Diatom Diversity on the Skin of Frozen Historic Loggerhead Sea Turtle Specimens
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Sample Preparation
2.3. Data Analyses
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frankovich, T.A.; Sullivan, M.J.; Stacy, N. Three new species of Tursiocola (Bacillariophyta) from the skin of the West Indian manatee (Trichechus manatus). Phytotaxa 2015, 204, 33–48. [Google Scholar] [CrossRef][Green Version]
- Frankovich, T.A.; Ashworth, M.P.; Sullivan, M.J.; Theriot, E.C.; Stacy, N. Epizoic and apochlorotic Tursiocola species (Bacillariophyta) from the skin of Florida manatees (Trichechus manatus latirostris). Protist 2018, 169, 539–568. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, M.E.; Cefarelli, A.O.; Fazio, A.; Bordino, P.; Romero, O.E. Bennettella ceticola (Nelson ex Bennett) Holmes on the skin of Franciscana dolphin (Pontoporia blainvillei) of the Argentinean Sea: An emendation of the generic description. Diatom Res. 2018, 33, 485–497. [Google Scholar] [CrossRef]
- Majewska, R.; Goosen, W.E. For better, for worse: Manatee-associated Tursiocola (Bacillariophyta) remain faithful to their host. J. Phycol. 2020, 56, 1019–1027. [Google Scholar] [CrossRef]
- Majewska, R.; Santoro, M.; Bolaños, F.; Chaves, G.; De Stefano, M. Diatoms and other epibionts associated with Olive Ridley (Lepidochelys olivacea) sea turtles from the Pacific Coast of Costa Rica. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Majewska, R.; Van de Vijver, B.; Nasrolahi, A.; Ehsanpour, M.; Afkhami, M.; Bolaños, F.; Iamunno, F.; Santoro, M.; De Stefano, M. Shared epizoic taxa and differences in diatom community structure between green turtles (Chelonia mydas) from distant habitats. Microb. Ecol. 2017, 74, 969–978. [Google Scholar] [CrossRef]
- Robinson, N.J.; Majewska, R.; Lazo-Wasem, E.A.; Nel, R.; Paladino, V.; Rojas, L.; Zardus, J.D.; Pinou, T. Epibiotic diatoms are universally present on all sea turtle species. PLoS ONE 2016, 11, e0157011. [Google Scholar] [CrossRef]
- Azari, M.; Farjad, Y.; Nasrolahi, A.; De Stefano, M.; Ehsanpour, M.; Dobrestov, S.; Majewska, R. Diatoms on sea turtles and floating debris in the Persian Gulf (Western Asia). Phycologia 2020, 59, 292–304. [Google Scholar] [CrossRef]
- Kaleli, A.; Car, A.; Witkowski, A.; Krzywda, M.; Riaux-Gobin, C.; Solak, C.N.; Kaska, Y.; Zgłobicka, I.; Płociński, T.; Wróbel, R.; et al. Biodiversity of carapace epibiont diatoms in loggerhead sea turtles (Caretta caretta Linnaeus 1758) in the Aegean Sea Turkish coast. PeerJ 2020, 8, e9406. [Google Scholar] [CrossRef]
- Van de Vijver, B.; Robert, K.; Majewska, R.; Frankovich, T.A.; Panagopoulou, A.; Bosak, S. Geographical variation in the diatom communities associated with loggerhead sea turtles (Caretta caretta). PLoS ONE 2020, 15, e0236513. [Google Scholar] [CrossRef]
- Frankovich, T.A.; Sullivan, M.J.; Stacy, N.I. Tursiocola denysii sp. nov. (Bacillariophyta) from the neck skin of loggerhead sea turtles (Caretta caretta). Phytotaxa 2015, 234, 227–236. [Google Scholar] [CrossRef][Green Version]
- Frankovich, T.A.; Ashworth, M.P.; Sullivan, M.J.; Veselá, J.; Stacy, I. Medlinella amphoroidea gen. et sp. nov. Phytotaxa 2016, 272, 101–114. [Google Scholar] [CrossRef]
- Majewska, R.; Kociolek, J.P.; Thomas, E.W.; de Stefano, M.; Santoro, M.; Bolaños, F.; Van De Vijver, B. Chelonicola and Poulinea, two new gomphonemoid diatom genera (Bacillariophyta) living on marine turtles from costa rica. Phytotaxa 2015, 233, 236–250. [Google Scholar] [CrossRef]
- Majewska, R.; De Stefano, M.; Ector, L.; Bolaños, F.; Frankovich, T.A.; Sullivan, M.J.; Ashworth, M.P.; Van De Vijver, B. Two new epizoic Achnanthes species (Bacillariophyta) living on marine turtles from Costa Rica. Bot. Mar. 2017, 60, 303–318. [Google Scholar] [CrossRef]
- Majewska, R.; De Stefano, M.; Van de Vijver, B. Labellicula lecohuiana, a new epizoic diatom species living on green turtles in Costa Rica. Nov. Hedwigia Beihefte 2018, 146, 23–31. [Google Scholar] [CrossRef]
- Majewska, R.; Bosak, S.; Frankovich, T.A.; Ashworth, M.P.; Sullivan, M.J.; Robinson, N.J.; Lazo-Wasem, E.A.; Pinou, T.; Nel, R.; Manning, S.R.; et al. Six new epibiotic Proschkinia (Bacillariophyta) species and new insights into the genus phylogeny. Eur. J. Phycol. 2019, 54, 609–631. [Google Scholar] [CrossRef]
- Majewska, R.; Robert, K.; Van de Vijver, B.; Nel, R. A new species of Lucanicum (Cyclophorales, Bacillariophyta) associated with loggerhead sea turtles from South Africa. Bot. Lett. 2020, 167, 7–14. [Google Scholar] [CrossRef]
- Riaux-Gobin, C.; Witkowski, A.; Kociolek, J.P.; Ector, L.; Chevallier, D.; Compère, P. New epizoic diatom (Bacillariophyta) species from sea turtles in the Eastern Caribbean and South Pacific. Diatom Res. 2017, 32, 109–125. [Google Scholar] [CrossRef]
- Riaux-Gobin, C.; Witkowski, A.; Chevallier, D.; Daniszewska-Kowalczyk, G. Two new Tursiocola species (Bacillariophyta) epizoic on green turtles (chelonia mydas) in French Guiana and eastern Caribbean. Fottea 2017, 17, 150–163. [Google Scholar] [CrossRef]
- Riaux-Gobin, C.; Witkowski, A.; Kociolek, J.P.; Chevallier, D. Navicula dermochelycola sp. nov., presumably an exclusively epizoic diatom on sea turtles Dermochelys coriacea and Lepidochelys olivacea from French Guiana. Ocean. Hydrob. Stud. 2020, 49, 132–139. [Google Scholar] [CrossRef]
- Kaleli, A.; Krzywda, M.; Wıtkowskı, A.; Rıaux-Gobın, C.; Solak, C.N.; Zgłobıcka, I.; Płocıńskı, T.; Grzonka, J.; Kurzydłowskı, K.J.; Car, A.; et al. New sediment dwelling and epizoic species of Olifantiella (Bacillariophyceae), with an account on the genus ultrastructure based on Focused Ion Beam nanocuts. Fottea 2018, 18, 212–226. [Google Scholar] [CrossRef]
- Robert, K.; Bosak, S.; Van De Vijver, B. Catenula exigua sp. nov., a new marine diatom (Bacillariophyta) species from the Adriatic Sea. Phytotaxa 2019, 414, 113–118. [Google Scholar] [CrossRef]
- Van De Vijver, B.; Bosak, S. Planothidium kaetherobertianum, a new marine diatom (Bacillariophyta) species from the Adriatic Sea. Phytotaxa 2019, 425, 105–112. [Google Scholar] [CrossRef]
- Majewska, R. Tursiocola neliana sp. nov (Bacillariophyceae) epizoic on South African leatherback sea turtles (Dermochelys coriacea) and new observations on the genus Tursiocola. Phytotaxa 2020, 453, 1–5. [Google Scholar]
- Wyneken, J. The External Morphology, Musculoskeletal System, and Neuro-Anatomy of Sea Turtles. In The Biology of Sea Turtles; Lohmann, K.J., Musick, J.A., Wyneken, J., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: New York, NY, USA, 2003; Volume 2, pp. 39–78. ISBN 0-8493-1123-3. [Google Scholar]
- Vitt, L.J.; Caldwell, J.P. Herpetology: An Introductory Biology of Amphibians and Reptiles, 4th ed.; Academic Press: San Diego, CA, USA, 2014; ISBN 978-0-12-386919-7. [Google Scholar]
- Wahl, M. Marine epibiosis. I. Fouling and antifouling: Some basic aspects. Mar. Ecol. Prog. Ser. 1989, 58, 175–189. [Google Scholar] [CrossRef]
- Ingels, J.; Valdes, Y.; Pontes, L.P.; Silva, A.C.; Neres, P.F.; Corrêa, G.V.; Silver-Gorges, I.; Fuentes, M.M.; Gillis, A.; Hooper, L.; et al. Meiofauna life on loggerhead sea turtles-diversely structured abundance and biodiversity hotspots that challenge the meiofauna paradox. Diversity 2020, 12, 203. [Google Scholar] [CrossRef]
- Wu, S.C.; Bergey, E.A. Diatoms on the carapace of common snapping turtles: Luticola spp. dominate despite spatial variation in assemblages. PLoS ONE 2017, 12, e0171910. [Google Scholar] [CrossRef]
- Majewska, R.; Ashworth, M.P.; Lazo-Wasem, E.; Robinson, N.J.; Rojas, L.; Van de Vijver, B.; Pinou, T. Craspedostauros alatus sp. nov., a new diatom (Bacillariophyta) species found on museum sea turtle specimens. Diat. Res. 2018, 33, 229–240. [Google Scholar] [CrossRef]
- Hasle, G.R.; Syvertsen, E.E. Marine Diatoms. In Tomas CR: Identifying Marine Phytoplankton; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Witkowski, A.H.; Lange-Bertalot, H.; Metzeltin, D. Diatom Flora of Marine Coasts. In Annotated Diatom Micrographs, 1st ed.; Lange-Bertalot, H., Ed.; Koeltz Scientific Books: Koenigstein, Germany, 2000; ISBN 3904144103. [Google Scholar]
- Álvarez-Blanco, I.; Blanco, S. Benthic Diatoms from Mediterranean Coasts; Schweizerbart Science Publishers: Stuttgart, Germany, 2014; ISBN 9783443570514. [Google Scholar]
- Hofmann, G.; Werum, M.; Lange-Bertalot, H. Diatomeen im Süßwasser-Benthos von Mitteleuropa: Bestimmungsflora Kieselalgen für die Ökologische Praxis, 2nd ed.; Lange-Bertalot, H., Ed.; Koeltz Scientific Books: Koenigstein, Germany, 2013; ISBN 9783874294317. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. Available online: http://www.algaebase.org (accessed on 29 June 2020).
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E Ltd.: Plymouth, UK, 2008; p. 214. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2015; p. 296. [Google Scholar]
- Round, F.E.; Crawford, R.M.; Mann, D.G. The Diatoms: Biology & Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
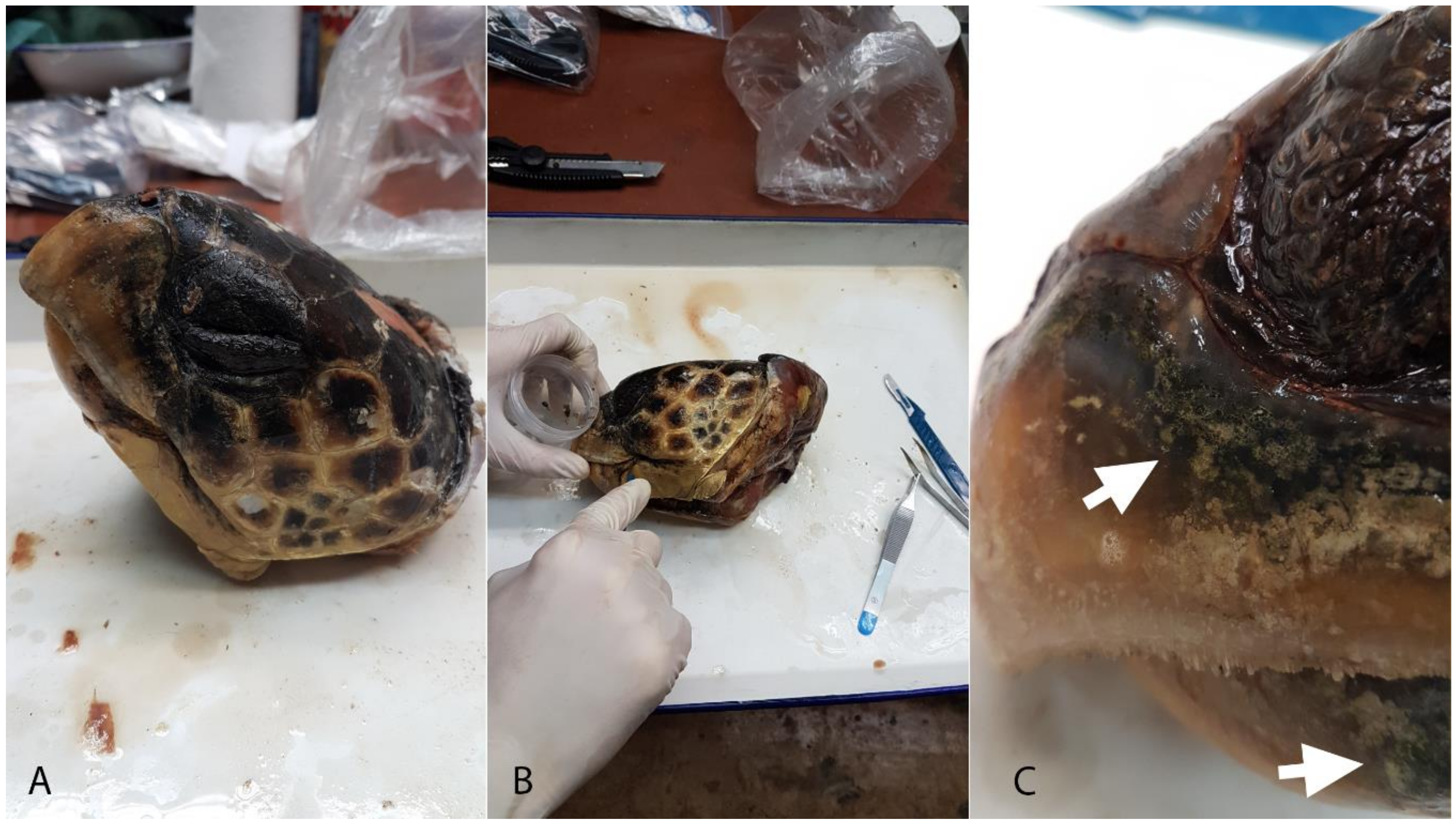
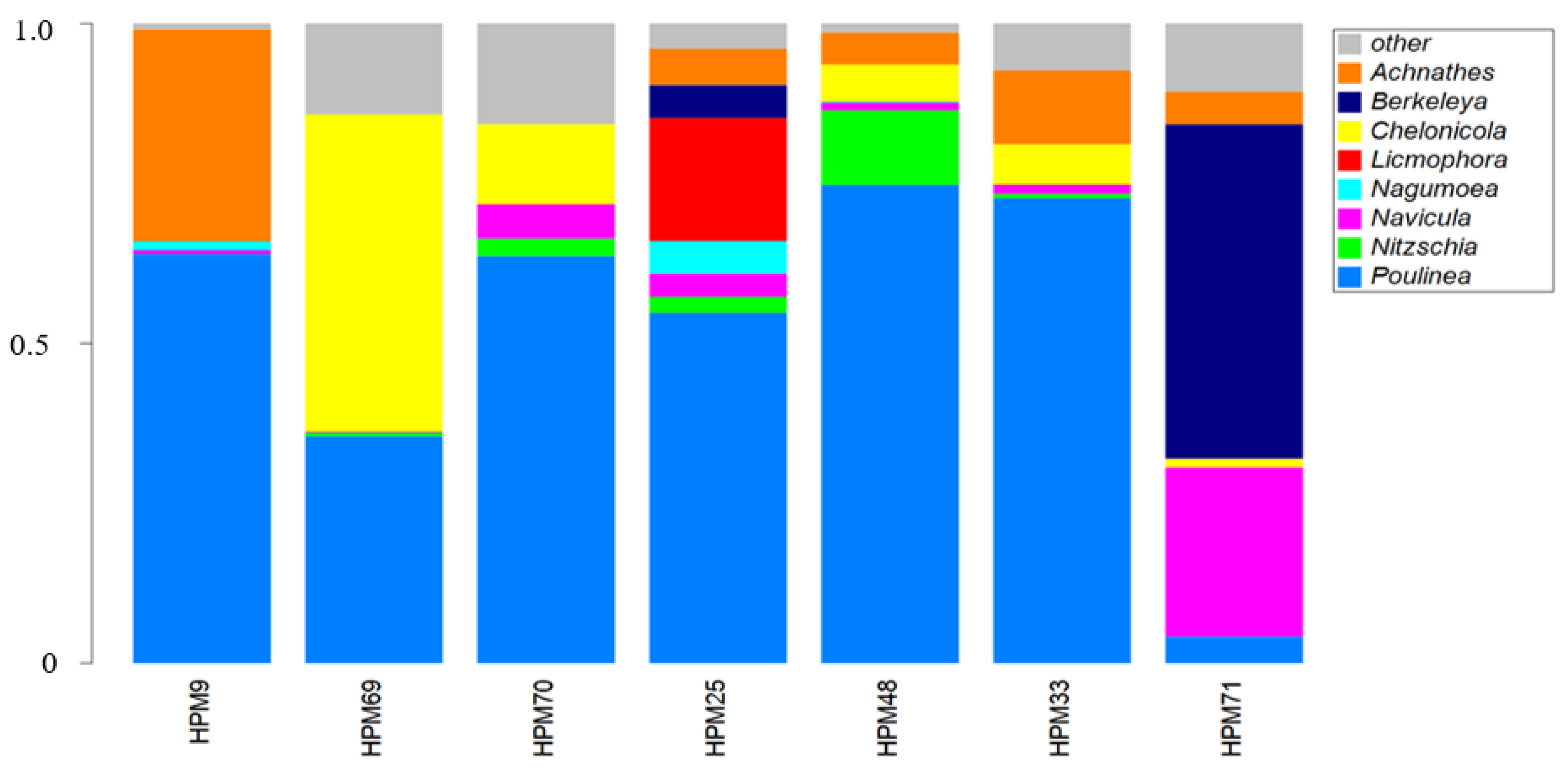
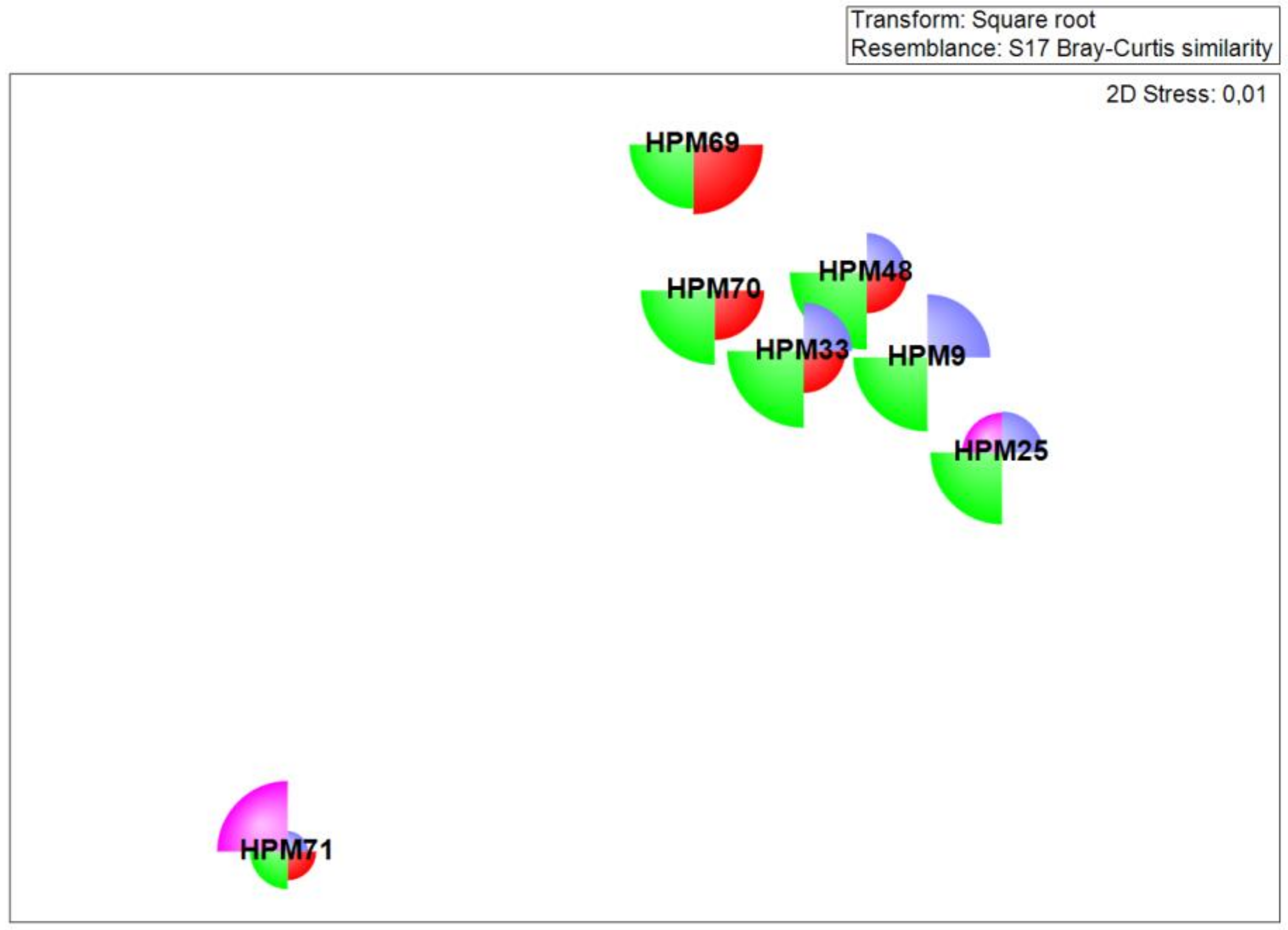
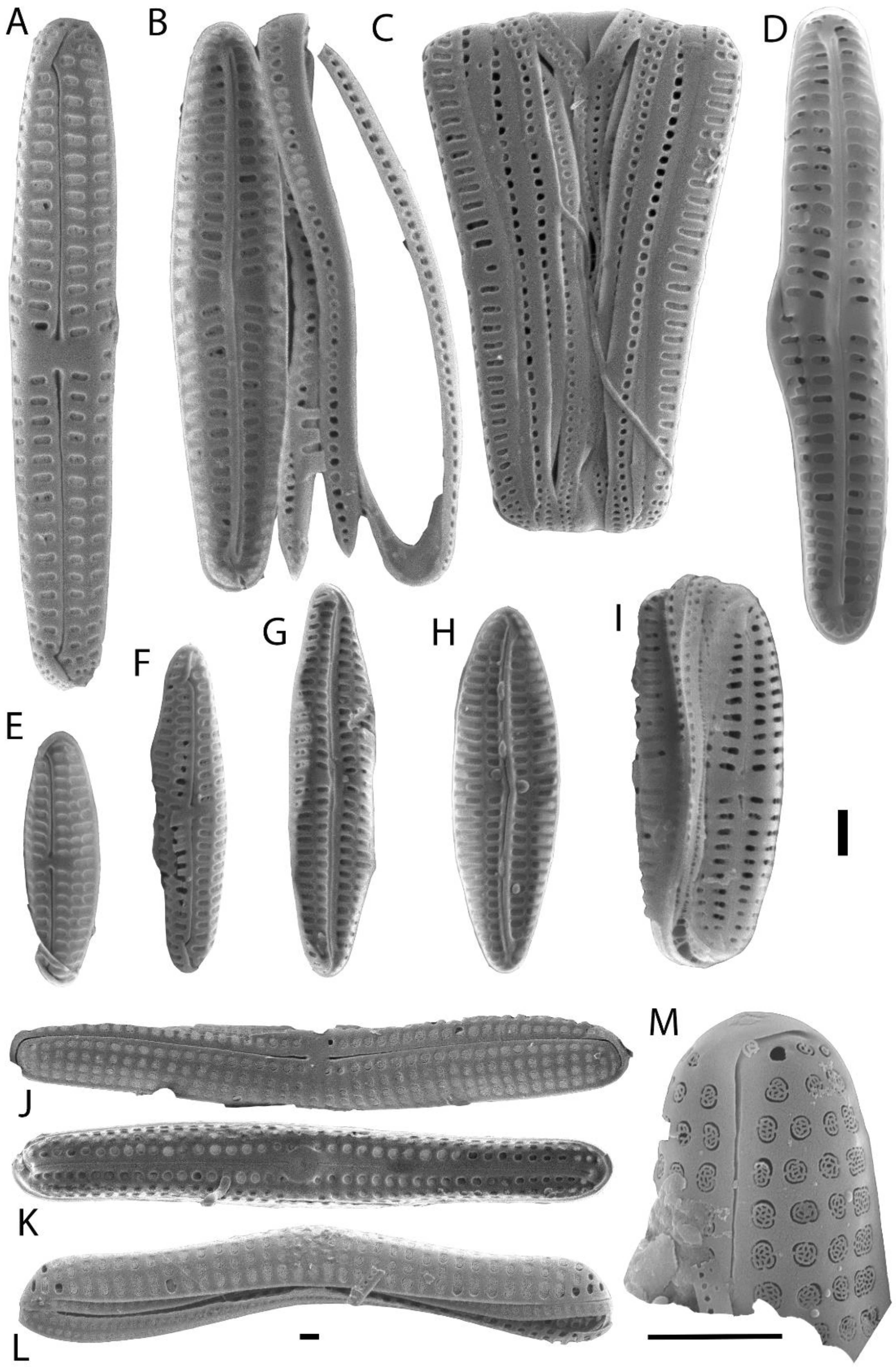
| Sample Code | Locality | Date | Cause of Death | SCCL (cm) | CCW (cm) | Sex |
|---|---|---|---|---|---|---|
| HPM3 | Mali Lošinj, (Croatia) | 10 May 2002 | stranded | 60.7 | 56.8 | ? |
| HPM9 * | Piran Bay (Slovenia) | 1995 | stationary net | 26.6 | 25.0 | male |
| HPM23 | Neretva, Komin (Bosnia and Herzegovina) | 21 June 2001 | stationary gill nets | 57.5 | 53.0 | female |
| HPM24 | Dugi otok (Croatia) | 4 February 2002 | floating in the sea | 58.6 | 51.5 | female |
| HPM25 * | Palagruža (Croatia) | 23 April 2002 | floating in the sea | 84.5 | 75.0 | female |
| HPM31 | Prevlaka, Konavle, (Croatia) | 20 September 2002 | longline | 41.4 | 37.1 | male |
| HPM33 * | Lokrum, (Croatia) | 15 August 2002 | gill net | 40.4 | 37.0 | female |
| HPM44 | Zabodarski Bay, Lošinj (Croatia) | 1 December 2003 | stranded in the beach | 63.0 | 58.8 | female |
| HPM48 * | Poreč (Croatia) | 19 October 2002 | stranded | 79.2 | 69.2 | female |
| HPM67 | Pula (Croatia) | 1 June 2003 | floating in the sea | 58.2 | 53.2 | male |
| HPM68 | North Adriatic (Croatia) | unknown | unknown | 47.7 | 41.8 | male |
| HPM69 * | Medulin (Croatia) | 21 May 2004 | stranded | 51.3 | 46.3 | female |
| HPM70 * | Krk (Croatia) | 2 June 2004 | unknown | 38.2 | 35.5 | female |
| HPM71 * | Mali Lošinj, (Croatia) | 19 May 2004 | unknown | 32.7 | 28.8 | male |
| Turtle Sample Code | S | N | d | J’ | H’ |
|---|---|---|---|---|---|
| HPM9 | 7 | 413 | 1.00 | 0.41 | 0.80 |
| HPM69 | 30 | 414 | 4.81 | 0.43 | 1.45 |
| HPM70 | 35 | 432 | 5.60 | 0.44 | 1.57 |
| HPM25 | 20 | 325 | 3.27 | 0.55 | 1.64 |
| HPM48 | 15 | 421 | 2.32 | 0.39 | 1.05 |
| HPM33 | 19 | 286 | 3.18 | 0.38 | 1.12 |
| HPM71 | 19 | 291 | 3.17 | 0.52 | 1.53 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanjer, L.; Majewska, R.; Van de Vijver, B.; Gračan, R.; Lazar, B.; Bosak, S. Diatom Diversity on the Skin of Frozen Historic Loggerhead Sea Turtle Specimens. Diversity 2020, 12, 383. https://doi.org/10.3390/d12100383
Kanjer L, Majewska R, Van de Vijver B, Gračan R, Lazar B, Bosak S. Diatom Diversity on the Skin of Frozen Historic Loggerhead Sea Turtle Specimens. Diversity. 2020; 12(10):383. https://doi.org/10.3390/d12100383
Chicago/Turabian StyleKanjer, Lucija, Roksana Majewska, Bart Van de Vijver, Romana Gračan, Bojan Lazar, and Sunčica Bosak. 2020. "Diatom Diversity on the Skin of Frozen Historic Loggerhead Sea Turtle Specimens" Diversity 12, no. 10: 383. https://doi.org/10.3390/d12100383
APA StyleKanjer, L., Majewska, R., Van de Vijver, B., Gračan, R., Lazar, B., & Bosak, S. (2020). Diatom Diversity on the Skin of Frozen Historic Loggerhead Sea Turtle Specimens. Diversity, 12(10), 383. https://doi.org/10.3390/d12100383






