Abstract
Rodent assemblages have ecological importance in ecosystem functioning and protected area management. Our study examines the patterns of assemblages of rodents across four habitat types (i.e., Miombo woodland, Acacia woodland, grasslands and farmlands) in the savanna environment. Capture-mark-recapture (CMR) methods were applied for data collection across the Chembe Bird Sanctuary (CBS) landscape. The Non-metric Multi-Dimensional Scaling (NMDS) was used for exploratory data analysis, followed by Analysis of Variance (ANOVA) and Tukey–Kramer’s Honestly Significant Difference (HSD) post-hoc tests. The rodent assemblages in CBS significantly differed between the non-farmlands (i.e., Miombo woodland, Acacia woodland and grasslands) and farmlands. There were: (1) zero rodent diversity in farmlands, dominated completely by a pest species, M. natalensis; and (2) different rodent assemblages in three non-farmland habitat types. We suggest that rodent assemblages should be mediated by conservation planning and multi-stakeholder collaboration beyond the protected area boundaries to contribute to a working CBS landscape positively.
1. Introduction
Small mammals, defined as mammalian fauna with body mass less than 200 g, such as rodents [1], play an important ecological role in shaping the structure, composition and diversity of landscapes [2]. Rodents (Order: Rodentia) contribute positively to plant cover, and soil chemical and physical properties [3,4], and impact on landscapes through pollination and dispersal of seeds and fungal spores [5,6]. The rodents form a prey base to predators, such as snakes and birds of prey [7] and insects [8]. The rodents play a critical role in the food web in the savanna, as numerous predators depend on them as a source of food [9]. The rodent assemblage may be affected by the amount of vegetation cover protecting rodents from predators, and food availability for their survival in different habitat types, including farmlands [10,11,12]. The rodent diversity would be affected by the functional spatial heterogeneity of habitats they explore and occupy [13]. Such environmental factors, such as fire, vegetation types, and season, may proffer cues to rodents to utilise, or not, a particular space in time for food and denning [14,15,16,17]. Seasons may affect the availability of dietary items, such as seeds, flowers, and fruits, for rodents [12]. Therefore, the use of different habitat types by rodents may be indicative of the landscape integrity [18] or ecosystem changes [19], based on the species assemblages [20].
Human-dominated landscapes can potentially reduce the assemblages and diversity of fauna [21], through the land conversion of natural landscapes to agriculture [22]. However, small mammals in agricultural areas may utilise vegetation remnants [23], and available food, which contribute to increased biodiversity across the landscapes [24,25]. At a landscape level, consisting of several ecosystems, such as wetland/grasslands, woodland and agro-ecosystems, rodents are expected to adapt their behaviour to avoid predation, and effectively search for food, mates, shelter and a suite of environmental conditions for their survival [26], thereby affecting their distribution. For instance, the rodents will endeavour to maximise their foraging benefits under given conditions in the landscapes, as predicted by Optimum Foraging Theory (OFT) [27], in combination with predation risk avoidance suggested by Optimal Escape Theory [28]. In the case of anti-predator responses of rodents, they are expected to increase during the breeding seasons (i.e., wet seasons) or during the post-parturient period when adult females are caring for their offspring, facing elevated predation risks for their progeny and themselves [29].
There is still a dearth of information on small mammal assemblages in tropical Africa, particularly for habitats in the Zambezian bioregion [30]. In this study, our focus was on the patterns of rodent assemblages across the CBS landscapes. Knowledge of rodent assemblages in an ecologically sensitive landscape, such as CBS, can be useful to conservation planning across conservation–agricultural interfaces. Anthropogenic activities, such as natural land (e.g., natural forested areas) conversions to various land uses, including agriculture, are ever-increasing and contributing to habitat fragmentation and biodiversity loss [22,31]. Nevertheless, in some cases, the conversion of natural lands to agricultural fields leads to increased biodiversity (e.g., [32]).
We assumed that differences in rodent assemblages could be associated with seasonal variations in food availability (i.e., food deprivation or abundance) and vegetation cover [14,33,34,35]. Seasonal differences in physiognomic vegetation in various habitat types may also affect the rodent assemblages [36]. Other factors that may affect rodent assemblages include lunar phases and illumination, influencing prey–predator relationships [37] and wildfires [15]. Our key research question was: what are the patterns of rodent assemblages across CBS landscape? We hypothesise that rodent assemblages vary between farmlands and non-farmlands.
2. Data and Methods
2.1. Study Area
This study was conducted in CBS (27.9955° E, 12.8302° S; 539 ha) and its adjacent area, located in Kalulushi District, Zambia (Figure 1). The study site lies in the Zambezian bioregion [30,38]. The area’s mean rainfall is 1200 mm per annum, and temperatures range between 18 and 30 °C [39]. Inside the CBS, there is Lake Chembe and outside the CBS are many wetlands, in the form of streams and ponds. The CBS is fenced and kept natural, precluding several anthropogenic activities, such as land clearing.
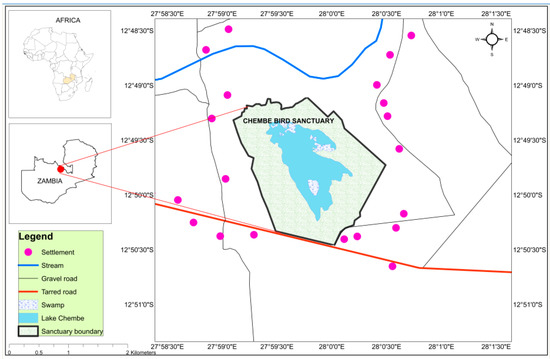
Figure 1.
Locational map of the study area of Chembe Bird Sanctuary, Kalulushi, Zambia.
The area is predominately Miombo woodland, characterised by Julbenadia, Brachystegia and Isobelinia tree species. The other vegetation types are Acacia woodland, mostly comprising Acacia tortilis and A. polyacantha, and open grasslands, constituting Setaria spp. and Hyparrhenia spp. Several termite (Macrotermes) mounds characterise both Miombo and Acacia woodlands. In addition, there are farmlands outside the CBS and these are owned by subsistence farmers. These farmers commonly cultivate maize (Zea mays). However, they also occasionally grow other crop varieties inter alia; sorghum (Sorghum bicolor), cassava (Manihot esculenta), sweet potatoes (Ipomoea batatas), beans (Phaseolus vulgaris) and groundnuts (Arachis hypogaea). These crops are among the dietary items consumed by some rodents. In the study area, there are a number of birds of prey, such as Barn owls, Tyto alba; spotted eagle owls, Bubo africanus; pearl spotted owls, Cilaucidium perlatum; and giant eagle owls, Bubo lacteus [40], which predate on rodents. The rodent-predator snakes known to inhabit the CBS landscape include Puff adders (Bitis arietans arietans), Cape vine snakes (Thelotornis capensisoatesii), Stripped grass snakes (Psammophylax tritaeniatus) and Common house snakes (Lamprophis fuliginosus).
2.2. Data Collection Protocols
Data were collected and recorded on assemblages of rodents across four habitat types of the CBS landscape (Figure 2; Table 1), under ethical approval code number DNPW/101/9/77, issued on 3 June 2018 by Department of National Parks and Wildlife (DNPW). The habitat types were classified based on the vegetation cover characteristics. The capture-mark-recapture (CMR) method adopted as a proxy method for deriving abundance [41], was applied in four different habitat types (i.e., Miombo woodland, Acacia woodland, grasslands and farmlands), along ten (10) transects, using 336 Sherman live traps over 3, 360 trap nights during wet (December) and dry (July–August) seasons of 2018 and 2019, respectively. The habitat types were considered as strata for data collection. Both grids and transects (Figure 2) were used for specific purposes: (1) the 200 m × 200 m grids [42] were laid out for determination of where to sample from in the landscape; (2) transects (lines) were applied to guide the systematic setting of traps. The transects comprised either 42 or 28 traps, resulting in transect lengths of 420 m or 280 m, totalling 840 trap nights for each habitat types in 2018 and 2019, respectively (Table 1). Due to Acacia woodland mosaics in the Miombo woodland on the south-western part of CBS, one transect was longer than others to compensate for Acacia woodland mosaics (Figure 2). Transects in the landscape originated from random positions within the randomly selected grids and proceeded in random directions (Figure 2).
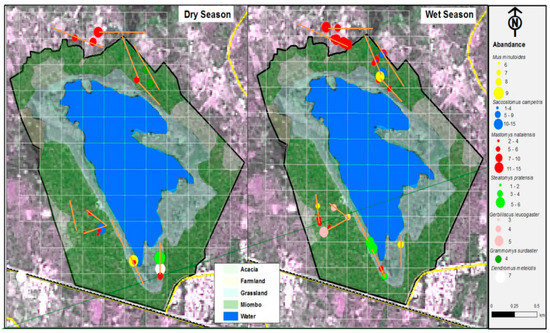
Figure 2.
Distribution of rodents and their abundance in dry and wet seasons across four habitat types in the Chembe Bird Sanctuary landscape, Zambia, 2018, and 2019. Points in Figure 2 represent the trap sites where captures (samples) were made, and the sizes of points signify the number of rodent captures. The transects along which samples were collected are shown as solid brown lines.

Table 1.
Numbers of captured rodents in wet and dry seasons across habitat types in Chembe Bird Sanctuary landscape, Zambia, in 2018–2019.
A trap session per season consisted of 5-trap nights, conducted in wet and dry seasons, respectively, across the four habitats in 2018 and 2019. The number of Sherman live traps per transect, and numbers of transects per habitat are depicted in Table 1. Sherman live traps on each transect were separated by 10 m from each other, considering the local scale heterogeneity [43]. As baiting is commonly practised as an effective way of rodent captures due to its olfactory effect [42], peanut butter mixed with maize mealie meal in the form of a paste was used for rodent baiting in the Sherman live traps. Being nocturnal animals, the traps for rodents were laid at sunset and inspected for catches at sunrise the next morning. By laying them late in the day, tampering by vervet monkeys (Chlorocebus pygerythrus) was avoided. When inspected, only two of the empty traps had each at a single instance, attracted ants (Order: Hymenoptera) in them after laying them overnight on the transects.
Further, the captured rodents were marked with ear-notches, followed by determination of their sex, age, body weights and morphometrics prior to releasing them [44,45], and were considered sufficient to aid identification of the recaptured individuals in the subsequent captures. The captured rodents were weighed in-situ using Pesola spring balances with a specification of a maximum specimen mass of 300 g allowable. The target rodents weigh less than 300 g (mean = 40.58 ± 2.24 g; n = 377). In addition, tail, ear and hindfoot lengths of rodents were measured using callipers and applied to ascertain the identity of the recaptured rodents. The visual sex determination was conducted by examining the genital organs of captured rodents visually while age was determined by visual examination of fur colour, texture and body size. The Garmin-ETrex 30 Global Positioning System (GPS) sets were used to collect geographic data.
2.3. Data Analysis
To derive patterns (i.e., similarities and differences) among rodent communities, we conducted a multivariate analysis of Bray–Curtis dissimilarity matrices by Non-metric Multi-Dimensional Scaling (NMDS) ordination. The input data were the transect IDs, habitat types and counts of captures per species. There were 5 and 6 taxa used in the analysis for the wet and dry season, respectively. Data were normalised a priori by conversion from absolute abundance to relative abundance to maintain their robustness. NMDS analyses were conducted using Vegan package in R-statistical software, version 3.6.1 [46]. Stress values were generated for model validation. We assumed homogeneity of multivariate dispersion of rodents, and tested it using distance-based ANOVA test to reveal deviations of rodent assemblages from individual habitat types [47,48].
Comparison of derived Shannon–Weiner indices of the diversity of rodents was conducted by converting to their diversity equitability ([49,50]; Equations (1) and (2)). In Equation (1), the Shannon–Weiner index (H’) was derived from sample species of rodents, where Pi is the proportion of captured rodents in the samples of a particular number, i, of individuals per habitat type, lnPi is the natural logarithm of the proportion of the captured rodents while S is the number of sampling units. In Equation (2), diversity equitability of Pielou (J’) is used to enable comparisons of the diversity of rodents between habitat types, where H’max is the natural logarithm of the number of species [ln(S)]. The diversity and its equitability values were calculated using 5-night trap sessions per season. A one-way Analysis of Variance (ANOVA) was based on a completely randomised design, established on randomly selected grids used in the setting of transects for data collection [51]. Further, the Minimum Number Alive (MNA) was determined from the rodent capture counts. MNA referred to the number of individual rodents caught in a 5-night trap session, plus those that were not caught during that particular session but were caught both previously and subsequently [52]. The number of recaptured rodents was not considered in the analyses of assemblages and diversity. The ANOVA was followed by Tukey–Kramer’s Honestly Significant Difference (HSD) post-hoc tests at α = 0.05 for multiple comparisons of means to determine which habitat types had significantly different rodent assemblages across seasons. Data analyses were performed in R statistical software, version 3.6.1 [46].
3. Results
A total of 377 individuals of seven sympatric rodent species were captured during the wet and dry surveys (Figure 2; Table 1). The captured rodent species include five murids (family: Muridae): Natal multimammate rat, Mastomys natalensis; Southern African pygmy mouse, Mus minutoides; Fat mouse, Steatomys pratensis; Bushveld gerbil, Gerbilliscus leucogaster; and African woodland thicket rat, Grammomys surdaster. The other two rodent species were nesomyids (family: Nesomyidae): Southern African pouched mouse, Saccostomus campestris; and Gray climbing mouse, Dendromus melanotis. M. natalensis was the most abundant species (n = 248; 65.8%) across seasons and habitat types. Most of the rodent captures were observed in the wet seasons (n = 276; 73.2%). In dry seasons, the rodent assemblages appear to be more clumped (i.e., the concentration of species in particular habitat types) than in the wet seasons across habitat types, except in farmlands dominated by M. natalensis (Figure 3a). On the other hand, the rodent assemblages seem random (i.e., pattern assortment with no particular order across habitat types) during dry seasons (Figure 3b). For the rodent assemblages in both wet and dry seasons, the stress values are <0.1 with dimensions k = 2, lying within acceptable limits as an indication of well-preserved original dissimilarities in a reduced number of dimensions (Figure 4a,b). There seem to be higher variability in rodent assemblages in wet seasons [F(3,38) = 3.659, p = 0.021] compared to dry seasons when the dispersion is very weak [F(3,21) = 0.499, p = 0.687] (Figure 3). In both wet and dry seasons, all species except M. natalensis showed avoidance of the farmlands. Further, in the wet season, 52.9% (n = 146) were associated with flood-prone spaces, while in the dry season, all captured rodents were captured in flood-prone spaces. Several captured rodents (n = 65; 84.4%) in Miombo woodland were in proximity with termite mounds (<10 m). The number of recaptured rodents ranged from 4.8% to 11.1% in wet seasons and 3.1% to 14.3% in dry seasons (Table 1). There were varying minimum numbers of rodents across seasons and habitat types (Table 1).
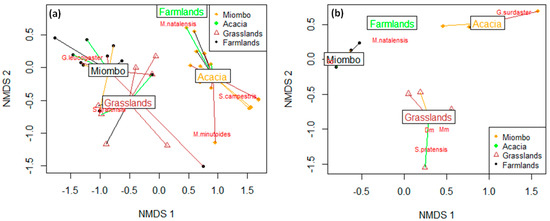
Figure 3.
Species–habitat associations of rodent community in the Chembe Bird Sanctuary landscape, Zambia, in 2018–2019 in wet (a) and dry (b) seasons, respectively, derived from Non-metric Multi-Dimensional Scaling. The terminal points in Figure 3a,b reveal networks of different rodent samples in particular habitats. Line colours show the similarity of species presence in a particular habitat in relation to other habitats. In Figure 3b, Sc in Miombo woodland represents S. campestris, while Dm and Mm in grasslands represent D. melanotis and M. minutoides, respectively.
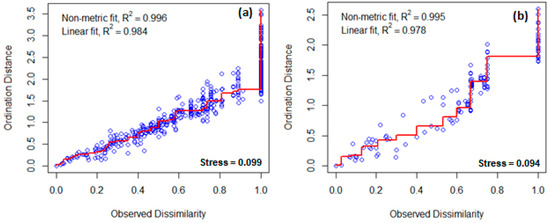
Figure 4.
Stress plots (Shepard’s diagrams) showing the fit of configuration in two dimensions (k = 2) with original data patterns of counts in the rodent communities in the Chembe Bird Sanctuary landscape, Zambia, in 2018–2019 in wet (a) and dry (b) seasons, respectively. The hollow circles (points) and red lines (i.e., monotonic step lines) represent rodent counts and the model fits, respectively.
The diversity of rodents varied significantly between farmlands and other habitat types in wet and dry seasons, respectively [F(3,38) = 5.127, p = 0.004; F(3,21) = 0.758, p = 0.035]. Comparison of Figure 5a,b depict higher mean diversity in the wet seasons than in the dry seasons, depicting similar patterns across habitat types. The diversity of rodent species varied significantly between out-CBS farmlands and counterpart in-CBS habitat types, while there were insignificant variations among in-CBS habitats (Figure 5a,b).
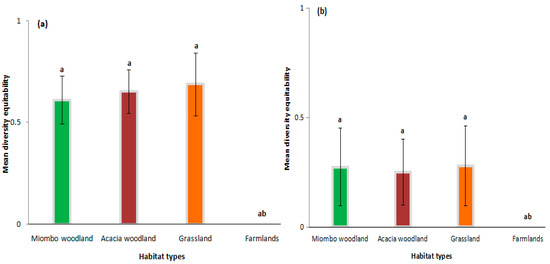
Figure 5.
Mean diversity of rodents across different habitat types in Chembe Bird Sanctuary landscape, Zambia, in 2018–2019 in wet (a) and dry (b) seasons, respectively. The error bars represent the standard errors in the mean diversity of rodents. Letters a, b, and ab above the bar graphs denote significant differences of mean diversity of rodents among different habitat types.
4. Discussion
This study reveals dynamic patterns of rodent assemblages across seasons and habitats in the CBS landscape (Figure 3 and Figure 5; Table 1). Although specific behavioural (activity) data per species is outside the scope of this study, it can be speculated based on patterns of rodent assemblages in CBS landscape in this study that seasonality in the rodent assemblages might have been affected by a number of factors, inter alia: (1) vegetation cover for protection from predators and food availability, which are usually more abundant in wet seasons than in dry seasons [14,53]; (2) as a result of food availability and vegetation cover, rodents prefer breeding and raising offspring in the wet seasons to the dry seasons [54,55,56]; (3) rodents may experience torpor during the dry seasons to conserve energy [57,58]; (4) rodents would also cache food for use when in short supply in the dry seasons or food deficient years, which could further limit demand for foraging [59,60]; and (5) some species, such as D. melanotis would prefer dry grasses to fresh ones [9].
Compared with their conspecifics, M. natalensis is most abundant in this study and occurring in all the habitat types, probably due to their being an omnivorous species and generalists [61]. M. natalensis was dominant in all habitats except in grasslands where it was less common, probably due to it being a generalist forager [62] and the presence of other environmental resources, such as vegetation cover for protection from predators. Apart from M. natalensis, all the other rodents in CBS depicted some form of habitat specialisation (Table 1) and, thus, spatial niche partitioning. However, rodents other than M. natalensis have also been recorded to occur in human-modified rural agricultural-fallow mosaic landscapes in Africa [63]. Spatial niche partitioning is an important means of co-occurrence of rodent species in a landscape [64]. In particular, the diversity of specialized species is likely to be negatively impacted upon by farmlands due to the limited choice of food and homogeneity of habitat that limits their survival across time and space [65]. For instance, S. pratensis specialised in utilising the grasslands, similar to the observation by [66], and they were relatively many during the dry seasons when they were supposed to be hibernating. This could be indicative of some behavioural change worth further study. Similarly, M. minutoides was recorded in all habitats except on farmlands, while S. campestris utilised the woodland (i.e., Miombo and Acacia) and can be evolutionarily linked to such environments [67]. G. leucogaster, G. surdaster, and D. melanotis exclusively utilised the Miombo woodland, Acacia woodland and grasslands, respectively. Though Aethomys chrysophilus were expected to be abundant in the African miombo woodland [38], none were captured and recorded during this study. G. surdaster appears to prefer thickets, such as Acacia woodland for their vegetation cover from predation and nesting sites [68]. As rodents respond to seasonal changes in the habitat types by adjusting their foraging behaviours, captures could also reduce in dry seasons as also observed by [64]. Further, the increase in diversity of rodents in the wet seasons coincides with the heightened food caching activity during the food abundant seasons [69]. The hoarding behaviour in rodents would be linked to productivity in a particular landscape (e.g., [70]), and available suitable food types [59].
Further, the rodent MNA seemed to vary across species, habitat types and seasons (Table 1). Like in other studies on rodents in Africa [71,72], M. natalensis had the highest populations, which peaked in the wet season. Acacia and farmlands had relatively higher M. natalensis populations than other habitat species, probably due to supportive habitat attributes, such as food availability. Varying conditions in particular habitat type might lead to changing rodent populations, which would also manifest from sporadic movements of rodents from one habitat to another [50].
The diversity of rodents in the Miombo woodland in CBS can be attributed to several factors, such as vegetation cover and a variety of food items across seasons [73,74]. While ordinarily Miombo woodland is expected to have relatively low fauna productivity, the resource-rich termite mounds in Miombo woodland could have contributed to increased abundance and richness of rodents [75], as food abundance facilitates breeding and survival in rodents [76]. The lower diversity in farmlands than in different habitat types in CBS could be a result of mono-specific cropping which favours generalist species [61], and usually seasonal cereal crops, which form restrictive single food types to specialised rodent species. Temporal variations in food availability can have negative effects on rodents and their predators [77]. While vegetation cover could have attracted rodents to Acacia woodland, grasslands with massive ground vegetation cover were interspersed by some flood areas, which are potentially resource and food-rich areas capable of attracting rodents as well [78].
5. Conservation Implications
Conservation of rodent populations is critical in small ecologically compressed and isolated sanctuaries, embedded in a human-dominated landscape, such as CBS where several predators, such as snakes, and birds of prey depend on them. Such an ecologically isolated sanctuary has the potential to conserve dwindling biodiversity [79]. The responsible wildlife agency and land owners around the CBS can benefit from the insights unravelled in this study, where rodents can be used as a general model group for the proposition of management steps towards protection of diversity in the area by (1) not increasing the area of farmlands into CBS and (2) protecting the mosaic of all present natural habitats. For instance, to sustain high rodent assemblages and diversity, more mosaics of natural and non-natural, agricultural and fallow, different habitat types are needed beyond CBS boundaries while land owners integrate practices that encourage balancing rodent conservation and crop protection [63,80,81]. This requires the broad-based participation of multiple stakeholders to assist with conservation planning and maximising expanses of natural areas under a form of protection to increase the potential for species persistence [82,83].
Foraging behaviour can also provide vital information for pest management, as in the case of M. natalensis that may be utilising the nearby farmlands as sink areas, and as such future research could comprehensively focus on the effects of farmlands and fallow fields on rodent assemblages in comparison with non-farmlands. Previously, ecological based rodent management has been proposed to eradicate rodents, such as M. natalensis, by trappings and the removal of vegetation cover and food sources [72], but we counter-propose that while methods of achieving food security need to be further explored or implemented, care should be taken in employing such methods in agrarian areas adjoining conservation areas. In return, local communities living adjacent to the protected area should be sufficiently supported to benefit from wildlife utilisation through various ecotourism businesses to sustain their support over wildlife conservation [84].
6. Conclusions
Our study has demonstrated that even small isolated conservation areas are important for maintaining terrestrial small mammal assemblages and diversity. Seasons and habitat types seem to be among the key factors affecting the rodent assemblages and diversity in the CBS, influencing the species co-existence and habitat preferences. The suppressed rodent assemblages and diversity could negatively be impacted upon by stochastic factors, such as environmental changes, leading to species extirpation. Therefore, we propose that such interactions should be mediated by conservation planning and multi-stakeholder collaboration, through dialogue and sensitisation of landowners/farmers bordering the conservation areas.
Author Contributions
Conceptualization, V.R.N. and N.N.; Methodology, V.R.N. and N.N.; Analysis, V.R.N.; Investigation, V.R.N., N.N., M.S., D.P. and K.M.; Resources, V.R.N., N.N., M.S., D.P., and Y.M.; Draft Preparation, V.R.N.; Internal Review and Editing, V.R.N., N.N., M.S., D.P., Y.M., M.R., and K.M.; Visualization, V.R.N., M.S., and D.P.; Project Administration, V.R.N. and N.N.; Funding Acquisition, Y.M., and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partly supported by the JSPS grant 18H00763 (2018-20).
Acknowledgments
The Copperbelt University (CBU), and DNPW authorised this study. The anonymous reviewers are thanked for their contribution to the improvement of manuscript quality. We are also grateful to Conrald Kunda, Remmy Kopeka and Clausin Chulu for their participation in the data collection.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; and in the decision to publish the results.
References
- Kurten, E.L. Cascading effects of contemporaneous defaunation of tropical forest communities. Biol. Conserv. 2013, 163, 22–32. [Google Scholar] [CrossRef]
- Johnson, S.; Burgoyne, P.; Harder, L.D.; Dotterl, S. Mammal pollinators lured by the scent of a parasitic plant. Proc. R. Soc. B 2011, 278, 2303–2310. [Google Scholar] [CrossRef] [PubMed]
- Galiano, D.; Kubiak, B.B.; Overbeck, G.E.; de Freitas, T.R.O. Effects of rodents on plant cover, soil hardness, and soil nutrient content: A case study on tuco-tucos (Ctenomys minutes). Acta Theriol. 2014, 59, 583–587. [Google Scholar] [CrossRef]
- Yong, S.K.; Jalaludin, N.H.; Brau, E.; Shamsudin, N.N.; Heo, C.C. Changes in soil nutrients (ammonia, phosphate and nitrate) associated with rat carcass decomposition under tropical climate conditions. Soil Res. 2019, 57, 482–488. [Google Scholar] [CrossRef]
- Flores-Peredo, R.; Sánchez-Velásquez, L.; Galindo-González, J.; Morales-Mávil, J. Post-dispersed pine seed removal and its effect on seedling establishment in a Mexican Temperate Forest. Plant. Ecol. 2011, 212, 1037–1046. [Google Scholar] [CrossRef]
- Stephens, R.B.; Rowe, R.J. The underappreciated role of rodent generalists in fungal spore-dispersal networks. Ecology 2020. [Google Scholar] [CrossRef] [PubMed]
- Labuschagne, L.; Swanepoel, L.H.; Taylor, P.J.; Belmain, R.R.; Keith, M. Are avian predators effective biological control agents for rodent pest management in agricultural systems? Biol. Control 2016, 101, 94–102. [Google Scholar] [CrossRef]
- Moretti, T.; Benedito, R.O.; Thyssen, P.; Russ Solis, D. Insects on decomposing carcasses of small rodents in a secondary forest in Southeastern Brazil. Eur. J. Entomol. 2008, 105, 691–696. [Google Scholar] [CrossRef]
- Byrom, A.; Ruscoe, W.; Nkwabi, A.; Metzger, K.; Forrester, G.; Craft, M.; Durant, S.; Makacha, S.; Bukombe, J.; Mchetto, J.; et al. Small mammal diversity and population dynamics in the greater Serengeti ecosystem. In Serengeti IV: Sustaining Biodiversity in a Coupled Human-Natural System; Sinclair, A.R.E., Metzger, K.L., Mduma, S.A.R., Fryxell, J.M., Eds.; University of Chicago Press: Chicago, IL, USA, 2015; pp. 323–357. [Google Scholar] [CrossRef]
- Manning, J.; Edge, W. Small mammals responses to fine woody depris and forest fuel reduction in southwest Oregon. J. Wildl. Manag. 2008, 72, 625–632. [Google Scholar] [CrossRef]
- Mulungu, L.S.; Borremans, B.; Ngowo, V.; Mdangi, M.E.; Katakweba, A.S.; Tesha, P.; Mrosso, F.P.; Mchomvu, M.; Kilonzo, B.S. Comparative study of movement patterns of Mastomys natalensis in irrigated rice and fallow fields in eastern Tanzania. Afr. J. Ecol. 2015, 53, 473–479. [Google Scholar] [CrossRef]
- Sellers, L.; Long, R.; Baldwin, R.A.; Jay-Russell, M.; Li, X.; Atwill, E.R.; Engeman, R.M. Impact of field border plantings on rodents and food safety concerns. Proc. Vertebr. Pest. Conf. 2016, 27, 150–155. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, M.; Zhang, H.; Yao, N.; Wang, Y.; Zhang, Y.; Wang, Z. Complex spatial patterns delay the food discovery and locating of rodents. Integr. Zool. 2020. [Google Scholar] [CrossRef]
- Flores-Peredo, R.; Vázquez-Domínquez, Y.G. Influence of vegetation type and season on rodent assemblage in a Mexican temperate forest mosaic. Therya 2016, 3, 357–369. [Google Scholar] [CrossRef]
- Namukonde, N.; Daniel, K.; Jörg, U.G. Differential effects of fire on small mammal communities in Busanga plain, Zambia. Trop. Conserv. Sci. 2017, 10, 1–13. [Google Scholar] [CrossRef]
- Babale, A.; Joshua, A.; Elena, M.; Pierre-Michel, F. Forest disturbance and seasonal food availability influence a conditional seed dispersal mutualism. Biotropica 2018, 50, 750–757. [Google Scholar] [CrossRef]
- Garrido-García, J.A.; Nieto-Lugilde, D.; Alba-Sánchez, F.; Soriguer, R.C. Agricultural intensification during the late Holocene rather than climatic aridification drives the population dynamics and the current conservation status of Microtus cabrerae, an endangered Mediterranean rodent. J. Biogeogr. 2018, 45, 448–460. [Google Scholar] [CrossRef]
- Avenant, N. The potential utility of rodents and other small mammals of indicators of ecosystem integrity of South African grasslands. Wildl. Res. 2011, 38, 626–639. [Google Scholar] [CrossRef]
- Ofori, B.Y.; Attuquayefio, D.K.; Owusu, E.H.; Musah, Y.; Ntiamoa-Baidu, Y. Spatio-temporal variation in small mammal species richness, relative abundance and body mass reveal changes in a coastal wetland ecosystem in Ghana. Environ. Monit. Assess. 2016, 188, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Milstead, B.; Meserve, P.; Campanella, A.; Previtali, K.; Gutierrez, R. Spatial ecology of small mammals in north-central Chile: Role of precipitation and refuges. J. Mammal. 2007, 88, 1532–1538. [Google Scholar] [CrossRef]
- Hurst, Z.M.; McCleery, R.A.; Collier, B.A.; Fletcher, R.J., Jr.; Silvy, N.J.; Taylor, P.J.; Monadjem, A. Dynamic edge effects in small mammal communities across a conservation-agricultural interface in Swaziland. PLoS ONE 2013, 8, e74520. [Google Scholar] [CrossRef] [PubMed]
- Balestrieri, A.; Remont, L.; Morotti, L.; Saino, N.; Prigioni, C.; Guidali, F. Multilevel habitat preferences of Apodemus syvaticus and Clethrionomys glareolus in an intensively cultivated agricultural landscape. Ethol. Ecol. Evol. 2017, 29, 38–53. [Google Scholar] [CrossRef]
- Ellis, E.; Ramankutty, N. Putting people in the map: Anthropogenic biomes of the world. Front. Ecol. Environ. 2008, 6, 439–447. [Google Scholar] [CrossRef]
- Monadlem, A.; Mahlaba, T.; Dlamini, N.; Eiscb, S.; Belmain, S. Impact of crop cycle on movement patterns of pest rodent species between fields and houses in Africa. Wildl. Res. 2011, 38, 603–609. [Google Scholar] [CrossRef]
- Nikolić, T.; Radišić, D.; Ćosić, N.; Díaz-Delgado, R.; Milić, D.; Vujić, A.; Ćirović, D. Landscape heterogeneity effects on keystone rodent species: Agro-ecological zoning for conservation of open grasslands. Biodivers. Conserv. 2019. [Google Scholar] [CrossRef]
- Yadok, B.G.; Pech, R.; Chapman, H. Perception of predation risk by African giant pouched rats (Cricetomys sp. nov) is higher in forest-edge microhabitats. Behav. Process. 2019, 168, 103953. [Google Scholar] [CrossRef] [PubMed]
- Pyke, G.H. Optimal foraging theory: A critical review. Annu. Rev. Ecol. Evol. Syst. 1984, 15, 523–575. [Google Scholar] [CrossRef]
- Cooper, W.E., Jr.; Frederick, W.G. Optimal flight initiation distance. J. Theor. Biol. 2007, 224, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Ciuti, S.; Pipia, A.; Ghiandai, F.; Grignolio, S.; Apollonio, M. The key role of lamb presence in affecting flight response in Sardinian mouflon (Ovis orientalis musimon). Behav. Process. 2008, 77, 408–412. [Google Scholar] [CrossRef]
- Linder, H.P.; de Klerk, H.M.; Born, J.; Burgess, N.D.; Fjeldsa, J.; Rahbek, C. The partitioning of Africa: Statistically defined biogeographical regions in sub-Saharan Africa. J. Biogeogr. 2012, 39, 1189–1205. [Google Scholar] [CrossRef]
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Biodiversity Synthesis; World Resources Institute: Washington, DC, USA, 2005. [Google Scholar]
- Konečný, A.; Koubek, P.; Bryja, J. Indications of higher density and abundance of small rodents in human-influenced Sudanian savannah than in the Niokolo Koba National Park (Senagal). Afr. J. Ecol. 2010, 48, 718–726. [Google Scholar] [CrossRef]
- Nicolas, V.; Colyn, M. Seasonal variations in population and community structure of small rodents in a tropical forest of Gabon. Can. J. Zool. 2003, 81, 1034–1046. [Google Scholar] [CrossRef]
- Addisu, A.; Bekele, A. Habitat preferences, seasonal abundance and diets of rodents in Alage, southern Ethiopia. Afr. J. Ecol. 2013, 52, 284–291. [Google Scholar] [CrossRef]
- Ramírez-Bautista, A.; Williams, J.N. The importance of productivity and seasonality for structuring small rodent diversity across a tropical elevation gradient. Oecologia 2019, 190, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Magige, F.; Senzota, R. Abundance and diversity of rodents at the human-wildlife interface in Western Serengeti, Tanzania. Afr. J. Ecol. 2006, 44, 371–378. [Google Scholar] [CrossRef]
- Johnson, M.; De León, Y. Effect of an invasive plant and moonlight on rodent foraging behavior in a coastal dune ecosystem. PLoS ONE 2015, 10, e0117903. [Google Scholar] [CrossRef] [PubMed]
- Mazoch, V.; Mikula, O.; Bryja, J.; Konvičková, H.; Russo, I.-R.; Verheyen, E.; Šumbera, R. Phylogeography of a widespread sub-Saharan murid rodent Aethomys chrysophilus: The role of geographic barriers and paleoclimate in the Zambezian bioregion. Mammalia 2018, 82, 373–387. [Google Scholar] [CrossRef]
- Conservation Farming Unit. Conservation Farming and Conservation Agriculture Handbook for Hoe Farmers in Agro-Ecological Regions I and IIa; Conservation Farming Unit: Lusaka, Zambia, 2009. [Google Scholar]
- Nyirenda, V.R.; Musonda, F.; Kambole, S.; Tembo, S. Peasant farmer–raptor conflicts around Chembe Bird Sanctuary, Zambia, Central Africa: Poultry predation, ethno–biology, land use practices and conservation. Anim. Biodivers. Conserv. 2017, 40, 121–132. [Google Scholar] [CrossRef]
- Anthony, N.M.; Ribic, C.A.; Bautz, R.; Garland, T., Jr. Comparative effectiveness of Longworth and Sherman live traps. Wildl. Soc. Bull. 2005, 33, 1018–1026. [Google Scholar] [CrossRef]
- Andrzejewski, R. The home-range concept in rodents revised. Acta Theriol. 2002, 47, 81–101. [Google Scholar] [CrossRef]
- Foord, S.H.; Swanepoel, L.H.; Evans, S.W.; Schoeman, C.S.; Erasmus, B.; Schoeman, M.C.; Keith, M.; Smith, A.; Mauda, E.V.; Maree, N.; et al. Animal taxa contrast in their scale-dependent responses to land use change in rural Africa. PLoS ONE 2018, 13, e0194336. [Google Scholar] [CrossRef]
- Bergeron, P.; Réale, D.; Humphries, M.M.; Garant, D. Anticipation and tracking of pulsed resources drive population dynamics in eastern chipmunks. Ecology 2011, 92, 2027–2034. [Google Scholar] [CrossRef]
- Hansen, N.; Hughes, N.K.; Byrom, A.E.; Banks, P.B. Population recovery of alien black rats Rattus rattus: A test of reinvasion theory. Austral. Ecol. 2020, 45, 291–304. [Google Scholar] [CrossRef]
- R Core Development Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Anderson, M.J. Distance-based test for homogeneity of multivariate dispersions. Biometrics 2006, 62, 245–253. [Google Scholar] [CrossRef]
- Gijbels, I.; Omelka, M. Testing for homogeneity of multivariate dispersions using dissimilarity measures. Biometrics 2013, 69, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Brady, M.J.; Slade, N.A. Diversity of a grassland rodent community at varying temporal scales: The role of ecologically dominant species. J. Mammal. 2001, 82, 974–983. [Google Scholar] [CrossRef]
- Mortelliti, A.; Boitani, L. Patterns of rodent species diversity and abundance in a Kenyan relict tropical rainforest. Biodivers. Conserv. 2006, 15, 1425–1440. [Google Scholar] [CrossRef]
- Dag, O.; Dolgun, A.; Konar, N.M. Onewaytests: An R package for one-way tests in independent group designs. R J. 2018, 10, 175–199. [Google Scholar] [CrossRef]
- Krebs, C.J. Demographic changes in fluctuating populations of Microtus californicus. Ecol. Monogr. 1966, 36, 239–273. [Google Scholar] [CrossRef]
- Sunyer, P.; Munoz, A.; Mazerolle, M.; Bonal, R.; Espelta, J.M. Wood mouse population dynamics: Interplay among seed abundance seasonality, shrub cover and wild boar interference. Mamm. Biol. 2016, 81, 372–379. [Google Scholar] [CrossRef]
- Massawe, A.W.; Makundi, R.H.; Mulungu, L.S.; Katakweba, A.; Shayo, T.N. Breeding dynamics of rodent species inhabiting farm–fallow mosaic fields in Central Tanzania. Afr. Zool. 2012, 47, 128–137. [Google Scholar] [CrossRef]
- Gasperini, S.; Mortelliti, A.; Bartolommei, P.; Bonacchi, A.; Manzo, E.; Cozzolino, R. Effects of forest management on diversity and survival in three forest rodent species. For. Ecol. Manag. 2016, 382, 151–160. [Google Scholar] [CrossRef]
- Hoset, K.S.; Villers, A.; Wistbacka, R.; Selonen, V. Pulsed food resources, but not forest cover, determine lifetime reproductive success in a forest-dwelling rodent. J. Anim. Ecol. 2017, 86, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Ellison, G.T.H.; Skinner, J.D. The influence of ambient temperature on spontaneous daily torpor in pouched mice (Saccostomus campestris: Rodentia: Cricetidae) from southern Africa. J. Therm. Biol. 1992, 17, 25–31. [Google Scholar] [CrossRef]
- Smith, S.E.; Ramos, F.A.; Refinetti, R.; Farthing, J.P.; Paterson, P.G. Proteinenergy malnutrition induces an aberrant acute-phase response and modifies the circadian rhythm of core temperature. Appl. Physiol. Nutr. Metab. 2013, 38, 844–853. [Google Scholar] [CrossRef]
- Charron, I.; Cabanac, M. Influence of pellet size on rat’s hoarding behaviour. Physiol. Behav. 2004, 82, 447–451. [Google Scholar] [CrossRef]
- Yadok, B.G.; Forget, P.-M.; Gerhard, D.; Chapman, H. Low fruit-crop years of Carapa oreophila drive increased seed removal and predation by scatter hoarding rodents in a West African forest. Acta Oecol. 2019, 99, 103448. [Google Scholar] [CrossRef]
- Mamba, M.; Fasel, N.; Mahlaba, T.A.M.; Austin, J.D.; McCleery, R.A.; Monadjem, A. Influence of sugarcane plantations on the population dynamics and community structure of small mammals in a savanna-agricultural landscape. Glob. Ecol. Conserv. 2019, 82, 250–260. [Google Scholar] [CrossRef]
- Mulungu, L.S.; Mahlaba, T.; Massawe, A.; Kennis, J.; Crauwels, D.; Eiseb, E.; Monadjem, A.; Makundi, R.; Katakweba, A.; Leirs, H.; et al. Dietary preferences of the multimammate mouse (Mastomys natalensis, Smith 1832) across different habitats and seasons in Tanzania and Swaziland. Wildl. Res. 2011, 38, 640–646. [Google Scholar] [CrossRef]
- Taylor, P.J.; Downs, S.; Monadjem, A.; Eiseb, S.J.; Mulungu, L.S.; Massawe, A.W.; Mahlaba, T.A.; Kirsten, F.; Maltitz von, E.; Malebane, P.; et al. Experimental treatment-control studies of ecologically based rodent management in Africa: Balancing conservation and pest management. Wildl. Res. 2012, 39, 51–61. [Google Scholar] [CrossRef]
- Püttker, T.; Barros, C.S.; Pinotti, B.T.; Bueno, A.A.; Pardini, R. Co-occurrence patterns of rodents at multiple spatial scales: Competitive release of generalists following habitat loss? J. Mammal. 2019, 100, 1229–1242. [Google Scholar] [CrossRef]
- Rocha, C.R.; Ribeiro, R.; Marinho-Filho, J. Influence of temporal variation and seasonality on population dynamics of three sympatric rodents. Mamm. Biol. 2017, 84, 20–29. [Google Scholar] [CrossRef]
- Plavsic, M.J. Seasonal dynamics of macrohabitat use by small mammals in the Okavango Delta, Boswana: Implications for landscape-level disturbance resilience. Afr. J. Ecol. 2015, 53, 44–53. [Google Scholar] [CrossRef]
- Mikula, O.; Šumbera, R.; Aghová, T.; Mbau, J.S.; Katakweba, A.S.; Sabuni, C.A.; Bryja, J. Evolutionary history and species diversity of African pouched mice (Rodentia: Nesomyidae: Saccostomus). Zool. Scripta 2016, 45, 595–617. [Google Scholar] [CrossRef]
- Bryja, J.; Šumbera, R.; Peterhans, J.C.K.; Aghová, T.; Bryjová, A.; Mikula, O.; Nicholas, V.; Denys, C.; Verheyen, E.K. Evolutionary history of the thicket rats (genus Grammomys) mirrors the evolution of African forests since the late Miocene. J. Biogeogr. 2017, 44, 182–194. [Google Scholar] [CrossRef]
- Rusch, U.D.; Midgley, J.J.; Anderson, B. Seasonal fluctuations in rodent seed catching and consumption behavior in fynbos shrublands: Implications for fire management. S. Afr. J. Bot. 2014, 93, 217–221. [Google Scholar] [CrossRef]
- McCain, C.M.; King, S.R.B.; Szewczyk, T.; Beck, J. Small mammal species richness is directly linked to regional productivity, but decoupled from food resources, abundance, or habitat complexity. J. Biogeogr. 2018, 45, 2533–2545. [Google Scholar] [CrossRef]
- Makundi, R.H.; Massawe, A.W.; Mulungu, L.S. Breeding seasonality and population dynamics of three rodent species in the Magamba Forest Reserve, Western Usambara Mountains, north-eastern Tanzania. Afr. J. Ecol. 2006, 45, 17–21. [Google Scholar] [CrossRef]
- Massawe, A.W.; Mulungu, L.S.; Makundi, R.H.; Dlamini, N.; Eiseb, S.; Kirsten, F.; Mahlaba, T.; Malebane, P.; von Maltitz, E.; Monadjem, A.; et al. Belmain. Spatial and temporal population dynamics of rodents in three geographically different regions: Implications for ecologically-based rodent management. Afr. Zool. 2011, 46, 393–405. [Google Scholar] [CrossRef]
- Adams, A.; Yihune, M. Species composition, relative abundance and habitat association of rodents in Yekoche Forest, East Gojjam, Ethiopia. Int. J. Biodivers. Conserv. 2016, 8, 216–223. [Google Scholar] [CrossRef]
- Larsen, A.L.; Homyack, J.A.; Bently-Wigley, T.; Miller, D.A.; Kalcounis-Rueppell, M.C. Effects of habitat modification on cotton rat population dynamics and rodent community structure. For. Ecol. Manag. 2016, 376, 238–246. [Google Scholar] [CrossRef]
- Fleming, P.A.; Loveridge, J.P. Miombo woodland termite mounds: Resource islands for small vertebrates? J. Zool. 2003, 259, 161–168. [Google Scholar] [CrossRef]
- Mlyashimbi, E.C.M.; Marien, J.; Kimaro, D.N.; Tarimo, A.J.P.; Isabirye, M.; Makundi, R.H.; Massawe, A.W.; Mdangi, M.E.; Kifumba, D.; Nakiyemba, A.; et al. Relationships between seasonal changes in diet of Multimammate rat and its breeding patterns in sermi-arid areas in Tanzania. Cogent Food Agric. 2018, 4, 1507–1553. [Google Scholar] [CrossRef]
- Martin, J.M.; Branch, L.C.; Rain, R.N.; Beyeler, S.C. Temporal instability of agricultural habitat reduces reproductive success of Barn owls (Tyto alba). Auk 2010, 127, 909–916. [Google Scholar] [CrossRef]
- Madsen, T.; Ujvari, B.; Shine, R.; Buttemer, W.; Olsson, M. Size matters: Extraordinary rodent abundance on an Australian tropical flood plain. Austral. Ecol. 2006, 31, 361–365. [Google Scholar] [CrossRef]
- Damschen, E.I.; Brudvig, L.A.; Burt, M.A.; Fletcher, R.J.; Haddad, N.M.; Levey, D.J.; Orrock, J.L.; Resasco, J.; Tewksbury, J.J. Ongoing accumulation of plant diversity through habitat connectivity in an 18-year experiment. Science 2019, 365, 1478–1480. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, M.L. Species Diversity in Space and Time; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 2001; Volume 1. [Google Scholar]
- Allan, J.R.; Possingham, H.P.; Atkinson, S.C.; Waldron, A.; Di Marco, M.; Adams, V.M.; Butchart, S.H.M.; Venter, O.; Maron, M.; Williams, B.A.; et al. Conservation attention necessary across at least 44% of Earth’s terrestrial area to safeguard biodiversity. bioRxiv 2019, 839977. [Google Scholar] [CrossRef]
- Dinerstein, E.; Vynne, C.; Sala, E.; Joshi, A.R.; Fernando, S.; Lovejoy, T.E.; Mayorga, J.; Olson, D.; Asner, G.P.; Baillie, J.E.M. A global deal for nature: Guiding principles, milestones, and targets. Sci. Adv. 2019, 5, eaaw2869. [Google Scholar] [CrossRef] [PubMed]
- Snyman, S. The role of ecotourism employment in poverty reduction and community perceptions of conservation and tourism in Southern Africa. J. Sustain. Tour. 2012, 20, 395–416. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).