1. Introduction
The insect pollination is one of the most important services that sustains biodiversity and food production. Out of the most important commercial crops 84% are insect pollinated [
1]. Honeybees (
Apis mellifera L.) are very important for increasing the quality of fruits and seeds of many wild and cultivated plant species [
2,
3]. For example, older research in Romania [
4] showed the following pollinators’ participation in stone tree pollination: honeybees—76.6%; bumblebees—7.6%; flies—3.7%; ants—3.6%; beetles—3.5%; wild bees—2.5%; wasps—0.5%; and other insects—2%.
Due to their complex biology (social life, reproduction, nutrition etc.) and intimate connection to climatic and vegetation conditions, honeybees are a natural biosensor of environmental quality [
5,
6,
7,
8,
9]. Some of their special behaviors make honeybees a special pollinator: they exclusively feed on nectar and pollen; there is a high number of individuals in a colony, which leads to large quantities of food storage; they have the big ray of forage flight (0–5 km); their “flower fidelity” behavior makes them an efficient pollinator for a certain plant species at a certain moment; they visit many flowers based on the quality and quantity of nectar secretion; they have good orientation, memory and communication regarding food sources; they thermoregulate their nests, which helps overwintering; and they have the possibility to manage and transport their colonies to different crops.
In the context of developing technologies based on electronic sensors combined with the Internet of Things (IoT) and big data storage, honeybees could become even better suited to be equipped and used to monitor different physical and biological aspects of their colonies in order to help beekeepers’ management, to avoid high honeybee losses, and to understand the different environmental factors that are causing the declining of their species [
9,
10,
11,
12,
13,
14,
15,
16]. Thus, from the perspective of managing qualities and their sensitivity to environmental contaminants, the honeybee colony represents an important bio tool to understand and even quantify the impact of different aggressive factors that intersect with its complex social life.
Honeybee depopulation and mortalities in the past two decades have become very important worldwide issues. Though the causes of these issues are quite diverse [
17,
18,
19], there have been numerous articles on the environmental factors and pesticides. A series of research studies showed that in experimental and field conditions, honeybees’ exposure to treated crops registered the following levels of residues: thiacloprid residues were in averages of 75.1 ng/g in pollen and 6.5 ng/g in honey, clothianidin residues were in averages of 9.4 ng/g in pollen and 1.9 ng/g in honey, and imidacloprid residues were in average of 19.7 ng/g in pollen and 6 ng/g in honey [
20]. Thiacloprid was also frequently detected in honey samples up to concentrations of 200 ng/g [
21], which is the maximum residue level that is accepted for human consumption. In another study [
22] the occurrence of pesticides was more frequent in pollen and beeswax, and imidacloprid and fipronil were detected mostly in all matrices. A recent study that was focused on worldwide honey samples [
23] showed that 75% of honey samples contained at least one neonicotinoid in quantifiable amounts, the total concentration of the measured neonicotinoids being 1.8 ng/g on average. In another piece of research [
24], 97% of neonicotinoids found in pollen came from wildflowers, which grow near treated crops. The presence of more than one pesticide from different categories, in the same sample, is another issue that has been highlighted in much research worldwide that has shown that honeybees are chronically exposed to neonicotinoids and fipronil, and their metabolites in the 1–100 ng/g (ppb) range [
20,
25,
26]. In one study [
27], sunflowers and corn tassels contained values of 10 ng/g of imidacloprid in average, which explained why the pollens from these crops were contaminated at levels of a few ng/g. Another study [
28] showed that the concentrations of imidacloprid found in sunflower and corn pollens collected in the pollen traps were, respectively, about 1.5 and 4.5 times less than the concentration of imidacloprid found in the same types of pollen that were directly collected from flowers, thus highlighting the potential hazard of neonicotinoids to honeybees through contaminated pollen and nectar. One survey [
29] showed that colonies located in a corn-dominated area registered greater colony mortality by 3.51 times more than in corn crop-free locations. In the same study, it was shown that 54% of analyzed samples contained clothianidin, and 31% contained both clothianidin and thiamethoxam.
Studies have shown that these substances affect the honeybee organism at nanograms levels (1 ng = 10
−9), with 0.10 ng imidacloprid/bee being the lowest concentration that had detrimental effects on honeybees in laboratory conditions [
30].
Following the scientific data of numerous studies regarding their toxicity by lethal and sublethal effects, based on European Food Safety Authority scientific reports [
31], the European Union (EU) (2018/783/784/785/29.05.2018) banned the use of three neonicotinoids (imidacloprid, clothianidin, and thiamethoxam) in fields. However, a series of countries were approved to use these substances by emergency authorization, Romania being the country which continuously used the forbidden neonicotinoids, at the country level, based on emergency authorizations.
In this context, it is important to mention that Romanian agriculture is one of the main economic sectors that is continuously developing thanks to the favorable geographic and climatic conditions. This situation has led to an increase of land surfaces that are cultivated with industrial crops (sunflower, rape, and corn) and their inputs. In the same time, beekeeping has been a very important sub-domain of agriculture that is favored by natural conditions and stimulated by the organizational national structure under Romanian Beekeepers Association, founded in 1958.
Since Romania’s admission into the EU (2007), the developing beekeeping sector has been encouraged by the national beekeeping program and some other agricultural programs that are ruled by European and national legislation. As a result, the number of hives has constantly increased, with the total number of hives being 11.7% of the total EU number with a production of honey of 13% of the total EU honey production in 2018 (these data were published at
https://ec.europa.eu/agriculture/honey/programmes_en).
If rape and sunflower are very well known in importance for beekeeping, corn crops (
Zea mays) are not so well highlighted as they are considered a wind pollinated crop. However, even pollen morphology reflects an adaptation to wind pollination, as its nutritional properties make it an important attractant for honeybees and other pollinating insects. Its male flower (the tassel) offers large amount of pollen that can be very attractive during the period of sunflower honey flow when crops are nearby. This source of pollen is of great importance as its flowering period overlaps on that when winter honeybees’ generations are reared, so it substantially contributes to the quality of wintering, both by honeybee quality as well as by pollen storage that is consumed in the early stage of brood rearing in the next season. As a consequence, the contamination of corn pollen with neonicotinoids may have a great negative impact on honeybees [
32,
33,
34,
35,
36,
37] in the sunflower honey flow period, as a lot of corn fields are closer to the sunflower crop fields—this is a reason why corn crops were included in this study.
Taking into account policy context and certain beekeepers’ complaints regarding honeybees’ depopulations [
38], a research project was funded in October 2017–October 2018 by the Ministry of Agriculture and Rural Development for the first time in Romania. The aim of this project was to establish the realistic-field exposure levels to neonicotinoids in certain areas that are intensively cultivated with oilseed rape, corn and sunflower. To carry out this research, an integrative approach was developed in order to follow up the honeybee colonies and to collect and prepare representative samples in order to evaluate the exposure of honeybees to the neonicotinoids used in intensive crops.
2. Materials and Methods
This research was carried out in the following phases:
- (1)
Field identification in different agricultural areas.
- (2)
Honeybee colony preparation and transportation to different envisaged crop fields.
- (3)
Sample collection and specific preparation.
- (4)
Sample preservation, codification, packing, and sending to the accredited laboratories according to specific requirements.
- (5)
The neonicotinoid analyses and results reports performed by the accredited laboratories.
2.1. Field Identification in Different Agricultural Areas
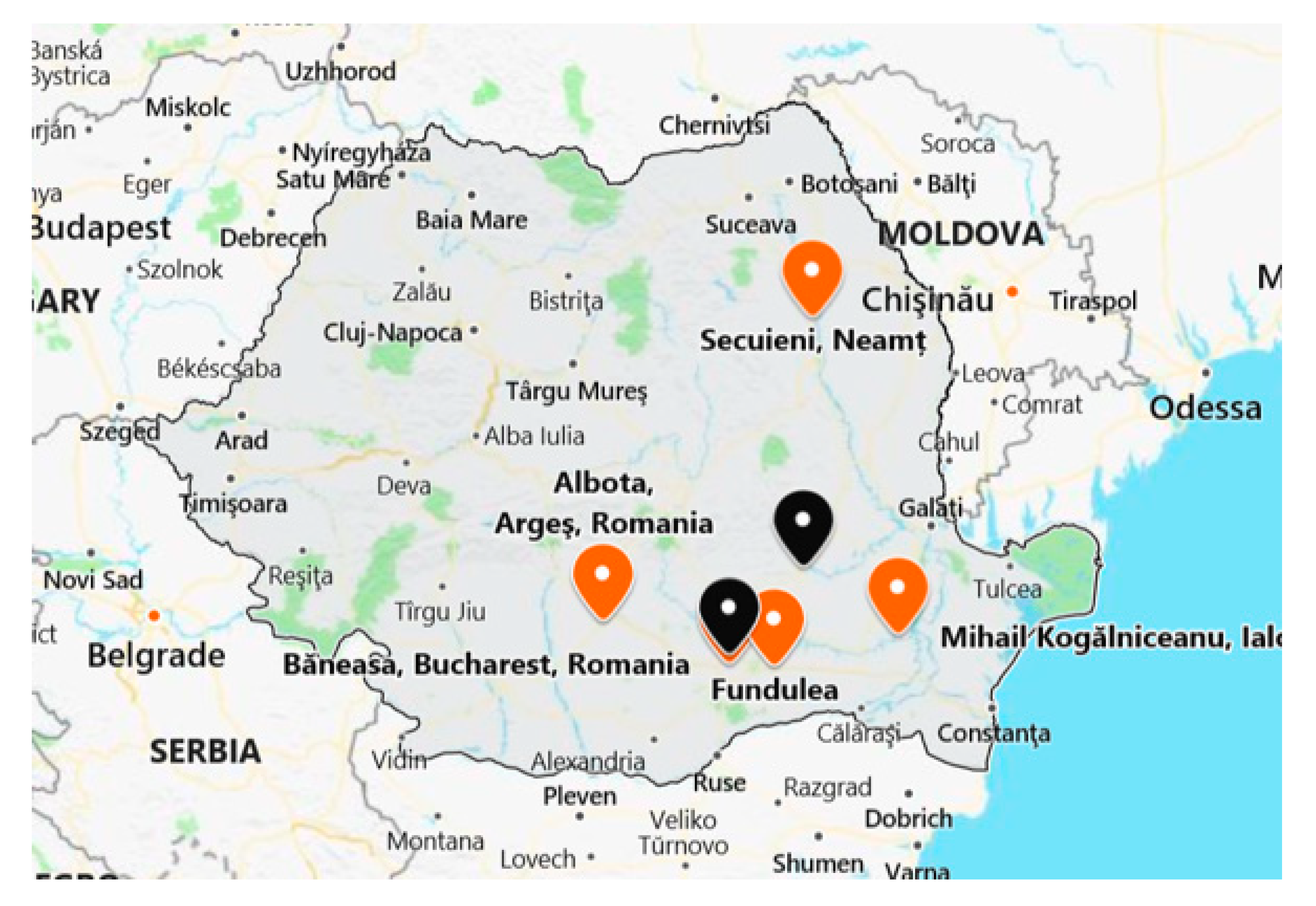
To implement the protocol and carry out the analyses regarding the exposure of honeybee colonies to neonicotinoids, three fields were selected in different areas of intensive agriculture in the southeastern and eastern parts of Romania, being provided by two different agricultural research stations and one institute that belongs to the Romanian Academy for Agricultural and Forestry Sciences “Gheorghe Ionescu Sisesti”. These were located in Neamt county (Statiunea de Cercetare Dezvoltare Agricola—SCDA Secuieni, 46°51′45″ N, 26°49′42″ E), Arges county (Statiunea de Cercetare Dezvoltare Agricola—SCDA Albota, 44°46′54″ N, 24°49′31″ E), and Calarasi county (Institutul National de Cercetare Dezvoltare Agricola—INCDA Fundulea, 44°27′10″ N, 26°30′55″ E). Besides these, one farmers’ association was involved in this monitoring study: The Corn Producers’ Association (Asociatia Producatorilor de Porumb din Romania—APPR) located in Ialomita county Mihail Kogalniceanu, Tandarei (44°40′39.67″ N, 27°42′14.71″ E) (
Figure 1).
These centers provided different surfaces of land (a minimum of 1 ha and a maximum of 100 ha) that were cultivated with targeted crops (rape, corn and sunflower) and treated with field-prescribed doses of active substances/products by seed dressing, regarding the three neonicotinoids (imidacloprid, clothianidin, and thiamethoxam) that are the subject of interdiction in the European Union. In addition to these locations, a series of samples were collected from two apiaries belonging to the Institute for Beekeeping Research and Development Bucharest, apiaries being located in different areas in southeastern Romania (Baneasa-Bucuresti—44°29′33″ N, 26°04′45″ E, Buzau—45°10′ N, 26°49′ E), as well as from private beekeepers (Fundulea—44°27′10″ N, 26°30′55″ E, Otopeni—44°32′ N, 26°6′ E,) (
Figure 1), in order to evaluate the presence of neonicotinoids in different areas with intensive agriculture but without any information about the use of phytosanitary treatments.
The experimental honeybee colonies were located near the treated crops (0–100 m), depending on the configuration of the land, at approximatively 10% blooming, in order to attract the honeybees for nectar or pollen collection and initiate the “fidelity flower” behavior.
2.2. Honeybee Colony Preparation and Transportation to Different Envisaged Crop Fields
In order to monitor the honey/pollen flows and collect representative samples, the experimental honeybee colonies were prepared. In this regard, every location was supplied with two honeybee colonies (normal colonies with queen, brood and food storages), established on 10 Dadant frames each, well covered with honeybees, and equipped with entrance type pollen collectors and foundation frames for honey collection in order to let honeybees build combs in the honey flow conditions to decrease the risk of contaminating the collected honey by older combs.
Out of these colonies, one honeybee colony per each location was equipped with an electronic hive (Simbee®,
http://www.simbee.ro/) that consisted of a special module of sensors and data collectors: two ambient temperature and humidity sensors, an internal sensor for honeybee colony temperature, and a weight sensor scale.
The monitoring hives transmitted the collected data to its database every 10 min, thus registering the activity of bee colonies regarding the monitoring parameters and helping to understand the existence or lack of honey flows in the flight area, as well as suspicions about a possible decline of population and its development status that are connected with phytosanitary treatments.
2.3. Sample Collection and Specific Preparation
2.3.1. The Honeybee Samples
These samples were collected from dying or live honeybees, depending on the encountered situation. The dying honeybees were collected out from the front of hive and, when faced a lack of dead or dying honeybees, we collected live honeybees (foragers) from the entrance, following the honey or pollen flow, by using a car aspirator. The samples were immediately confined to a bag and put in a car freezer.
As during the experiments, acute and lethal effects on honeybees were not directly observed in most of the cases, the honeybee sampling consisted of the collection of live forage honeybees from the entrance of the hives in order to increase the probability of finding neonicotinoids in honeybees that carry freshly contaminated nectar or pollen.
2.3.2. The Honey and Pollen Samples
After 7–10 days from the beginning of the honey flow, honey samples were collected from specially prepared and introduced frames in the monitored hives that were preserved in refrigerators.
The collected honeybee pollens were taken out from collectors and preserved in specific low temperature conditions (between −10 and 4 °C) daily, depending on local situation, until their mono-floral analyses and special preparing samples, which was the case for samples sent to the French Agency for Food, Environmental and Occupational Health and Safety (ANSES) laboratory.
In order to identify the source of different contaminants by using the honeybee as a sampler, one problem was represented by the big ray of forage flight from the hive (0–5 km), which covers a big surface of land and seldom obtains multi-floral products, e.g., honey, pollen, and beebread. In the correct monitoring of a specific crop, the problem lies in collecting its specific mono-floral samples, as this has a big impact on residue data identification and interpretation.
Thus, to understand the floral componence and to establish the mono-floral honeys from the targeted field, the samples collected out from the rape and sunflower crops were analyzed in the framework of the chemistry laboratory of the Institute for Beekeeping Research and Development, based on specific standardized methods of melissopalinology used in the evaluation of honey types (
Table 1). These analyses are very important in the context of the neonicotinoid residues analysis because they confirm whether the samples are sufficiently relevant for the purpose of the study.
Due a lack of mono-floral envisaged samples because of climatic conditions (drought or heavy rains specific to the 2018 season) in the present study, we also used honey collected during the studied crop flowering that was classified as multi-floral honeys but also contained the targeted honey in different percentages.
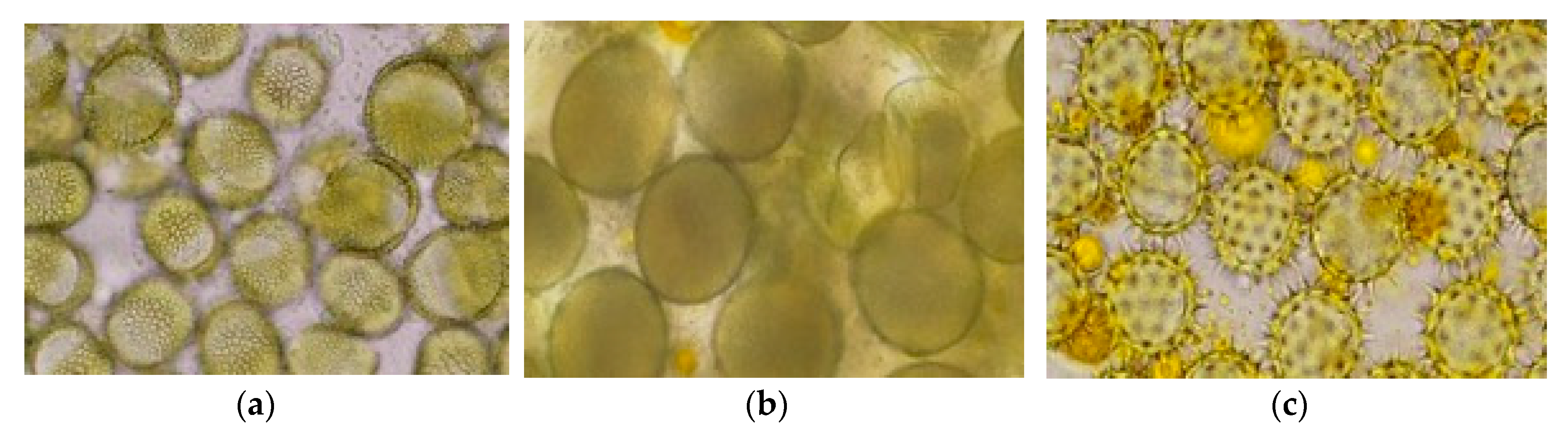
Regarding the mono-floral pollen samples and taking into account the variability of pollens usually collected by honeybees, most of the pollens collected by specific entrance collectors in the rape and sunflower period were multi-floral. To have relevant, envisaged mono-floral pollen samples for neonicotinoid analyses, based on the minimum required quantities (when possible), we manually selected the pollen pellets of rape, corn and sunflower (
Figure 2a,b) in the laboratory conditions based on the pellet aspect and color, with the selection being randomly confirmed by microscopy based on pollen characteristics (
Figure 3a–c).
Pollen mono-floral selection, a time consuming activity, was possible only when the minimum sample quantity requested by laboratory was low. The minimum quantities of the samples requested by the two laboratories were a minimum of 10 g at the ANSES laboratory and a minimum of 250 g at Quality Services International (QSI) laboratory. The minimum quantity of 10 grams (e.g., ANSES laboratory) permitted a better approach in the sample preparation, thus providing a better analysis of the pollen origin regarding the plant species. The process of collection and selection of representative samples was a key stage in the neonicotinoid identification and quantification.
Following these selection steps, 50 samples were prepared and sent to the laboratories, as follows: honeybees—10 samples; honey—15 samples; and pollen—25 samples.
2.4. Sample Preservation, Codification, Packing and Sending to the Accredited Laboratories According to Specific Requirements
After the collection and transportation to the central laboratory of the Institute for Beekeeping Research and Development, all the samples were preserved at −18 °C.
For identification, samples were coded with a unique code, e.g., R-P-F-2 = Rape-Pollen-Fundulea-2nd sample (R = rape; FS = sunflower; P = corn; M= honey; P= pollen; and A= honeybees). The samples, which were prepared according to the specific requirements of analyzing laboratories, were packed in special containers and sent to the following two European accredited laboratories: thirty samples were sent to an EU Reference laboratory—French Agency for Food, Environmental and Occupational Health and Safety (ANSES), France, and 20 samples were sent to Quality Services International (QSI), Germany. The shipping was performed in special freezing packing for biological material.
The two laboratories were chosen in order to diversify and better understand the requirements of the accredited laboratories regarding the preparation of samples for analyses. Generally, the samples were distributed between the two laboratories depending on the collected quantity correlated with melissopalinological analysis.
The results of neonicotinoid analyses performed by the accredited laboratories are presented in
Table 2 and
Table 3, together with their level of detection and quantification on five neonicotinoids—acetamiprid, clothianidin, thiamethoxam, imidacloprid and thiacloprid. The analyses were done with the liquid chromatography method coupled with tandem mass spectrometry (LC-MS/MS) at both laboratories.
3. Results
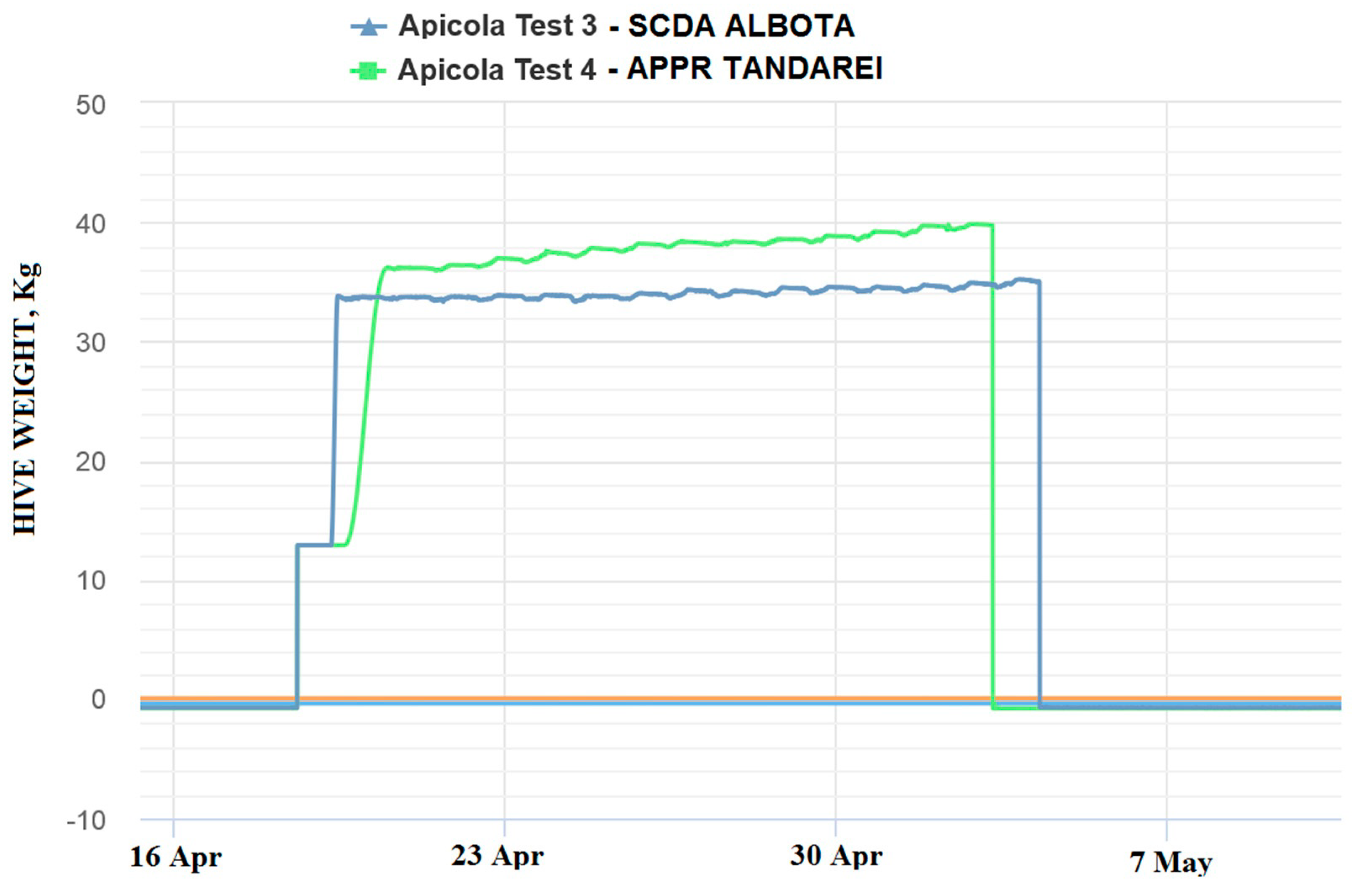
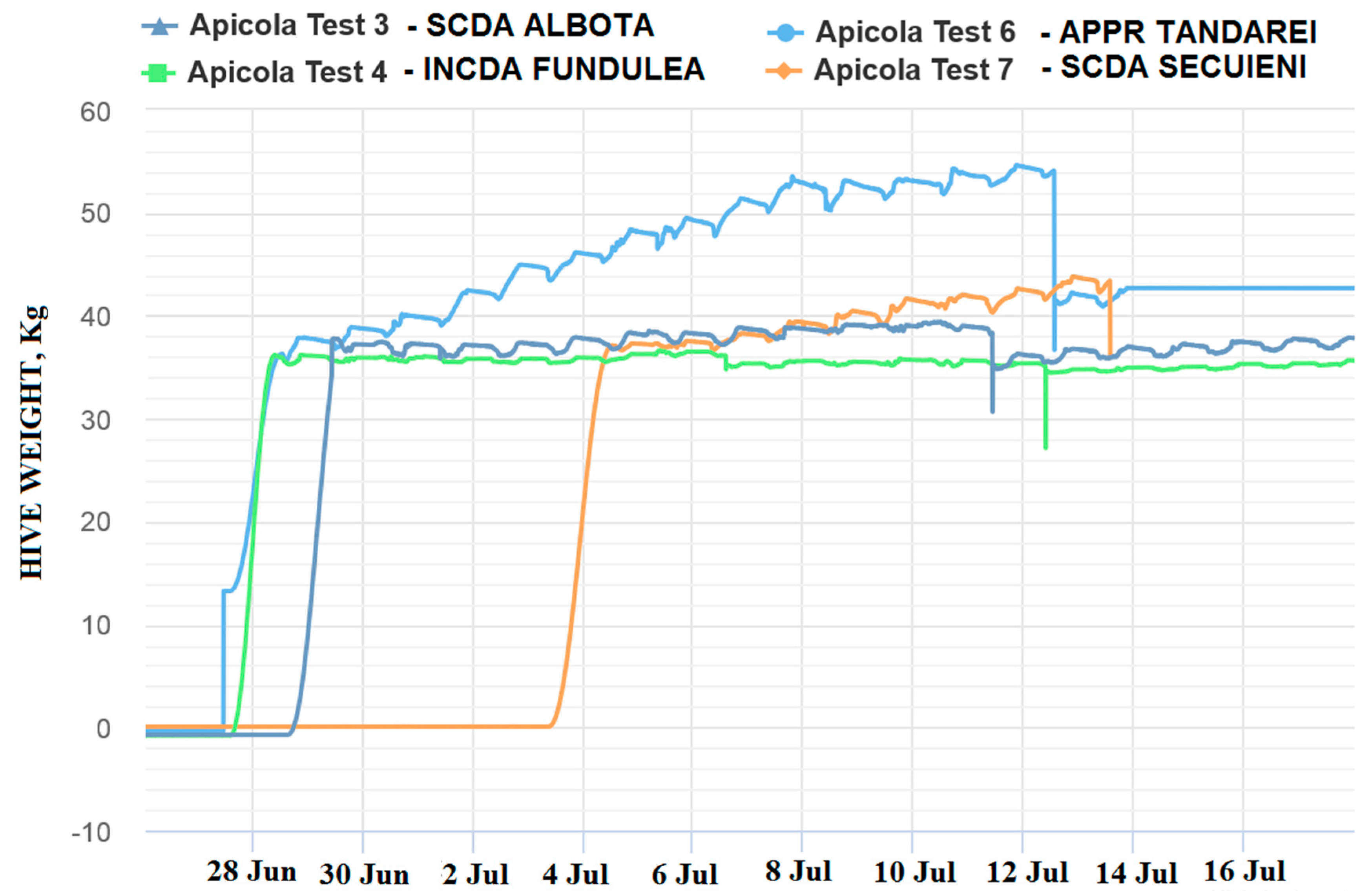
The 2018 beekeeping season was generally a weak active season for Romania in terms of honey production, (via oilseed rape and sunflower), this situation being registered in the monitored locations by means of the electronic hives. The weak beekeeping season had a negative impact on honey sample collection. The weight gain represented the first indicator of honeybee colony activity and, mainly, of the existence of a nectar flow from targeted fields that is necessary for honey sample collections. This evaluation offered preliminary information about the probability of collecting samples from the targeted fields, but this information needed to be correlated and confirmed by melissopalinological studies on collected samples. The weight gain in the monitored hives is shown in the following images (
Figure 4 and
Figure 5):
As can be noticed from the gain weight registered by the electronic hive, the general activity of honeybee colonies and the development of the colonies in honey flow conditions were relatively low, with the causes being not well understood.
Concerning the results on honeybees, it is important to mention that in general, in the Fundulea, Tandarei and Baneasa locations, a weak activity was noticed. Additionally noticed were signs of depopulations and/or honeybees in front of the hives with specific symptoms of acute toxicity (walking on the ground, paralysis, and dying) during the oilseed rape honey flow period. The results showed that imidacloprid was present in a concentration of 0.1 ng/bee, as well as under the limit of quantification (LOQ) in these locations.
The presented protocol that was used to collect and prepare honey and pollen samples for neonicotinoid residues analyses, included an important step: melissopalinological analyses for the identification the mono-floral honeys (
Table 1) and mono-floral pollens selection. Following these preliminary analyses, all rape honey samples were framed in the standards of mono-floral honeys, and the results showed that these samples were set according to the standard internal laboratory references for rape honey (minimum 40% rape pollen grains). However, in the case of sunflower honeys, only one of the collected samples was framed in the internal standards of typical sunflower honey.
These results indicate that the analyzed honey samples in the case of multi-floral honeys (e.g., sunflower honey) could not correctly reflect the residues of neonicotinoids found in the envisaged flora, and this led to their sub-evaluation.
The results on the neonicotinoid residues analyzed on different matrices and laboratories are highlighted in
Table 2.
The raw data show that 48% of the total samples (n = 50) sent to the two laboratories contained one or more, detected or quantified, neonicotinoid residues, with the quantifiable residues being found in 38% of samples.
It can be emphasized that, in the case of the EU reference laboratory (ANSES), 43.3% of the total analyzed samples (n = 30) contained registered, quantifiable residues of one or more neonicotinoids, and 33.3% contained registered, detectable amounts of one or more residues of neonicotinoids. In the QSI laboratory, 30% of the total analyzed samples (n = 20) contained registered, quantifiable residues.
One very important aspect needs to be mentioned regarding the analytical references of the two laboratories (
Table 3) for the residue quantification limit (LOQ), which could influence the results and their interpratation; for example, in the honey analyses, the different residue LOQs (ANSES = 1.0–4.0 ng/g, QSI = 1.0–5.0 ng/g) need to be analyzed on different active substances between the two laboratories, while for the pollen analyses, the LOQs (ANSES = 0.5–1 ng/g, QSI = 10.0 ng/g) are more favorable in ANSES laboratory.
Taking into account the LOQ of the residue analysis in the involved laboratories, the following neonicotinoids were found in quantifiable levels: imidacloprid in honeybee samples, acetamiprid and thiacloprid in honey samples, and acetamiprid, imidacloprid and thiacloprid in pollen samples.
The minimum–maximum levels of the quantified neonicotinoids were:
- (1)
0.1 ng/bee imidacloprid in honeybee samples in the oilseed rape blooming period.
- (2)
1.2–3.7 ng/g thiacloprid in oilseed rape honey samples.
- (3)
1.8–7.3 ng/g acetamiprid in oilseed rape honey samples.
- (4)
1.3–31.1 ng/g imidacloprid in oilseed rape pollen samples.
- (5)
1.1–1.4 ng/g imidacloprid in corn pollen samples.
- (6)
2.0 ng/g thiacloprid in corn pollen samples.
- (7)
1.1–795.1 ng/g thiacloprid in oilseed rape pollen samples.
Regarding the three neonicotinoids (imidacloprid, clothianidin and thiamethoxam) that are banned at European level but are the subject of derogation in Romania, the following percentages of residues were found on different matrices and crops in the two laboratories:
- (1)
In honeybees collected from oilseed rape crops, imidacloprid was in 50% of the total collected samples (n = 6) and was found in 33.3% of the samples, being quantified at 0.1 ng in the ANSES laboratory;
- (2)
In the honey collected from oilseed rape crops, imidacloprid was found under the LOQ in 16.6% of the total samples (n = 6) analyzed in the ANSES laboratory;
- (3)
In honey collected from sunflower crops, imidacloprid was found under the LOQ in 25.0% of the total samples (n = 4) analyzed in the ANSES laboratory;
- (4)
In pollen collected from oilseed rape crops, imidacloprid was found in 16.6% (n = 1, 13 ng/g) of the total samples (n = 6) analyzed in the QSI laboratory;
- (5)
In pollen collected from oilseed rape crops, imidacloprid was found in concentrations of between 10.6 and 31.1 ng/g in 100% of the total samples (n = 3) analyzed in the ANSES laboratory;
- (6)
In pollen collected from corn crops, imidacloprid was found in concentrations between 1.1 and 1.4 ng/g in 100% of the total samples (n = 3) analyzed in the ANSES laboratory.
Out of all analyzed active substances, clothianidin was not found in any sample.
Concerning the monitored crops, the percentages of samples with one or more quantifiable neonicotinoid residues in different matrices are presented as it follows:
- (1)
In the oilseed rape crops, neonicotinoid residues were found in 33.3% of the honeybee samples (n = 6), in 87.5% of the honey samples (n = 8), and in 77.7% of the pollen samples (n = 9).
- (2)
In the corn crops, neonicotinoid residues were found in 100% of the pollen samples (n = 3).
With only one exception found in oilseed rape pollen (795.1 ng/g thiacloprid), all residues were under the maximum residue limit (MRL) established for human consumption.
Looking to the percentages of samples with one or more neonicotinoid residues over the detection limit in different matrices and crops, the situation is presented as follows:
- (1)
In the oilseed rape crops, one or more neonicotinoid residues over the detection limit were found in 66.6% of the honeybee samples (n = 6), in 87.5% of the honey samples (n = 8), and in 77.7% of the pollen samples (n = 9).
- (2)
In the corn crops, one or more neonicotinoid residues over the detection limit were found in 100% of the pollen samples (n = 3).
- (3)
In the sunflower crops, one or more neonicotinoid residues over the detection limit were found in 42.8% of the total honey samples (n = 7).
One can notice the presence of neonicotinoids in detectable amounts in three of the four sunflower honey samples analyzed in the ANSES laboratory, while in sunflower pollen from the same location (Albota, Tandarei, and Secuieni), neonicotinoids were not detected, even in the pollen samples that were mono-floral selected. An inverse situation was noticed in one oilseed rape sample analyzed in the same laboratory (ANSES), where neonicotinoid residues were not identified in honey (R-M-F-1) from the same location (Fundulea), but they were very well identified in a pollen sample (R-P-F-1). This result shows the importance of analyzing residues in both matrices of honey and pollen collected from the same hive.
Another aspect of residue analysis is related to the presence in detectable and/or quantifiable amounts of different active substances of neonicotinoids in the same sample that could conduct the so-called “cocktail” effect. In this study, this was the case of oilseed rape honey (50%) and pollen samples (100%), as well as in corn samples (33.3%), though only in the samples analyzed in the ANSES laboratory.
Another important aspect that is worth mentioning is that in every sample preparation, the corn and sunflower pollens pellets were separated from the same multi-floral sample with different levels of mixtures with other pollens (not analyzed). Out of the obtained results, one can highlight that the neonicotinoid residues found in corn pollens did not influence the sunflower pollen residues because all corn pollens registered different levels of residues and sunflower pollen was free of residue from an analytical point of view. This fact indicates us that neonicotinoids from a pollen pellet do not contaminate other pollen pellets.
Taking into account the eight locations where the samples were collected from, the results showed that every location had at least one sample with detected or quantified neonicotinoids in honey or pollen, so the distribution of neonicotinoids in the environment was relatively large in the areas with intensive agriculture.
4. Discussion
The present study aimed to improve the evaluation methodologies for analyzing honeybees’ exposure to pesticides in order to better identify and quantify the contamination of honeybees’ food resources from the main treated crops, as well as to have a first image of their presence and quantification in Romania in some treated crops. Thus, the most important levels of neonicotinoid residues, in the vegetation conditions of the 2018 season, were identified in oilseed rape honeybees, honey, and pollens, as well as in corn pollen.
In view of these results, the analysis of neonicotinoids in these matrices is very important for establishing a basic exposure level of honeybees to pesticides in a specific area at a certain moment.
The identification and quantification of neonicotinoids in any sample could be a combined result of many factors, but the collection and sample preparation until their analysis, as well as the analysis methodology with the lowest LOD (limit of detection) and the lowest LOQ, are of great importance.
In order to identify and quantify the neonicotinoids, the obtained results in the two laboratories also showed the importance of the mono-floral sample preparation of honeys and pollens.
One interesting finding concerned the presence of different neonicotinoids in detectable and/or quantifiable amounts in all matrices that were obtained from oilseed rape crops, while some neonicotinoids (acetamiprid, imidacloprid and thiacloprid) were only found in detectable amounts in sunflower honey. The low level of neonicotinoid residues obtained in some sunflower honey samples can be explained by the fact that sunflower honey is actually a multi-floral honey, so the contamination residues were diluted by different sources of nectars. However, the lack of neonicotinoids in the selected sunflower pollen samples remains questionable, and further research is necessary.
Following the identification of neonicotinoids (imidacloprid) in all the three corn pollen samples and in view of the fact that honeybees collect high quantities of corn pollen in areas with intensive crops, this study can be seen as offering an important overview of the importance of this crop for honeybee nutrition.
What is remarkable here, from the field observation, is the fact that corn tassels were intensely visited by honeybees, even when the nearby sunflower crops are in full bloom. This shows that corn tassels are an important source of pollen for honeybee colonies, as is sunflower pollen. For this reason, the corn crops represent important sources of nutrients, not only by guttation water but also by pollen, so its contamination with pesticides could affect honeybees during the whole vegetation period.
The obtained results are similar with those found in the literature [
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
39] that have shown that the residues of neonicotinoids in honeys and pollens are found in the range of a few nanograms per gram. Their lethal or sublethal effects on honeybees depend on many factors [
28] such as daily consumption, seasonal conditions, the activity and strength of the colony, the age and the duration of exposure, and health state. For example, the literature data show that the lethal toxicity of imidacloprid is in the range of a few picograms if ingestion is repetitive for minimum eight days and of a few nanograms if the ingestion is for one-to-two days [
40]; however, its sublethal effects, such as learning and orientation ability modifications, could appear at a concentration of 0.1 ng/bee [
30], which was the case in our study in real conditions.
Taking into account the toxic and cumulative effects of neonicotinoids, based on irreversible bind on the nicotinic acetylcholine receptors (nAChRs), as well as the toxic effects of their metabolites proven by research [
41,
42,
43,
44,
45], the found concentrations pose serious risks to honeybee health in the short and long term. One such study [
44] that was done over a short period of time (10–30 days) showed, by extrapolation, that the daily ingestion of about 0.005 ng/day of imidacloprid produced important lethal toxic effects (LT50) in 150 days. If a bee consumes around 0.02 g honey per day [
28], a concentration of 0.25 ppb in honey (which is not a quantifiable amount (see LOQ,
Table 3)) can cause long-term mortalities (over 150 days), as it happens in the winter. These low residue quantities can be consistently supplied by residues in storage pollens (beebread) in the late winter-to-early spring period when colonies begin rearing their brood, and this situation can explain the collapse of colonies due to a long exposure to sublethal effects.
Some research has gone even deeper, demonstrating that the use of neonicotinoids can lead to a wider range of sub-lethal effects on honeybees as a result of very low concentrations of neonicotinoids. Thus, if lethal acute effects can be rapidly noticed by the rapid decrease of the population or mortalities in front of the hive, sub-lethal effects can be difficult to observe, as colonies generally have problems of development or slow mortality for longer periods (autumn, winter, and early spring depopulation), inducing a variety of behavioral dysfunctions. Many of these dysfunctions affect orientation, memory, communication [
46,
47,
48], foraging and flight [
49,
50], the olfactory sense [
51], the glandular system and respiratory rhythm [
52,
53], reproduction [
54,
55], global temperature and metabolism [
56,
57], sensitivity to diseases, and immunity reduction [
58,
59,
60,
61,
62,
63,
64].
Taking into account the high level of residue of thiacloprid found in one sample (four times more than the maximum residual limit for human consumption), it is important to show the risks that the EFSA mentioned in its report published in 2019 [
65], such as: “delayed effects or relevant sub-lethal effects on bees at relatively low concentrations cannot be excluded” and “thiacloprid presents important risks for human health.”
Honeybees are a very important biosensor that can be managed to obtain information about the environment. Nonetheless, the samples collected by honeybees need to be melissopalinologically analyzed when it comes to the necessity of analyzing a specific crop or plant species.
The collection, preparation and preservation of samples should be done so that to reflect the pesticide residues in nectar and pollens at the time of their collection by honeybees. This is of great importance in pesticide exposure studies in order to identify the real residues of neonicotinoids at detection or quantification levels and their risks to honeybees.
The monitoring of honey flows by electronic hives, even if not very important for sample collection and preparation, is the first indicator about honey flow, weather conditions, depopulations or other activities of honeybee colonies that give preliminary information on weight gain and honeybee populations (e.g., swarming). This basic electronic system could be completed by a specific electronic device that could measure, with high accuracy, entrance activity in order to highlight any slight modification in the number of foragers and their loss in the field over the normal levels of depopulation. In this sense, a series of new research projects are necessary to quantify the number of outgoing and incoming bees at the entrance and by these data processing measures in order to offer important information about abnormal depopulations and to send specific alerts in a useful time. Through this approach, it is possible to collect important information about realistic field depopulations and to collect relevant samples for neonicotinoid residues at depopulation moments in order to better analyze the impact of pesticide field exposure on honeybee health.
Thus, the registration of honey flow monitoring data by electronic means, the detection of general colony dynamic activities by electronic sensors, the collection and preparation of mono-floral honeys or pollens, the use of small samples for analyses in order to facilitate their mono-floral preparation for the further specialized laboratory analyses, and the good preservation of samples from the collection moment to the laboratory analyses all contribute to an effective system for neonicotinoid identification.
5. Conclusions
Considering the fact that, generally, neonicotinoid residues are found in honeybee colonies at low levels (e.g., ng/bee and ng/g), it is very important to have a reliable methodology to collect representative samples for neonicotinoids analyses.
In this regard, the good preparation and follow up of honeybee colonies and honey/pollen flows by using classic methods or techniques, new technologies (electronic sensors and the IoT), the validation of the mono-floral envisaged samples by specific mellissopalinological analyses, and their preservation at low temperatures from collection until neonicotinoid residues analysis are very important steps.
The presented approach for monitoring neonicotinoid residues in honeybee colonies could help to maximize the chances for their identification and quantification in different monitoring studies or evaluations, in order to better evaluate the exposure of honeybees to the neonicotinoids used in different real field melliferous crops.
As could be seen from the levels of neonicotinoid residues found in different locations in Romania, their presence in all the sampled locations correlated with the worldwide scientific evidence on their lethal or sublethal toxic effects in chronic exposure, demonstrate the existence of important risks for honeybee health and local beekeeping.
Author Contributions
Conceptualization, E.C., A.S., G.O.V. and D.C.; data curation, E.C. and A.S.; formal analysis, E.C., A.S., G.O.V., D.C., T.C. and R.A.S.; funding acquisition, E.C. and A.S.; investigation, E.C., A.S., G.O.V., D.C., T.C. and R.A.S.; methodology, E.C., A.S., G.O.V., D.C., T.C. and R.A.S.; project administration, A.S.; resources, E.C., A.S., G.O.V., D.C., T.C. and R.A.S.; validation, E.C., A.S., G.O.V., D.C., T.C. and R.A.S.; visualization, E.C., A.S., G.O.V. and D.C.; writing—original draft, E.C., A.S. and G.O.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Agriculture and Rural Development, Romania, grant number ADER 4.1.5/2017.
Acknowledgments
We acknowledge administrative and technical support received by the Institute for Beekeeping Research and Development, Bucharest, Romania. The authors thank to the reviewers for the constructive analysis of the article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Ethics Statement
We declare this study does not need approval of any institution and/or governmental agency that regulates research with animals.
References
- Allsopp, M.H.; De Lange, W.J.; Veldtman, R. Valuing insect pollination services with cost of replacement. PLoS ONE 2008, 3, e3128. [Google Scholar] [CrossRef] [PubMed]
- Corbet, S.; Williams, I.; Osborne, J. Bees and the pollination of crops and wild flowers in the european community. Bee World 1991, 72, 47–59. [Google Scholar] [CrossRef]
- Morse, R.A.; Calderone, N.W. The value of honey bees as pollinators of U.S. crops in 2000. Bee Cult. Mag. 2000, 128, 1–15. [Google Scholar]
- Cârnu, I. Plante Melifere; Editura Ceres: Bucharest, Romania, 1972. [Google Scholar]
- Celli, G.; Maccagnani, B. Honey bees as bioindicators of environmental pollution. Bull. Insectology 2003, 56, 137–139. [Google Scholar]
- Negri, I.; Mavris, C.; Di Prisco, G.; Caprio, E.; Pellecchia, M. Honey bees (Apis mellifera, L.) as active samplers of airborne particulate matter. PLoS ONE 2015, 10, e0132491. [Google Scholar] [CrossRef] [PubMed]
- Bargańska, Ż.; Ślebioda, M.; Namieśnik, J. Honey bees and their products: Bioindicators of environmental contamination. Crit. Rev. Environ. Sci. Technol. 2016, 46, 235–248. [Google Scholar] [CrossRef]
- Barmaz, S.; Potts, S.G.; Vighi, M. A novel method for assessing risks to pollinators from plant protection products using honey bees as a model species. Ecotoxicology 2010, 19, 1347–1359. [Google Scholar] [CrossRef]
- Bromenshenk, J.J.; Henderson, C.B.; Seccomb, R.A.; Welch, P.M.; Debnam, S.E.; Firth, D.R. Bees as biosensors: Chemosensory ability, honey bee monitoring systems, and emergent sensor technologies derived from the pollinator syndrome. Biosensors 2015, 5, 678–711. [Google Scholar] [CrossRef]
- Arcega-Rustia, D.J.; Ngo, T.N.; Ta-Te, L. An IoT based information system for honeybees in and out activity with beehive environmental condition monitoring. In Proceedings of the International Symposium on Machinery and Mechatronics for Agricultural and Biosystems Engineering 2016, Niigata, Japan, 23–25 May 2016; Available online: https://www.researchgate.net/publication/320134391 (accessed on 2 November 2019).
- Debauche, O.; El Moulat, M.; Mahmoudi, S.; Boukraa, S.; Manneback, P.; Lebeau, F. Web monitoring of bee health for researchers and beekeepers based on the internet of things. Procedia Comput. Sci. 2018, 130, 991–998. [Google Scholar] [CrossRef]
- Gil-Lebrero, S.; Quiles-Latorre, F.J.; Ortiz-López, M.; Sánchez-Ruiz, V.; Gámiz-López, V.; Luna-Rodríguez, J.J. Honey bee colonies remote monitoring system. Sensors 2017, 17, 55. [Google Scholar] [CrossRef]
- Meikle, W.G.; Holst, N. Application of continuous monitoring of honeybee colonies. Apidologie 2015, 46, 10–22. [Google Scholar] [CrossRef]
- Pesovic, U.; Randic, S.; Stamenkovic, Z. Design and implementation of hardware platform for monitoring honeybee activity. In Proceedings of the 4th International Conference on Electrical, Electronics and Computing Engineering, IcETRAN 2017, Kladovo, Serbia, 5–8 June 2017. [Google Scholar]
- Siceanu, A.; Căuia, E.; Rădoi, C.; Gureșoaie, I.; Svasta, P.; Vulpe, V.; Davidescu, M.; Ionescu, C. Electronic equipment to monitorize some biological process of economic importance in honeybee colony and its environment. Sci. Pap. Anim. Sci. Biotechnol. 2007, 40, 134–140. [Google Scholar]
- Siceanu, A. Sistemul de stup intelligent Simfony (The intelligent hive Simfony system). Rom. Apic. 2017, 5, 24–25. [Google Scholar]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Gray, A.; Brodschneider, R.; Adjlane, N.; Ballis, A.; Brusbardis, V.; Charrière, J.-D.; Chlebo, R.; Coffey, M.F.; Cornelissen, B.; Amaro da Costa, C.; et al. Loss rates of honey bee colonies during winter 2017/18 in 36 countries participating in the COLOSS survey, including effects of forage sources. J. Apic. Res. 2019. [Google Scholar] [CrossRef]
- Potts, S.G.; Roberts, S.P.M.; Dean, R.; Marris, G.; Brown, M.A.; Jones, R.; Neumann, P.; Settele, J. Declines of managed honey bees and beekeepers in Europe. J. Apic. Res. 2010, 49, 15–22. [Google Scholar] [CrossRef]
- Sanchez-Bayo, F.; Goka, K. Pesticide Residues and Bees—A Risk Assessment. PLoS ONE 2014, 9, e94482. [Google Scholar] [CrossRef]
- Blacquiere, T.; Smagghe, G.; van Gestel, C.M.; Mommaerts, V. Neonicotinoids in bees: A review on concentrations, side-effects and risk assessment. Ecotoxicology 2012, 21, 973–992. [Google Scholar] [CrossRef]
- Chauzat, M.-P.; Martel, A.-C.; Cougoule, N.; Porta, P.; Lachaize, J.; Zegane, S.; Aubert, M.; Carpentier, P.; Faucon, J.-P. An assessment of honey bee colony matrices, Apis mellifera (Hymenoptera: Apidae) to monitor pesticide presence in continental France. Environ. Toxicol. Chem. 2011, 30, 103–111. [Google Scholar] [CrossRef]
- Mitchell, E.A.D.; Mulhauser, B.; Mulot, M.; Mutabazi, A.; Glauser, G.; Aebi, A. A worldwide survey of neonicotinoids in honey. Science 2017, 358, 109–111. [Google Scholar] [CrossRef]
- Botías, C.; David, A.; Horwood, J.; Abdul-Sada, A.; Nicholls, E.; Hill, E.; Goulson, D. Neonicotinoid residues in wildflowers, a potential route of chronic exposure for bees. Environ. Sci. Technol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Böhme, F.; Bischoff, G.; Zebitz, C.P.W.; Rosenkranz, P.; Wallner, K. Pesticide residue survey of pollen loads collected by honeybees (Apis mellifera) in daily intervals at three agricultural sites in South Germany. PLoS ONE 2018, 13, e0199995. [Google Scholar] [CrossRef] [PubMed]
- Bonmatin, J.M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Long, E.; Marzaro, M.; Mitchell, E.A.D.; et al. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Bonmatin, J.M.; Moineau, R.; Charvet, C.; Fleche, M.E.; Colin, E.; Bengsch, R. A LC/APCI-MS/MS Method for analysis of imidacloprid in soils, in plants, and in pollens. Anal. Chem. 2003, 75, 2027–2033. [Google Scholar] [CrossRef]
- Rortais, A.; Arnold, G.; Halm, M.-P.; Touffet-Briens, F. Modes of honeybees exposure to systemic insecticides: Estimated amounts of contaminated pollen and nectar consumed by different categories of bees. Apidologie 2005. [Google Scholar] [CrossRef]
- Samson-Robert, O.; Labrie, G.; Chagnon, M.; Fournier, V. Planting of neonicotinoid-coated corn raises honey bee mortality and sets back colony development. PeerJ 2017, 5, e3670. [Google Scholar] [CrossRef]
- Guez, D.; Suchail, S.; Gauthier, M.; Maleszka, R.; Belzunces, L.P. Contrasting effects of imidacloprid on habituation in 7- and 8-day-old honeybees (Apis mellifera). Neurobiol. Learn. Mem. 2001, 76, 183–191. [Google Scholar] [CrossRef]
- European Food Safety Authority. Evaluation of the data on clothianidin, imidacloprid and thiamethoxam for the updated risk assessment to bees for seed treatments and granules in the EU. EFSA Support. Publ. 2018. [Google Scholar] [CrossRef]
- Bonmatin, J.M.; Marchand, P.A.; Charvet, R.; Moineau, I.; Bengsch, E.R.; Colin., M.E. Quantification of imidacloprid uptake in maize crops. J. Agric. Food Chem. 2005, 53, 5336–5341. [Google Scholar] [CrossRef]
- Siceanu, A.; Căuia, E. Analize de neonicotinoide în România—Rezultatele parțiale obținute în urma derulării unui proiect de cercetare (Analyses of neonicotinoids in Romania—The preliminary results obtained following a research project). Rom. Apic. 2018, 10, 10–16. [Google Scholar]
- Siceanu, A.; Căuia, E. Analize de neonicotinoide în România—Rezultate finale si concluzii (Analyses of neonicotinoids in Romania—The final results and conclusions). Rom. Apic. 2018, 11, 3–9. [Google Scholar]
- Tsvetkov, N.; Samson-Robert, O.; Sood, K.; Patel, H.S.; Malena, D.A.; Gajiwala, P.H.; Maciukiewicz, P.; Fournier, V.; Zayed, A. Chronic exposure to neonicotinoids reduces honey-bee health near corn crops. Science 2017. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hernández, L.; Hernández-Domínguez, D.; Martín, M.T.; Nozal, M.J.; Higes, M.; Bernal Yagüe, J.L. Residues of neonicotinoids and their metabolites in honey and pollen from sunflower and maize seed dressing crops. J. Chromatogr. A 2016, 1428, 220–227. [Google Scholar] [CrossRef]
- European Food Safety Authority. Statement on the assessment of the scientific information from the Italian project “APENET” investigating effects on honeybees of coated maize seeds with some neonicotinoids and fipronil. EFSA J. 2012, 10, 2792. [Google Scholar] [CrossRef]
- Online Petition for Honeybee Colones Losses in Romania: 2015. Available online: https://www.petitieonline.com/sos_albina_romaneasc (accessed on 25 July 2019).
- The European Academies’ Science Advisory Council. Ecosystem Services, Agriculture and Neonicotinoids; EASAC Policy Report; German National Academy of Sciences Leopoldina: Halle (Saale), Germany, 2015; ISBN 978-3-8047-3437-1. Available online: www.easac.eu (accessed on 27 November 2017).
- Suchail, S.; Guez, D.; Belzunces, L. Discrepancy between acute and chronic toxicity induced by imidacloprid and its metabolites in Apis mellifera. Environ. Toxicol. Chem. 2001, 20, 2482–2486. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, M.; Casida, J.E. Neonicotinoid insecticide toxicology: Mechanisms of selective action. Annu. Rev. Pharm. Toxicol. 2005, 45, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, I.; Yabuta, G.; Tomizawa, M.; Saito, T.; Miyamoto, T.; Kagabu, S. Molecular mechanism for selective toxicity of nicotinoids and neonicotinoids. J. Pestic. Sci. 1995, 20, 33–40. [Google Scholar] [CrossRef]
- Suchail, S.; Guez, D.; Belzunces, L. Characteristics of imidacloprid toxicity in two Apis mellifera subspecies. Env. Toxicol. Chem. 2000, 19, 1901–1905. [Google Scholar] [CrossRef]
- Rondeau., G.; Sánchez-Bayo, F.; Tennekes, H.A.; Decourtye, A.; Ramírez-Romero, R.; Desneux, N. Delayed and time-cumulative toxicity of imidacloprid in bees, ants and termites. Sci. Rep. 2014, 4, 5566. [Google Scholar] [CrossRef]
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. 2015, 22, 5–34. [Google Scholar] [CrossRef]
- Bortolotti, L.; Montanari, R.; Marcelino, J.; Medrzycki, P.; Maini, S.; Porrini, C. Effects of sub-lethal imidacloprid doses on the homing rate and foraging activity of honey bees. Bull. Insectol. 2003, 56, 63–67. [Google Scholar]
- Decourtye, A.; Armengaud, C.; Renou, M.; Devillers, J.; Cluzeau, S.; Gauthier, M.; Pham-Delegue, M.H. Imidacloprid impairs memory and brain metabolism in the honeybee (Apis Mellifera L.). Pestic. Biochem. Phys. 2004, 78, 83–92. [Google Scholar] [CrossRef]
- Fischer, J.; Muller, T.; Spatz, A.-K.; Greggers, U.; Grunewald, B.; Menzel, R. Neonicotinoids interfere with specific components of navigation in honeybees. PLoS ONE 2014, 9, e91364. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Chen, W.; Dong, S.; Liu, X.; Wang, Y.; Nieh, J.C. Imidacloprid alters foraging and decreases bee avoidance of predators. PLoS ONE 2014, 9, e102725. [Google Scholar] [CrossRef] [PubMed]
- Tosi, S.; Burgio, G.; Nieh, J.C. A common neonicotinoid pesticide, thiamethoxam, impairs honey bee flight ability. Nat. Sci. Rep. 2017, 7, 1201. [Google Scholar] [CrossRef]
- Yang, E.C.; Chang, H.C.; Wu, W.Y.; Chen, Y.W. Impaired olfactory associative behavior of honeybee workers due to contamination of imidacloprid in the larval stage. PLoS ONE 2012, 7, e49472. [Google Scholar] [CrossRef]
- Baines, D.; Wilton, E.; Pawluk, A.; Gorter, M.; Chomistek, N. Neonicotinoids act like endocrine disrupting chemicals in newly-emerged bees and winter bees. Nat. Sci. Rep. 2017, 7, 10979. [Google Scholar] [CrossRef]
- Hatjina, F.; Papaefthimiou, C.; Charistos, L.; Dogaroglu, T.; Bouga, M.; Emmanouil, C.; Arnold, G. Sublethal doses of imidacloprid decreased size of hypopharyngeal glands and respiratory rhythm of honeybees in vivo. Apidologie 2013. [Google Scholar] [CrossRef]
- Wu-Smart, J.; Spivak, M. Sub-lethal effects of dietary neonicotinoid insecticide exposure on honey bee queen fecundity and colony development, www.nature.com/scientificreports. Sci. Rep. 2016, 6, 32108. [Google Scholar] [CrossRef]
- Straub, L.; Villamar-Bouza, L.; Bruckner, S.; Chantawannakul, P.; Gauthier, L.; Khongphinitbunjong, K.; Retschnig, G.; Troxler, A.; Vidondo, B.; Neumann, P.; et al. Neonicotinoid insecticides can serve as inadvertent insect contraceptives. Proc. R. Soc. B Biol. Sci. 2016. [Google Scholar] [CrossRef]
- Lu, C.; Warchol, K.M.; Callahan, R.A. Sub-lethal exposure to neonicotinoids impaired honey bees, winterization before proceeding to colony collapse disorder. Bull. Insectol. 2014, 67, 125–130. [Google Scholar]
- Meikle, W.G.; Adamczyk, J.J.; Weiss, M.; Gregorc, A. Effects of bee density and sublethal imidacloprid exposure on cluster temperatures of caged honey bees. Apidologie 2018, 49, 581–593. [Google Scholar] [CrossRef]
- Alaux, C.; Brunet, J.-L.; Dussaubat, C.; Mondet, F.; Tchamitchan, S.; Cousin, M.; Brillard, J.; Baldy, A.; Belzunces, L.P.; Le Conte, Y. Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera). Environ. Microbiol. 2009. [Google Scholar] [CrossRef]
- Aufauvre, J.; Biron, D.G.; Vidau, C.; Fontbonne, R.; Roude, M.; Diogon, M.; Viguès, B.; Belzunces, L.P.; Delbac, F.; Blot, N. Parasite-insecticide interactions: A case study of Nosema ceranae and fipronil synergy on honeybee. Nat. Sci. Rep. 2012, 2, 326. [Google Scholar] [CrossRef] [PubMed]
- Hernández, L.J.; Krainer, S.; Engert, A.; Schuehly, W.; Riessberger-Gallé, U.; Crailsheim, K. Sublethal pesticide doses negatively affect survival and the cellular responses in American foulbrood-infected honeybee larvae. Sci. Rep. 2017, 7, 40853. [Google Scholar] [CrossRef]
- Pettis, J.S.; VanEngelsdorp, D.; Johnson, J.; Dively, G. Pesticide exposure in honey bees results in increased levels of the gut pathogen Nosema. Naturwissenschaften 2012, 99, 153–158. [Google Scholar] [CrossRef]
- Vidau, C.; Diogon, M.; Aufauvre, J.; Fontbonne, R.; Viguès, B.; Brunet, J.-L.; Texier, C.; Biron, D.G.; Blot, N.; El Alaoui, H.; et al. Exposure to sublethal doses of fipronil and thiacloprid highly increases mortality of honeybees previously infected by Nosema Ceranae. PLoS ONE 2011, 6, e21550. [Google Scholar] [CrossRef]
- Van der Sluijs, J.P.; Simon-Delso, N.; Goulson, D.; Maxim, L.; Bonmatin, J.-M.; Belzunces, L.P. Neonicotinoids, bee disorders and the sustainability of pollinator services. Curr. Opin. Environ. Sustain. 2013. [Google Scholar] [CrossRef]
- Di Prisco, G.; Cavaliere, V.; Annoscia, D.; Varricchio, P.; Caprio, E.; Nazzi, F.; Gargiulo, G.; Pennacchio, F. Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proc. Natl. Acad. Sci. USA 2013, 110, 18466–18471. [Google Scholar] [CrossRef]
- European Food Safety Authority. Conclusion on the peer review of the pesticide risk assessment of the active substance thiacloprid. EFSA J. 2019, 17, 5595. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).