Zooplankton Community Response to Seasonal Hypoxia: A Test of Three Hypotheses
Abstract
1. Introduction
2. Methods
2.1. Field Collections
2.2. Laboratory Analysis
2.3. Statistical Analyses
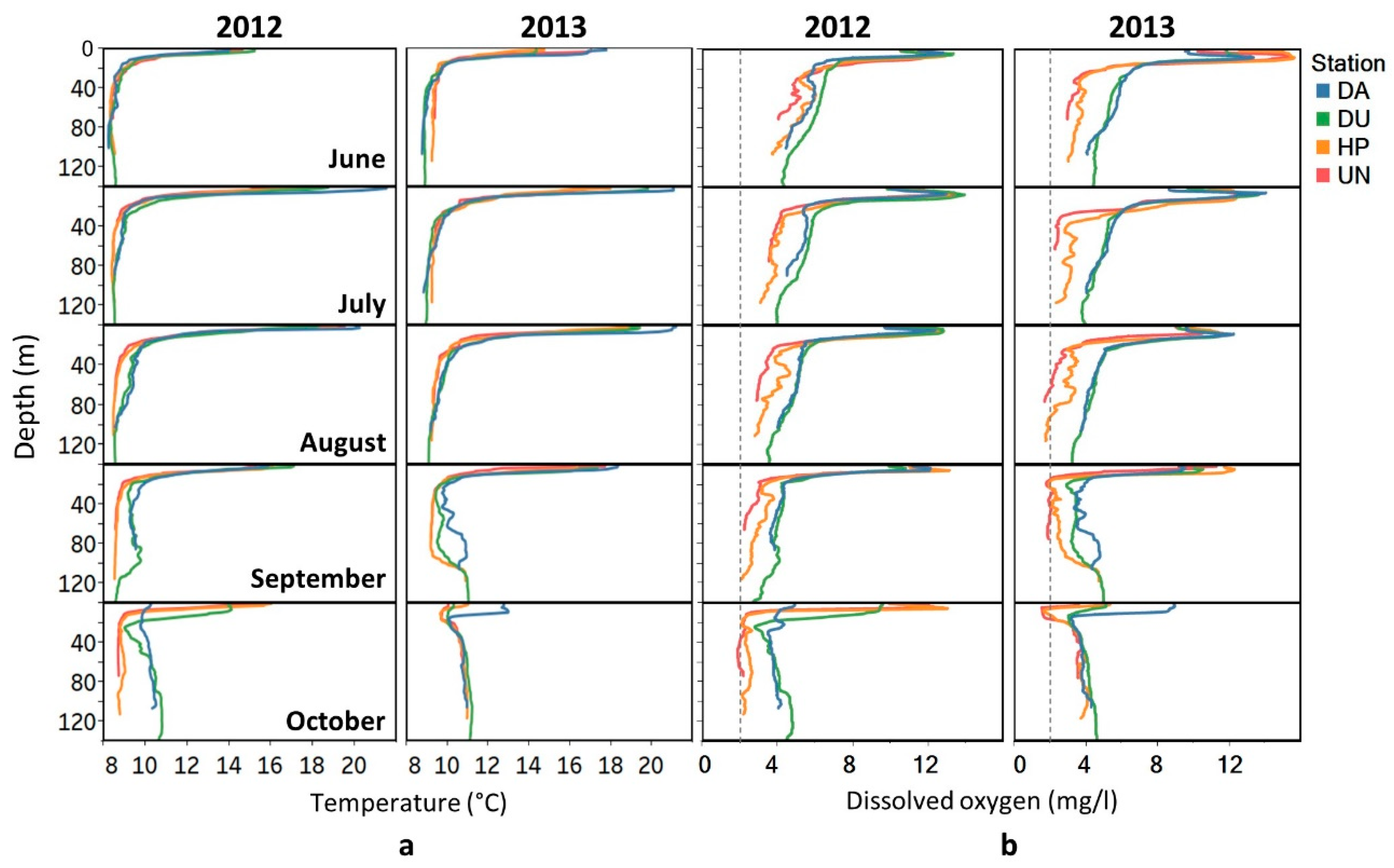
3. Results
3.1. Environmental Conditions
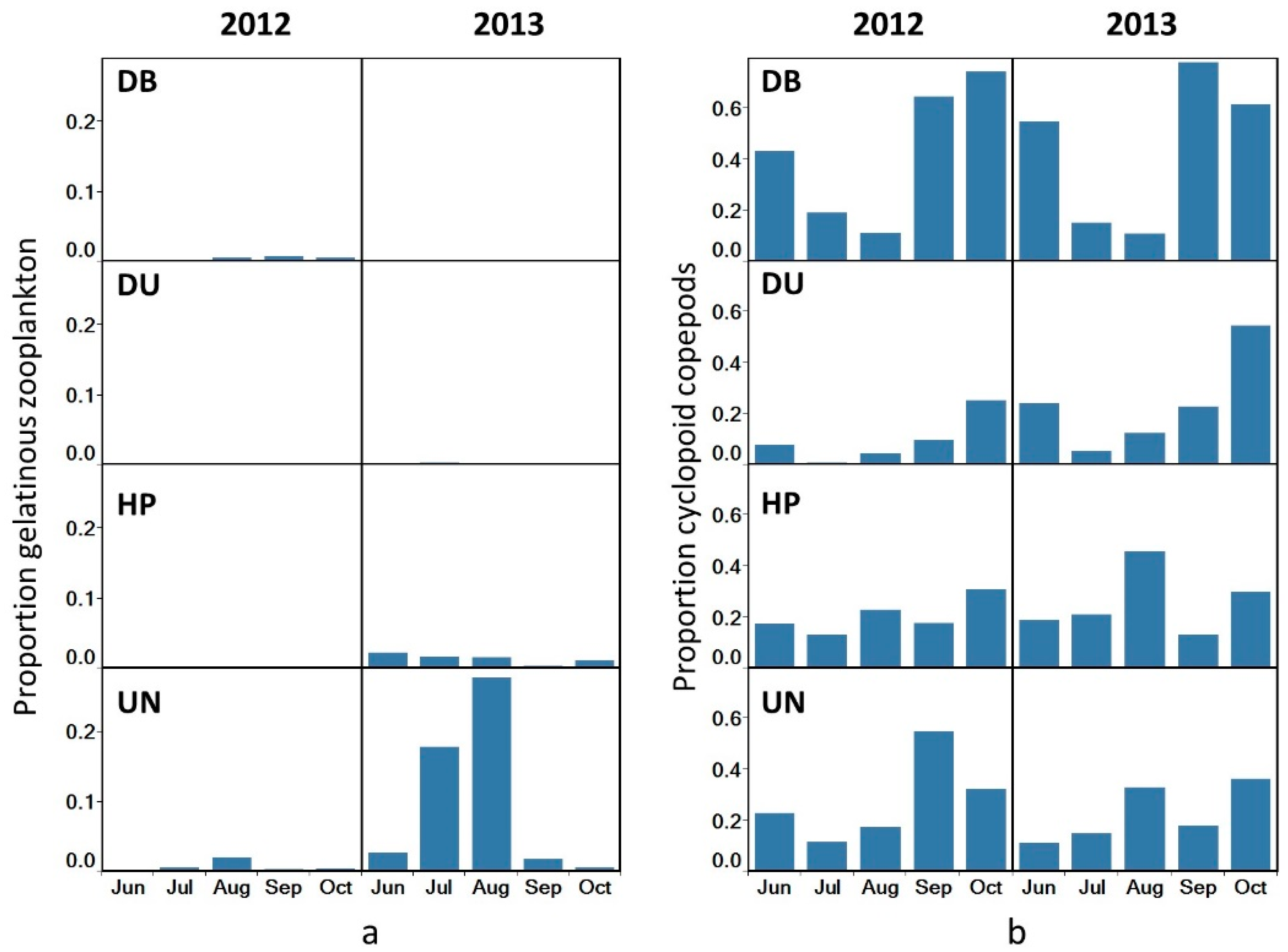
3.2. Zooplankton Community Composition
3.3. Species Dominance
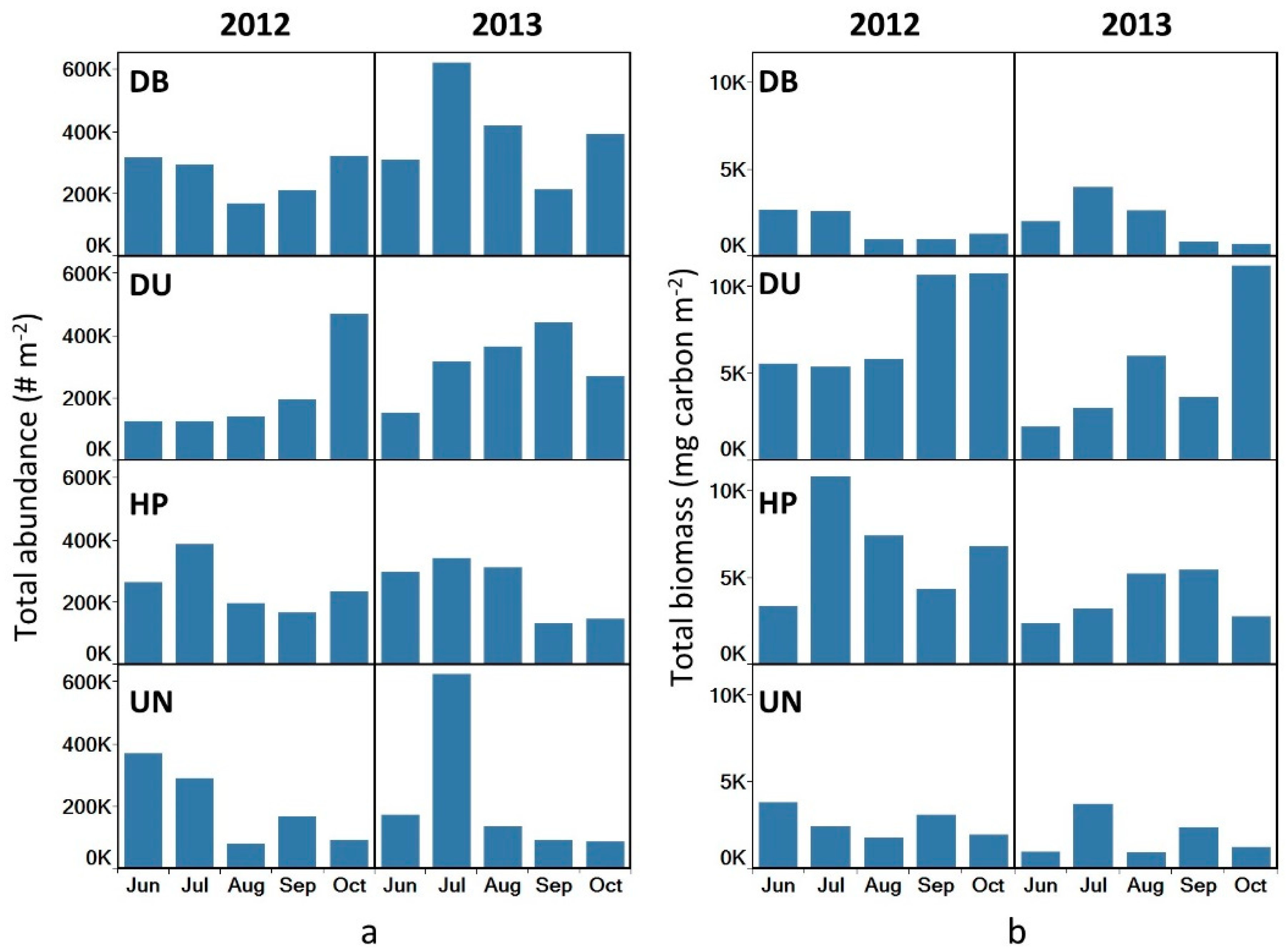
3.4. Total Abundance and Biomass
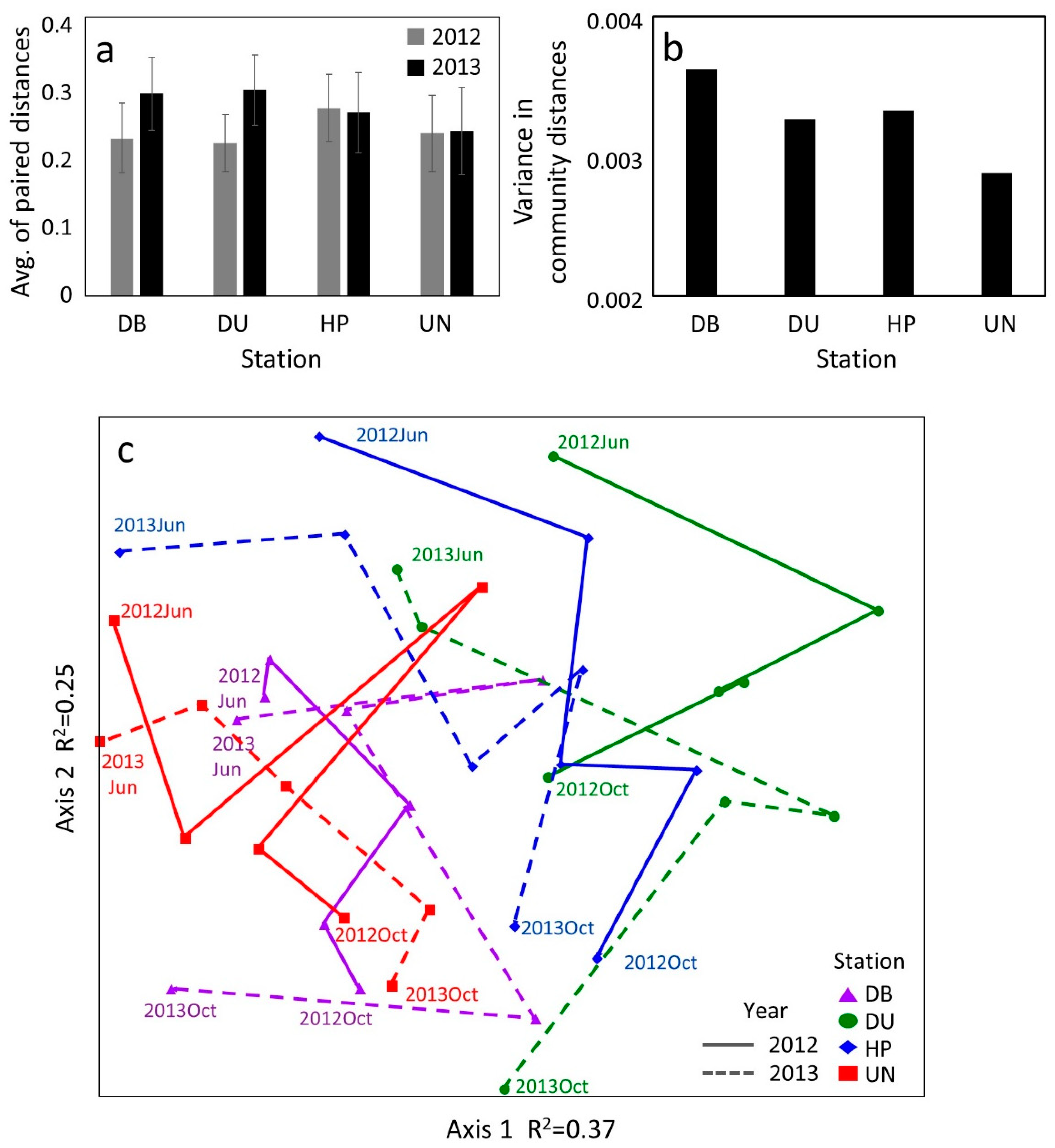
3.5. Zooplankton Community Stability
4. Discussion
4.1. Species Dominance
4.1.1. Gelatinous vs. Crustacean Zooplankton
4.1.2. Cyclopoid vs. Calanoid Dominance
4.2. Abundances and Biomass
4.3. Community Structure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Breitburg, D.; Levin, L.A.; Oschlies, A.; Grégoire, M.; Chavez, F.P.; Conley, D.J.; Garçon, V.; Gilbert, D.; Gutiérrez, D.; Isensee, K.; et al. Declining oxygen in the global ocean and coastal waters. Science 2018, 359, eaam7240. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.R.; Gauzens, A.L.; Rhinehart, W.K.; White, J.R. Effects of low oxygen waters on Chesapeake Bay zooplankton. Limnol. Oceanogr. 1993, 38, 1603–1614. [Google Scholar] [CrossRef]
- Stalder, L.C.; Marcus, N.H. Zooplankton responses to hypoxia: Behavioral patterns and survival of three species of calanoid copepods. Mar. Biol. 1997, 127, 599–607. [Google Scholar] [CrossRef]
- Miller, D.C.; Poucher, S.L.; Coiro, L. Determination of lethal dissolved oxygen levels for selected marine and estuarine fishes, crustaceans, and a bivalve. Mar. Biol. 2002, 140, 287–296. [Google Scholar]
- Invidia, M.; Sei, S.; Gorbi, G. Survival of the copepod Acartia tonsa following egg exposure to near anoxia and to sulfide at different pH values. Mar. Ecol. Prog. Ser. 2004, 276, 187–196. [Google Scholar] [CrossRef]
- Grodzins, M.A.; Ruz, P.M.; Keister, J.E. Effects of oxygen depletion on field distributions and laboratory survival of the marine copepod Calanus pacificus. J. Plankton Res. 2016, 38, 1412–1419. [Google Scholar]
- Ruz, P.M.; Hidalgo, P.; Yáñez, S.; Escribano, R.; Keister, J.E. Egg production and hatching success of Calanus chilensis and Acartia tonsa in the northern Chile upwelling zone (23°S), Humboldt Current System. J. Mar. Syst. 2015, 148, 200–212. [Google Scholar] [CrossRef]
- Keister, J.E.; Houde, E.D.; Breitburg, D.L. Effects of bottom-layer hypoxia on abundances and depth distributions of organisms in Patuxent River, Chesapeake Bay. Mar. Ecol. Prog. Ser. 2000, 205, 43–59. [Google Scholar] [CrossRef]
- Pierson, J.J.; Roman, M.R.; Kimmel, D.G.; Boicourt, W.C.; Zhang, X. Quantifying changes in the vertical distribution of mesozooplankton in response to hypoxic bottom waters. J. Exp. Mar. Bio. Ecol. 2009, 381, S74–S79. [Google Scholar] [CrossRef]
- Keister, J.E.; Tuttle, L.B. Effects of bottom-layer hypoxia on spatial distributions and community structure of mesozooplankton in a sub-estuary of Puget sound, Washington, U.S.A. Limnol. Oceanogr. 2013, 58, 667–680. [Google Scholar] [CrossRef]
- De Robertis, A.; Eiane, K.; Rau, G. Eat and run: Anoxic feeding and subsequent aerobic recovery by Orchomene obtusus in Saanich Inlet, British Columbia, Canada. Mar. Ecol. Prog. Ser. 2001, 219, 221–227. [Google Scholar] [CrossRef]
- Taylor, J.C.; Rand, P.S. Spatial overlap and distribution of anchovies (Anchoa spp.) and copepods in a shallow stratified estuary. Aquat. Living Resour. 2003, 16, 191–196. [Google Scholar] [CrossRef]
- Zhang, H.; Ludsin, S.A.; Mason, D.M.; Adamack, A.T.; Brandt, S.B.; Zhang, X.; Kimmel, D.G.; Roman, M.R.; Boicourt, W.C. Hypoxia-driven changes in the behavior and spatial distribution of pelagic fish and mesozooplankton in the northern Gulf of Mexico. J. Exp. Mar. Bio. Ecol. 2009, 381, S80–S90. [Google Scholar] [CrossRef]
- Uye, S. Replacement of large copepods by small ones with eutrophication of embayments: Cause and consequence. Hydrobiologia 1994, 292, 513–519. [Google Scholar] [CrossRef]
- Roman, M.R.; Brandt, S.B.; Houde, E.D.; Pierson, J.J. Interactive effects of hypoxia and temperature on coastal pelagic zooplankton and fish. Front. Mar. Sci. 2019, 6, 1–18. [Google Scholar] [CrossRef]
- Purcell, J.E.; Breitburg, D.L.; Decker, M.B.; Graham, W.M.; Youngbluth, M.J.; Raskoff, K.A. Pelagic Cnidarians and Ctenophores in Low Dissolved Oxygen Environments: A Review. In Coastal Hypoxia: Consequences for Living Resources and Ecosystems; American Geophyical Union: Washington, DC, USA, 2001; pp. 77–100. [Google Scholar]
- Condon, R.H.; Decker, M.B.; Purcell, J.E. Effects of low dissolved oxygen on survival and asexual reproduction of scyphozoan polyps (Chrysaora quinquecirrha). Hydrobiologia 2001, 451, 89–95. [Google Scholar] [CrossRef]
- Richardson, A.J.; Bakun, A.; Hays, G.C.; Gibbons, M.J. The jellyfish joyride: Causes, consequences and management responses to a more gelatinous future. Trends Ecol. Evol. 2009, 24, 312–322. [Google Scholar] [CrossRef]
- Rutherford, L.D.; Thuesen, E.V. Metabolic performance and survival of medusae in estuarine hypoxia. Mar. Ecol. Prog. Ser. 2005, 294, 189–200. [Google Scholar] [CrossRef]
- Thuesen, E.V.; Rutherford, L.D.; Brommer, P.L.; Garrison, K.; Gutowska, M.A.; Towanda, T. Intragel oxygen promotes hypoxia tolerance of scyphomedusae. J. Exp. Biol. 2005, 208, 2475–2482. [Google Scholar] [CrossRef]
- Breitburg, D.L.; Loher, T.; Pacey, C.A.; Gerstein, A. Varying effects of low dissolved oxygen on trophic interactions in an estuarine food web. Ecol. Monogr. 1997, 67, 489–507. [Google Scholar] [CrossRef]
- Decker, M.B.; Breitburg, D.L.; Purcell, J.E. Effects of low dissolved oxygen on zooplankton predation by the ctenophore Mnemiopsis leidyi. Mar. Ecol. Prog. Ser. 2004, 280, 163–172. [Google Scholar] [CrossRef]
- Purcell, J.E.; Sturdevant, M.V. Prey selection and dietary overlap among zooplanktivorous jellyfish and juvenile fishes in Prince William Sound, Alaska. Mar. Ecol. Prog. Ser. 2001, 210, 67–83. [Google Scholar] [CrossRef]
- Uye, S. Production ecology of marine planktonic copepods. Bull. Plankt. Soc. Jpn. 1984, 30, 44–55. [Google Scholar]
- Castellani, C.; Robinson, C.; Smith, T.; Lampitt, R.S. Temperature affects respiration rate of Oithona similis. Mar. Ecol. Prog. Ser. 2005, 285, 129–135. [Google Scholar] [CrossRef]
- Paffenhöfer, G.A. Oxygen consumption in relation to motion of marine planktonic copepods. Mar. Ecol. Prog. Ser. 2006, 317, 187–192. [Google Scholar] [CrossRef]
- Almeda, R.; Alcaraz, M.; Calbet, A.; Saiz, E. Metabolic rates and carbon budget of early developmental stages of the marine cyclopoid copepod Oithona davisae. Limnol. Oceanogr. 2011, 56, 403–414. [Google Scholar] [CrossRef]
- Elliott, D.T.; Pierson, J.J.; Roman, M.R. Relationship between environmental conditions and zooplankton community structure during summer hypoxia in the northern Gulf of Mexico. J. Plankton. Res. 2012, 34, 602–613. [Google Scholar] [CrossRef]
- Cote, D.; Gregory, R.S.; Morris, C.J.; Newton, B.H.; Schneider, D.C. Elevated habitat quality reduces variance in fish community composition. J. Exp. Mar. Bio. Ecol. 2013, 440, 22–28. [Google Scholar] [CrossRef]
- Babson, A.L.; Kawase, M.; MacCready, P. Seasonal and interannual variability in the circulation of Puget Sound, Washington: A box model study. Atmosphere-Ocean 2006, 44, 29–45. [Google Scholar] [CrossRef]
- Brandenberger, J.M.; Louchouarn, P.; Crecelius, E.A. Natural and post-urbanization signatures of hypoxia in two basins of Puget Sound: Historical reconstruction of redox sensitive metals and organic matter inputs. Aquat. Geochem. 2011, 17, 645–670. [Google Scholar] [CrossRef]
- Newton, J.; Bassin, C.; Devol, A.; Kawase, M.; Ruef, W.; Warner, M.; Hannafious, D.; Rose, R. Hypoxia in Hood Canal: An overview of status and contributing factors. In Proceedings of the 2007 Georgia Basin Puget Sound Reseach Conference, Vancouver, BC, Canada, 26–29 March 2007. [Google Scholar]
- Carpenter, J.H. The accuracy of the Winkler method for dissolved oxygen analysis. Limnol. Oceanogr. 1965, 10, 135–140. [Google Scholar] [CrossRef]
- Carpenter, J.H. The Chesapeake Bay Institute technique for the Winkler dissolved oxygen method. Limnol. Oceanogr. 1965, 10, 141–143. [Google Scholar] [CrossRef]
- Kafanov, A.I.; Fedotov, P.A. Relation between body length and weight in some amphipods of the litoral of Vityaz’ Bay (Sea of Japan). Sov. J. Mar. Biol. 1982, 8, 190–196. [Google Scholar]
- Hirota, R.; Fukuda, Y. Dry weight and chemical composition of the larval forms of crabs (Decapoda: Brachyura). Bull. Plankt. Soc. Jpn. 1985, 32, 149–153. [Google Scholar]
- Webber, M.K.; Roff, J.C. Annual biomass and production of the oceanic copepod community off Discovery Bay, Jamaica. Mar. Biol. 1995, 123, 481–495. [Google Scholar] [CrossRef]
- Lavaniegos, B.E.; Ohman, M.D. Coherence of long-term variations of zooplankton in two sectors of the California Current System. Prog. Oceanogr. 2007, 75, 42–69. [Google Scholar] [CrossRef]
- Uye, S. Length-weight relationships of important zooplankton from the Inland Sea of Japan. J. Oceanogr. Soc. Jpn. 1982, 38, 149–158. [Google Scholar] [CrossRef]
- Chisholm, L.; Roff, J. Size-weight relationships and biomass of tropical neritic copepods off Kingston, Jamaica. Mar. Biol. 1990, 106, 71–77. [Google Scholar] [CrossRef]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; McCune, B., Ed.; MJM Software Design: Gleneden Beach, OR, USA, 2002; ISBN 0-9721290-0-6. [Google Scholar]
- McCune, B.; Mefford, M.J. PC-ORD. Multivariate Analysis of Ecological Data; Wild Blueberry Media: Corvallis, OR, USA, 2016. [Google Scholar]
- Herrmann, B.; Keister, J.E.; Winans, A.K. Species composition and distribution of jellyfish in a seasonally hypoxic estuary, Hood Canal, Washington. Diversity 2019. under review. [Google Scholar]
- Mills, C.E. Jellyfish blooms: Are populations increasing globally in response to changing ocean conditions? Hydrobiologia 2001, 451, 55–68. [Google Scholar] [CrossRef]
- Purcell, J.E. Jellyfish and ctenophore blooms coincide with human proliferations and environmental perturbations. Ann. Rev. Mar. Sci. 2012, 4, 209–235. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, Y.P. Recent changes in the trophic structure of the Black Sea. Fish. Oceanogr. 1992, 1, 180–189. [Google Scholar] [CrossRef]
- Park, G.S.; Marshall, H.G. Estuarine relationships between zooplankton community structure and trophic gradients. J. Plankton Res. 2000, 22, 121–136. [Google Scholar] [CrossRef]
- Breitburg, D.L. Behavioral response of fish larvae to low dissolved oxygen concentrations in a stratified water column. Mar. Biol. 1994, 120, 615–625. [Google Scholar] [CrossRef]
- Ishii, H.; Ohba, T.; Kobayashi, T. Effects of low dissolved oxygen on planula settlement, polyp growth and asexual reproduction of Aurelia aurita. Plankt. Benthos Res. 2008, 3, 107–113. [Google Scholar] [CrossRef]
- Farrell, A.P.; Richards, J.G. Defining Hypoxia: An Integrative Synthesis of the Responses of Fish to Hypoxia. In Fish Physiology; Academic Press: London, UK, 2009; Volume 27, pp. 487–503. [Google Scholar]
- Purcell, J.E. Feeding and growth of the siphonophore Muggiaea atlantica (Cunningham 1893). J. Exp. Biol. Ecol. 1982, 62, 39–54. [Google Scholar] [CrossRef]
- Purcell, J.E.; Decker, M.B. Effects of climate on relative predation by scyphomedusae and ctenophores on copepods in Chesapeake Bay during 1987–2000. Limnol. Oceanogr. 2005, 50, 376–387. [Google Scholar] [CrossRef]
- Essington, T.E. Unpublished Data. Available online: https://www.bco-dmo.org/dataset/718698 (accessed on 1 December 2018).
- Gallienne, C.P. Is Oithona the most important copepod in the world’s oceans? J. Plankton Res. 2001, 23, 1421–1432. [Google Scholar] [CrossRef]
- Kiørboe, T. What makes pelagic copepods so successful? J. Plankton Res. 2011, 33, 677–685. [Google Scholar] [CrossRef]
- Paffenhöfer, G.-A. On the ecology of marine cyclopoid copepods (Crustacea, Copepoda). J. Plankton Res. 1993, 15, 37–55. [Google Scholar] [CrossRef]
- Verberk, W.C.E.P.; Bilton, D.T.; Calos, P.; Spicer, J.I. Oxygen supply in aquatic ectotherms: Partial pressure and solubility together explain biodiversity and size patterns. Ecology 2011, 92, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Castellani, C.; Irigoien, X.; Harris, R.P.; Lampitt, R.S. Feeding and egg production of Oithona similis in the North Atlantic. Mar. Ecol. Prog. Ser. 2005, 288, 173–182. [Google Scholar] [CrossRef]
- Cornwell, L.E.; Findlay, H.S.; Fileman, E.S.; Smyth, T.J.; Hirst, A.G.; Bruun, J.T.; McEvoy, A.J.; Widdicombe, C.E.; Castellani, C.; Lewis, C.; et al. Seasonality of Oithona similis and Calanus helgolandicus reproduction and abundance: Contrasting responses to environmental variation at a shelf site. J. Plankton Res. 2018, 40, 295–310. [Google Scholar] [CrossRef]
- Sabatini, M.; Kiørboe, T. Egg production, growth and development of the cyclopoid copepod Oithona similis. J. Plankton Res. 1994, 16, 1329–1351. [Google Scholar] [CrossRef]
- Nielsen, T.G.; Sabatini, M. Role of cyclopoid copepods Oithona spp. in North Sea plankton communities. Mar. Ecol. Prog. Ser. 1996, 139, 79–93. [Google Scholar] [CrossRef]
- Roman, M.R.; Pierson, J.J.; Kimmel, D.G.; Boicourt, W.C.; Zhang, X. Impacts of hypoxia on zooplankton spatial distributions in the Northern Gulf of Mexico. Estuaries Coasts 2012, 35, 1261–1269. [Google Scholar] [CrossRef]
- Kehayias, G.; Aposporis, M. Zooplankton variation in relation to hydrology in an enclosed hypoxic bay (Amvrakikos Gulf, Greece). Mediterr. Mar. Sci. 2014, 15, 554–568. [Google Scholar] [CrossRef]
- Sato, M.; Horne, J.K.; Parker-Stetter, S.L.; Essington, T.E.; Keister, J.E.; Moriarty, P.E.; Li, L.; Newton, J. Impacts of moderate hypoxia on fish and zooplankton prey distributions in a coastal fjord. Mar. Ecol. Prog. Ser. 2016, 560, 57–72. [Google Scholar] [CrossRef]
- Li, L.; Keister, J.E.; Essington, T.E.; Newton, J. Vertical distributions and abundances of life stages of the euphausiid Euphausia pacifica in relation to oxygen and temperature in a seasonally hypoxic fjord. J. Plankton Res. 2019, 41, 188–202. [Google Scholar] [CrossRef]
- Houde, E.D. Emerging from Hjort’s shadow. J. Northwest Atl. Fish. Sci. 2008, 41, 53–70. [Google Scholar] [CrossRef]
- Horppila, J.; Liljendahl-Nurminen, A.; Malinen, T.; Salonen, M.; Tuomaala, A.; Uusitalo, L.; Vinni, M. Mysis relicta in a eutrophic lake: Consequences of obligatory habitat shifts. Limnol. Oceanogr. 2003, 48, 1214–1222. [Google Scholar] [CrossRef]
- Vanderploeg, H.A.; Ludsin, S.A.; Cavaletto, J.F.; Höök, T.O.; Pothoven, S.A.; Brandt, S.B.; Liebig, J.R.; Lang, G.A. Hypoxic zones as habitat for zooplankton in Lake Erie: Refuges from predation or exclusion zones? J. Exp. Mar. Bio. Ecol. 2009, 381, S92–S107. [Google Scholar] [CrossRef]
- Parker-Stetter, S.L.; Horne, J.K. Nekton distribution and midwater hypoxia: A seasonal, diel prey refuge? Est. Coast. Shelf Sci. 2008, 88, 1–6. [Google Scholar] [CrossRef]
- Ekau, W.; Auel, H.; Portner, H.O.; Gilbert, D. Impacts of hypoxia on the structure and processes in pelagic communities (zooplankton, macro-invertebrates and fish). Biogeosciences 2010, 7, 1669–1699. [Google Scholar] [CrossRef]
- Zaneveld, J.R.; McMinds, R.; Thurber, R.V. Stress and stability: Applying the Anna Karenina principle to animal microbiomes. Nat. Microbiol. 2017, 2. [Google Scholar] [CrossRef]
- Gonze, D.; Coyte, K.Z.; Lahti, L.; Faust, K. Microbial communities as dynamical systems. Curr. Opin. Microbiol. 2018, 44, 41–49. [Google Scholar] [CrossRef]
- Ritter, C.; Montagna, P.A. Seasonal hypoxia and models of benthic response in a Texas bay. Estuaries 1999, 22, 7–20. [Google Scholar] [CrossRef]
- Dauer, D.M. Biological criteria, environmental health and estuarine macrobenthic community structure. Mar. Pollut. Bull. 1993, 26, 249–257. [Google Scholar] [CrossRef]
- Rabalais, N.N.; Díaz, R.J.; Levin, L.A.; Turner, R.E.; Gilbert, D.; Zhang, J. Dynamics and distribution of natural and human-caused hypoxia. Biogeosciences 2010, 7, 585–619. [Google Scholar] [CrossRef]
- Decker, M.B.; Breitburg, D.L.; Marcus, N.H. Geographical differences in behavioral responses to hypoxia: Local adaptation to an anthropogenic stressor? Ecol. Appl. 2003, 13, 1104–1109. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keister, J.E.; Winans, A.K.; Herrmann, B. Zooplankton Community Response to Seasonal Hypoxia: A Test of Three Hypotheses. Diversity 2020, 12, 21. https://doi.org/10.3390/d12010021
Keister JE, Winans AK, Herrmann B. Zooplankton Community Response to Seasonal Hypoxia: A Test of Three Hypotheses. Diversity. 2020; 12(1):21. https://doi.org/10.3390/d12010021
Chicago/Turabian StyleKeister, Julie E., Amanda K. Winans, and BethElLee Herrmann. 2020. "Zooplankton Community Response to Seasonal Hypoxia: A Test of Three Hypotheses" Diversity 12, no. 1: 21. https://doi.org/10.3390/d12010021
APA StyleKeister, J. E., Winans, A. K., & Herrmann, B. (2020). Zooplankton Community Response to Seasonal Hypoxia: A Test of Three Hypotheses. Diversity, 12(1), 21. https://doi.org/10.3390/d12010021



