 0.06 | 0.01 | NA | −0.28 | 6 | 47.2 | 2.91 |
| Averaged Model | 0.55 | 0.07 | −0.01 | 0.06 | −0.28 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffmann, L.; Edwards, W.; York, P.H.; Rasheed, M.A. Richness of Primary Producers and Consumer Abundance Mediate Epiphyte Loads in a Tropical Seagrass System. Diversity 2020, 12, 384. https://doi.org/10.3390/d12100384
Hoffmann L, Edwards W, York PH, Rasheed MA. Richness of Primary Producers and Consumer Abundance Mediate Epiphyte Loads in a Tropical Seagrass System. Diversity. 2020; 12(10):384. https://doi.org/10.3390/d12100384
Chicago/Turabian StyleHoffmann, Luke, Will Edwards, Paul H. York, and Michael A. Rasheed. 2020. "Richness of Primary Producers and Consumer Abundance Mediate Epiphyte Loads in a Tropical Seagrass System" Diversity 12, no. 10: 384. https://doi.org/10.3390/d12100384
APA StyleHoffmann, L., Edwards, W., York, P. H., & Rasheed, M. A. (2020). Richness of Primary Producers and Consumer Abundance Mediate Epiphyte Loads in a Tropical Seagrass System. Diversity, 12(10), 384. https://doi.org/10.3390/d12100384





