Control of Varroa destructor Mite Infestations at Experimental Apiaries Situated in Croatia
Abstract
:1. Introduction
2. Materials and Methods
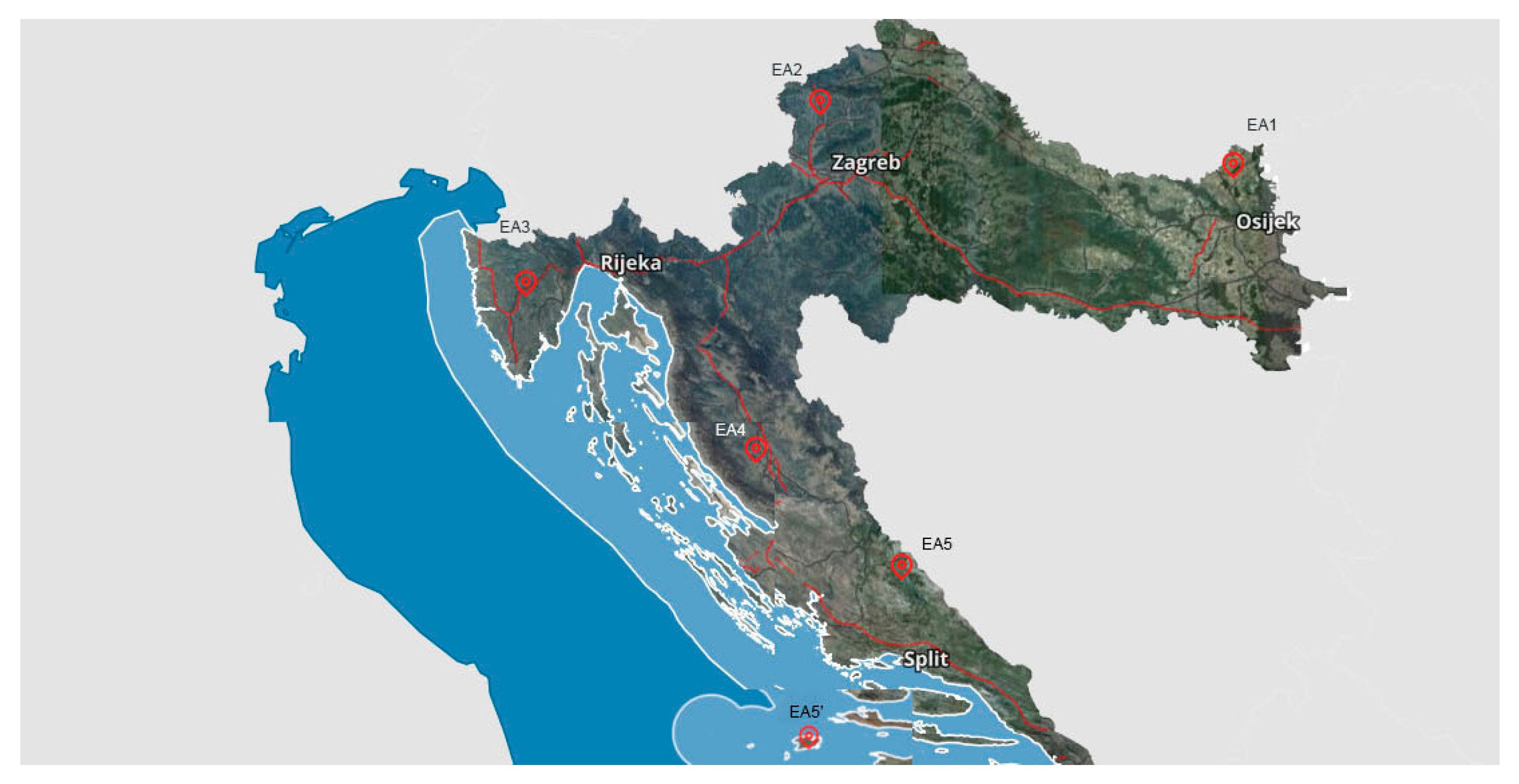
2.1. Locations of Experimental Apiaries and Field Trail Design
2.2. Drugs and Treatments
2.3. Meteorological Conditions
2.4. Estimating the Strength of Honeybee Colonies
2.5. Colony Examinations, Mite Counts and Treatments Efficacy
2.6. Data Analysis
3. Results
3.1. Meteorological Conditions
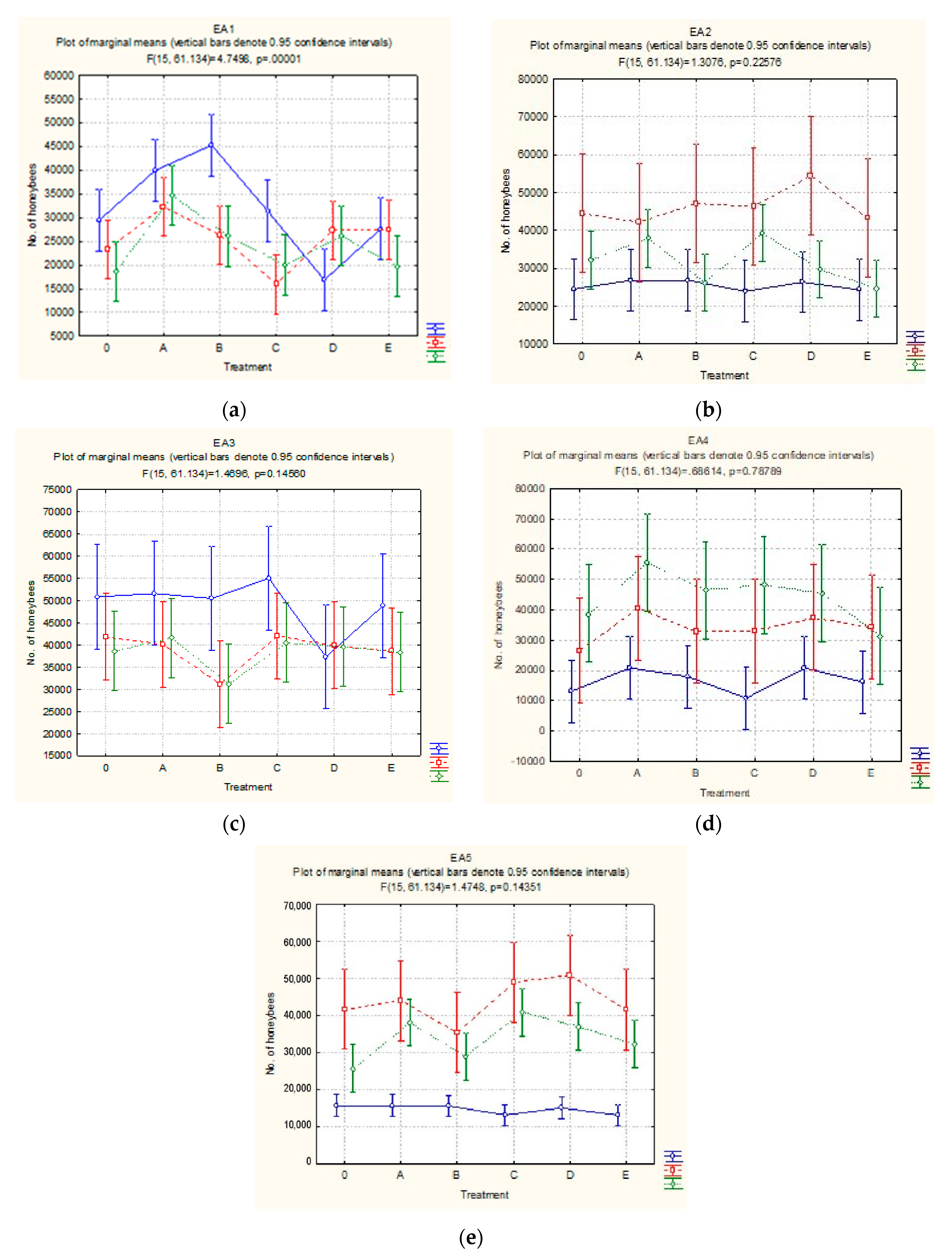
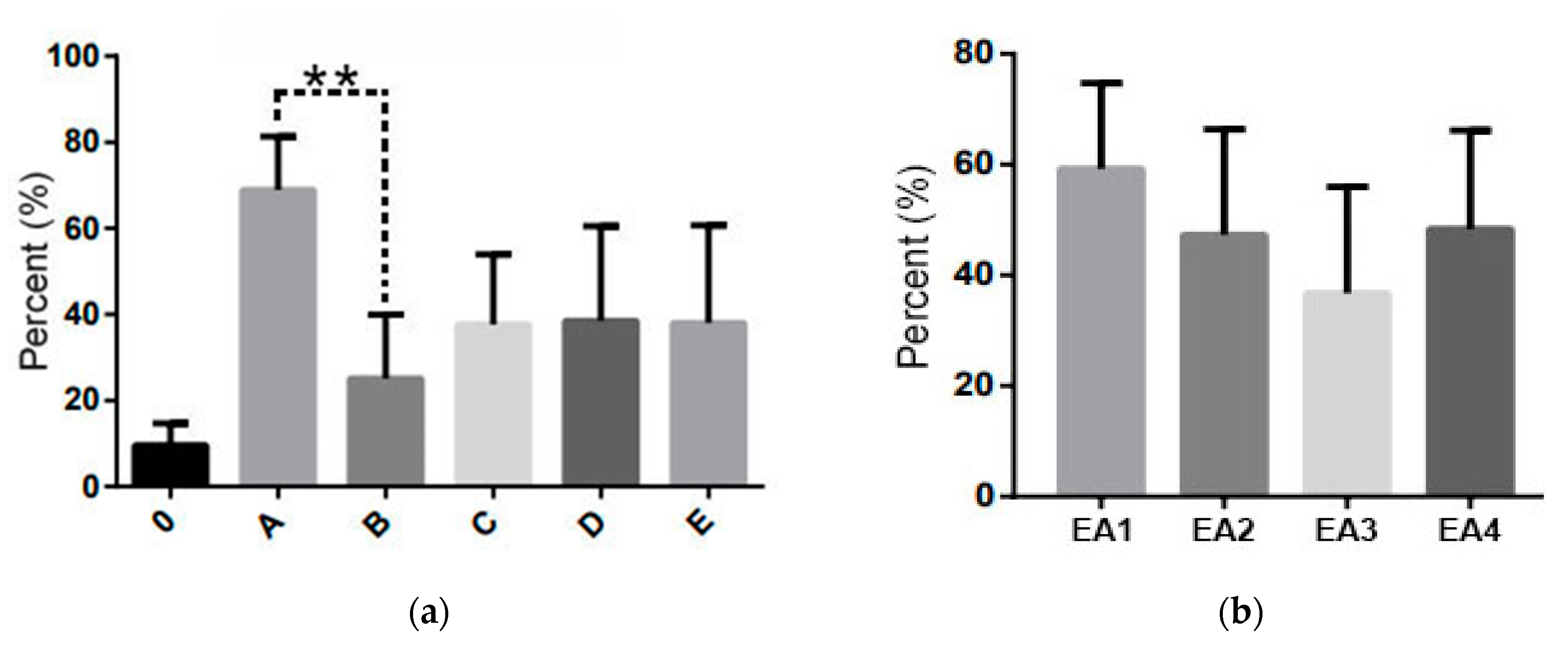
3.2. Estimating the Strength of Honeybee Colonies
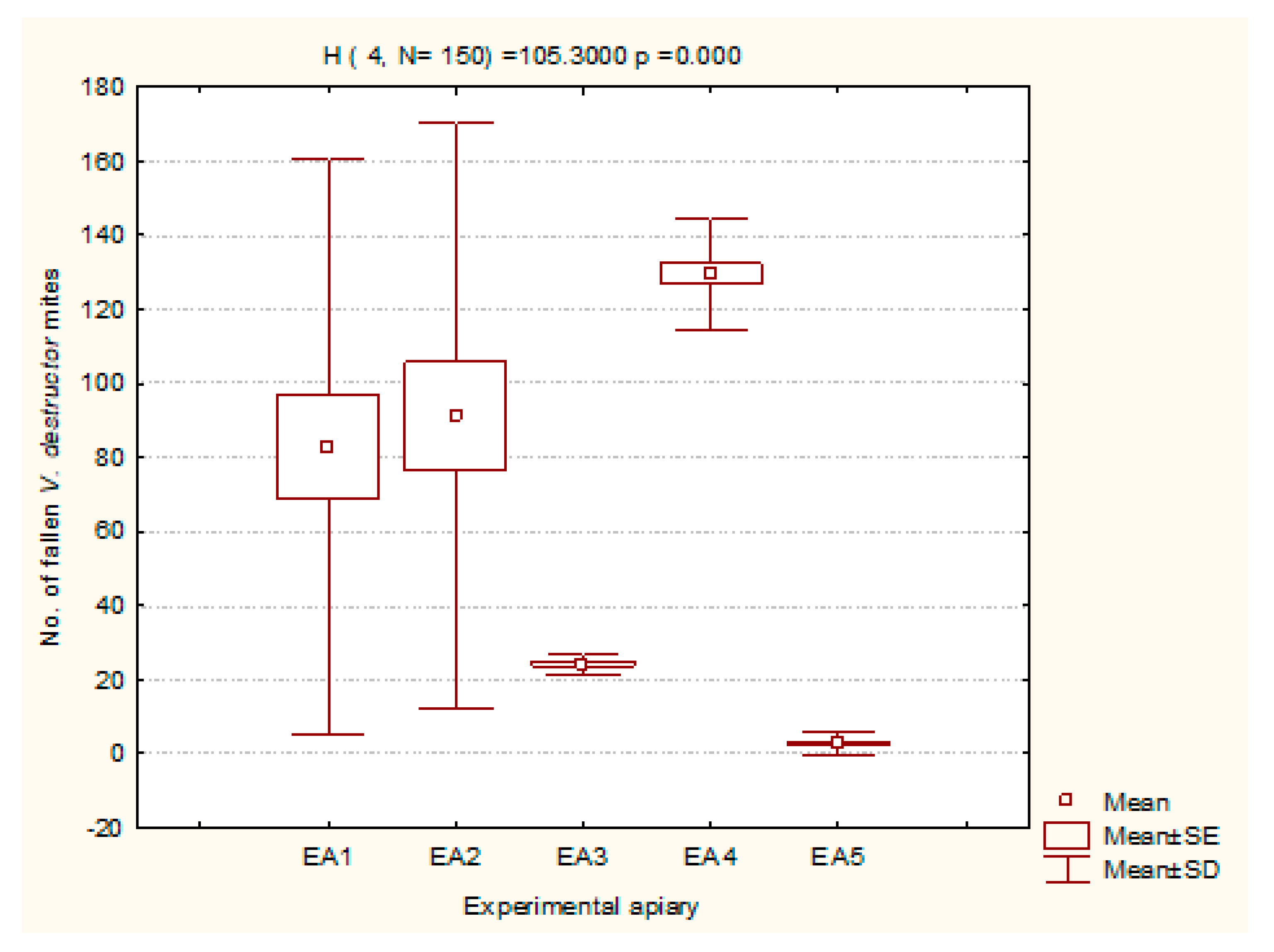
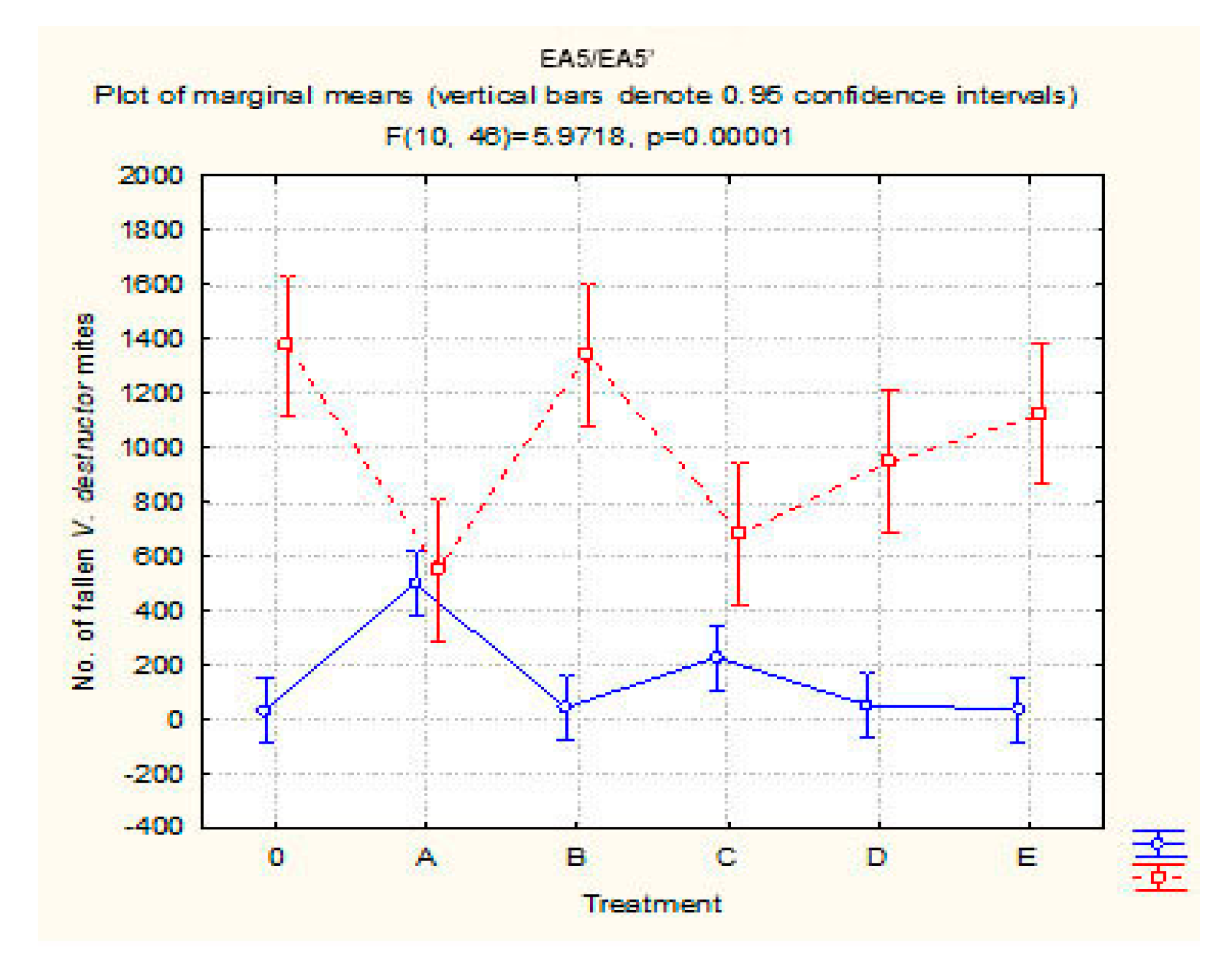
3.3. V. destructor Mite Fall Prior to, during, and after Varroacidal Treatments
3.3.1. Pretreatment Period
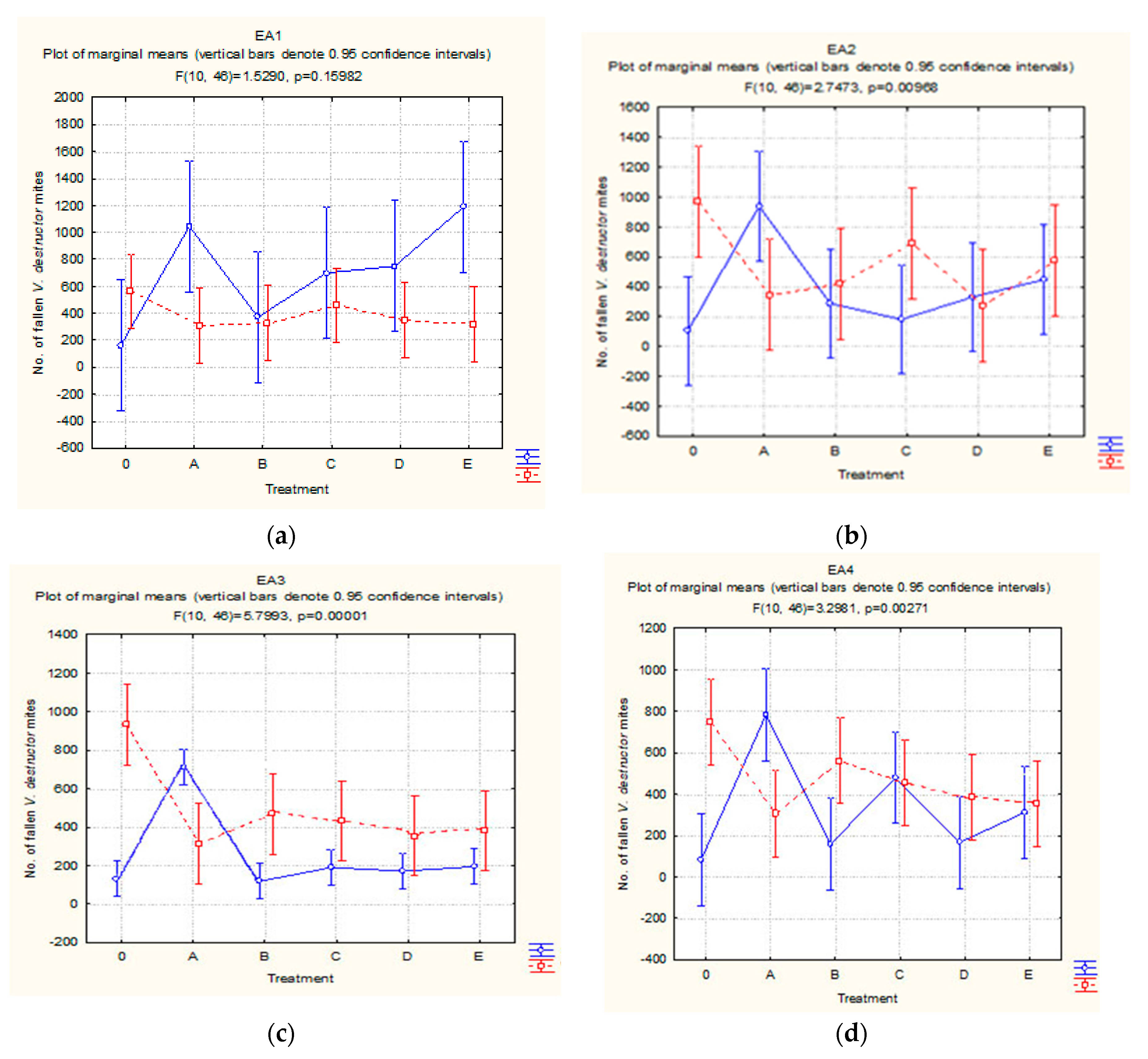
3.3.2. Treatment Period
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poots, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Rollin, O.; Benelli, G.; Benvenuti, S.; Decourtye, A.; Wratten, S.D.; Canale, A.; Desneux, N. Weed-insect pollinator networks as bio-indicators of ecological sustainability in agriculture. A review. Agron. Sustain. Dev. 2016, 36, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Goulson, D.; Nicholls, E.; Botias, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef] [PubMed]
- Kluser, S.; Neumann, P.; Chauzat, M.P.; Pettis, J.S. UNEP Emerging Issues: Global Honey Bee Colony Disorder and other Threats to Insect Pollinators. United Nations Environment Programme 2010. Available online: https://europa.eu/capacity4dev/unep/document/global-bee-colony-disorders-and-other-threats-insect-pollinators (accessed on 30 October 2019).
- Smith, K.M.; Loh, E.H.; Rostal, M.K.; Zambrana-Torrelio, C.M.; Mndiola, L.; Daszak, P. Pathogens, pests, and economics: Drivers of honey bee colony declines and losses. EcoHealth 2014, 10, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa destructor feeds primarily on honey bee fat body tissue not haemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef] [Green Version]
- Ryabov, E.V.; Wood, G.R.; Fannon, J.M.; Moore, J.D.; Bull, J.C.; Chandler, D.; Mead, A.; Burroughs, N.; Evans, D.J. A Virulent Strain of Deformed Wing Virus (DWV) of Honeybees (Apis mellifera) Prevails after Varroa destructor-Mediated, or in Vitro, Transmission. PLoS Pathog. 2014, 10, e1004230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazzi, F.; Brown, S.P.; Annoscia, D.; Del Piccolo, F.; Di Prisco, G.; Varricchio, P.; Della Vedova, G.; Cattonaro, F.; Caprio, E.; Pennacchio, F. Synergistic parasite-pathogen interactions mediated by host immunity can drive the collapse of honey bee colonies. PLoS Pathog. 2012, 8, e1002735. [Google Scholar] [CrossRef] [Green Version]
- Nazzi, F.; Le Conte, Y. Ecology of Varroa destructor, the Major Ectoparasite of the Western Honey Bee, Apis mellifera. Annu. Rev. Entomol. 2016, 61, 417–432. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Cox-Foster, D.L. Impact of an ectoparasite on the immunity and pathology of an invertebrate: Evidence for host immunosuppression and viral amplification. Proc. Natl. Acad. Sci. USA 2005, 102, 7470–7475. [Google Scholar] [CrossRef] [Green Version]
- Gilioli, G.; Simonetto, A.; Hatina, F.; Sperandio, G. Multi-dimensional modelling tools supporting decision-making for the beekeeping sector. IFAC PapersOnLine 2018, 51, 144–149. [Google Scholar] [CrossRef]
- Rortais, A.; Arnold, G.; Dorne, J.-L.; More, S.J.; Sperandio, G.; Streissl, F.; Szentes, C.; Verdonck, F. Risk assessment of pesticides and other stressors in bees: Principles, data gaps and perspectives from the European Food Safety Authority. Sci. Total Environ. 2017, 587, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. National Beekeeping Program for Period 2017–2019; Ministry of Agriculture: Zagreb, Croatia, 2016. [Google Scholar]
- Anonymous. National Beekeeping Program for Period 2020–2022; Ministry of Agriculture: Zagreb, Croatia, 2019. [Google Scholar]
- Anonymous. Program for Varroosis Control and Eradication; Ministry of Agriculture: Croatia, 2017. Available online: http://www.veterinarstvo.hr/default.aspx?id=140 (accessed on 2 November 2019).
- Anonymous. Croatian National Regulation on Animal Protection Measures against Infectious and Parasitic Diseases and on Related Financing for 2019; Ministry of Agriculture: Croatia, 2019. N.N. 5/2019. Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2019_01_5_92.html (accessed on 2 November 2019).
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef] [PubMed]
- Frey, E.; Rosenkranz, P. Autumn invasion rates of Varroa destructor (Mesostigmata: Varroidae) into honey bee (Hymenoptera: Apidae) colonies and the resulting increase in mite populations. J. Econ. Entomol. 2014, 107, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Seeley, T.D.; Smith, M.L. Crowding honeybee colonies in apiaries can increase their vulnerability zo the deadly ectoparasite Varroa destructor. Apidologie 2015, 46, 716–727. [Google Scholar] [CrossRef] [Green Version]
- Floris, I.; Satta, A.; Garau, V.L.; Melis, M.; Cabras, P.; Aloul, N. Effectiveness, persistence, and residue of amitraz plastic strips in the apiary control of Varroa destructor. Apidologie 2001, 32, 577–585. [Google Scholar] [CrossRef] [Green Version]
- Sammataro, D.; Untalan, P.; Guerrero, F.; Finley, J. The resistance of varroa mites (Acari: Varroidae) to acaricides and the presence of esterase. Int. J. Acarol. 2005, 31, 67–74. [Google Scholar] [CrossRef]
- Maggi, M.D.; Ruffinengo, S.R.; Negri, P.; Eguaras, M.J. Resistance phenomena to amitraz from populations of the ectoparasitic mite Varroa destructor of Argentina. J. Parasitol. Res. 2010, 107, 1189–1192. [Google Scholar] [CrossRef]
- Howis, M.; Nowakowski, P. Varroa destructor removal efficiency using Beevital Clean preparation. J. Apic. Sci. 2009, 53, 15–20. [Google Scholar]
- Rodriguez-Dehaibes, S.R.; Parido Sedas, V.T.; Luna-Olivars, G.; Villanuva-Jimenez, J.A. Two commercial formulations of natural compounds for Varroa destructor (Acari: Varroidae) control on Africanized bees under tropical climatic conditions. J. Apic. Res. 2017, 56, 58–62. [Google Scholar] [CrossRef]
- Tlak Gajger, I.; Sušec, P. Efficacy of varroacidal food additive appliance during summer treatment of honeybee colonies (Apis mellifera). Vet. Arhiv 2019, 89, 87–96. [Google Scholar] [CrossRef]
- Tlak Gajger, I.; Tomljanović, Z.; Stanisavljević, L. An environmentally friendly approach to the control of Varooa destructor mite and Nosema ceranae disease in Carnolian honeybee (Apis mellifera carnica) colonies. Arch. Biol. Sci. 2013, 65, 1585–1592. [Google Scholar] [CrossRef] [Green Version]
- Anonymous. Guideline on Veterinary Medicinal Products Controlling Varroa Destructor Parasitosis in Bees. EMA— European Medicines Agency, EMA/CVMP/EWP/459883/2008, Committee for Medicinal Products for Veterinary Use (CVMP). Available online: http://www.ema.europaeu/docs/en_GB/document_library/Scientific_guideline/2010/11/WC500099137.pdf (accessed on 2 November 2019).
- Delaplane, K.S.; Van Der Steen, J.; Guzman-Novoa, E. Standard methods for estimating strength parameters of Apis mellifera colonies. J. Apic. Res. 2013, 52, 1–12. [Google Scholar] [CrossRef]
- Tlak Gajger, I.; Tomljanovic, Z.; Petrinec, Z. Monitoring health status of Croatian honey bee colonies and possible reasons for winter losses. J. Apic. Res. 2010, 49, 107–108. [Google Scholar] [CrossRef]
- Mutinelli, F. Veterinary medicinal products to control Varroa destructor in honey bee colonies (Apis mellifera) and related EU legislation—An update. J. Apic. Res. 2016, 55, 78–88. [Google Scholar] [CrossRef]
- Žorat, T. The Efficiency of Acaricides used in Summer Treatment of Honeybee Colonies against Varroosis. Graduating Thesis; University of Zagreb Faculty of Veterinary Medicine: Zagreb, Croatia, 10 October 2015. [Google Scholar]
- Van Dooremalen, C.; Gerritsen, L.; Cornelissen, B.; van der Steen, J.J.M.; van Langevelde, F.; Blacquière, T. Winter Survival of Individual Honey Bees and Honey Bee Colonies Depends on Level of Varroa destructor Infestation. PLoS ONE 2012, 7, e36285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregorc, A.; Smodis Škerl, I.M. Combating Varroa destructor in Honeybee Colonies Using Flumethrin or Fluvalinate. Acta Vet. Brno 2007, 76, 309–314. [Google Scholar] [CrossRef]
- Gregorc, A.; Planinc, I. Use of Thymol formulations, Amitraz, and Oxalic Acid for the control of the varroa mite in honey bee (Apis mellifera carnica) colonies. J. Apic. Sci. 2012, 56, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Blacquiere, T.; Altreuther, G.; Krieger, K.J. Evaluation of the Efficacy and Safety of Flumethrin 275 mg Bee-hive Strips (PolyVar Yellow®) against Varroa destructor in Naturally Infested Honey Bee Colonies in a Controlled Study. Parasitol. Res. 2017, 116, 109–122. [Google Scholar] [CrossRef] [Green Version]
- Moro, A.; Mutinelli, F. Field evaluation of Maqs® and Api-Bioxal® for late summer control of Varroa mite infestation in Northeastern Italy. J. Apic. Res. 2018, 53–61. [Google Scholar] [CrossRef]
- Wilkinson, D.; Smith, G.C. A model of the mite parasite, Varroa destructor, on honeybees (Apis mellifera) to investigate parameters important to mite population growth. Ecol. Model. 2002, 148, 263–275. [Google Scholar] [CrossRef]
| Apiary | Pretreatment Mite Fall n = 150 | CheckMite+ A n = 5/apiary | Apiguard B n = 5/apiary | Bayvarol C n = 5/apiary | Thymovar D n = 5/apiary | ApiLife Var E n = 5/apiary | Oxalic Acid A,B,C,D,E,O |
|---|---|---|---|---|---|---|---|
| EA1 | 1.6–24.7 | 25.7–5.9 | 25.7–22.8 | 25.7–5.9 | 25.7–5.9 | 25.7–22.8 | 28.11–6.12 |
| EA2 | 1.6–17.7 | 17.7–28.8 | 17.7–14.8 | 17.7–28.8 | 17.7–28.8 | 17.7–14.8 | 29.11–7.12 |
| EA3 | 1.6–15.7 | 16.7–27.8 | 16.7–13.8 | 16.7–27.8 | 16.7–27.8 | 16.7–13.8 | 11.12–18.12 |
| EA4 | 1.6.–16.7 | 17.7–28.8 | 17.7–14.8 | 17.7–28.8 | 17.7–28.8 | 17.7–14.8 | 1.12–8.12 |
| EA5 | 1.6–14.7 | 15.7–26.8 | 15.7–12.8 | 15.7–26.8 | 15.7–26.8 | 15.7–12.8 | _ |
| Meteorological Circumstances (Average Values Per Month) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Month | Air Temperature (°C) | Relative Air Moisture (%) | Number of Days with Rain | ||||||||||||
| EA1 | EA2 | EA3 | EA4 | EA5 | EA1 | EA2 | EA3 | EA4 | EA5 | EA1 | EA2 | EA3 | EA4 | EA5 | |
| Mar | 9.5 | 9.9 | 8.9 | 7.0 | 9.5 | 74 | 65 | 67 | 69 | 68 | 9 | 8 | 5 | 10 | 9 |
| Apr | 13.2 | 12.9 | 11.9 | 10.5 | 12.6 | 74 | 73 | 74 | 72 | 71 | 11 | 12 | 8 | 10 | 15 |
| May | 16.1 | 14.9 | 14.4 | 12.9 | 15.1 | 73 | 69 | 72 | 66 | 69 | 11 | 18 | 3 | 7 | 14 |
| Jun | 20.5 | 19.2 | 19.6 | 17.7 | 20.1 | 67 | 74 | 66 | 67 | 66 | 3 | 9 | 2 | 4 | 10 |
| Jul | 21.8 | 20.7 | 20.0 | 18.6 | 21.1 | 74 | 76 | 78 | 73 | 73 | 3 | 10 | 6 | 10 | 12 |
| Aug | 20.8 | 19.1 | 19.4 | 18.3 | 20.9 | 76 | 79 | 79 | 72 | 73 | 6 | 11 | 8 | 2 | 7 |
| Sep | 17.0 | 15.6 | 15.7 | 13.7 | 16.7 | 82 | 86 | 81 | 84 | 79 | 10 | 17 | 11 | 13 | 14 |
| Oct | 13.3 | 12.8 | 13.0 | 11.2 | 13.7 | 82 | 85 | 83 | 79 | 76 | 9 | 9 | 8 | 7 | 10 |
| Nov | 8.3 | 8.6 | 11.0 | 8.5 | 10.9 | 87 | 89 | 87 | 86 | 84 | 3 | 17 | 16 | 13 | 14 |
| EA | Experimental Group | Number of Fallen V. destructor Mites (S/W) | % of Treatment Efficacy | ||
|---|---|---|---|---|---|
| - | - | Mean | Min. | Max. | |
| EA1 | A | 1044/286.4 | 430/237 | 1967/449 | 78.50 |
| B ǂǂǂ | 371.6/660.5 | 149/616 | 975/705 | 36.00 | |
| C ǂ | 699.2/546 | 168/490 | 1112/609 | 56.20 | |
| D ǂǂ | 780/509.33 | 283/357 | 1605/774 | 60.50 | |
| E ǂǂǂ | 1188.8/642 | 429/604 | 2046/680 | 65.00 | |
| EA2 | A | 950/326.6 | 381/135 | 2217/671 | 74.42 |
| B | 287.8/421.2 | 65/177 | 744/658 | 40.60 | |
| C | 200.4/680.8 | 96/111 | 326/1038 | 22.75 | |
| D | 332.2/273.6 | 152/85 | 664/638 | 54.90 | |
| E | 448.4/572.8 | 48/100 | 1177/1195 | 43.90 | |
| EA3 | A | 724/314 | 603/138 | 802/435 | 69.75 |
| B | 118.4/489.2 | 97/233 | 159/635 | 19.49 | |
| C | 190.4/433.4 | 115/231 | 346/625 | 30.52 | |
| D | 171.6/379.6 | 114/168 | 333/589 | 31.13 | |
| E | 194.6/397 | 102/168 | 442/602 | 32.90 | |
| EA4 | A | 783.2/264.4 | 125/278 | 1207/382 | 74.77 |
| B | 195/542.4 | 43/384 | 381/789 | 26.45 | |
| C | 480.2/405.2 | 279/240 | 766/563 | 54.24 | |
| D | 167.2/406.6 | 48/180 | 373/594 | 41.13 | |
| E ǂ | 312.6/380.7 | 30/126 | 1119/594 | 45.09 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tlak Gajger, I.; Svečnjak, L.; Bubalo, D.; Žorat, T. Control of Varroa destructor Mite Infestations at Experimental Apiaries Situated in Croatia. Diversity 2020, 12, 12. https://doi.org/10.3390/d12010012
Tlak Gajger I, Svečnjak L, Bubalo D, Žorat T. Control of Varroa destructor Mite Infestations at Experimental Apiaries Situated in Croatia. Diversity. 2020; 12(1):12. https://doi.org/10.3390/d12010012
Chicago/Turabian StyleTlak Gajger, Ivana, Lidija Svečnjak, Dragan Bubalo, and Tomislav Žorat. 2020. "Control of Varroa destructor Mite Infestations at Experimental Apiaries Situated in Croatia" Diversity 12, no. 1: 12. https://doi.org/10.3390/d12010012
APA StyleTlak Gajger, I., Svečnjak, L., Bubalo, D., & Žorat, T. (2020). Control of Varroa destructor Mite Infestations at Experimental Apiaries Situated in Croatia. Diversity, 12(1), 12. https://doi.org/10.3390/d12010012






