Effects of Temperature and pH on the Egg Production and Hatching Success of a Common Korean Copepod
Abstract
1. Introduction
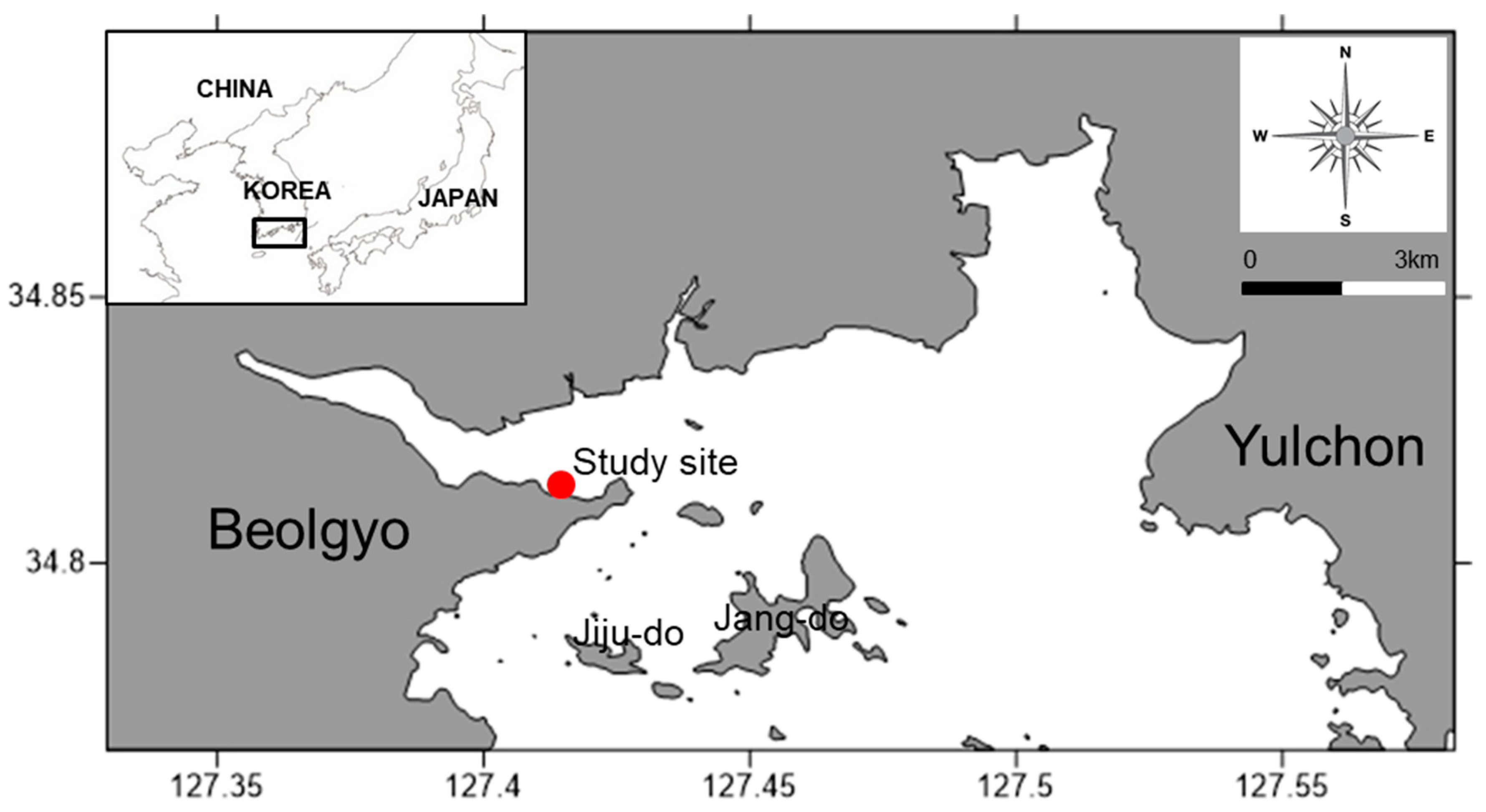
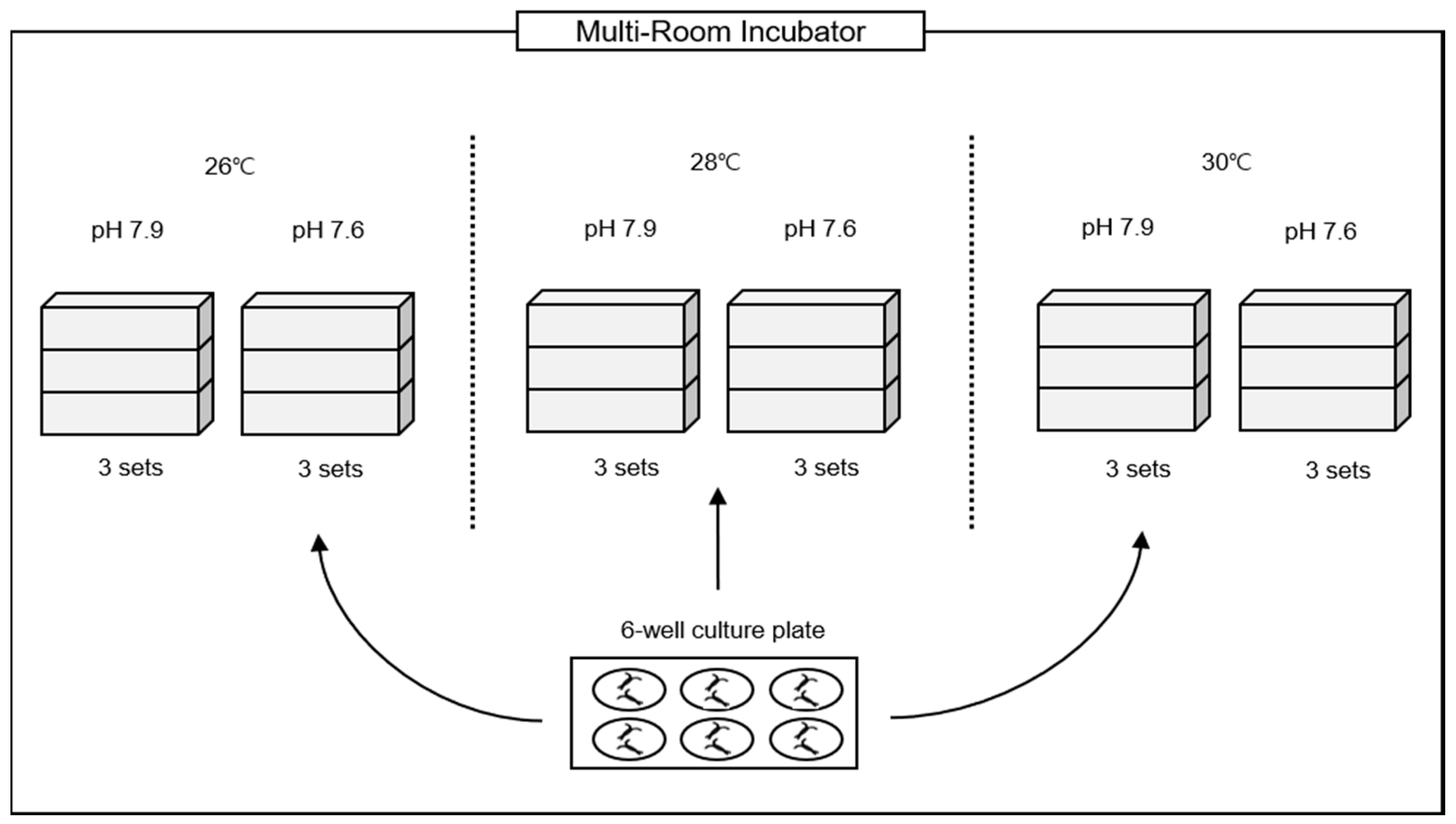
2. Materials and Methods
3. Results
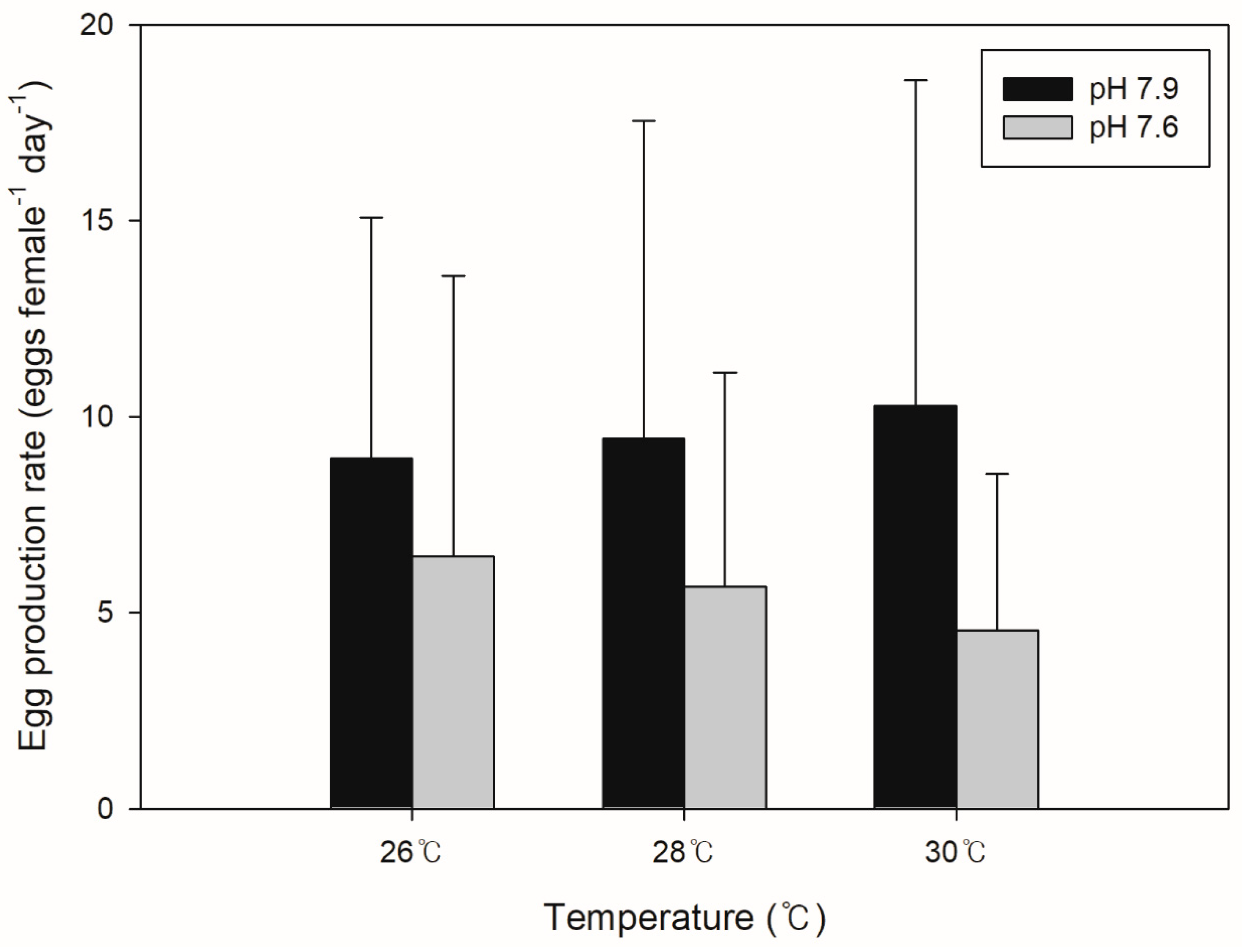
3.1. Egg Production Rate (EPR)
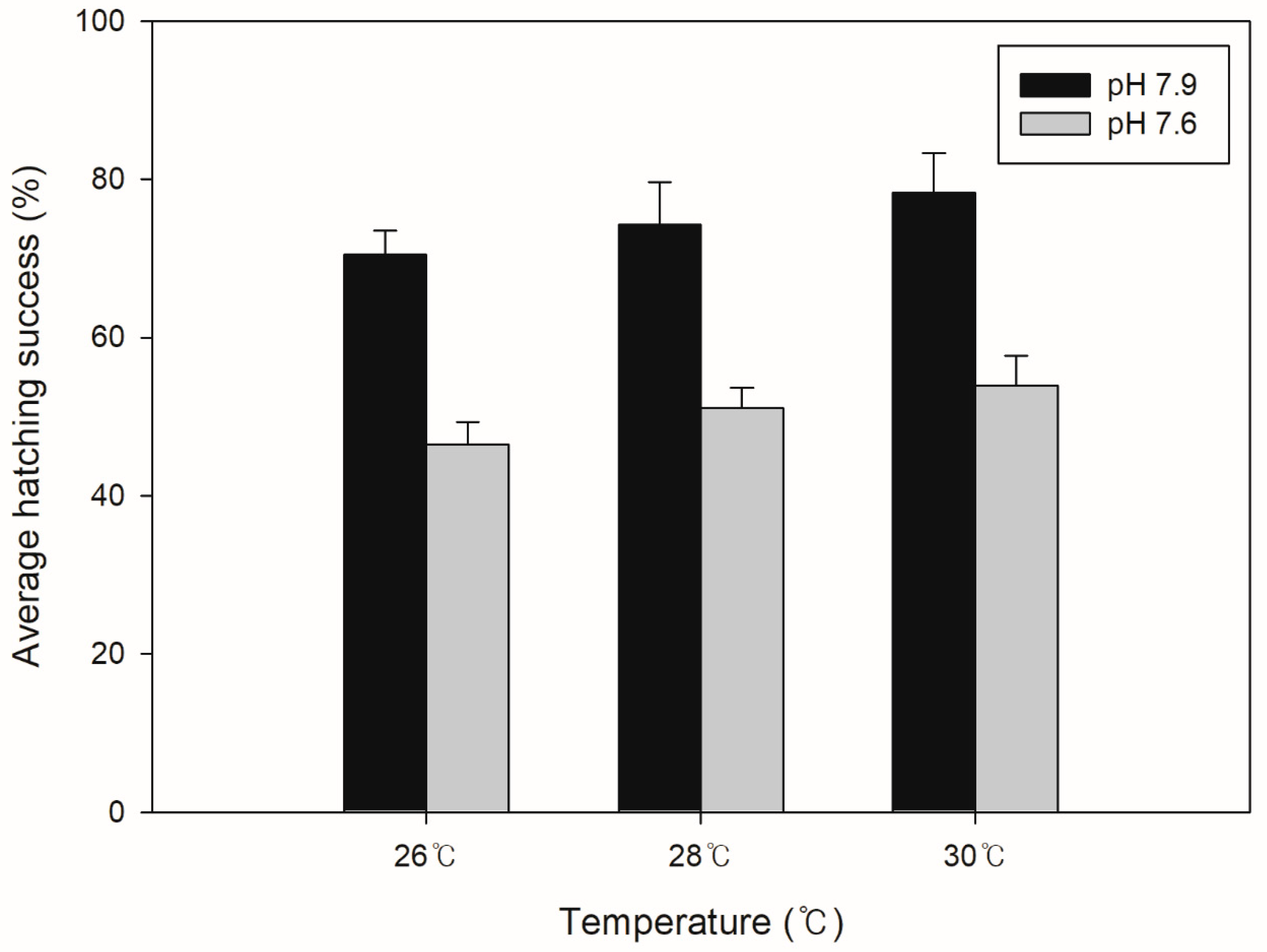
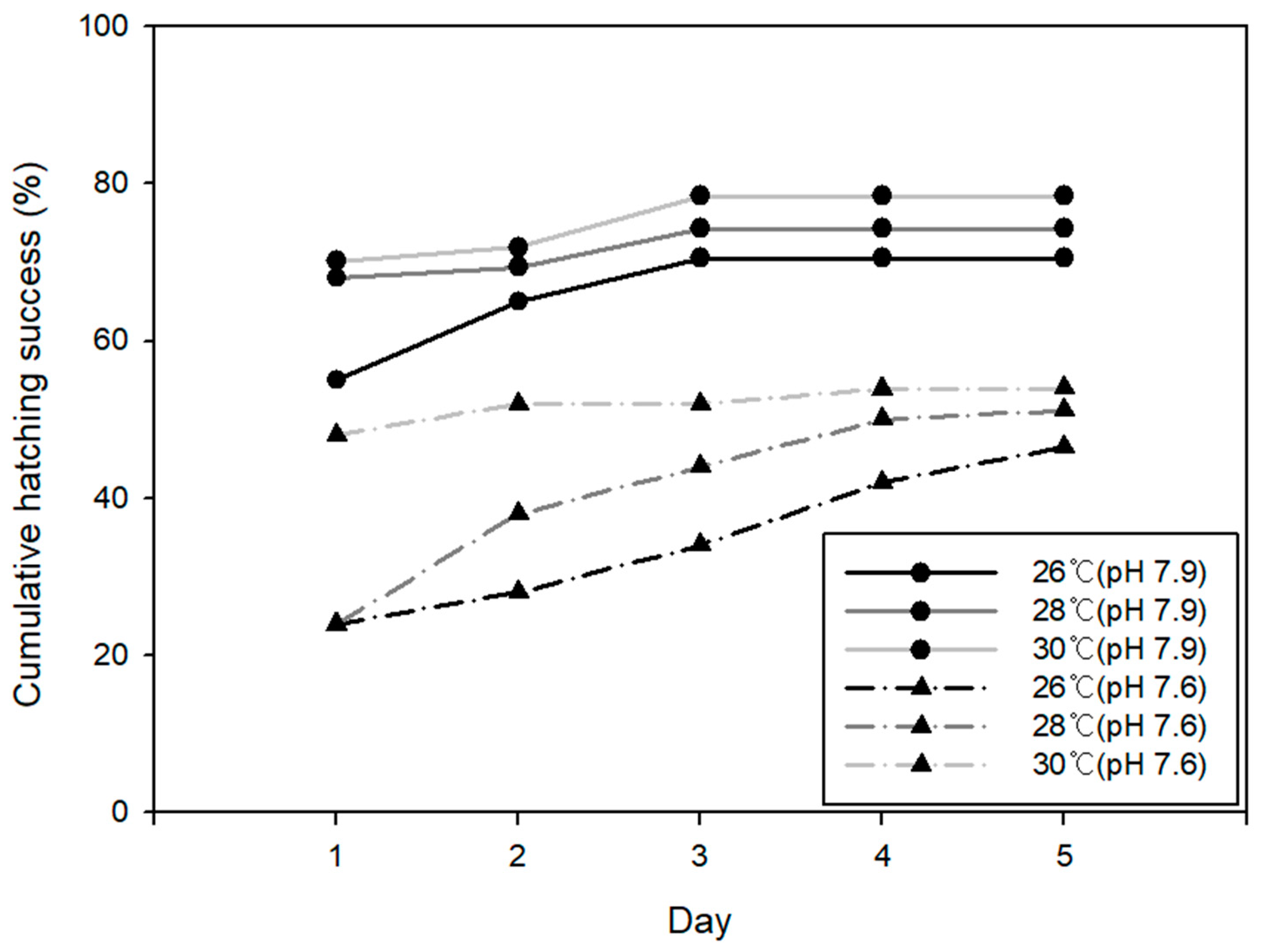
3.2. Egg Hatching Success (EHS)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Collins, M.; Knutti, R.; Arblaster, J.; Dufresne, J.-L.; Fichefet, T.; Friedlingstein, P.; Gao, X.; Gutowski, W.J.; Johns, T.; Krinner, G.; et al. Long-term climate change: Projections, commitments and irreversibility. In Climate Change 2013—The Physical Science Basis: Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; pp. 1029–1136. [Google Scholar]
- Munday, P.L.; Dixson, D.L.; McCormick, M.I.; Meekan, M.; Ferrari, M.C.; Chivers, D.P. Replenishment of fish populations is threatened by ocean acidification. Proc. Natl. Acad. Sci. USA 2010, 107, 12930–12934. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, G.E.; Dixson, D.L.; Domenici, P.; McCormick, M.I.; Sørensen, C.; Watson, S.A.; Munday, P.L. Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nat. Clim. Chang. 2012, 2, 201–204. [Google Scholar] [CrossRef]
- Kurihara, H.; Shimode, S.; Shirayama, Y. Effects of raised CO2 concentration on the egg production rate and early development of two marine copepods (Acartia steueri and Acartia erythraea). Mar. Pollut. Bull. 2004, 49, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Feely, R.A.; Sabine, C.L.; Lee, K.; Berelson, W.; Kleypas, J.; Fabry, V.J.; Millero, F.J. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 2004, 305, 362–366. [Google Scholar] [CrossRef]
- Michaelidis, B.; Ouzounis, C.; Paleras, A.; Pörtner, H.O. Effects of long-term moderate hypercapnia on acid–base balance and growth rate in marine mussels Mytilus galloprovincialis. Mar. Ecol. Prog. Ser. 2005, 293, 109–118. [Google Scholar] [CrossRef]
- Anthony, K.R.N.; Kline, D.I.; Diaz-Pulido, G.; Dove, S.; Hoegh-Guldberg, O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl. Acad. Sci. USA 2008, 105, 17442–17446. [Google Scholar] [CrossRef]
- Michaelidis, B.; Spring, A.; Pörtner, H.O. Effects of long-term acclimation to environmental hypercapnia on extracellular acid–base status and metabolic capacity in Mediterranean fish Sparus aurata. Mar. Biol. 2007, 150, 1417–1429. [Google Scholar] [CrossRef]
- Park, C.; Kim, K.H.; Moon, H.N.; Yeo, I.K. The physiological responses of spotted seahorse Hippocampus kuda to low-pH water. J. Life Sci. 2017, 27, 826–833. [Google Scholar] [CrossRef]
- Pond, D.; Harris, R.; Head, R.; Harbour, D. Environmental and nutritional factors determining seasonal variability in the fecundity and egg viability of Calanus helgolandicus in coastal waters off Plymouth, UK. Mar. Ecol. Prog. Ser. 1996, 143, 45–63. [Google Scholar] [CrossRef]
- Devreker, D.; Souissi, S.; Seuront, L. Development and mortality of the first naupliar stages of Eurytemora affinis (Copepoda, Calanoida) under different conditions of salinity and temperature. J. Exp. Mar. Biol. Ecol. 2004, 303, 31–46. [Google Scholar] [CrossRef]
- Holste, L.; Peck, M.A. The effects of temperature and salinity on egg production and hatching success of Baltic Acartia tonsa (Copepoda: Calanoida): A laboratory investigation. Mar. Biol. 2006, 148, 1061–1070. [Google Scholar] [CrossRef]
- Peck, M.A.; Holste, L. Effects of salinity, photoperiod and adult stocking density on egg production and egg hatching success in Acartia tonsa (Calanoida: Copepoda): Optimizing intensive cultures. Aquaculture 2006, 255, 341–350. [Google Scholar] [CrossRef]
- Kang, H.K.; Kang, Y.J. Egg production of the copepod Acartia steueri in Ilkwang Bay, Southeastern Coast of Korea. J. Korean Fish. Soc. 1998, 31, 288–295. [Google Scholar]
- Miralto, A.; Ianora, A.; Buttino, I.; Romano, G.; Di Pinto, M. Egg production and hatching success in North Adriatic Sea populations of the copepod Acartia clausi. Chem. Ecol. 2002, 18, 117–125. [Google Scholar] [CrossRef]
- Jonasdottir, S.H.; Kiorboe, T. Copepod recruitment and food composition: Do diatoms affect hatching success? Mar. Biol. 1996, 125, 743–750. [Google Scholar] [CrossRef]
- Halsband, C.; Hirche, H.J. Reproductive cycles of dominant calanoid copepods in the North Sea. Mar. Ecol. Prog. Ser. 2001, 209, 219–229. [Google Scholar] [CrossRef]
- Diversity and Geographic Distribution of Marine Planktonic Copepods. Available online: http://copepodes.obs-banyuls.fr/en (accessed on 2 August 2020).
- Castro-Longoria, E. Egg production and hatching success of four Acartia species under different temperature and salinity regimes. J. Crustac. Biol. 2003, 23, 289–299. [Google Scholar] [CrossRef]
- Shayegan, M.; Esmaeili, F.A.; Agh, N.; Jani, K.K. Effects of salinity on egg and fecal pellet production, development and survival, adult sex ratio and life span in the calanoid copepod Acartia tonsa: A laboratory study. Chin. J. Oceanol. Limnol. 2016, 34, 709–718. [Google Scholar] [CrossRef]
- Engström-Öst, J.; Holmborn, T.; Brutemark, A.; Hogfors, H.; Vehmaa, A.; Gorokhova, E. The effects of short-term pH decrease on the reproductive output of the copepod Acartia bifilosa–a laboratory study. Mar. Freshw. Behav. Physiol. 2014, 47, 173–183. [Google Scholar] [CrossRef]
- Camus, T.; Zeng, C. Effects of photoperiod on egg production and hatching success, naupliar and copepodite development, adult sex ratio and life expectancy of the tropical calanoid copepod Acartia sinjiensis. Aquaculture 2008, 280, 220–226. [Google Scholar] [CrossRef]
- Turner, J.T.; Ianora, A.; Miralto, A.; Laabir, M.; Esposito, F. Decoupling of copepod grazing rates, fecundity and egg-hatching success on mixed and alternating diatom and dinoflagellate diets. Mar. Ecol. Prog. Ser. 2001, 220, 187–199. [Google Scholar] [CrossRef]
- Vehmaa, A.; Brutemark, A.; Engström-Öst, J. Maternal effects may act as an adaptation mechanism for copepods facing pH and temperature changes. PLoS ONE 2012, 7, e48538. [Google Scholar] [CrossRef] [PubMed]
- Zervoudaki, S.; Frangoulis, C.; Giannoudi, E.; Krasakopoulou, E. Effects of low pH and raised temperature on egg production, hatching and metabolic rates of a Mediterranean copepod species (Acartia clausi) under oligotrophic conditions. Mediterr. Mar. Sci. 2013, 15, 74–83. [Google Scholar] [CrossRef][Green Version]
- Youn, S.H.; Choi, J.K. Distribution pattern of zooplankton in the Han River estuary with respect to tidal cycle. Ocean Sci. J. 2008, 43, 135–146. [Google Scholar] [CrossRef]
- Moon, S.Y.; Seo, M.H.; Shin, Y.; Soh, H.Y. Seasonal variation of mesozooplankton communities in the semi-enclosed Muan bay, Korea. Ocean Polar Res. 2012, 34, 1–18. [Google Scholar] [CrossRef]
- Park, E.O.; Suh, H.L.; Soh, H.Y. Spatio-temporal distribution of Acartia (Copepoda: Calanoida) species along a salinity gradient in the Seomjin River estuary, South Korea. J. Nat. Hist. 2015, 49, 2799–2812. [Google Scholar] [CrossRef]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical and Biological Methods for Seawater Analysis; Pergamon Press: Oxford, UK, 1984; p. 173. [Google Scholar]
- Guisande, C.; Riveiro, I.; Maneiro, I. Comparisons among the amino acid composition of females, eggs and food to determine the relative importance of food quantity and food quality to copepod reproduction. Mar. Ecol. Prog. Ser. 2002, 202, 135–142. [Google Scholar] [CrossRef]
- Shin, K.; Jang, M.C.; Jang, P.K.; Ju, S.J.; Lee, T.K.; Chang, M. Influence of food quality on egg production and viability of the marine planktonic copepod Acartia omorii. Prog. Oceanogr. 2003, 57, 265–277. [Google Scholar] [CrossRef]
- Devreker, D.; Souissi, S.; Winkler, G.; Forget-Leray, J.; Leboulenger, F. Effects of salinity, temperature and individual variability on the reproduction of Eurytemora affinis (Copepoda; Calanoida) from the Seine estuary: A laboratory study. J. Exp. Mar. Biol. Ecol. 2009, 368, 113–123. [Google Scholar] [CrossRef]
- Kang, H.K.; Lee, C.R.; Kim, D.; Yoo, S. Effect of enhanced pCO2 and temperature on reproduction and survival of the copepod Calanus sinicus. Ocean Polar Res. 2016, 38, 303–314. [Google Scholar] [CrossRef][Green Version]
- Uye, S.I. Fecundity studies of neritic calanoid copepods Acartia clausi Giesbrecht and Acartia steueri Smirnov—A simple empirical-model of daily egg-production. J. Exp. Mar. Biol. Ecol. 1981, 50, 255–271. [Google Scholar] [CrossRef]
- Ambler, J.W. Effect of food quantity and quality on egg production of Acartia tonsa Dana from East Lagoon, Galveston, Texas. Estuar. Coast. Shelf Sci. 1986, 23, 183–193. [Google Scholar] [CrossRef]
- Liang, D.; Uye, S. Population dynamics and production of the planktonic copepods in a eutrophic inlet of the Inland Sea of Japan. II. Acartia omorii. Mar. Biol. 1996, 125, 109–117. [Google Scholar] [CrossRef]
- Jung, Y.; Kang, H.K.; Kang, Y.J. In situ egg production rate of the planktonic copepod Acartia steueri in Ilkwang Bay, southeastern coast of Korea. J. Plankton Res. 2004, 26, 1547–1553. [Google Scholar] [CrossRef]
- Zhang, D.; Li, S.; Wang, G.; Guo, D. Impacts of CO2-driven seawater acidification on survival, egg production rate and hatching success of four marine copepods. Acta Oceanol. Sin. 2011, 30, 86–94. [Google Scholar] [CrossRef]
- Roddie, B.D.; Leakey, R.J.G.; Berry, A.J. Salinity-temperature tolerance and osmoregulation in Eurytemora affinis (Poppe) (Copepoda: Calanoida) in relation to its distribution in the zooplankton of the upper reaches of the Forth estuary. J. Exp. Mar. Biol. Ecol. 1984, 79, 191–211. [Google Scholar] [CrossRef]
- Støttrup, J.G.; Jensen, J. Influence of algal diet on feeding and egg-production of the calanoid copepod Acartia tonsa Dana. J. Exp. Mar. Biol. Ecol. 1990, 141, 87–105. [Google Scholar] [CrossRef]
- Karlsson, K.; Puiac, S.; Winder, M. Life-history responses to changing temperature and salinity of the Baltic Sea copepod Eurytemora affinis. Mar. Biol. 2018, 165, 30. [Google Scholar] [CrossRef]
- Mayor, D.J.; Matthews, C.; Cook, K.; Zuur, A.F.; Hay, S. CO2-induced acidification affects hatching success in Calanus finmarchicus. Mar. Ecol. Prog. Ser. 2007, 350, 91–97. [Google Scholar] [CrossRef]
- McConville, K.; Halsband, C.; Fileman, E.S.; Somerfield, P.J.; Findlay, H.S.; Spicer, J.I. Effects of elevated CO2 on the reproduction of two calanoid copepods. Mar. Pollut. Bull. 2013, 73, 428–434. [Google Scholar] [CrossRef]
- Uye, S.I. Resting egg production as a life history strategy of marine planktonic copepods. Bull. Mar. Sci. 1985, 37, 440–449. [Google Scholar]
- Chen, F.; Marcus, N.H. Subitaneous, diapause, and delayed-hatching eggs of planktonic copepods from the northern Gulf of Mexico: Morphology and hatching success. Mar. Biol. 1997, 127, 587–597. [Google Scholar] [CrossRef]
- Santella, L.; Ianora, A. Subitaneous and diapause eggs in Mediterranean populations of Pontella mediterranea (Copepoda: Calanoida): A morphological study. Mar. Biol. 1990, 105, 83–90. [Google Scholar] [CrossRef]
- Belmonte, G. Diapause egg production in Acartia (Paracartia) latisetosa (Crustacea, Copepoda, Calanoida). Boll. Zool. 1992, 59, 363–366. [Google Scholar] [CrossRef]
- Belmonte, G. Resting eggs in the life cycle of Acartia italica and A. adriatica (Copepoda, Calanoida, Acartiidae). Crustaceana 1997, 70, 114–117. [Google Scholar] [CrossRef]
- Belmonte, G.; Puce, M. Morphological aspects of subitaneous and resting eggs from Acartia josephinae (Calanoida). Hydrobiologia 1994, 292/293, 131–135. [Google Scholar] [CrossRef]
- Onoue, Y.; Toda, T.; Ban, S. Morphological features and hatching patterns of eggs in Acartia steueri (Crustacea, Copepoda) from Sagami Bay, Japan. Hydrobiologia 2004, 511, 17–25. [Google Scholar] [CrossRef]
- Nakajima, R.; Yoshida, T.; Sakaguchi, S.O.; Othman, B.H.R.; Toda, T. Spiny but subitaneous eggs: Egg morphology and hatching in Acartia copepods in the tropics. Zool. Stud. 2019, 58, e5. [Google Scholar] [CrossRef]
- Breitburg, D.L.; Salisbury, J.; Bernhard, J.M.; Cai, W.J.; Dupont, S.; Doney, S.C.; Kroeker, K.J.; Levin, W.C.; Long, L.M.; Miller, S.H. And on top of all that Coping with ocean acidification in the midst of many stressors. Oceanography 2015, 28, 48–61. [Google Scholar] [CrossRef]
- Cripps, G.; Lindeque, P.; Flynn, K.J. Have we been underestimating the effects of ocean acidification in zooplankton? Glob. Chang. Biol. 2014, 20, 3377–3385. [Google Scholar] [CrossRef]
- Dam, H.G. Evolutionary adaptation of marine zooplankton to global change. Annu. Rev. Mar. Sci. 2013, 5, 349–370. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, H.; Ishimatsu, A. Effects of high CO2 seawater on the copepod (Acartia tsuensis) through all life stages and subsequent generations. Mar. Pollut. Bull. 2008, 56, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Weydmann, A.; Søreide, J.E.; Kwasniewski, S.; Widdicombe, S. Influence of CO2-induced acidification on the reproduction of a key Arctic copepod Calanus glacialis. J. Exp. Mar. Biol. Ecol. 2012, 428, 39–42. [Google Scholar] [CrossRef]
- Vehmaa, A.; Hogfors, H.; Gorokhova, E.; Brutemark, A.; Holmborn, T.; Engström-Öst, J. Projected marine climate change: Effects on copepod oxidative status and reproduction. Ecoll. Evol. 2013, 3, 4548–4557. [Google Scholar] [CrossRef] [PubMed]
- Cripps, G.; Lindeque, P.; Flynn, K. Parental exposure to elevated pCO2 influences the reproductive success of copepods. J. Plankton Res. 2014, 36, 1165–1174. [Google Scholar] [CrossRef]
- Vehmaa, A.; Almén, A.K.; Brutemark, A.; Paul, A.; Riebesell, U.; Furuhagen, S.; Engstrom-Ost, J. Ocean acidification challenges copepod phenotypic plasticity. Biogeosciences 2016, 13, 6171–6182. [Google Scholar] [CrossRef]
| Temperature (°C) | Salinity | pH | Chl-a (µgL−1) | |
|---|---|---|---|---|
| In this study area | 28.08 | 27.02 | 7.92 | 3.02 |
| pH 7.9 | pH 7.6 | |||||
|---|---|---|---|---|---|---|
| Cell Number | 26 °C | 28 °C | 30 °C | 26 °C | 28 °C | 30 °C |
| 1–1 | 12 | 3 | 17 | 0 | 4 | 1 |
| 1–2 | 7 | 1 | 10 | 17 | 5 | 3 |
| 1–3 | 6 | 5 | 9 | 18 | 4 | 10 |
| 1–4 | 7 | 9 | 6 | 9 | 13 | 5 |
| 1–5 | 2 | 7 | 14 | 2 | 1 | 5 |
| 1–6 | 0 | 10 | 5 | 18 | 0 | 6 |
| 2–1 | 9 | 20 | 12 | 0 | 6 | 9 |
| 2–2 | 21 | 0 | 9 | 16 | 7 | 0 |
| 2–3 | 5 | 0 | 0 | 7 | 8 | 7 |
| 2–4 | 13 | 22 | 4 | 2 | 2 | 2 |
| 2–5 | 16 | 3 | 13 | 0 | 0 | 7 |
| 2–6 | 15 | 4 | 8 | 1 | 0 | 7 |
| 3–1 | 3 | 1 | 6 | 7 | 12 | 4 |
| 3–2 | 9 | 24 | 13 | 0 | 18 | 0 |
| 3–3 | 20 | 13 | 0 | 3 | 14 | 0 |
| 3–4 | 2 | 16 | 11 | 1 | 6 | 14 |
| 3–5 | 5 | 20 | 10 | 15 | 2 | 0 |
| 3–6 | 9 | 12 | 38 | 0 | 0 | 2 |
| Total eggs | 161 | 170 | 185 | 116 | 102 | 82 |
| SD (±) | 6.13 | 8.10 | 8.29 | 7.15 | 5.46 | 3.99 |
| Average | 8.94 | 9.44 | 10.28 | 6.44 | 5.67 | 4.56 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.H.; Choi, S.Y.; Seo, M.H.; Lee, S.J.; Soh, H.Y. Effects of Temperature and pH on the Egg Production and Hatching Success of a Common Korean Copepod. Diversity 2020, 12, 372. https://doi.org/10.3390/d12100372
Lee EH, Choi SY, Seo MH, Lee SJ, Soh HY. Effects of Temperature and pH on the Egg Production and Hatching Success of a Common Korean Copepod. Diversity. 2020; 12(10):372. https://doi.org/10.3390/d12100372
Chicago/Turabian StyleLee, Eun Hye, Seo Yeol Choi, Min Ho Seo, Seok Ju Lee, and Ho Young Soh. 2020. "Effects of Temperature and pH on the Egg Production and Hatching Success of a Common Korean Copepod" Diversity 12, no. 10: 372. https://doi.org/10.3390/d12100372
APA StyleLee, E. H., Choi, S. Y., Seo, M. H., Lee, S. J., & Soh, H. Y. (2020). Effects of Temperature and pH on the Egg Production and Hatching Success of a Common Korean Copepod. Diversity, 12(10), 372. https://doi.org/10.3390/d12100372





