Conservation Status of Brachycephalus Toadlets (Anura: Brachycephalidae) from the Brazilian Atlantic Rainforest
Abstract
1. Introduction
2. Material and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frost, D.R. Amphibian Species of the World: An Online Reference, Version 6.0. 2019. Available online: http://research.amnh.org/herpetology/amphibia/index.html (accessed on 22 June 2019).
- Köhler, K.; Vieites, D.R.; Bonett, R.M.; Garcia, F.H.; Glaw, F.; Steinke, D.; Vences, M. New Amphibians and Global Conservations: A boost in species discoveries in a highly endangered vertebrate group. BioScience 2005, 55, 693–696. [Google Scholar] [CrossRef]
- Tapley, B.; Michaels, C.J.; Gumbs, R.; Böhm, M.; Luedtke, J.; Pearce-Kelly, P.; Rowley, J.J.L. The disparity between species description and conservation assessment: A case study in taxa with high rates of species discovery. Biol. Conserv. 2018, 220, 209–214. [Google Scholar] [CrossRef]
- Wake, D.B.; Vredenburg, V.T. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl. Acad. Sci. USA 2008, 105, 11466–11473. [Google Scholar] [CrossRef] [PubMed]
- IUCN. The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org (accessed on 28 June 2019).
- Stuart, S.; Chanson, J.; Cox, N. Status and trends of amphibian declines and extinctions worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef] [PubMed]
- Skerrat, L.F.; Berger, L.; Speare, R.; Cashins, S.; McDonald, K.R. Spread of Chytridiomycosis Has Caused the Rapid Global Decline and Extinction of Frogs. EcoHealth 2007, 4, 126. [Google Scholar] [CrossRef]
- Stuart, S.N.; Hoffmann, M.; Chanson, J.S.; Cox, N.A.; Berridge, R.J.; Ramani, P.; Young, B.E. Threatened Amphibians of the World; Lynx Editions: Barcelona, Spain, 2008; pp. 1–151. [Google Scholar]
- Lips, K.P.R.; Burrowes, P.A.; Mendelson, J.R., III; Parra-Olea, G. Amphibian declines in Latins America: A synthesis. Biotropica 2005, 37, 222–226. [Google Scholar] [CrossRef]
- Ruland, F.; Jeschke, J.M. Threat-dependent traits of endangered frogs. Biol. Conserv. 2017, 206, 310–313. [Google Scholar] [CrossRef]
- Ruggeri, J.; Ribeiro, L.P.; Pontes, M.R.; Toffolo, C.; Candido, M.; Carriero, M.M.; Zanella, N.; Sousa, R.L.M.; Toledo, L.F. First case of wild amphibians infected with Ranavirus in Brazil. J. Wildlife Dis. 2019, 55. Preprint. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Dov-Por, F. Sooretema, the Atlantic Rain Forest of Brazil; SPB Academic Publishing: The Hague, The Netherlands, 1992; Volume 128. [Google Scholar]
- MMA. Mapa de Vegetação Nativa na Área de Aplicação da Lei no. 11.428/2006—Lei da Mata Atlântica (ano base 2009); Ministério do Meio Ambiente: Brasília, Brazil, 2015; pp. 1–85.
- Rezende, C.L.; Scarano, F.R.; Assad, E.D.; Joly, C.A.; Metzger, J.P.; Strassburg, B.B.N.; Tabarelli, M.; Fonseca, G.A.; Mittermeier, R.A. From hotspots to hopespot: An opportunity for the Brazilian Atlantic Forest. Perspect. Ecol. Conserv. 2018, 16, 204–214. [Google Scholar] [CrossRef]
- Ministério do Meio Ambiente. Livro Vermelho da Fauna Brasileira Ameaçada de Extinção—Sumário Executivo; Instituto Chico Mendes de Conservação da Biodiversidade—ICMBio: Brasília, Brazil, 2016; pp. 1–76.
- Izecksohn, E. Novo gênero e nova espécie de Brachycephalidae do Estado do Rio de Janeiro, Brasil. Boletim do Museu Nacional Zoologia 1971, 280, 1–12. [Google Scholar]
- Giaretta, A.A.; Sawaya, R.J. Second species of Psyllophryne (Anura: Brachycephalidae). Copeia 1998, 1998, 985–987. [Google Scholar] [CrossRef]
- Pombal, J.P., Jr. Oviposição e desenvolvimento de Brachycephalus ephippium (Spix) (Anura, Brachycephalidae). Rev. Brasil. Zool. 1999, 16, 967–976. [Google Scholar] [CrossRef]
- Napoli, M.F.; Caramaschi, U.; Cruz, C.A.G.; Dias, I.R. A new species of flea-toad, genus Brachycephalus Fitzinger (Amphibia: Anura: Brachycephalidae), from the Atlantic Rainforest of southern Bahia, Brazil. Zootaxa 2011, 2739, 33–40. [Google Scholar] [CrossRef]
- Yeh, J. The effect of miniaturized body size on skeletal morphology in frogs. Evolution 2002, 56, 628–641. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.A.; de Souza Castro, M.; Pires Júnior, O.R.; Maciel, N.M.; Schwartz, E.N.F.; Sebben, A. Princípios bioativos da pele de anfíbios: Panorama atual e perspectivas. In Herpetologia no Brasil II; Nascimento, L.B., Oliveira, M.E., Eds.; Sociedade Brasileira de Herpetologia: Belo Horizonte, Brasil, 2007; pp. 146–168. [Google Scholar]
- Bornschein, M.R.; Firkowski, C.R.; Belmonte-Lopes, R.; Corrêa, L.; Ribeiro, L.F.; Morato, S.A.A.; Antoniazzi, R.L., Jr.; Reinert, B.L.; Meyer, A.L.S.; Cini, F.A.; et al. Geographical and altitudinal distribution of Brachycephalus (Anura: Brachycephalidae) endemic to the Brazilian Atlantic Rainforest. PeerJ 2016, 4, e2490. [Google Scholar] [CrossRef] [PubMed]
- Bornschein, M.R.; Teixeira, L.; Ribeiro, L.F. New record of Brachycephalus fuscolineatus Pie, Bornschein, Firkowski, Belmonte-Lopes & Ribeiro, 2015 (Anura, Brachycephalidae) from Santa Catarina state, Brazil. Check List 2019, 15, 379–385. [Google Scholar]
- Pie, M.R.; Ribeiro, L.F.; Confetti, A.E.; Nadaline, M.J.; Bornschein, M.R. A new species of Brachycephalus (Anura: Brachycephalidae) from southern Brazil. PeerJ 2018, 6, e5683. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.F.; Bornschein, M.R.; Belmonte-Lopes, R.; Firkowski, C.R.; Morato, S.A.A.; Pie, M.R. Seven new microendemic species of Brachycephalus (Anura: Brachycephalidae) from southern Brazil. PeerJ 2015, 3, e1011. [Google Scholar] [CrossRef] [PubMed]
- Pie, M.R.; Meyer, A.L.S.; Firkowski, C.R.; Ribeiro, L.F.; Bornschein, M.R. Understanding the mechanisms underlying the distribution of microendemic montane frogs (Brachycephalus spp., Terrarana: Brachycephalidae) in the Brazilian Atlantic Rainforest. Ecol. Model. 2013, 250, 165–176. [Google Scholar] [CrossRef]
- Firkowski, C.R.; Bornschein, M.R.; Ribeiro, L.F.; Pie, M.R. Species delimitation, phylogeny and evolutionary demography of co-distributed, montane frogs in the southern Brazilian Atlantic Forest. Mol. Phylogenet. Evol. 2016, 100, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Pie, M.R.; Faircloth, B.C.; Bornschein, M.R.; McComarck, J.E. Phylogenomics of montane frogs of the Brazilian Atlantic Forest is consistent with isolation in sky islands followed by climatic stability. Biol. J. Linn. Soc. 2018, 125, 72–82. [Google Scholar] [CrossRef]
- Pombal, J.P., Jr. A posição taxonômica das “variedades” de Brachycephalus ephippium (Spix, 1824) descritas por Miranda-Ribeiro, 1920 (Amphibia, Anura, Brachycephalidae). Bol. Mus. Nac. Zool. 2010, 526, 1–12. [Google Scholar]
- Silvano, D.; Heyer, R.; Caramaschi, U. Brachycephalus nodoterga. The IUCN Red List of Threatened Species 2004: e.T54454A11149387. Available online: https://www.iucnredlist.org/species/54454/11149387 (accessed on 13 July 2019).
- Silvano, D.; Garcia, P.; Segalla, M.V. Brachycephalus pernix. The IUCN Red List of Threatened Species 2004, e.T54455A11149530. Available online: https://www.iucnredlist.org/species/54455/11149530 (accessed on 13 July 2019).
- Silvano, D.; Caramaschi, U. Brachycephalus hermogenesi. The IUCN Red List of Threatened Species 2010, e.T29487A9501270. Available online: https://www.iucnredlist.org/species/29487/9501270 (accessed on 13 July 2019).
- Angulo, A. Brachycephalus ferruginus. The IUCN Red List of Threatened Species 2006, e.T135912A4229152. Available online: https://www.iucnredlist.org/species/135912/4220152 (accessed on 13 July 2019).
- Angulo, A. Brachycephalus alipioi. The IUCN Red List of Threatened Species 2008, e.T135774A4199662. Available online: https://www.iucnredlist.org/species/135774/4199662 (accessed on 13 July 2019).
- Angulo, A. Brachycephalus pombali. The IUCN Red List of Threatened Species 2008, e.T135830A4208137. Available online: https://www.iucnredlist.org/species/135830/4208137 (accessed on 13 July 2019).
- Caramaschi, U.; Carvalho-e-Silva, S.P. Brachycephalus vertebralis. The IUCN Red List of Threatened Species 2004, e.T54456A11134718. Available online: https://www.iucnredlist.org/species/54456/11134718 (accessed on 13 July 2019).
- Sluys, M.V.; Rocha, C.F. Brachycephalus ephippium. The IUCN Red List of Threatened Species 2010: e.T54453A11149233. Available online: https://www.iucnredlist.org/species/54453/11149233 (accessed on 13 July 2019).
- Stuart, S. Brachycephalus brunneus. The IUCN Red List of Threatened Species 2006, e.T61745A12553521. Available online: https://www.iucnredlist.org/species/61746/12553521 (accessed on 13 July 2019).
- Stuart, S. Brachycephalus izecksohni. The IUCN Red List of Threatened Species 2006, e.T61747A12553638. Available online: https://www.iucnredlist.org/species/61747/12553638 (accessed on 13 July 2019).
- Telles, A.M.; Carvalho-e-Silva, S.P. Brachycephalus didactylus. The IUCN Red List of Threatened Species 2004, e.T54452A11148997. Available online: https://www.iucnredlist.org/species/54452/11148997 (accessed on 13 July 2019).
- Haddad, C.F.B.; Machado, I.F.; Giovanelli, J.G.R.; Bataus, Y.S.L.; Ublig, V.M.; Batista, F.R.Q.; Cruz, C.A.G.; Conte, C.E.; Zank, C.; Strüsmann, C.; et al. Avaliação do Risco de Extinção de Brachycephalus nodoterga Miranda-Ribeiro, 1920. In Processo de Avaliação do Risco de Extinção da Fauna Brasileira; Instituto Chico Mentes de conservação da Biodiversidade—ICMbio: Brasília, Brazil, 2016. [Google Scholar]
- Haddad, C.F.B.; Machado, I.F.; Giovanelli, J.G.R.; Bataus, Y.S.L.; Uhlig, V.M.; Batista, F.R.Q.; Cruz, C.A.G.; Conte, C.E.; Zank, C.; Strüsmann, C.; et al. Avaliação do Risco de Extinção de Brachycephalus alipioi Pombal & Gasparini, 2006. In Processo de Avaliação do Risco de Extinção da Fauna Brasileira; Instituto Chico Mentes de conservação da Biodiversidade—ICMbio: Brasília, Brazil, 2016. [Google Scholar]
- Haddad, C.F.B.; Machado, I.F.; Giovanelli, J.G.R.; Bataus, Y.S.L.; Uhlig, V.M.; Batista, F.R.Q.; Cruz, C.A.G.; Conte, C.E.; Zank, C.; Strüsmann, C.; et al. Avaliação do Risco de Extinção de Brachycephalus vertebralis Pombal, 2001. In Processo de Avaliação do Risco de Extinção da Fauna Brasileira; Instituto Chico Mentes de conservação da Biodiversidade—ICMbio: Brasília, Brazil, 2016. [Google Scholar]
- Haddad, C.F.B.; Segalla, M.V.; Bataus, Y.S.L.; Uhlig, V.M.; Batista, F.R.Q.; Garda, A.; Hudson, A.A.; Cruz, C.A.G.; Strüsmann, C.; Brasileiro, C.A.; et al. Avaliação do Risco de Extinção de Brachycephalus pernix Pombal, Wistuba & Bornschein, 1998. In Processo de Avaliação do Risco de Extinção da Fauna Brasileira; Instituto Chico Mentes de conservação da Biodiversidade—ICMbio: Brasília, Brazil, 2016. [Google Scholar]
- Bland, L.M.; Colle, B.; Orme, C.D.L.; Bielby, J. Data uncertainty and the selectivity of extinction risk in freshwater invertebrates. Divers. Distrib. 2012, 18, 1211–1220. [Google Scholar] [CrossRef]
- Morais, A.R.; Siqueira, M.N.; Lemes, P.; Maciel, N.M.; De Marco, P.; Brito, D. Unraveling the conservations status of Data Deficient species. Biol. Conserv. 2013, 166, 98–102. [Google Scholar] [CrossRef]
- IUCN. IUCN Red List Categories and Criteria: Version 3.1., 2nd ed.; International Union for Conservation of Nature—IUCN: Gland, Switzerland; Cambridge, UK, 2012. [Google Scholar]
- Reinert, B.L.; Bornschein, M.R.; Firkowski, C. Distribuição, tamanho populacional, hábitat e conservação do bicudinho-do-brejo Stymphalornis acutirostris Bornschein, Reinert e Teixeira, 1995 (Thamnophilidae). Rev. Bras. Ornitol. 2007, 15, 493–519. [Google Scholar]
- Ribeiro, L.F.; Blackburn, D.C.; Stanley, E.L.; Pie, M.R.; Bornschein, M.R. Two new species of the Brachycephalus pernix group (Anura: Brachycephalidae) from the state of Paraná, southern Brazil. PeerJ 2017, 5, e3603. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.C.F.; Coco, L.; Pagotto, R.V.; Pralon, E.; Vrcibradic, D.; Pombal, J.P., Jr.; Rocha, C.F.D. Amphibia, Anura, Brachycephalus didactylus (Izecksohn, 1971) and Zachaenus parvulus (Girard, 1853): Distribution extension. Check List 2012, 8, 242–244. [Google Scholar] [CrossRef]
- Oliveira, J.C.F.; Pralon, E.; Coco, L.; Pagotto, R.V.; Rocha, C.F.D. Environmental humidity and leaf-litter depth affecting ecological parameters of a leaf-litter frog community in an Atlantic Rainforest area. J. Nat. Hist. 2013, 47, 2115–2124. [Google Scholar] [CrossRef]
- Siqueira, C.C.; Vrcibradic, D.; Almeida-Gomes, M.; Borges, V.N.T., Jr.; Almeida-Santos, P.; Almeida-Santos, M.; Ariani, C.V.; Guedes, D.M.; Goyannes-Araújo, P.; Dorigo, T.A.; et al. Density and richness of the leaf litter frogs of an Atlantic Rainforest area in Serra dos Órgãos, Rio de Janeiro State, Brazil. Zoologia 2009, 26, 97–102. [Google Scholar] [CrossRef]
- Almeida-Santos, M.; Siqueira, C.C.; Van Sluys, M.; Rocha, C.D.F. Ecology of the Brazilian flea frog Brachycephalus didactylus (Terrana: Brachycephalidae). J. Hepertol. 2011, 45, 251–255. [Google Scholar]
- Siqueira, C.C.; Vrcibradic, D.; Nogueira-Costa, P.; Martins, A.R.; Dantas, L.; Gomes, V.L.R.; Bergallo, H.G.; Rocha, C.F.D. Environmental parameters affecting the structure of leaf-litter frog (Amphibia: Anura) communities in tropical forests: A case study from an Atlantic Rainforest area in southeastern Brazil. Zoologia 2014, 31, 147–152. [Google Scholar] [CrossRef]
- Carvalho-e-Silva, A.M.T.; Silva, G.R.; Carvalho-e-Silva, S.P. Anuros da Reserva Rio das Pedras, Mangaratiba, RJ, Brasil. Biota Neotrop. 2008, 8, 199–209. [Google Scholar] [CrossRef]
- Rocha, C.F.D.; Vrcibradic, D.; Kiefer, M.C.; Almeida-Gomes, M.; Borges-Junior, V.N.T.; Menezes, V.A.; Ariani, C.V.; Pontes, J.A.L.; Goyannes-Araújo, P.; Marra, R.V.; et al. The leaf-litter frog community from Reserva Rio das Pedras, Mangaratiba, Rio de Janeiro State, Southeastern Brazil: Species richness, composition and densities. North West. J. Zool. 2013, 9, 151–156. [Google Scholar]
- Pombal, J.P., Jr. A new species of Brachycephalus (Anura: Brachycephalidae) from Atlantic Forest of southeastern Brazil. Amphib. Reptil. 2001, 22, 179–185. [Google Scholar] [CrossRef]
- Ribeiro, L.F.; Alves, A.C.R.; Haddad, C.F.B. Two new species of Brachycephalus Günther, 1858 from the state of Paraná, southern Brazil (Amphibia, Anura, Brachycephalidae). Bol. Mus. Nac. Zool. 2005, 519, 1–18. [Google Scholar]
- Alves, A.C.R.; Ribeiro, L.F.; Haddad, C.F.B.; Reis, S.F. Two new species of Brachycephalus (Anura: Brachycephalidae) from the Atlantic Forest in Paraná State, southern Brasil. Hepertologica 2006, 62, 221–233. [Google Scholar] [CrossRef]
- Alves, A.C.R.; Sawaya, R.J.; Reis, S.F.; Haddad, C.F.B. New species of Brachycephalus (Anura: Brachycephalidae) from the Atlantic Rain Forest in São Paulo State, Southeastern Brazil. J. Hepertol. 2009, 43, 212–219. [Google Scholar] [CrossRef]
- Da Silva, H.R.; Campos, L.A.; Sebben, A. The auditory region of Brachycephalus and its bearing on the monophyly of the genus (Anura: Brachycephalidae). Zootaxa 2007, 1422, 59–68. [Google Scholar] [CrossRef]
- Verdade, V.K.; Rodrigues, M.T.; Cassimiro, J.; Pavan, D.; Liou, N.; Lange, M. Advertisement call, vocal activity, and geographic distribution of Brachycephalus hermogenesi (Giaretta and Sawaya, 1998) (Anura, Brachycephalidae). J. Herpetol. 2008, 42, 542–549. [Google Scholar] [CrossRef]
- Clemente-Carvalho, R.G.B.; Antoniazzi, M.M.; Jared, C.; Haddad, C.F.B.; Alvez, A.C.R.; Rocha, H.S.; Pereira, G.R.; Oliveira, D.F.; Lopes, R.T.; Reis, S.F. Hyperossification in miniaturized toadlets of the genus Brachycephalus (Amphibia: Anura: Brachycephalidae): Microscopic structure and macroscopic patterns of variation. J. Morphol. 2009, 270, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Campos, L.A. Sistemática Filogenética do Gênero Brachycephalus Ftzinger, 1826 (Anura Brachycephalidae) Com Base Em Dados Morfológicos. Ph.D. Thesis, Universidade de Brasília, Brasília, Brazil, 2011. [Google Scholar]
- Pombal, J.P., Jr.; Izecksohn, E. Uma nova espécie de Brachycephalus (Anura, Brachycephalidae) do Estado do Rio de Janeiro. Pap. Av. Zool. 2011, 51, 443–451. [Google Scholar] [CrossRef]
- Siqueira, C.C.; Vrcibradic, D.; Dorigo, T.A.; Rocha, C.F.D. Anurans from two high-elevation areas of Atlantic Forest in the state of Rio de Janeiro, Brazil. Zoologia 2011, 28, 457–464. [Google Scholar] [CrossRef]
- Rocha, C.F.D.; Van Sluys, M.; Alves, M.A.S.; Bergallo, H.G.; Vrcibradic, D. Activity of leaf-litter frogs: When should frogs be sampled? J. Herpetol. 2000, 34, 285–287. [Google Scholar] [CrossRef]
- Rocha, C.F.D.; Van Sluys, M.; Alves, M.A.S.; Bergallo, H.G.; Vrcibradic, D. Estimates of forest floor litter frog communities: A comparison of two methods. Austral. Ecol. 2001, 26, 14–21. [Google Scholar] [CrossRef]
- Van Sluys, M.; Vrcibradic, D.; Alves, M.A.S.; Bergallo, H.G.; Rocha, C.F.D. Ecological parameters of the leaf-litter frog community of an Atlantic Rainforest area at Ilha Grande, Rio de Janeiro state, Brazil. Austral. Ecol. 2007, 32, 254–260. [Google Scholar] [CrossRef]
- Pimenta, B.V.S.; Bérnils, R.S.; Pombal, J.P., Jr. Amphibia, Anura, Brachycephalidae, Brachycephalus hermogenesi: Filling gap and geographic distribution map. Check List 2007, 3, 277–279. [Google Scholar] [CrossRef][Green Version]
- Condez, T.H.; Monteiro, J.P.C.; Comitti, E.J.; Garcia, P.C.A.; Amaral, I.B.; Haddad, C.F.B. A new species of flea-toad (Anura: Brachycephalidae) from southern Atlantic Forest, Brazil. Zootaxa 2016, 4083, 40–56. [Google Scholar] [CrossRef]
- Firkowski, C.R. Diversification and microendemism in montane refugia from the Brazilian Atlantic Forest. Master’s Thesis, Universidade Federal do Paraná, Curitiba, Brazil, 2013. [Google Scholar]
- Bornschein, M.R.; Ribeiro, L.F.; Blackburn, D.C.; Stanley, E.L.; Pie, M.R. A new species of Brachycephalus (Anura: Brachycephalidae) from Santa Catarina, southern Brazil. PeerJ 2016, 4, e2629. [Google Scholar] [CrossRef]
- Monteiro, J.P.C.; Condez, T.H.; Garcia, P.C.A.; Comitti, E.J.; Amaral, I.B.; Haddad, C.F.B. A new species of Brachycephalus (Anura, Brachycephalidae) from the coast of Santa Catarina State, southern Atlantic Forest, Brazil. Zootaxa 2018, 4407, 483–505. [Google Scholar] [CrossRef]
- Teixeira, L.; Ribeiro, L.F.; Côrrea, L.; Confetti, A.E.; Pie, M.R.; Bornschein, M.R. A second record of the recently described Brachycephalus albolineatus Bornschein, Ribeiro, Blackburn, Stanley & Pie, 2016 (Anura, Brachycephalidae). Check List 2018, 14, 1013–1016. [Google Scholar]
- Pereira, M.S.; Candaten, A.; Milani, D.; Oliveira, F.B.; Gardelin, J.; Rocha, C.F.D.; Vrcibradic, D. Geographic distribution: Brachycephalus hermogenesi. Herpetol. Rev. 2010, 41, 506. [Google Scholar]
- Santos-Pereira, M.; Candaten, A.; Milani, D.; Oliveira, F.B.; Gardelin, J.; Rocha, C.F.D. Seasonal variation in the leaf-litter frog community (Amphibia: Anura) from an Atlantic Forest area in the Salto Morato Natural Reserve, southern Brazil. Zoologia 2011, 28, 755–761. [Google Scholar] [CrossRef]
- Santos-Pereira, M.; Milani, D.; Barata-Bittencourt, L.F.; Iapp, T.M.; Rocha, C.F.D. Anuran species of the Salto Morato Nature Reserve in Paraná, southern Brazil: Review of the species list. Check List 2016, 12, 1907. [Google Scholar] [CrossRef][Green Version]
- Pombal, J.P., Jr.; Gasparini, J.L. A new Brachycephalus (Anura: Brachycephalidae) from the Atlantic Rainforest of Espírito Santo, southeastern Brazil. S. Am. J. Herpet. 2006, 1, 87–93. [Google Scholar] [CrossRef]
- Clemente-Carvalho, R.G.B.; Klaczko, J.; Perez, S.R.; Alves, A.C.R.; Haddad, C.F.B.; Reis, S.F. Molecular phylogenetic relationships and phenotypic diversity in miniaturized toadlets, genus Brachycephalus (Amphibia: Anura: Brahycephalidae). Mol. Phylogenet. Evol. 2011, 61, 79–89. [Google Scholar] [CrossRef]
- Clemente-Carvalho, R.B.G.; Giaretta, A.A.; Condez, T.H.; Haddad, C.F.B.; Reis, S.F. A new species of miniaturized toadlet, genus Brachycephalus (Anura: Brachycephalidae), from the Atlantic Forest of southeastern Brazil. Herpetologica 2012, 68, 365–374. [Google Scholar] [CrossRef]
- Miranda-Ribeiro, A. Os Brachycephalideos do Museu Paulista (com tres especies novas). Rev. Mus. Paulista 1920, 12, 306–318. [Google Scholar]
- Condez, T.H.; Clemente-Carvalho, R.B.G.; Haddad, C.F.B.; Reis, S.F. A new species of Brachycephalus (Anura: Brachycephalidae) from the highlands of the Atlantic Forest, southeastern Brazil. Herpetologica 2014, 70, 89–99. [Google Scholar] [CrossRef]
- Moura, M.R.; Motta, A.P.; Fernandes, V.D.; Feio, R.N. Herpetofauna from Serra do Brigadeiro, an Atlantic Forest remain in the state of Minas Gerais, southeastern Brazil. Biota Neotrop. 2012, 12, 209–235. [Google Scholar] [CrossRef]
- Guimarães, C.S.; Luz, S.; Rocha, P.C.; Feio, R.N. The dark side of pumpkin toadlet: A new species of Brachycephalus (Anura: Brachycephalidae) from Serra do Brigadeiro, southeastern Brazil. Zootaxa 2017, 4258, 327–344. [Google Scholar] [CrossRef]
- Pombal, J.P., Jr.; Sazima, I.; Haddad, C.F.B. Breeding behavior of the pumpkin toadlet, Brachycephalus ephippium (Brachycephalidae). J. Herpetol. 1994, 28, 516–519. [Google Scholar] [CrossRef]
- Giaretta, A.A. Diversidade e Densidade de Anuros de Serapilheira Num Gradiente Altitudinal na Mata Atlântica Costeira. Ph.D. Thesis, Universidade Estadual de Campinas, Campinas, Brazil, 1999. [Google Scholar]
- Giaretta, A.A.; Facure, K.G.; Sawaya, R.J.; Meyer, J.H.M.; Chemin, N. Diversity and abundance of litter frogs in a montane forest of Southeastern Brazil: Seasonal and altitudinal changes. Biotropica 1999, 31, 669–674. [Google Scholar] [CrossRef]
- Giaretta, A.A.; Sawaya, R.J.; Machado, G.; Araújo, M.S.; Facure, K.G.; Medeiros, H.F.; Nunes, R. Diversity and abundance of litter frogs at altitudinal sites at Serra do Japi, Southeastern Brazil. Rev. Brasil. Zool. 1997, 14, 341–346. [Google Scholar] [CrossRef]
- Clemente-Carvalho, R.B.G.; Monteiro, L.R.; Bonato, V.; Rocha, H.S.; Pereira, G.R.; Oliveira, D.F.; Lopes, R.T.; Haddad, C.F.B.; Martins, E.G.; Reis, S.F. Geographic variation in cranial shape in the Pumpkin Toadlet (Brachycephalus ephippium): A geometric analysis. J. Herpetol. 2008, 42, 176–185. [Google Scholar] [CrossRef]
- Clemente-Carvalho, R.G.B.; Alves, A.C.R.; Perez, S.I.; Haddad, C.F.B.; Reis, S.F. Morphological and molecular variation in the Pumpkin Toadlet Branchycephalus ephippium (Anura: Brachycephalidae). J. Herpetol. 2011, 45, 94–99. [Google Scholar] [CrossRef]
- Zaher, H.; Aguiar, E.; Pombal, J.P. Paratelmatobius gaigeae (Cochran, 1938) rediscovered (Amphibia, Anura, Leptodactylidae). Arquiv. Mus. Nac. 2005, 63, 321–328. [Google Scholar]
- Dixo, M.; Verdade, V.K. Herpetofauna de serrapilheira da Reserva Florestal de Morro Grande, Cotia (SP). Biota Neotrop. 2006, 6, 1–20. [Google Scholar] [CrossRef]
- Siqueira, C.C.; Vrcibradic, D.; Rocha, C.F.D. Altitudinal records of data-deficient and threatened frog species from the Atlantic Rainforest of the Serra dos Órgãos mountains, in southeastern Brazil. Braz. J. Biol. 2013, 73, 229–230. [Google Scholar] [CrossRef]
- Dorigo, T.A.; Siqueira, C.C.; Vrcibradic, D.; Maia-Carneiro, T.; Almeida-Santos, M.; Rocha, C.F.D. Ecological aspects of the pumpkin toadlet, Brachycephalus garbeanus Miranda-Ribeiro, 1920 (Anura: Neobatrachia: Brachycephalidae), in a highland forest of southeastern Brazil. J. Nat. Hist. 2012, 46, 2497–2507. [Google Scholar] [CrossRef]
- Pombal, J.P., Jr.; Wistuba, E.M.; Bornschein, M.R. A new species of brachycephalid (Anura) from the Atlantic Rain Forest of Brazil. J. Herpetol. 1998, 32, 70–74. [Google Scholar] [CrossRef]
- Haddad, C.F.B.; Alves, A.C.R.; Clemente-Carvalho, R.B.G.; Reis, S.F. A new species of Brachycephalus from the Atlantic Rain Forest in São Paulo state, southeastern Brazil (Amphibia: Anura: Brachycephalidae). Copeia 2010, 410–420. [Google Scholar] [CrossRef]
- Abegg, A.D.; Ortiz, F.R.; Rocha, B.; Condes, T.H. A new record for Brachycephalus nodoterga (Amphibia, Anura Brachycephalidae) in the state of São Paulo, Brazil. Check List 2015, 11, 1769. [Google Scholar] [CrossRef][Green Version]
- Ribeiro, R.S. Ecologia Alimentar das Quatro Espécies Dominantes da Anurofauna de Serapilheira Em Um Gradiente Altitudinal na Ilha de São Sebastião, SP. Master Thesis, Universidade Estadual Paulista “Júlio de Mesquita Filho”, Rio Claro, São Paulo, Brazil, 2006. [Google Scholar]
- Campos, L.A.; Silva, H.R.; Sebben, A. Morphology and development of additional bony elements in the genus Brachycephalus (Anura: Brachycephalidae). Biol. J. Linn. Soc. 2010, 99, 752–767. [Google Scholar] [CrossRef]
- Oliveira, E.G. História Natural de Brachycephalus pitanga no Núcleo Santa Virgínia, Parque Estadual da Serra do Mar, Estado de São Paulo. Master Thesis, Universidade Estadual Paulista “Júlio de Mesquita Filho”, Rio Claro, Brazil, 2013. [Google Scholar]
- Tandel, M.C.F.F.; Loibel, S.; Oliveira, E.G.; Haddad, C.F.B. Diferenciação de 3 tipos de vocalizações (cantos) na espécie Brachycephalus pitanga. Revista da Estatística da Universidade Federal de Ouro Preto 2014, 3, 374–386. [Google Scholar]
- Araújo, C.B.; Guerra, T.J.; Amatuzzi, M.C.O.; Campos, L.A. Advertisement and territorial calls of Brachycephalus pitanga (Anura: Brachycephalidae). Zootaxa 2012, 3302, 66–67. [Google Scholar] [CrossRef]
- Bornschein, M.R.; Ribeiro, L.F.; Rollo, M.M., Jr.; Confetti, A.E.; Pie, M.R. Advertisement call of Brachycephalus albolineatus (Anura: Brachycephalidae). PeerJ 2018, 6, e5273. [Google Scholar] [CrossRef]
- Fontoura, P.L.; Ribeiro, L.F.; Pie, M.R. Diet of Brachycephalus brunneus (Anura: Brachycephalidae) in the Atlantic Rainforest of Paraná, southern Brazil. Zoologia 2011, 28, 687–689. [Google Scholar] [CrossRef]
- Pie, M.R.; Ströher, P.R.; Bornschein, M.R.; Ribeiro, L.F.; Faircloth, B.C.; Mccormack, J.E. The mitochondrial genome of Brachycephalus brunneus (Anura: Brachycephalidae), with comments on the phylogenetic position of Brachycephalidae. Biochem. Syst. Ecol. 2017, 71, 26–31. [Google Scholar] [CrossRef][Green Version]
- Monteiro, J.P.C.; Condez, T.H.; Garcia, P.C.A.; Haddad, C.F.B. The advertisement calls of two species of Brachycephalus (Anura: Brachycephalidae) from southern Atlantic Forest, Brazil. Zootaxa 2018, 4415, 183–188. [Google Scholar] [CrossRef]
- Wistuba, E.M. História Natural de Brachycephalus pernix Pombal, Wistuba e Bornschein, 1998 (Anura) no Morro Anhangava, Município de Quatro Barras, Estado do Paraná. Ph.D. Thesis, Universidade Federal do Paraná, Curitiba, Brazil, 1998. [Google Scholar]
- Pires, O.R., Jr.; Sebben, A.; Schwartz, E.F.; Morales, R.A.V.; Bloch, C., Jr.; Schwartz, C.A. Further report of the occurrence of tetrodotoxin and new analogues in the Anuran family Brachycephalidae. Toxicon 2005, 45, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.F.; Ströher, P.R.; Firkowski, C.R.; Cini, F.A.; Bornschein, M.R.; Pie, M.R. Brachycephalus pernix (Anura: Brachycephalidae), a new host of Ophiotaenia (Eucestoda: Proteocephalidea). Herpetol. Notes 2014, 7, 291–294. [Google Scholar]
- Pie, M.R.; Ribeiro, L.F. A new species of Brachycephalus (Anura: Brachycephalidae) from the Quiriri mountain range of southern Brazil. PeerJ 2015, 3, e1179. [Google Scholar] [CrossRef] [PubMed]
- Garey, M.V.; Lima, A.M.X.; Hartmann, M.T.; Haddad, C.F.B. A new species of miniaturized toadlet, genus Brachycephalus (Anura: Brachycephalidae), from southern Brazil. Herpetologica 2012, 68, 266–271. [Google Scholar] [CrossRef]
- Bornschein, M.R.; Rollo, M.M., Jr.; Pie, M.R.; Confetti, A.E.; Ribeiro, L.F. Redescription of the advertisement call of Brachycephalus tridactylus (Anura: Brachycephalidae). Phyllomedusa 2019, 18, 3–12. [Google Scholar] [CrossRef]
- Cunha, A.K.; Oliveira, I.S.; Hartmann, M.T. Anurofauna da Colônia Castelhanos, na Área de Proteção Ambiental de Guaratuba, Serra do Mar paranaense, Brasil. Biotemas 2010, 23, 123–134. [Google Scholar]
- De Oliveira, A.K.C.; Oliveira, I.S.; Hartmann, M.T.; Silva, N.R.; Toledo, L.F. Amphibia, Anura, Brachycephalidae, Brachycephalus hermogenesi (Giaretta and Sawaya, 1998): New species record in the state of Paraná, southern Brazil and geographic distribution map. Check List 2011, 7, 17–18. [Google Scholar] [CrossRef]
- Mariotto, L.R. Anfíbios de Um Gradiente Altitudinal Em Mata Atlântica. Master Thesis, Universidade Federal do Paraná, Curitiba, Brazil, 2014. [Google Scholar]
- Condez, T.H.; Sawaya, R.J.; Dixo, M. Herpetofauna dos remanescentes de Mata Atlântica da região de Tapiraí e Piedade, SP, sudeste do Brasil. Biota Neotrop. 2009, 9, 157–185. [Google Scholar] [CrossRef]
- Verdade, V.K.; Rodrigues, M.T.; Pavan, D. Anfíbios Anuros da Região da Estação Biológica do Alto da Serra de Paranapiacaba. Patrimônio da Reserva Biológica do Alto da Serra de Paranapiacaba: A antiga Estação Biológica do Alto da Serra; Governo do Estado de São Paulo, Secretaria do Meio Ambiente: São Paulo, Brazil, 2009; pp. 579–603. [Google Scholar]
- Trevine, V.; Forlani, M.C.; Haddad, C.F.B.; Zaher, H. Herpetofauna of Paranapiacaba: Expanding our knowledge on a historical region in the Atlantic forest of southeastern Brazil. Zoologia 2014, 31, 126–146. [Google Scholar] [CrossRef]
- Collins, J.P. Amphibian decline and extinction: What we know and what we need to learn. Dis. Aquat. Organ. 2010, 92, 93–99. [Google Scholar] [CrossRef]
- Walter, H. Zonas de Vegetación y Clima: Breve Exposición Desde el Punto de Vista Causal y Global; Omega: Barcelona, Espanha, 1977; pp. 1–245. [Google Scholar]
- Walsh, R.P.D. Climate. In The Tropical Rain Forest; Richards, P.W., Ed.; Cambridge University Press: Cambridge, UK, 1979; pp. 159–205. [Google Scholar]
- Roderjan, C.V. O Gradiente da Floresta Ombrófila Densa no Morro Anhangava, Quatro Barras, PR—Aspectos Climáticos, Pedológicos e Fitossociológicos. Ph.D. Thesis, Universidade Federal do Paraná, Curitiba, Brazil, 1994. [Google Scholar]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef] [PubMed]
- Bornschein, M.R.; Firkowski, C.R.; Baldo, D.; Ribeiro, L.F.; Belmonte-Lopes, R.; Corrêa, L.; Morato, S.A.A.; Pie, M.R. Three new species of phytotelm-breeding Melanophryniscus from the Atlantic Rainforest of southern Brazil (Anura: Bufonidae). PLoS ONE 2015, 10, e0142791. [Google Scholar] [CrossRef] [PubMed]
- Nadaline, M.J.; Ribeiro, L.F.; Teixeira, L.; Vannuchi, F.S.; Bornschein, M.R. New record of Melanophryniscus biancae Bornschein, Baldo, Pie, Firkowski, Ribeiro & Corrêa, 2015 (Anura: Bufonidae) from Paraná, Brazil, with comments on its phytotelm-breeding ecology. Check List, in press.
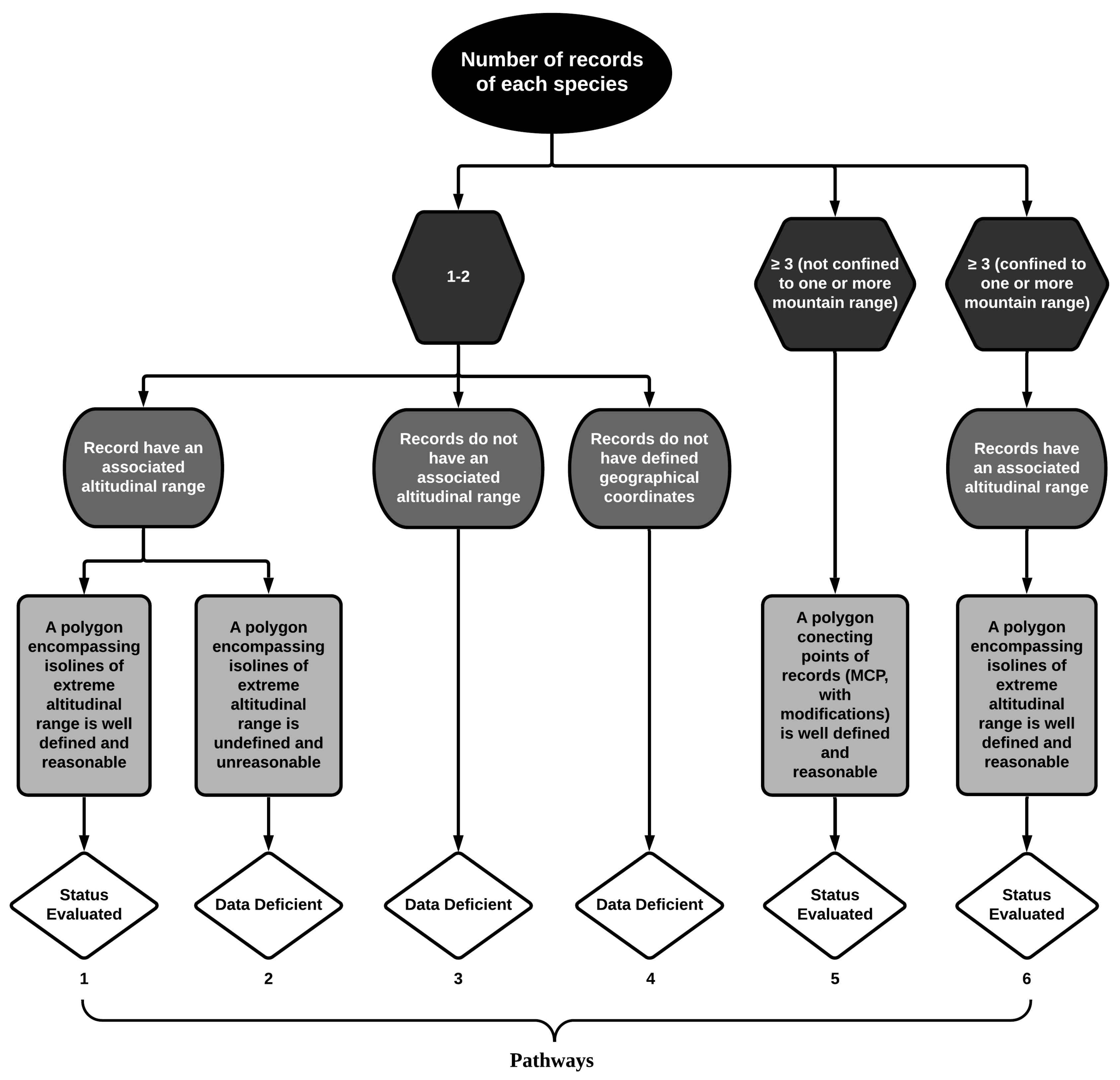
| Species | Group | Locality and State | Altitude 1 | Source |
|---|---|---|---|---|
| B. didactylus | didactylus | Monumento Natural Serra das Torres (21°00’04” S, 41°13’17” W), municipality of Atílio Vivácqua, Espírito Santo | 600–900? | [51] as B. didactylus; [52] as B. didactylus |
| B. didactylus | didactylus | Fazenda Santa Bárbara (22°25’17” S, 42°35’01” W), Parque Estadual dos Três Picos, municipality of Cachoeiras de Macacu, Rio de Janeiro | 500–800 | [53] as B. didactylus; [54] as B. didactylus |
| B. didactylus | didactylus | Reserva Ecológica de Guapiaçu (22°24’00” S, 42°44’00” W), municipality of Cachoeiras de Macacu, Rio de Janeiro | 300–520 | [55] as B. didactylus] |
| B. didactylus | didactylus | Reserva Ecológica Rio das Pedras (22°59’00” S, 44°06’45” W), municipality of Mangaratiba, Rio de Janeiro | 200–1110 | [23] as B. didactylus; [54] as B. didactylus; [56] as B. didactylus; [57] as B. didactylus |
| B. didactylus | didactylus | Sacra Família do Tinguá (22°29’11” S, 43°36’18” W), municipality of Engenheiro Paulo de Frontin, Rio de Janeiro | 600 | [17] as B. didactylus; [27] as B. didactylus; [58] as B. didactylus; [59] as B. didactylus; [60] as B. didactylus; [61] as B. didactylus; [62] as B. didactylus; [63] as B. didactylus; [64] as B. didactylus; [65] as B. didactylus; [66] as B. didactylus |
| B. didactylus | didactylus | Theodoro de Oliveira (first position: 22°22’11” S, 42°33’25” W), Parque Estadual dos Três Picos, municipality of Nova Friburgo, Rio de Janeiro | 1100–1400? | [23] as B. didactylus; [67] as B. didactylus |
| B. didactylus | didactylus | Tinguá (22°35’51” S, 43°24’54” W), municipality of Nova Iguaçu, Rio de Janeiro | 35 | [17] as B. didactylus |
| B. didactylus | didactylus | Vila Dois Rios (23°11’01” S, 44°12’23” W), Ilha Grande, municipality of Angra dos Reis, Rio de Janeiro | 220–240 | [23] as B. didactylus; [68] as B. didactylus; [69] as B. didactylus; [70] as B. didactylus |
| B. hermogenesi | didactylus | Corcovado (23°28’20” S, 45°11’41” W), municipality of Ubatuba, São Paulo | 30–250 | This study, [18] as B. hermogenesi; [23] as B. hermogenesi; [25] as B. hermogenesi collected at Picinguaba; [27] as B. hermogenesi; [63] as B. hermogenesi |
| B. hermogenesi | didactylus | Estação Biológica de Boracéia (23°39’10” S, 45°53’05” W), municipality of Salesópolis, São Paulo | 825–900 | [23] as B. hermogenesi; [27] as B. hermogenesi; [63] as B. hermogenesi; [71] as B. hermogenesi; [72] as B. hermogenesi |
| B. hermogenesi | didactylus | Fazenda Capricórnio (23°23’27” S, 45°04’26” W), municipality of Ubatuba, São Paulo | 60 | [18] as B. hermogenesi; [23] as B. hermogenesi; [27] as B. hermogenesi; [63] as B. hermogenesi; [72] as B. hermogenesi |
| B. hermogenesi | didactylus | Morro Cuscuzeiro (23°17’50”S, 44°47’21” W), on the border of municipalities of Paraty, Rio de Janeiro, and Ubatuba, São Paulo | 730–1090 | This study |
| B. hermogenesi | didactylus | Morro do Corcovado (23°27’06” S, 45°12’03” W), Parque Estadual da Serra do Mar, municipality of Ubatuba, São Paulo | 250–1060 | This study |
| B. hermogenesi | didactylus | Municipality of Paraibuna (c. 23°23’34” S, 45°39’42” W), São Paulo | ? | [72] as B. hermogenesi |
| B. hermogenesi | didactylus | Núcleo Cunha (23°15’48”S, 45°02’39”W), Parque Estadual da Serra do Mar, municipality of Cunha, São Paulo | 1045–1140 | This study |
| B. hermogenesi | didactylus | Núcleo Picinguaba (23°22’21”S, 44°49’53”W), Parque Estadual da Serra do Mar, municipality of Ubatuba, São Paulo | 0–700 | [18] as B. hermogenesi; [23] as B. hermogenesi]; [27] as B. hermogenesi; [29] as B. hermogenesi; [63] as B. hermogenesi; [64] as B. hermogenesi; [71] as B. hermogenesi; [72] as B. hermogenesi |
| B. hermogenesi | didactylus | Sertão da Cutia (not located), municipality of Ubatuba, São Paulo | ? | [72] as B. hermogenesi |
| B. hermogenesi | didactylus | Trilha do Corisco (23°16’38” S, 44°46’39” W), municipality of Paraty, Rio de Janeiro | 350–725 | This study |
| B. hermogenesi | didactylus | Trilha do Ipiranga 50 m from the Rio Ipiranga (23°20’41” S, 45°08’21” W), Núcleo Santa Virgínia, Parque Estadual da Serra do Mar, municipality of São Luiz do Paraitinga, São Paulo | 920–940 | This study |
| B. pulex | didactylus | Serra Bonita (15°23’28” S, 39°33’59” W), municipality of Camacan, Bahia | 800–930 | [20] as B. pulex |
| B. sulfuratus | didactylus | Base of the Serra Água Limpa (24°28’52” S, 48°47’12” W), municipality of Apiaí, São Paulo | 920 | [23] as Brachycephalus sp. 1; [25] as B. sulfuratus; [28] without species identification; [50] as B. sulfuratus; [73] without species identification; [74] as B. sulfuratus |
| B. sulfuratus | didactylus | Biquinha (24°17’43” S, 47°36’26” W), municipality of Juquiá, São Paulo | 40 | This study |
| B. sulfuratus | didactylus | Braço do Norte (26°07’29” S, 48°43’48” W), municipality of Itapoá, Santa Catarina | 240 | [75] as B. sulfuratus |
| B. sulfuratus | didactylus | Caratuval, near the Parque Estadual das Lauráceas (24°51’17” S, 48°43’43” W), municipality of Adrianópolis, Paraná | 900 | [23] as Brachycephalus sp. 1; [25] as B. sulfuratus; [27] as Brachycephalus sp. nov. 1; [28] without species identification; [50] as B. sulfuratus; [73] without species identification; [74] as B. sulfuratus |
| B. sulfuratus | didactylus | Caratuval, Parque Estadual das Lauráceas (24°51’14” S, 48°42’01” W), municipality of Adrianópolis, Paraná | 890 | [23] as Brachycephalus sp. 1; [27] as Brachycephalus sp. nov. 1 |
| B. sulfuratus | didactylus | Castelo dos Bugres (26°13’47” S, 49°03’20” W), municipality of Joinville, Paraná | 790–860 | [23] as Brachycephalus sp. 1; [27] as Brachycephalus sp. nov. 1; [72] as B. sulfuratus; [75] as B. sulfuratus |
| B. sulfuratus | didactylus | Centro de Estudos e Pesquisas Ambientais da Univille (26°13’39” S, 48°41’31” W), Vila da Glória, Distrito do Saí, municipality of São Francisco do Sul, Santa Catarina | 125 | [72] as B. sulfuratus |
| B. sulfuratus | didactylus | Corvo (25°20’17” S, 48°54’56” W), municipality of Quatro Barras, Paraná | 930 | [23] as Brachycephalus sp. 1; [25] as B. sulfuratus; [27] as Brachycephalus sp. nov. 1; [28] without species identification; [29] as B. sulfuratus; [50] as B. sulfuratus; [73] without species identification; [74] as B. sulfuratus |
| B. sulfuratus | didactylus | Estância Hidroclimática Recreio da Serra (25°27’14” S, 49°00’28” W), Serra da Baitaca, municipality of Piraquara, Paraná | 1150–1205 | This study |
| B. sulfuratus | didactylus | Fazenda Thalia (25°30’58” S, 49°40’12” W), municipality of Balsa Nova, Paraná | 1025 | [23] as Brachycephalus sp. 1; [25] as B. sulfuratus; [27] as Brachycephalus sp. nov. 1; [28] without species identification; [50] as B. sulfuratus; [73] without species identification; [74] as B. sulfuratus |
| B. sulfuratus | didactylus | near the Jurupará dam (23°56’30” S, 47°23’45” W), municipality of Piedade, São Paulo | 690 | [25] as B. sulfuratus |
| B. sulfuratus | didactylus | Mananciais da Serra (25°29’32” S, 48°59’33” W), municipality of Piraquara, Paraná | 970–1050 | [23] as Brachycephalus sp. 1; [25] as B. sulfuratus; [27] as Brachycephalus sp. nov. 1; [50] as B. sulfuratus; [74] as B. sulfuratus |
| B. sulfuratus | didactylus | Morro Anhangava (25°22’51” S, 49°01’26” W), municipality of Quatro Barras, Paraná | 915 | [72] as B. sulfuratus; [75] as B. sulfuratus |
| B. sulfuratus | didactylus | Morro do Cantagalo (26°10’31” S, 48°42’44” W), Vila da Glória, Distrito do Saí, municipality of São Francisco do Sul, Santa Catarina | 160 | [72] as B. sulfuratus |
| B. sulfuratus | didactylus | Morro do Garrafão (26°28’23” S, 49°15’57” W), municipality of Corupá, Santa Catarina | 500–530 | [25] as B. sulfuratus; [76] as B. sulfuratus |
| B. sulfuratus | didactylus | Morro Garuva (26°02’29” S, 48°53’14” W), municipality of Garuva, Santa Catarina | 215–495 | This study |
| B. sulfuratus | didactylus | Núcleo Itutinga-Pilões (23°54’17” S, 46°29’22” W), Parque Estadual da Serra do Mar, municipality of Cubatão, São Paulo | 55 | This study |
| B. sulfuratus | didactylus | Parque Estadual da Ilha do Cardoso (25°06’53” S, 47°55’40” W), municipality of Cananéia, São Paulo | 385 | [63] as possibly B. hermogenesi; [72] as B. sulfuratus |
| B. sulfuratus | didactylus | Parque Estadual Intervales (24°16’33” S, 48°25’04” W), municipality of Iporanga, São Paulo | 820 | This study |
| B. sulfuratus | didactylus | Recanto das Hortências (25°33’24” S, 48°59’38” W), municipality of São José dos Pinhais, Paraná | 975 | [23] as Brachycephalus sp. 1; [25] as B. sulfuratus; [50] as B. sulfuratus; [74] as B. sulfuratus |
| B. sulfuratus | didactylus | Reserva Particular do Patrimônio Natural Salto Morato (25°09’14” S, 48°18’06” W), municipality of Guaraqueçaba, Paraná | 40–880 | [23] as Brachycephalus sp. 1; [77] as B. hermogenesi; [78] as B. hermogenesi; [79] as B. hermogenesi |
| B. sulfuratus | didactylus | Salto do Inferno (25°00’02” S, 48°37’07” W), Rio Capivari, municipality of Bocaiúva do Sul, Paraná | 610 | [25] as B. sulfuratus; [50] as B. sulfuratus; [74] as B. sulfuratus |
| B. sulfuratus | didactylus | Serra do Guaraú (24°47’12” S, 48°07’11” W), on the border of the municipalities of Cajati and Jacupiranga, São Paulo | 680–835 | This study |
| B. sulfuratus | didactylus | Serra do Pico (26°08’31” S, 48°57’19” W), municipality of Joinville, Santa Catarina | 340–720 | This study |
| B. sulfuratus | didactylus | Torre Embratel (24°52’46” S, 48°15’27” W), municipality of Cajati, São Paulo | 960–990 | This study |
| B. sulfuratus | didactylus | Truticultura (26°01’33” S, 48°52’02” W), municipality of Garuva, Paraná | 90 | [23] as Brachycephalus sp. 1; [27] as Brachycephalus sp. nov. 1 |
| B. alipioi | ephippium | Fazenda Aoki or Fazenda dos Japoneses (20°28’24” S, 41°00’36” W), boundary of the municipalities of Vargem Alta and Domingos Martins, Espírito Santo | 1070–1100 | [27] as B. alipioi; [64] as B. alipioi; [66] as B. alipioi; [80] as B. alipioi; [81] as B. alipioi; [82] as B. alipioi |
| B. alipioi | ephippium | Forno Grande (20°31’41” S, 41°06’51” W), Parque Estadual de Forno Grande, municipality of Castelo, Espírito Santo | 1430? | [27] as B. alipioi |
| B. alipioi | ephippium | Alto Castelinho (20°30’34” S, 41°00’33” W), municipality of Vargem Alta, Espírito Santo | 1100 | This study, [25] as B. alipioi |
| B. bufonoides | ephippium | Serra de Macaé (22°18’02” S, 42°18’20” W), municipality of Nova Friburgo, Rio de Janeiro | 1100? | [30] as B. bufonoides; [66] as B. bufonoides; [83] as B. ephippium bufonoides |
| B. crispus | ephippium | Bacia B, Núcleo Cunha, Parque Estadual da Serra do Mar (23°15’15” S, 45°01’58” W), municipality of Cunha, São Paulo | 800–1190 | This study, [84] as B. crispus |
| B. darkside | ephippium | Mata do Pai Inácio (20°46′44″ S, 42°29′10″ W), Parque Estadual da Serra do Brigadeiro, municipality Miradouro, Minas Gerais | 1340 | [66] as B. ephippium; [85] as B. ephippium; [86] as B. darkside |
| B. darkside | ephippium | Trilha do Cruzeiro (20°52′41″ S, 42°31′15″ W), Parque Estadual da Serra do Brigadeiro, boundary of the municipalities of Ervália and Muriaé, Minas Gerais | 1265–1500 | [86] as B. darkside |
| B. ephippium | ephippium | Condomínio Ermida (23°14’13” S, 46°58’52” W), Serra do Japi, municipality of Jundiaí, São Paulo | 1225 | [27] as B. ephippium |
| B. ephippium | ephippium | Hotel Fazenda Pé da Serra (22°51’56” S, 45°31’40” W), municipality of Pindamonhangaba, São Paulo | 700 | [27] as B. ephippium |
| B. ephippium | ephippium | Lago Azul (22°27’23” S, 44°36’34” W), Parque Nacional do Itatiaia, municipality of Itatiaia, Rio de Janeiro | 750 | [27] as B. ephippium |
| B. ephippium | ephippium | Maromba (22°25’43” S, 44°37’11” W), Parque Nacional do Itatiaia, municipality of Itatiaia, Rio de Janeiro | 1125 | [27] as B. ephippium |
| B. ephippium | ephippium | Monteiro Lobato (22°57’07” S, 45°50’20” W), municipality of Monteiro Lobato, São Paulo | 700 | [66] as B. ephippium |
| B. ephippium | ephippium | Observatório de Capricórnio (22°53’54” S, 46°49’01” W), Serra das Cabras, Joaquim Egídio District, boundary of the municipalities of Campinas and Morungaba, São Paulo | 1085 | [19] as B. ephippium]; [27] as B. ephippium; [66] as B. ephippium; [87] as B. ephippium |
| B. ephippium | ephippium | Parque Municipal de Itapetinga (Grota Funda) (23°11’07” S, 46°31’47” W), municipality of Atibaia, São Paulo | 900–1250 | [27] as B. ephippium; [64] as B. ephippium; [81] as B. ephippium; [88] as B. ephippium; [89] as B. ephippium |
| B. ephippium | ephippium | Reserva Biológica da Serra do Japi (23°17’07” S, 47°00’05” W), Serra do Japi, boundary of the municipalities of Jundiaí and Cabreúva, São Paulo | 1000 | [27] as B. ephippium; [64] as B. ephippium; [66] as B. ephippium; [90] as B. ephippium |
| B. ephippium | ephippium | Reserva Ecológica do Trabiju (22°48’01” S, 45°32’03” W), Trabiju, municipality of Pindamonhangaba, São Paulo | 1000? | [66] as B. ephippium |
| B. ephippium | ephippium | Reserva Pedra Branca (22°56’22” S, 45°41’04” W), municipality of Tremembé, São Paulo | 890? | [66] as B. ephippium |
| B. ephippium | ephippium | Santo Antônio do Pinhal (22°49’28” S, 45°40’20” W), municipality of Santo Antônio do Pinhal, São Paulo | 1080 | [66] as B. ephippium |
| B. ephippium | ephippium | São Francisco Xavier (22°53’44” S, 45°58’04” W), municipality of São José dos Campos, São Paulo | 1000 | [27] as B. ephippium; [66] as B. ephippium; [91] as B. ephippium; [92] as B. ephippium |
| B. ephippium | ephippium | Serra Negra (21°57’28” S, 43°47’20” W), municipality of Santa Bárbara do Monte Verde, Minas Gerais | ? | [23] as B. ephippium; [65] as BMV MG2 |
| B. ephippium | ephippium | Serra da Concórdia (22°20’30” S, 43°44’04” W), Parque Estadual Serra da Concórdia, Barão de Juparanã, municipality of Valença, Rio de Janeiro | 900? | [66] as B. ephippium |
| B. ephippium | ephippium | Alto do Soberbo (22°27’15” S, 42°59’21” W), municipality of Teresópolis, Rio de Janeiro | 1250 | [66] as B. ephippium |
| B. ephippium | ephippium | Comary (22°27’22” S, 42°58’24” W), municipality of Teresópolis, Rio de Janeiro | 990 | [66] as B. ephippium |
| B. ephippium | ephippium | Floresta dos Macacos (22°58’15” S, 43°15’24” W), municipality of Rio de Janeiro, Rio de Janeiro | 450? | [66] as B. ephippium |
| B. ephippium | ephippium | Garrafão (22°28’04” S, 43°01’52” W), municipality of Guapimirim, Rio de Janeiro | 1785? | [66] as B. ephippium |
| B. ephippium | ephippium | Pedra Branca (22°55’55” S, 43°28’23” W), Serra da Pedra Branca, municipality of Rio de Janeiro, Rio de Janeiro | 1000 | [58] as B. ephippium; [66] as B. ephippium |
| B. ephippium | ephippium | Represa do Rio Grande (22°55’58” S, 43°26’36” W), Parque Estadual da Pedra Branca, municipality of Rio de Janeiro, Rio de Janeiro | 150? | [27] as B. ephippium; [66] as B. ephippium |
| B. ephippium | ephippium | Reserva Ecológica Rio das Pedras (22°59’00” S, 44°06’45” W), municipality of Mangaratiba, Rio de Janeiro | 200–1110 | [56] as B. ephippium |
| B. ephippium | ephippium | Riacho Beija-flor (22°27’04” S, 43°00’04” W), Parque Nacional da Serra dos Órgãos, municipality of Teresópolis, Rio de Janeiro | 1195 | [27] as B. ephippium |
| B. ephippium | ephippium | Rocio District (22°28’23” S, 43°14’38” W), municipality of Petrópolis, Rio de Janeiro | 950 | [27] as B. ephippium |
| B. ephippium | ephippium | Serra do Tinguá (22°35’31” S, 43°28’16” W), municipality of Nova Iguaçu, Rio de Janeiro | 950? | [66] as B. ephippium |
| B. ephippium | ephippium | Vale da Revolta (22°26’17” S, 42°56’19” W), municipality of Teresópolis, Rio de Janeiro | 1035 | [66] as B. ephippium |
| B. ephippium | ephippium | Varginha (22°24’34” S, 42°52’11” W), municipality of Teresópolis, Rio de Janeiro | 825? | [66] as B. ephippium |
| B. ephippium | ephippium | Bonito (22°42’51” S, 44°34’39” W), Serra da Bocaina, municipality of São José do Barreiro, São Paulo | 1660? | [66] as B. ephippium |
| B. ephippium | ephippium | Estação Ecológica de Bananal (22°48’05” S, 44°22’12” W), Serra da Bocaina, municipality of Bananal, São Paulo | 1200? | [93] as B. ephippium |
| B. ephippium | ephippium | Lídice District (22°50’01” S, 44°11’32” W), municipality of Rio Claro, Rio de Janeiro | 650? | [58] as B. ephippium; [66] as B. ephippium |
| B. ephippium | ephippium | Pedra Branca (23°10’38” S, 44°47’19” W), Serra da Bocaina, municipality of Parati, Rio de Janeiro | 630? | [58] as B. ephippium; [66] as B. ephippium |
| B. ephippium | ephippium | Reserva Florestal de Morro Grande (23°42’08” S, 46°58’22” W), municipality of Cotia, São Paulo | 990? | [94] as B. ephippium |
| B. garbeanus | ephippium | Alto Caledônia (22°20’10” S, 42°33’20” W), municipality of Nova Friburgo, Rio de Janeiro | 1070? | [66] as B. garbeanus |
| B. garbeanus | ephippium | Baixo Caledônia (22°21’33” S, 42°34’12” W), municipality of Nova Friburgo, Rio de Janeiro | 1600–1900 | [66] as B. garbeanus; [67] as B. garbeanus; [95] as B. garbeanus; [96] as B. garbeanus |
| B. garbeanus | ephippium | Macaé de Cima (22°21’37” S, 42°17’50” W), municipality of Nova Friburgo, Rio de Janeiro | 1130 | [27] as B. garbeanus; [64] as B. ephippium; [66] as B. garbeanus; [81] as B. garbeanus; [91] as B. ephippium; [92] as B. ephippium |
| B. garbeanus | ephippium | Morro São João (22°22’47” S, 42°30’34” W), municipality of Nova Friburgo, Rio de Janeiro | 1550? | [66] as B. garbeanus |
| B. garbeanus | ephippium | Serra de Macaé (22°18’02” S, 42°18’20” W), municipality of Nova Friburgo, Rio de Janeiro | 1100? | [30] as B. garbeanus; [66] as B. garbeanus; [83] as B. ephippium garbeanus |
| B. garbeanus | ephippium | Serra Nevada (22°21’46” S, 42°32’48” W), municipality of Nova Friburgo, Rio de Janeiro | 1190 | [66] as B. garbeanus |
| B. garbeanus | ephippium | Theodoro de Oliveira (second position: 22°21’48” S, 42°33’13” W), Parque Estadual dos Três Picos, municipality of Nova Friburgo, Rio de Janeiro | 1400 | [66] as B. garbeanus; [67] as B. garbeanus; [95] as B. garbeanus |
| B. guarani | ephippium | Morro Prumirim (23°20’50” S, 45°01’37” W), municipality of Ubatuba, São Paulo | 500–900 | [82] as B. guarani; [84] as B. guarani; [88] as Brachycephalus sp. |
| B. margaritatus | ephippium | Castelo Country Club (22°32’21” S, 43°13’08” W), municipality of Petrópolis, Rio de Janeiro | 980 | [66] as B. margaritatus |
| B. margaritatus | ephippium | Castelo Montebello (22°24’24” S, 42°58’06” W), municipality of Teresópolis, Rio de Janeiro | 920 | [66] as B. margaritatus |
| B. margaritatus | ephippium | Independência (22°32’58” S, 43°12’27” W), municipality of Petrópolis, Rio de Janeiro | 860 | [66] as B. margaritatus |
| B. margaritatus | ephippium | Morro Azul (22°28’34” S, 43°34’40” W), municipality of Engenheiro Paulo de Frontin, Rio de Janeiro | 620 | [65] as BPF RJ2; [66] as B. margaritatus |
| B. margaritatus | ephippium | Quitandinha (22°31’47” S, 43°12’26” W), municipality of Petrópolis, Rio de Janeiro | 925 | [66] as B. margaritatus |
| B. margaritatus | ephippium | Sacra Família do Tinguá (22°29’11” S, 43°36’18” W), municipality of Engenheiro Paulo de Frontin, Rio de Janeiro | 600 | [17] as B. ephippium; [27] as B. ephippium; [58] as Brachycephalus cf. ephippium; [66] as B. margaritatus |
| B. nodoterga | ephippium | Estação Biológica de Boracéia (second position: 23°38’00”S, 45°52’00”W), municipality of Salesópolis, São Paulo | 945 | [27] as B. nodoterga; [30] as B. nodoterga; [58] as B. nodoterga; [59] as B. nodoterga; [60] as B. nodoterga; [61] as B. nodoterga; [66] as B. nodoterga; [97] as B. nodoterga; [98] as B. nodoterga; [99] as B. nodoterga |
| B. nodoterga | ephippium | Fazenda Paiva Ramos (23°28’21” S, 46°47’25” W), municipality of Osasco, São Paulo | 820 | [99] as B. nodoterga |
| B. nodoterga | ephippium | Pico do Ramalho (23°51’42” S, 45°21’28” W), Ilha de São Sebastião, municipality of Ilhabela, São Paulo | 700–900 | [27] as B. nodoterga; [66] as B. nodoterga; [99] as B. nodoterga; [100] as Brachycephalus sp. aff. nodoterga |
| B. nodoterga | ephippium | Santana de Parnaíba (23°26’19” S, 46°56’06” W), municipality of Santana de Parnaíba, São Paulo | 730 | [99] as B. nodoterga |
| B. nodoterga | ephippium | Serra da Cantareira (23°27’13” S, 46°38’11” W), Parque Estadual da Cantareira, municipality of São Paulo, São Paulo | 850? | [30] as B. nodoterga; [59] as B. nodoterga; [60] as B. nodoterga; [61] as B. nodoterga; [64] as B. nodoterga; [66] as B. nodoterga; [81] as B. nodoterga; [82] as B. nodoterga, [83] as B. ephippium nodoterga; [84] as B. nodoterga, [98] as B. nodoterga; [99] as B. nodoterga |
| B. pitanga | ephippium | Fazenda Capricórnio (23°22’36” S, 45°04’07” W), municipality of Ubatuba, São Paulo | 450? | [27] as B. pitanga; [61] as B. pitanga; [65] as B. pitanga; [101] as Brachycephalus sp. 2 |
| B. pitanga | ephippium | Núcleo Santa Virgínia (23°19’23” S, 45°05’19” W), Parque Estadual da Serra do Mar, municipality of São Luis do Paraitinga, São Paulo | 980–1140 | [102] as B. pitanga; [103] as B. pitanga |
| B. pitanga | ephippium | SP 125—municipality of São Luís do Paraitinga (23°22’57” S, 45°09’59” W), São Paulo | 935–950 | [23] as B. pitanga |
| B. pitanga | ephippium | Trilha do Ipiranga 50 m from the Rio Ipiranga (23°20’39” S, 45°08’16” W), Núcleo Santa Virgínia, Parque Estadual da Serra do Mar, municipality of São Luis do Paraitinga, São Paulo | 900–960 | [27] as B. pitanga; [61] as B. pitanga; [64] as B. pitanga; [81] as B. pitanga; [102] as B. pitanga; [104] as B. pitanga |
| B. toby | ephippium | Morro do Corcovado (23°27’22” S, 45°11’53” W), Parque Estadual da Serra do Mar, municipality of Ubatuba, São Paulo | 750–1060 | This study, [27] as B. toby; [81] as B. toby; [82] as B. toby; [84] as B. toby; [98] as B. toby |
| B. vertebralis | ephippium | Morro Cuscuzeiro (23°17’51” S, 44°47’20” W), on the border of municipalities of Paraty, Rio de Janeiro, and Ubatuba, Sao Paulo | 760–1110 | This study, [27] as B. vertebralis; [81] as B. vertebralis; [84] as B. vertebralis |
| B. vertebralis | ephippium | Pedra Branca (23°10’38” S, 44°47’19” W), Serra da Bocaina, municipality of Parati, Rio de Janeiro | 630? | [27] as B. vertebralis; [30] as B. vertebralis; [58] as. B. vertebralis; [64] as B. vertebralis |
| B. actaeus | pernix | Braço do Norte (26°07′29″ S, 48°43′48″ W), municipality of Itapoá, Santa Catarina | 240 | [75] as B. actaeus |
| B. actaeus | pernix | Centro de Estudos e Pesquisas Ambientais da Univille (CEPA) (26°13’39” S; 48°41’31” W), Vila da Glória, Distrito do Saí, municipality of São Francisco do Sul, Santa Catarina | 120 | [75] as B. actaeus |
| B. actaeus | pernix | Estrada do Saí (26°12′06″ S; 48°41′37″ W), Distrito do Saí, municipality of São Francisco do Sul, Santa Catarina | 100 | [75] as B. actaeus |
| B. actaeus | pernix | Fazenda Morro Grande (26°17′47″ S; 48°37′10″ W), Morro Grande, Ilha de São Francisco, municipality of São Francisco do Sul, Santa Catarina | 60 | [75] as B. actaeus |
| B. actaeus | pernix | Fazenda Palmito Juriti (26°08′09″ S; 48°43′54″ W), municipality of Itapoá, Santa Catarina | 100–170 | [75] as B. actaeus |
| B. actaeus | pernix | Serra da Palha (26°17′50″ S; 48°40′28″ W), Laranjeiras, Ilha de São Francisco, municipality of São Francisco do Sul, Santa Catarina | 20–90 | [75] as B. actaeus |
| B. actaeus | pernix | Serra da Tiririca (26°07′42″ S, 48°44′32″ W), municipality of Itapoá, Santa Catarina | 170–530 | This study |
| B. albolineatus | pernix | Morro Azul (26°45′52″ S, 49°12′20″ W), on the border between the municipalities of Pomerode and Rio dos Cedros, Santa Catarina | 725–740 | This study |
| B. albolineatus | pernix | Morro Boa Vista (26°30’58” S, 49°03’14” W), on the border between the municipalities of Jaraguá do Sul and Massaranduba, Santa Catarina | 790–835 | [74] as B. albolineatus; [105] as B. albolineatus |
| B. albolineatus | pernix | Morro do Garrafão (26°30’58” S, 49°03’14” W), municipality of Corupá, Santa Catarina | 500–530 | [76] as B. albolineatus |
| B. albolineatus | pernix | Morro do Schmidt (26°39′55″ S, 49°12′55″ W), municipality of Pomerode, Santa Catarina | 810–870 | This study |
| B. auroguttatus | pernix | Pedra da Tartaruga (26°00’21”S, 48°55’25”W), municipality of Garuva, Santa Catarina | 1070–1100 | [23] as B. auroguttatus; [26] as B. auroguttatus; [28] as B. auroguttatus; [29] as B. auroguttatus; [73] without species identification |
| B. boticario | pernix | Morro do Cachorro (26°46’42” S, 49°01’57” W), boundary of the municipalities of Blumenau, Gaspar, and Luiz Alves, Santa Catarina | 685–795 | [23] as B. boticario; [26] as B. boticario; [28] as B. boticario; [29] as B. boticario; [73] without species identification |
| B. brunneus | pernix | Abrigo 1 (25°13’29” S, 48°51’17” W), municipality of Campina Grande do Sul, Paraná | 1440–1640 | This study, [28] as not identified; [29] as B. brunneus |
| B. brunneus | pernix | Camapuã (25°15’59” S, 48°50’16” W), Serra dos Órgãos, boundary of the municipalities of Campina Grande do Sul and Antonina, Paraná | 1595 | [27] as B. brunneus; [28] as B. brunneus]; [29] as B. brunneus; [73] without species identification; [106] as B. brunneus |
| B. brunneus | pernix | Caranguejeira (25°20’27” S, 48°54’31” W), Serra da Graciosa, municipality of Quatro Barras, Paraná | 1095–1110 | [23] as B. brunneus; [73] without species identification |
| B. brunneus | pernix | Caratuva (25°14’33” S, 48°50’04” W), Serra dos Órgãos, municipality of Campina Grande do Sul, Paraná | 1300–1770 | [27] as B. brunneus; [28] as B. brunneus; [29] as B. brunneus, [59] as B. brunneus; [64] as B. brunneus; [65] as B. brunneus; [66] as B. brunneus; including Pico Paraná; [73] without species identification; including Pico Paraná; [81] as B. brunneus; [106] as B. brunneus; [107] as B. brunneus |
| B. brunneus | pernix | Getúlio (25°14’18” S, 48°50’13” W), Serra dos Órgãos, municipality of Campina Grande do Sul, Paraná | 1310–1490 | [23] as B. brunneus; [27] as B. brunneus |
| B. brunneus | pernix | Mãe Catira (25°20’51” S, 48°54’25” W), Serra da Graciosa, municipality of Quatro Barras, Paraná | 1135–1405 | This study, [27] as Brachycephalus sp. nov. 2; [28] as not identified; [73] without species identification |
| B. coloratus | pernix | Estância Hidroclimática Recreio da Serra (25°27’14” S, 49°00’27” W), Serra da Baitaca, municipality of Piraquara, Paraná | 1145–1230 | [50] as B. coloratus |
| B. curupira | pernix | Morro do Canal (25°30’55” S, 48°58’56” W), municipality of Piraquara, Paraná | 1320 | This study, [23] as Brachycephalus sp. 4; [28] as not identified]; [73] without species identification |
| B. curupira | pernix | Morro do Vigia (25°30’33” S, 48°58’58” W), municipality of Piraquara, Paraná | 1250 | [23] as Brachycephalus sp. 4; [27] as Brachycephalus sp. nov. 3; [28] as not identified; [29] as B. curupira; [73] without species identification |
| B. curupira | pernix | Serra do Salto (25°42’07” S, 49°03’44” W), Malhada District, municipality of São José dos Pinhais, Paraná | 1095–1160 | [23] as Brachycephalus sp. 6; [27] as Brachycephalus sp. 2; [28] as not identified; [29] as B. curupira; [50] as B. curupira, [73] without species identification |
| B. ferruginus | pernix | Olimpo (25°27’03” S, 48°54’59” W), Serra do Marumbi, municipality of Morretes, Paraná | 965–1470 | [27] as B. ferruginus; [28] as B. ferruginus; [29] as B. ferruginus, [60] as B. ferruginus; [64] as B. ferruginus; [66] as B. ferruginus; [73] without species identification; [81] as B. ferruginus |
| B. fuscolineatus | pernix | Morro Braço da Onça (26°44’58” S, 48°55’41” W), municipality of Luiz Alves, Santa Catarina | 525–530 | [24] as B. fuscolineatus |
| B. fuscolineatus | pernix | Morro do Baú (26°47’58” S, 48°55’47” W), municipality of Ilhota, Santa Catarina | 640–790 | [26] as B. fuscolineatus; [27] as Brachycephalus sp. nov. 9; [28] as B. fuscolineatus; [29] as B. fuscolineatus; [73] without species identification |
| B. izecksohni | pernix | Torre da Prata, Serra da Prata (25°37’25” S, 48°41’31” W), boundary of the municipalities of Morretes, Paranaguá, and Guaratuba, Paraná | 980–1340 | [27] as B. izecksohni; [28] as B. izecksohni; [29] as B. izecksohni; [59] as B. izecksohni; [64] as B. izecksohni; [66] as B. izecksohni; [73] without species identification; [81] as B. izecksohni |
| B. leopardus | pernix | Morro dos Perdidos (25°53’22” S, 48°57’22” W), municipality of Guaratuba, Paraná | 1340–1420 | [26] as B. leopardus; [27] as Brachycephalus sp. nov. 4; [28] as B. leopardus; [73] without species identification |
| B. leopardus | pernix | Serra do Araçatuba (25°54’07” S, 48°59’47” W), municipality of Tijucas do Sul, Paraná | 1640–1645 | [26] as B. leopardus; [27] as Brachycephalus sp. nov. 4; [28] as B. leopardus; [73] without species identification |
| B. mariaeterezae | pernix | Reserva Particular do Patrimônio Natural Caetezal, top of the Serra Queimada (26°06’51” S, 49°03’45” W), municipality of Joinville, Santa Catarina | 1265–1270 | [26] as B. mariaeterezae; [27] as Brachycephalus sp. nov. 6; [28] as B. mariaeterezae; [29] as B. mariaeterezae; [73] without species identification |
| B. mirissimus | pernix | Morro Santo Anjo (26°37’41” S, 48°55’50” W), municipality of Massaranduba, Santa Catarina | 470–540 | [25] as B. mirissimus |
| B. olivaceus | pernix | Base of the Serra Queimada (26°04’57” S, 49°03’59” W), municipality of Joinville, Santa Catarina | 985 | [17] as Brachycephalus sp. nov. 7; [26] as B. olivaceus |
| B. olivaceus | pernix | Castelo dos Bugres (second position: 26°13’59”S, 49°03’13”W), municipality of Joinville, Santa Catarina | 800–835 | [26] as B. olivaceus; [27] as Brachycephalus sp. nov. 7; [28] as B. olivaceus; [73] without species identification; [108] as B. olivaceus |
| B. olivaceus | pernix | Morro do Boi (26°24’42” S, 49°12’59” W), municipality of Corupá, Santa Catarina | 650–920 | [23] as B. olivaceus; [27] as Brachycephalus sp. 3; [29] as B. olivaceus |
| B. olivaceus | pernix | Pico Jurapê (26°16′27″ S, 49°00′13″ W), municipality of Joinville, Santa Catarina | 650–780 | This study |
| B. pernix | pernix | Anhangava (25°23’19” S, 49°00’15” W), Serra da Baitaca, municipality of Quatro Barras, Paraná | 1135–1405 | [27] as B. pernix; [28] as B. pernix; [29] as B. pernix; [62] as B. pernix; [64] as B. pernix; [65] as B. pernix; [66] as B. pernix; [73] without species identification; [81] as B. pernix; [97] as B. pernix; [101] as B. pernix; [109] as B. pernix; [110] as B. pernix; [111] as B. pernix |
| B. pombali | pernix | Morro dos Padres (25°36’40” S, 48°51’22” W), Serra da Igreja, municipality of Morretes, Paraná | 1060–1300 | [27] as B. pombali; [28] as B. pombali; [29] as B. pombali; [60] as B. pombali; [64] as B. pombali; [73] without species identification; [81] as B. pombali |
| B. pombali | pernix | trail to Morro dos Padres (25°35’58” S, 48°51’57” W), municipality of Morretes, Paraná | 845–1060 | [27] as B. pombali |
| B. quiririensis | pernix | Serra do Quiriri (26°01’17” S, 48°59’47” W), municipality of Campo Alegre, Santa Catarina | 1240–1270 | [23] as B. quiririensis; [27] as Brachycephalus sp. nov. 5; [28] as B. quiririensis; [29] as B. quiririensis; [73] without species identification; [112] as B. quiririensis |
| B. quiririensis | pernix | Serra do Quiriri (first position: 26°01’42” S, 48°57’11” W; second position: 26°01’32” S, 48°58’24” W), municipality of Garuva, Santa Catarina | 1320–1380 | [27] as Brachycephalus sp. nov. 5; [108] as B. quiririensis |
| B. tridactylus | pernix | Serra do Morato (25°08’09” S, 48°17’59” W), Reserva Natural Salto Morato, municipality of Guaraqueçaba, Paraná | 805–910 | [23] as B. tridactylus; [28] as B. tridactylus; [113] as B. tridactylus; [114] as B. tridactylus |
| B. verrucosus | pernix | Morro da Tromba (26°12’44” S, 48°57’29” W), municipality of Joinville, Santa Catarina | 455–945 | [23] as B. verrucosus; [26] as B. verrucosus; [27], as Brachycephalus sp. nov. 8; [28] as B. verrucosus; [29] as B. verrucosus; [73] without species identification |
| B. atelopoide | ? | Piquete, São Paulo | ? | [30] as B. atelopoide; [83] as B. ephippium atelopoide] |
| Brachycephalus sp. (cf. B. sulfuratus) | didactylus | Alto Quiriri (26°05’34” S, 48°59’41” W), municipality of Garuva, Santa Catarina | 240 | [23] as Brachycephalus sp. 1; [27] as Brachycephalus sp. nov. 1 |
| Brachycephalus sp. (cf. B. sulfuratus) | didactylus | Colônia Castelhanos (25°47’58” S, 48°54’40” W), municipality of Guaratuba, Paraná | 290 | [23] as Brachycephalus sp. 1; [27] as Brachycephalus sp. nov. 1; [72] as B. sulfuratus; [115] as Brachycephalus aff. hermogenesi]; [116] as B. hermogenesi |
| Brachycephalus sp. (cf. B. sulfuratus) | didactylus | Dona Francisca (26°09’52” S, 48°59’23” W), municipality of Joinville, Santa Catarina | 150 | [23] as Brachycephalus sp. 1; [27] as Brachycephalus sp. nov. 1 |
| Brachycephalus sp. (B. sulfuratus or B. hermogenesi) | didactylus | Estação Ecológica Juréia-Itatins (c. 24°27’ S, 47°24’ W), municipality of Iguape, São Paulo | ? | [63] as B. hermogenesi |
| Brachycephalus sp. (cf. B. sulfuratus) | didactylus | Estrada do Rio do Júlio (26°17’02” S, 49°06’08” W), municipality of Joinville, Santa Catarina | 650 | [23] as Brachycephalus sp. 1; [117] as Brachycephalus sp. |
| Brachycephalus sp. (cf. B. sulfuratus) | didactylus | Fazenda Pico Paraná (25°13’29” S, 48°51’17” W), municipality of Campina Grande do Sul, Paraná | 1050–1085 | [23] as Brachycephalus sp. 1; [27] as Brachycephalus sp. nov. 1 |
| Brachycephalus sp. (cf. B. sulfuratus) | didactylus | Fazenda Primavera (24°53’08” S, 48°45’51” W), municipality of Tunas do Paraná, Paraná | 1060 | [23] as Brachycephalus sp. 1; [27] as Brachycephalus sp. nov. 1 |
| Brachycephalus sp. (B. sulfuratus or B. hermogenesi) | didactylus | Municipality of Ibiúna (c. 23°39’ S, 47°13’ W), São Paulo | ? | [72] as B. hermogenesi |
| Brachycephalus sp. (B. sulfuratus or B. hermogenesi) | didactylus | Municipality of Juquitiba (c. 23°56’ S, 47°04’ W), São Paulo | ? | [63] as B. hermogenesi; [72] as B. hermogenesi |
| Brachycephalus sp. (cf. B. hermogenesi) | didactylus | Municipality of Paraty (c. 23°13’07” S, 44°43’15” W), Rio de Janeiro | ? | [18] as B. hermogenesi |
| Brachycephalus sp. (B. sulfuratus or B. hermogenesi) | didactylus | Municipality of Peruíbe (24°18’ S, 46°59’ W), São Paulo | ? | [72] as B. hermogenesi |
| Brachycephalus sp. (B. sulfuratus or B. hermogenesi) | didactylus | Municipality of Piedade (c. 23°54’S, 47°25’ W), São Paulo | ? | [81] as B. hermogenesi; [118] as B. hermogenesi |
| Brachycephalus sp. (B. sulfuratus or B. hermogenesi) | didactylus | Municipality of Registro (c. 24°30’ S, 47°51’ W), São Paulo | ? | [72] as B. hermogenesi |
| Brachycephalus sp. (B. sulfuratus or B. hermogenesi) | didactylus | Municipality of Ribeirão Grande (c. 24°06’ S, 48°22’ W), São Paulo | ? | [63] as B. hermogenesi |
| Brachycephalus sp. (B. sulfuratus or B. hermogenesi) | didactylus | Municipality of Tapiraí (c. 23°57’55” S, 47°30’19” W), São Paulo | 870 | [63] as B. hermogenesi; [118] as B. hermogenesi |
| Brachycephalus sp. (B. sulfuratus or B. hermogenesi) | didactylus | Parque Estadual de Jacupiranga (c. 24°38’ S, 48°24’ W), municipality of Eldorado, São Paulo | ? | [72] as B. hermogenesi |
| Brachycephalus sp. (B. hermogenesi or B. sulfuratus) | didactylus | Parque Natural Municipal Nascentes de Paranapiacaba (23°46’10” S, 46°17’36” W), municipality of Santo André, São Paulo | 840 | [119] as B. hermogenesi |
| Brachycephalus sp. (cf. B. sulfuratus) | didactylus | Pico Agudinho (25°36’24” S, 48°43’33” W), Serra da Prata, municipality of Morretes, Paraná | 385 | [23] as Brachycephalus sp. 1; [27] as Brachycephalus sp. nov. 1 |
| Brachycephalus sp. (B. sulfuratus or B. hermogenesi) | didactylus | Reserva Betary (24°33’08” S, 48°40’49” W), municipality of Iporanga, São Paulo | 190 | This study |
| Brachycephalus sp. (B. hermogenesi or B. sulfuratus) | didactylus | Reserva Biológica do Alto da Serra de Paranapiacaba (23°46’40” S, 46°18’45” W), municipality of Santo André, São Paulo | 800–850 | [23] as B. hermogenesi; [63] as B. hermogenesi; [119] as B. hermogenesi |
| Brachycephalus sp. (B. sulfuratus or B. hermogenesi) | didactylus | Reserva Florestal de Morro Grande (23°42’08” S, 46°58’22” W), municipality of Cotia, São Paulo | 990? | [23] as B. hermogenesi, [63] as B. hermogenesi; [72] as B. hermogenesi; [94] as B. hermogenesi |
| Brachycephalus sp. (cf. B. sulfuratus) | didactylus | Sítio Ananias (25°47’08” S, 48°43’03” W), municipality of Guaratuba, Paraná | 25 | [23] as Brachycephalus sp. 1; [27] as Brachycephalus sp. nov. 1 |
| Brachycephalus sp. | ephippium | Paranapiacaba (23°46’30” S, 46°17’57” W), municipality of Santo André, São Paulo | 825 | [27] as Brachycephalus sp. 1; [66] as B. ephippium |
| Brachycephalus sp. | ephippium | Parque Natural Municipal Nascentes de Paranapiacaba (23°46’10” S, 46°17’36” W), municipality of Santo André, São Paulo | 800–1164? | [120] as Brachycephalus sp. |
| Brachycephalus sp. | ephippium | Penísula do Bororé (23°47’11” S, 46°38’45” W), Represa Billings, Grajaú District, municipality of São Paulo, São Paulo | 780 | [27] as Brachycephalus nodoterga; [99] as another species than B. nodoterga of [27] |
| Brachycephalus sp. | ephippium | Reserva Biológica do Alto da Serra de Paranapiacaba (23°46’40” S, 46°18’45” W), municipality of Santo André, São Paulo | 800 | [27] as Brachycephalus sp. 1 |
| Brachycephalus sp. | ephippium | Theodoro de Oliveira (first position: 22°22’11” S, 42°33’25” W), Parque Estadual dos Três Picos, municipality of Nova Friburgo, Rio de Janeiro | 1100–1200 | [67] as Brachycephalus sp.; [95] as Brachycephalus sp. nov. |
| Brachycephalus sp. | pernix | Pedra Branca do Araraquara (25°56’00” S, 48°52’50” W), Serra do Araraquara, municipality of Guaratuba, Paraná | 1000 | [23] as Brachycephalus sp. 5 |
| Brachycephalus sp. | pernix | Pico Paraná (25°15′10″ S, 48°48′32″ W), Serra dos Órgãos, municipality of Antonina, Paraná | 1880 | This study |
| Brachycephalus sp. | pernix | Serra Canasvieiras (25°36’58” S, 48°46’59” W), boundary of the municipalities of Guaratuba and Morretes, Paraná | 1080 | [23] as Brachycephalus sp. 5; [25] as B. sp. Canasvieiras; [28] as not identified; [73] without species identification |
| Brachycephalus sp. | pernix | Tupipiá (25°15’13” S, 48°48’20” W), Serra dos Órgãos, municipality of Antonina, Paraná | 1560 | This study, [27] as B. brunneus; [28] as B. brunneus; [29] as B. sp. Tupipiá, [73] without species identification |
| Brachycephalus sp. (cf. B. darkside juvenile) | ? | Parque Estadual da Serra do Brigadeiro (cf. 20°43′16″ S, 42°29′05″ W), municipality of Araponga, Minas Gerais | 1330? | [85] as Brachycephalus cf. didactylus |
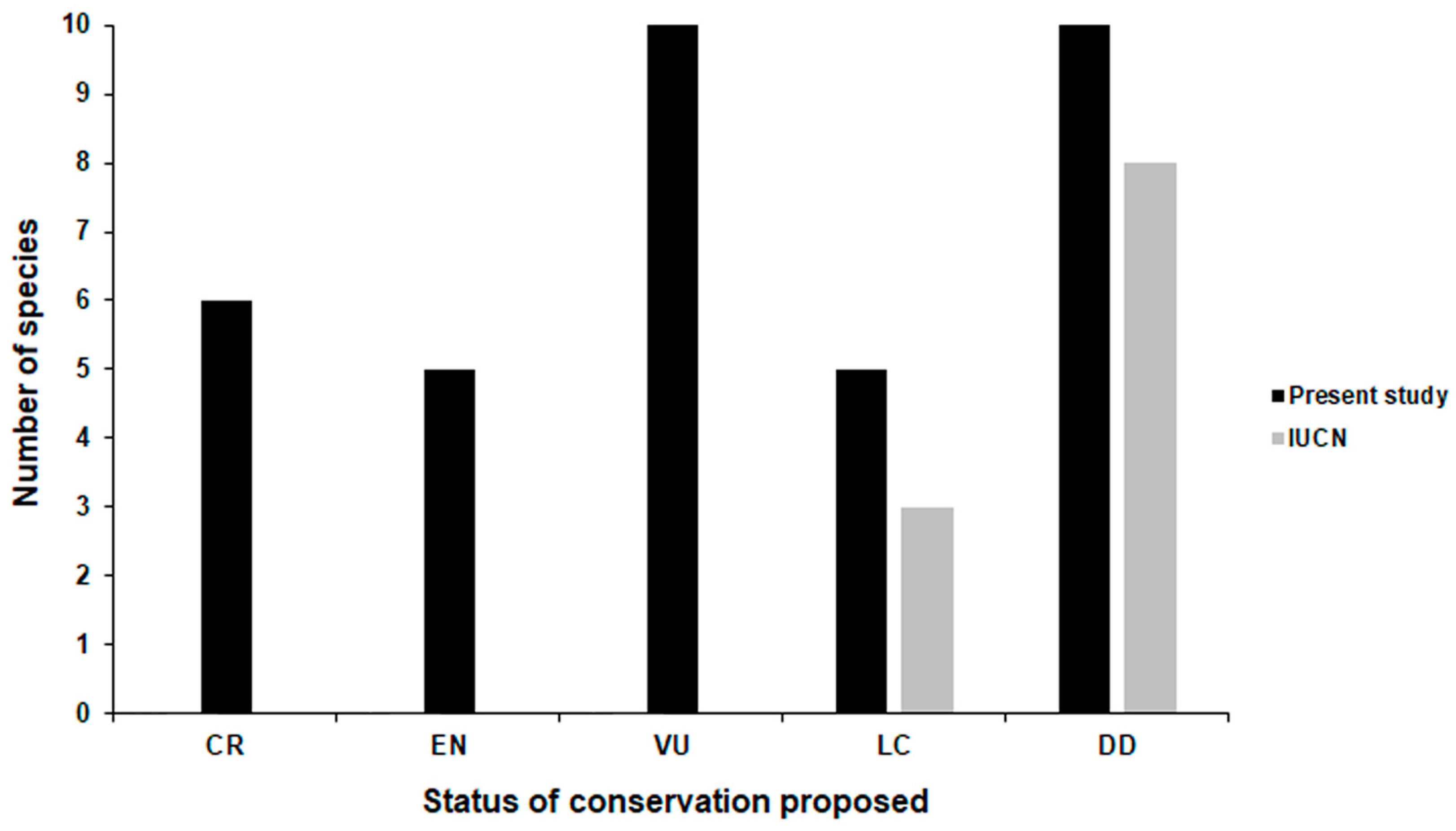
| Species 1 | Localities 1 | Altitudinal Range (m a.s.l.) 1,2 | Evaluation of EO (ha) | Flow Chart Pathway 3 | Population | Conservation Status—Criteria | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Previous 2 | This Study 2 | Locations 2 | Individuals 2, 3 | IUCN | MMA | Others | This Study | ||||
| B. didactylus group | |||||||||||
| B. didactylus | 8 | 35–1110 | --- | 702,983.4 | 5 | 4 | 79,655,049 | LC [41] | --- | --- | VU - B1ab(i,iii) |
| B. hermogenesi | 11 | 0–1090 | 567,589.9 [23] | 143,325.0 | 5 | 1 | ? | LC [33] | --- | --- | VU - B1ab(i,iii) |
| B. pulex | 1 | 800–930 | 488.2 [23] | 482.3 | 1 | 1 | ? | --- | --- | --- | VU - D2 |
| B. sulfuratus | 26 | 40–1205 | 778,458.4 [23] | 3,021,786.1 | 5 | 1 | 302,178,610 | --- | --- | --- | LC |
| B. ephippium group | |||||||||||
| B. alipioi | 3 | 1070–1100 | 38,950.0 [47], 27,930.0 [43] | 1,706.1 | 3 | 1 | ? | DD [35] | NT [43] | --- | CR - B1ab(i,iii) |
| B. bufonoides | 1 | ? | --- | ? | 4 | ? | ? | --- | --- | --- | DD |
| B. crispus | 1 | 800–1190 | ? | ? | 2 | 1 | ? | --- | --- | --- | DD |
| B. darkside | 2 | 1265–1500 | -- | 5,700.8 | 1 | 1 | ? | --- | --- | --- | CR - B1ab(i,iii) |
| B. ephippium | 31 | 200–1250 | ? | 1,792,535.1 | 5 | 6 | 13,336,461 | LC [38] | --- | --- | VU - B1ab(i,iii) |
| B. garbeanus | 7 | 1130–1900 | 12,268.0 [23] | 6,426.5 | 5 | 2 | ? | --- | --- | --- | EN - B1ab(i,iii)+2ab(ii,iii) |
| B. guarani | 1 | 500–900 | ? | ? | 2 | 1 | ? | --- | --- | --- | DD |
| B. margaritatus | 6 | 600–980 | 18,272.9 [23] | 10,710.5 | 5 | 2 | ? | --- | --- | --- | EN - B1ab(i,iii) |
| B. nodoterga | 5 | 700–900 | 9,690.0 [47], 108,280.0 [42] | 28.458.1 | 5 | 3 | ? | DD [31] | DD [42] | --- | VU - B1ab(i,iii) |
| B. pitanga | 4 | 900–1140 | 2,377.1 [23] | 2,245.1 | 5 | 1 | 29,157,136 | --- | --- | --- | LC |
| B. toby | 1 | 750–1060 | ? | ? | 2 | 1 | ? | --- | --- | --- | DD |
| B. vertebralis | 2 | 760–1110 | 161,990.0 [47], 18,580.0 [44] | ? | 2 | 2 | ? | DD [37] | DD [44] | --- | DD |
| B. pernix group | |||||||||||
| B. actaeus | 7 | 20–530 | --- | 15,841.6 | 6 | 2 | ? | --- | --- | --- | EN - B1ab(i,iii)+2ab(ii,iii) |
| B. albolineatus | 4 | 500–835 | 34.4 [74] | 2,784.4 | 5 | 12 | 1,076,087 | --- | --- | DD [74] | VU - B1ab(i,iii) |
| B. auroguttatus | 1 | 1070–1100 | --- | ? | 3 | 1 | --- | --- | --- | DD | |
| B. boticario | 1 | 685–795 | 11.1 [23] | 38.8 | 1 | 1 | ? | --- | --- | --- | CR - B1ab(i,iii)+2ab(ii,iii) |
| B. brunneus | 6 | 1095–1770 | 1,100.0 [47], 5,687.1 [23] | 5,385.6 | 6 | 2 | ? | DD [39] | --- | --- | LC |
| B. coloratus | 1 | 1145–1230 | --- | 37.4 | 1 | 1 | --- | --- | DD [50] | VU - D2 | |
| B. curupira | 3 | 1095–1320 | 2,211.54 [23] | 4,751.4 | 6 | 2 | 21,117,312 | --- | --- | DD [50] | LC |
| B. ferruginus | 1 | 965–1,470 | 38,950.0 [47], 5,475.5 [23] | 5,994.3 | 1 | 1 | ? | DD [34] | --- | --- | LC |
| B. fuscolineatus | 2 | 525–790 | 23.63 [23], 23.8 [24] | 23.8 | 1 | 2 | 119,000 | --- | --- | --- | CR - B1ab(i,iii)+2ab(ii,iii) |
| B. izecksohni | 1 | 980–1340 | 1,100.0 [47], 350.4 [23] | 378.3 | 1 | 1 | ? | DD [40] | --- | --- | VU - D2 |
| B. leopardus | 2 | 1340–1645 | 176.7 [23] | 363.1 | 1 | 3 | ? | --- | --- | --- | EN - B1ab(i,iii)+2ab(ii,iii) |
| B. mariaeterezae | 1 | 1265–1270 | --- | ? | 3 | 1 | ? | --- | --- | --- | DD |
| B. mirissimus | 1 | 470–540 | 56.8 [25] | 56.8 | 1 | 1 | 78,344 | --- | --- | CR [25] | CR - B1ab(i,iii)+2ab(ii,iii) |
| B. olivaceus | 4 | 650–985 | 12,531.6 [23] | 18,850.1 | 5 | 2 | ? | --- | --- | --- | EN - B1ab(i,iii)+2ab(ii,iii) |
| B. pernix | 1 | 1135–1405 | 1,950.0 [47], 432.1 [23], 400 [45] | 389.4 | 1 | 1 | ? | DD [32] | CR - B1ab(iii)+2ab(iii) [45] | --- | VU - D2 |
| B. pombali | 2 | 845–1300 | 31,300.0 [47] | ? | 2 | 1 | ? | DD [36] | --- | --- | DD |
| B.quiririensis | 2 | 1240–1380 | 1339.0 [23] | 629.0 | 1 | 1 | ? | --- | --- | --- | CR - B1ab(i,iii)+2ab(ii,iii) |
| B. tridactylus | 1 | 805–910 | 41.4 [23] | 41.4 | 1 | 1 | ? | --- | --- | --- | VU - D2 |
| B. verrucosus | 1 | 455–945 | --- | ? | 2 | 1 | ? | --- | --- | --- | DD |
| Incertae sedis | |||||||||||
| B. atelopoide | 1 | ? | --- | ? | 4 | ? | ? | --- | --- | --- | DD |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bornschein, M.R.; Pie, M.R.; Teixeira, L. Conservation Status of Brachycephalus Toadlets (Anura: Brachycephalidae) from the Brazilian Atlantic Rainforest. Diversity 2019, 11, 150. https://doi.org/10.3390/d11090150
Bornschein MR, Pie MR, Teixeira L. Conservation Status of Brachycephalus Toadlets (Anura: Brachycephalidae) from the Brazilian Atlantic Rainforest. Diversity. 2019; 11(9):150. https://doi.org/10.3390/d11090150
Chicago/Turabian StyleBornschein, Marcos R., Marcio R. Pie, and Larissa Teixeira. 2019. "Conservation Status of Brachycephalus Toadlets (Anura: Brachycephalidae) from the Brazilian Atlantic Rainforest" Diversity 11, no. 9: 150. https://doi.org/10.3390/d11090150
APA StyleBornschein, M. R., Pie, M. R., & Teixeira, L. (2019). Conservation Status of Brachycephalus Toadlets (Anura: Brachycephalidae) from the Brazilian Atlantic Rainforest. Diversity, 11(9), 150. https://doi.org/10.3390/d11090150




