Direct and Indirect Effects of Overstory Canopy and Sex-Biased Density Dependence on Reproduction in the Dioecious Shrub Shepherdia canadensis (Elaeagnaceae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Flower and Fruit Production
2.3. Pollen Supplementation
2.4. Statistical Analysis
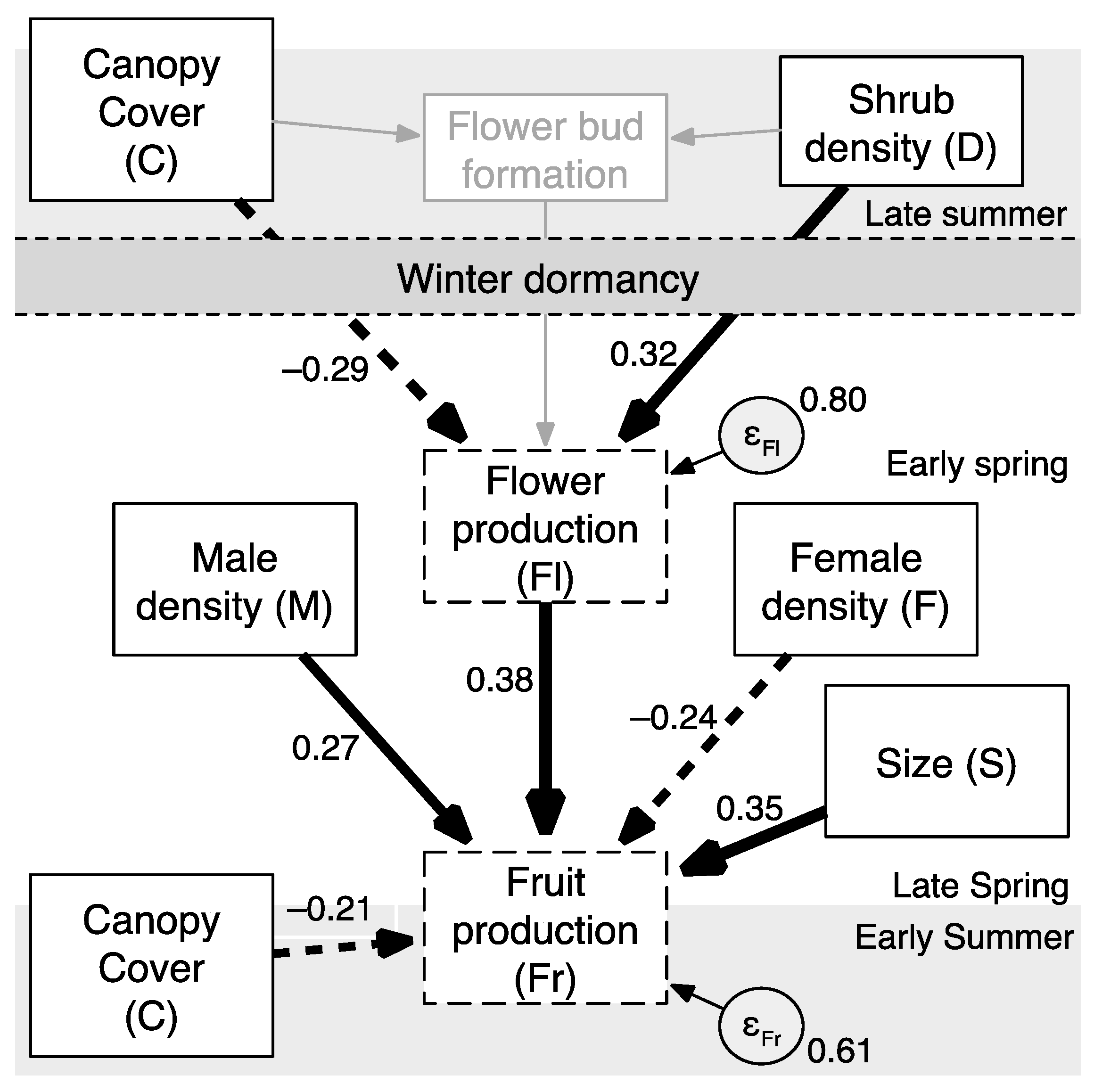
Fr = (−0.29C)(0.38) + (0.32D)(0.38) − 0.21C − 0.24F + 0.27M + 0.35S,
Fr = −0.11C + 0.12D − 0.21C − 0.24F + 0.27M + 0.35S,
3. Results
3.1. Flower and Fruit Production
3.2. Pollination Environment and Supplementation
4. Discussion
4.1. Plant Size
4.2. Positive Effect of Light Availability on Flower and Fruit Production
4.3. Intraspecific Density and Flower Production
4.4. Pollination Environment and Supplementation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Variable Normality
Appendix B
References
- Bartels, S.F.; Chen, H.Y.H. Is understory plant species diversity driven by resource quantity or resource heterogeneity? Ecology 2010, 91, 1931–1938. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Chen, H.Y.H.; Thomas, S.C.; Shahi, C. Linking resource availability and heterogeneity to understorey species diversity through succession in boreal forest of Canada. J. Ecol. 2018, 106, 1266–1276. [Google Scholar] [CrossRef]
- USDA. Shepherdia Canadensis (L.) Nutt.; United States Department of Agriculture Natural Resources Conservation Service: Washington, DC, USA, 2019.
- Walkup, C.J. Shepherdia canadensis. In Fire Effects Information System; United Sates Department of Agriculture, Forest Service, Fire Sciences Laboratory: Washington, DC, USA, 1991. [Google Scholar]
- Denny, C.K.; Stenhouse, G.B.; Nielsen, S.E. Scales of selection and perception: Landscape heterogeneity of an important food resource influences habitat use by a large omnivore. Wildl. Biol. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Krebs, C.J.; Cowcill, K.; Boonstra, R.; Kenney, A.J. Do changes in berry crops drive population fluctuations in small rodents in the southwestern Yukon? J. Mammal. 2010, 91, 500–509. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Nol, E.; Dorken, M.E. Spatial dynamics of pollination in dioecious Shepherdia canadensis (Elaeagnaceae). Plant Ecol. 2015, 216, 1213–1223. [Google Scholar] [CrossRef]
- Nielsen, S.E.; Munro, R.H.M.; Bainbridge, E.L.; Stenhouse, G.B.; Boyce, M.S. Grizzly bears and forestry II: Distribution of grizzly bear foods in clearcuts of west-central Alberta, Canada. For. Ecol. Manag. 2004, 199, 67–82. [Google Scholar] [CrossRef]
- Ashman, T.-L.; Knight, T.M.; Steets, J.A.; Amarasekare, P.; Burd, M.; Campbell, D.R.; Dudash, M.R.; Johnston, M.O.; Mazer, S.J.; Mitchell, R.J.; et al. Pollen limitation of plant reproduction: Ecological and evolutionary causes and consequences. Ecology 2004, 85, 2408–2421. [Google Scholar] [CrossRef]
- Kudo, G.; Ida, T.Y.; Tani, T. Linkages between phenology, pollination, photosynthesis, and reproduction in deciduous forest understory plants. Ecology 2008, 89, 321–332. [Google Scholar] [CrossRef]
- Stephenson, A.G. Flower and fruit abortion: Proximate causes and ultimate functions. Annu. Rev. Ecol. Syst. 1981, 12, 253–279. [Google Scholar] [CrossRef]
- Alaback, P.B. Dynamics of understory biomass in sitka spruce-western hemlock forests of southeast Alaska. Ecology 1982, 63, 1932–1948. [Google Scholar] [CrossRef]
- Eckerter, T.; Buse, J.; Förschler, M.; Pufal, G. Additive positive effects of canopy openness on European bilberry (Vaccinium myrtillus) fruit quantity and quality. For. Ecol. Manag. 2019, 433, 122–130. [Google Scholar] [CrossRef]
- Barbier, S.; Gosselin, F.; Balandier, P. Influence of tree species on understory vegetation diversity and mechanisms involved—A critical review for temperate and boreal forests. For. Ecol. Manag. 2008, 254, 1–15. [Google Scholar] [CrossRef]
- Kumar, P.; Chen, H.Y.H.; Searle, E.B.; Shahi, C. Dynamics of understorey biomass, production and turnover associated with long-term overstorey succession in boreal forest of Canada. For. Ecol. Manag. 2018, 427, 152–161. [Google Scholar] [CrossRef]
- Burnham, K.M.; Lee, T.D. Canopy gaps facilitate establishment, growth, and reproduction of invasive Frangula alnus in a Tsuga canadensis dominated forest. Biol. Invasions 2010, 12, 1509–1520. [Google Scholar] [CrossRef]
- Denny, C.; Nielsen, S. Spatial heterogeneity of the forest canopy scales with the heterogeneity of an understory shrub based on fractal analysis. Forests 2017, 8, 146. [Google Scholar] [CrossRef]
- Hamer, D. Buffaloberry [Shepherdia canadensis (L.) Nutt.] fruit production in fire-successional bear feeding sites. J. Range Manag. 1996, 49, 520–529. [Google Scholar] [CrossRef]
- Antos, J.A.; Allen, G.A. Patterns of reproductive effort in male and female shrubs of Oemleria cerasiformis: A 6-year study. J. Ecol. 1999, 87, 77–84. [Google Scholar] [CrossRef]
- Abe, S.; Motai, H.; Tanaka, H.; Shibata, M.; Kominami, Y.; Nakashizuka, T. Population maintenance of the short-lived shrub Sambucus in a deciduous forest. Ecology 2008, 89, 1155–1167. [Google Scholar] [CrossRef]
- Wheeler, C.T.; Akkermans, D.L.; Berry, A.M. Frankia and actinorhizal plants: A historical perspective. In Nitrogen-Fixing Actinorhizal Symbioses; Pawlowski, K., Newton, W.E., Eds.; Nitrogen Fixation: Origins, Applications, and Research Progress; Springer: Dordrecht, The Netherlands, 2008; Volume 6, pp. 1–24. [Google Scholar]
- Hayes, P.A.; Steeves, T.A.; Neal, B.R. An architectural analysis of Shepherdia canadensis and Shepherdia argentea: Patterns of shoot development. Can. J. Bot. 1989, 67, 1870–1877. [Google Scholar] [CrossRef]
- Krebs, C.J.; Boonstra, R.; Cowcill, K.; Kenney, A.J. Climatic determinants of berry crops in the boreal forest of the southwestern Yukon. Botany 2009, 87, 401–408. [Google Scholar] [CrossRef]
- Thilenius, F.J.; Evans, K.E.; Garrett, C.E. Shepherdia, buffaloberry. In Seeds of Woody Plants in the United States; Schopmeyer, C.S., Ed.; U.S. Forest Service, Department of Agriculture: Washington, DC, USA, 1974; pp. 771–773. [Google Scholar]
- Bañuelos, M.-J.; Obeso, J.-R. How is fruit production regulated in the dioecious fleshy-fruited shrub Rhamnus alpinus? Basic Appl. Ecol. 2005, 6, 249–259. [Google Scholar] [CrossRef]
- Timmerman-Erskine, M.; Boyd, R.S. Reproductive biology of the endangered plant Clematis socialis (Ranunculaceae). J. Torrey Bot. Soc. 1999, 126, 107–116. [Google Scholar] [CrossRef]
- Barkman, J.J. Canopies and microclimate of tree species mixtures. In The Ecology of Mixed Species Stands of Trees; Blackwell Scientific Publications: Oxford, UK, 1992; pp. 181–188. [Google Scholar]
- Sharpe, F. The biologically significant attributes of forest canopies to small birds. Northwest Sci. 1996, 70, 86–93. [Google Scholar]
- Boulanger-Lapointe, N.; Järvinen, A.; Partanen, R.; Herrmann, T.M. Climate and herbivore influence on Vaccinium myrtillus over the last 40 years in northwest Lapland, Finland. Ecosphere 2017, 8, e01654. [Google Scholar] [CrossRef]
- Westerbergh, A.; Saura, A. Gene flow and pollinator behaviour in Silene dioica populations. Oikos 1994, 71, 215. [Google Scholar] [CrossRef]
- Knight, T.M.; Steets, J.A.; Vamosi, J.C.; Mazer, S.J.; Burd, M.; Campbell, D.R.; Dudash, M.R.; Johnston, M.O.; Mitchell, R.J.; Ashman, T.-L. Pollen limitation of plant reproduction: Pattern and process. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 467–497. [Google Scholar] [CrossRef]
- Carlsson-Graner, U.; Elmqvist, T.; Agren, J.; Gardfjell, H.; Ingvarsson, P. Floral sex ratios, disease and seed set in dioecious Silene dioica. J. Ecol. 1998, 86, 79–91. [Google Scholar] [CrossRef]
- House, S.M. Pollination success in a population of dioecious rain forest trees. Oecologia 1993, 96, 555–561. [Google Scholar] [CrossRef]
- Steven, J.C.; Waller, D.M. Isolation affects reproductive success in low-density but not high-density populations of two wind-pollinated Thalictrum species. Plant Ecol. 2007, 190, 131–141. [Google Scholar] [CrossRef]
- Hargreaves, A.L.; Harder, L.D.; Johnson, S.D. Consumptive emasculation: The ecological and evolutionary consequences of pollen theft. Biol. Rev. 2009, 84, 259–276. [Google Scholar] [CrossRef]
- Peeters, L.; Totland, Ø. Wind to insect pollination ratios and floral traits in five alpine Salix species. Can. J. Bot. 1999, 77, 556–563. [Google Scholar]
- Schulz, K.; Zasada, J.; Nauertz, E. Annual, local, and individual variation in the inflorescence and fruit production of eastern leatherwood (Dirca palustris L. Thymelaeaceae). J. Torrey Bot. Soc. 2004, 131, 292–304. [Google Scholar] [CrossRef]
- Bierzychudek, P. Pollinators increase the cost of sex by avoiding female flowers. Ecology 1987, 68, 444–447. [Google Scholar] [CrossRef]
- Willmer, P. Pollination and Floral Ecology; Princeton University Press: Princeton, NJ, USA; Woodstock, UK, 2011. [Google Scholar]
- Borkent, C.J.; Harder, L.D. Flies (Diptera) as pollinators of two dioecious plants: Behaviour and implications for plant mating. Can. Entomol. 2007, 139, 235–246. [Google Scholar] [CrossRef]
- Kevan, P.G. Insect pollination of high arctic flowers. J. Ecol. 1972, 60, 831–847. [Google Scholar] [CrossRef]
- Ashman, T.-L.; Stanton, M. Seasonal variation in pollination dynamics of sexually dimorphic Sidalcea oregana ssp. spicata (Malvaceae). Ecology 1991, 72, 993–1003. [Google Scholar] [CrossRef]
- Charlesworth, D. Why are unisexual flowers associated with wind pollination and unspecialized pollinators? Am. Nat. 1993, 141, 481–490. [Google Scholar] [CrossRef]
- Eckhart, V.M. The effects of floral display on pollinator visitation vary among populations of Phacelia linearis (Hydrophyllaceae). Evol. Ecol. 1991, 5, 370–384. [Google Scholar] [CrossRef]
- Renner, S.S.; Ricklefs, R.E. Dioecy and its correlates in the flowering plants. Am. J. Bot. 1995, 82, 596–606. [Google Scholar] [CrossRef]
- Johnson, K.M.; Nielsen, S.E. Demographic effects on fruit set in the dioecious shrub Canada buffaloberry (Shepherdia canadensis). PeerJ 2014, 2, e526. [Google Scholar] [CrossRef]
- Ghazoul, J. Floral diversity and the facilitation of pollination. J. Ecol. 2006, 94, 295–304. [Google Scholar] [CrossRef]
- Haig, D.; Westoby, M. On limits to seed production. Am. Nat. 1988, 131, 757–759. [Google Scholar] [CrossRef]
- Knight, T.M.; Steets, J.A.; Ashman, T.-L. A quantitative synthesis of pollen supplementation experiments highlights the contribution of resource reallocation to estimates of pollen limitation. Am. J. Bot. 2006, 93, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Government of Canada. Canadian Climate Normals 1981–2010 Station Data; Government of Canada: Ottawa, ON, Canada, 2014.
- City of Edmonton. Terwillegar Park Concept Plan Study: Opportunities and Constraints Analysis; City of Edmonton: Edmonton, AB, Canada, 2007. [Google Scholar]
- Alberta Environmental Protection. The Parkland Natural Region of Alberta; Government of Alberta: Edmonton, AB, Canada, 1997.
- Downing, D.J.; Pettapiece, W.W. Natural Regions and Subregions of Alberta; Government of Alberta, Natural Regions Committee: Edmonton, AB, Canada, 2006.
- Fiala, A.C.S.; Garman, S.L.; Gray, A.N. Comparison of five canopy cover estimation techniques in the western Oregon Cascades. For. Ecol. Manag. 2006, 232, 188–197. [Google Scholar] [CrossRef]
- Lemmon, P.E. A spherical densiometer for estimating forest overstory density. For. Sci. 1956, 2, 314–320. [Google Scholar]
- StataCorp. Stata Multilevel Mixed-Effects Reference Manual (Release 13); Stata Press: College Station, TX, USA, 2013. [Google Scholar]
- StataCorp. Structural Equation Modeling Reference Manual (Release 13); Stata Press: College Station, TX, USA, 2013. [Google Scholar]
- Osunkoya, O.O. Population structure and breeding biology in relation to conservation in the dioecious Gardenia actinocarpa (Rubiaceae): A rare shrub of North Queensland rainforest. Biol. Conserv. 1999, 88, 347–359. [Google Scholar] [CrossRef]
- Kozlowski, T.T.; Pallardy, S.G. Growth Control in Woody Plants; Academic Press: Toronto, ON, USA, 1997; ISBN 978-0-12-424210-4. [Google Scholar]
- Glaettli, M.; Barrett, S.C.H. Pollinator responses to variation in floral display and flower size in dioecious Sagittaria latifolia (Alismataceae). New Phytol. 2008, 179, 1193–1201. [Google Scholar]
- Matsuhisa, S.; Ushimaru, A. Sexual dimorphism in floral longevity and flowering synchrony in relation to pollination and mating success in three dioecious Ilex species. Am. J. Bot. 2015, 102, 1187–1197. [Google Scholar] [CrossRef]
- Niesenbaum, R.A. Light or pollen: Seasonal limitations on female reproductive success in the understory shrub Lindera benzoin. J. Ecol. 1993, 81, 315–323. [Google Scholar] [CrossRef]
- Alvarez-Buylla, E.R.; Martinez-Ramos, M. Demography and allometry of Cecropia obtusifolia, a neotropical pioneer tree: An evaluation of the climax-pioneer paradigm for tropical rain forests. J. Ecol. 1992, 80, 275–290. [Google Scholar] [CrossRef]
- Amadeu, L.S.N.; Sampaio, M.B.; dos Santos, F.A.M. Influence of light and plant size on the reproduction and growth of small palm tree species: Comparing two methods for measuring canopy openness. Am. J. Bot. 2016, 103, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Paudel, S.; Battaglia, L.L. The role of light, soil and human factors on the probability of occurrence of an invasive and three native plant species in coastal transitions of coastal Mississippi, USA. J. Plant Ecol. 2015, 8, 491–500. [Google Scholar] [CrossRef]
- Sultan, S.E.; Wilczek, A.M.; Hann, S.D.; Brosi, B.J. Contrasting ecological breadth of co-occurring annual Polygonum species. J. Ecol. 1998, 86, 363–383. [Google Scholar] [CrossRef]
- Hudson, J.E.; Levia, D.F.; Hudson, S.A.; Bais, H.P.; Legates, D.R. Phenoseasonal subcanopy light dynamics and the effects of light on the physiological ecology of a common understory shrub, Lindera benzoin. PLoS ONE 2017, 12, e0185894. [Google Scholar] [CrossRef] [PubMed]
- De la Bandera, M.d.C.; Traveset, A.; Valladares, F.; Gulías, J. Gender, season and habitat: Patterns of variation in photosynthetic activity, growth and fecundity in Thymelaea velutina. Acta Oecol. Montrouge 2008, 34, 294–302. [Google Scholar] [CrossRef][Green Version]
- Valdéz, M. Frankia ecology. In Nitrogen-Fixing Actinorhizal Symbioses; Pawlowski, K., Newton, W.E., Eds.; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Hamer, D.; Herrero, S. Wildfire’s influence on grizzly bear feeding ecology in Banff National Park, Alberta. Int. Conf. Bear Res. Manag. 1987, 7, 179–186. [Google Scholar] [CrossRef]
- McLellan, B.N. Implications of a high-energy and low-protein diet on the body composition, fitness, and competitive abilities of black (Ursus americanus) and grizzly (Ursus arctos) bears. Can. J. Zool. 2011, 89, 546–558. [Google Scholar] [CrossRef]
- McLellan, B.N.; Hovey, F.W. The diet of grizzly bears in the Flathead River drainage of southeastern British Columbia. Can. J. Zool. 1995, 73, 704–712. [Google Scholar] [CrossRef]
- Munro, R.H.M.; Nielsen, S.E.; Price, M.H.; Stenhouse, G.B.; Boyce, M.S. Seasonal and diel patterns of grizzly bear diet and activity in west-central Alberta. J. Mammal. 2006, 87, 1112–1121. [Google Scholar] [CrossRef]
- Antonovics, J.; Levin, D.A. The ecological and genetic consequences of density-dependent regulation in plants. Annu. Rev. Ecol. Syst. 1980, 11, 411–452. [Google Scholar] [CrossRef]
- Huguet, V.; Batzli, J.M.; Zimpfer, J.F.; Gourbière, F.; Dawson, J.O.; Fernandez, M.P. Nodular symbionts of Shepherdia, Alnus, and Myrica from a sand dune ecosystem: Trends in occurrence of soilborne Frankia genotypes. Can. J. Bot. 2004, 82, 691–699. [Google Scholar] [CrossRef]
- Batzli, J.M.; Zimpfer, J.F.; Huguet, V.; Smyth, C.A.; Fernandez, M.; Dawson, J.O. Distribution and abundance of infective, soilborne Frankia and host symbionts Shepherdia, Alnus, and Myrica in a sand dune ecosystem. Can. J. Bot. 2004, 82, 700–709. [Google Scholar] [CrossRef]
- Visser, S.; Danielson, R.M.; Parkinson, D. Field performance of Elaeagnus commutata and Shepherdia canadensis (Elaeagnaceae) inoculated with soil containing Frankia and vesicular–arbuscular mycorrhizal fungi. Can. J. Bot. 1991, 69, 1321–1328. [Google Scholar] [CrossRef]
- Burleigh, S.H.; Dawson, J.O. Occurrence of Myrica-nodulating Frankia in Hawaiian volcanic soils. Plant Soil. 1994, 164, 283–289. [Google Scholar] [CrossRef]
- Huss-Danell, K. Actinorhizal symbioses and their N2 fixation. New Phytol. 1997, 136, 375–405. [Google Scholar] [CrossRef]
- Zitzer, S.F.; Dawson, J.O. Soil properties and actinorhizal vegetation influence nodulation of Alnus glutinosa and Elaeagnus angustifolia by Frankia. Plant Soil. 1992, 140, 197–204. [Google Scholar] [CrossRef]
- Nickel, A.; Pelz, O.; Hahn, D.; Saurer, M.; Siegwolf, R.; Zeyer, J. Effect of inoculation and leaf litter amendment on establishment of nodule-forming Frankia populations in soil. Appl. Environ. Microbiol. 2001, 67, 2603–2609. [Google Scholar] [CrossRef]
- Zimpfer, J.F.; Kennedy, G.J.; Smyth, C.A.; Hamelin, J.; Navarro, E.; Dawson, J.O. Localization of Casuarina -infective Frankia near Casuarina cunninghamiana trees in Jamaica. Can. J. Bot. 1999, 77, 1248–1256. [Google Scholar]
- Barea, J.M.; Azcon-Aguilar, C. Mycorrhizas and their significance in nodulating nitrogen-fixing plants. Adv. Agron. 1983, 36, 1–54. [Google Scholar]
- McClure, P.R.; Coker, G.T.; Schubert, K.R. Carbon dioxide fixation in roots and nodules of Alnus glutinosa: I: Role of phosphoenolpyruvate carboxylase and carbamyl phosphate synthetase in dark CO2 fixation, citrulline synthesis, and N2 fixation. Plant Physiol. 1983, 71, 652–657. [Google Scholar] [CrossRef]
- House, S.M. Population density and fruit set in three dioecious tree species in Australian tropical rain forest. J. Ecol. 1992, 80, 57–69. [Google Scholar] [CrossRef]
- Dauber, J.; Biesmeijer, J.C.; Gabriel, D.; Kunin, W.E.; Lamborn, E.; Meyer, B.; Nielsen, A.; Potts, S.G.; Roberts, S.P.M.; Sõber, V.; et al. Effects of patch size and density on flower visitation and seed set of wild plants: A pan-European approach. J. Ecol. 2010, 98, 188–196. [Google Scholar] [CrossRef]
- Labouche, A.-M.; Richards, S.A.; Pannell, J.R. Effects of pollination intensity on offspring number and quality in a wind-pollinated herb. J. Ecol. 2017, 105, 197–208. [Google Scholar] [CrossRef]
- Crossman, A.; Charlesworth, D. Breakdown of dioecy: Models where males acquire cosexual functions. Evolution 2014, 68, 426–440. [Google Scholar] [CrossRef]
- McCabe, L.M.; Colella, E.; Chesshire, P.; Smith, D.; Cobb, N.S. The transition from bee-to-fly dominated communities with increasing elevation and greater forest canopy cover. PLoS ONE 2019, 14, e0217198. [Google Scholar] [CrossRef]
- Miller-Rushing, A.J.; Primack, R.B. Global warming and flowering times in Thoreau’s concord: A community perspective. Ecology 2008, 89, 332–341. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Francia, R.S. An approximate analysis of variance test for normality. J. Am. Stat. Assoc. 1972, 67, 215–216. [Google Scholar] [CrossRef]
- Gould, W.; Rogers, W. sg3.4: Summary of tests of normality. Stata Tech. Bull. 1991, 1, 20–23. [Google Scholar]
- Gould, W. sg11.1: Quantile regression with bootstrapped standard errors. Stata Tech. Bull. 1992, 2, 19–21. [Google Scholar]
- StataCorp Swilk—Shapiro–Wilk and Shapiro–Francia Tests for Normality. Available online: http://www.stata.com/manuals13/rswilk.pdf (accessed on 17 January 2020).
- Royston, P. Estimating departure from normality. Stat. Med. 1991, 10, 1283–1293. [Google Scholar] [CrossRef]

| Variable | SE | Min | Max | ||
|---|---|---|---|---|---|
| Environment | |||||
| Canopy cover (%) | 66.9 | 2.2 | 5 | 91 | |
| Demography | |||||
| Density (female) (50 m−2) | 3.5 | 0.5 | 0 | 15 | |
| Density (male) (50 m−2) | 2.8 | 0.4 | 0 | 13 | |
| Density (total) (50 m−2) | 7.1 | 0.5 | 0 | 25 | |
| Size (height) (m) | 1.32 | 0.03 | 0.81 | 1.75 | |
| Reproduction | |||||
| Flower production (cm−1) | 2.6 | 0.2 | 0.4 | 5.5 | |
| Fruit production (cm−1) | 1.1 | 0.1 | 0.1 | 2.7 | |
| Fruit production (total) | 2530 | 258 | 199 | 9805 | |
| Path Factor | Path | β | SE | z | P |
|---|---|---|---|---|---|
| Canopy cover, direct effect (βFrC) | C–>Fr | –0.21 | 0.11 | –2.02 | 0.043 |
| Canopy cover, direct effect (βFlC) | C–>Fl | –0.29 | 0.11 | –2.63 | 0.009 |
| Canopy cover, indirect effect (βFrFlC) | C–>Fl * Fl–>Fr | –0.11 | 0.05 | –2.11 | 0.035 |
| Canopy cover, total effect (βC(total)Fr) | C–>Fl–>Fr + C–>Fr | –0.32 | 0.10 | –3.06 | 0.002 |
| Density dependence, direct effect (βFlD) | D–>Fl | 0.32 | 0.11 | 3.01 | 0.003 |
| Density dependence, indirect effect (βFrFlD) | D–>Fl * Fl–>Fr | 0.12 | 0.05 | 2.26 | 0.024 |
| Internal constraints, direct effect (βFrFl) | Fl–>Fr | 0.38 | 0.10 | 3.61 | <0.001 |
| Internal constraints, direct effect (βFrS) | S–>Fr | 0.35 | 0.10 | 3.50 | <0.001 |
| Pollen competitor, direct effect (βFrF) | F–>Fr | –0.24 | 0.12 | –1.99 | 0.047 |
| Pollen donor, direct effect (βFrM) | M–>Fr | 0.27 | 0.12 | 2.18 | 0.029 |
| Error | e.Fl–>Fl | 0.80 | 0.09 | – | – |
| Error | e.Fr–>Fr | 0.61 | 0.09 | – | – |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bateman, T.J.; Nielsen, S.E. Direct and Indirect Effects of Overstory Canopy and Sex-Biased Density Dependence on Reproduction in the Dioecious Shrub Shepherdia canadensis (Elaeagnaceae). Diversity 2020, 12, 37. https://doi.org/10.3390/d12010037
Bateman TJ, Nielsen SE. Direct and Indirect Effects of Overstory Canopy and Sex-Biased Density Dependence on Reproduction in the Dioecious Shrub Shepherdia canadensis (Elaeagnaceae). Diversity. 2020; 12(1):37. https://doi.org/10.3390/d12010037
Chicago/Turabian StyleBateman, Tyler J., and Scott E. Nielsen. 2020. "Direct and Indirect Effects of Overstory Canopy and Sex-Biased Density Dependence on Reproduction in the Dioecious Shrub Shepherdia canadensis (Elaeagnaceae)" Diversity 12, no. 1: 37. https://doi.org/10.3390/d12010037
APA StyleBateman, T. J., & Nielsen, S. E. (2020). Direct and Indirect Effects of Overstory Canopy and Sex-Biased Density Dependence on Reproduction in the Dioecious Shrub Shepherdia canadensis (Elaeagnaceae). Diversity, 12(1), 37. https://doi.org/10.3390/d12010037






