Ecological and Conservation Correlates of Rarity in New World Pitvipers
Abstract
1. Introduction
2. Materials and Methods
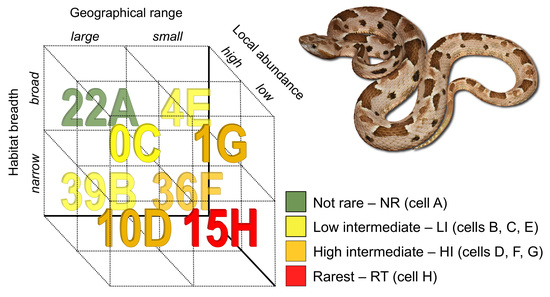
2.1. Rarity Patterns
2.2. Predicting Rarity
2.3. Rarity and Other Assessments of Extinction Risk or Prioritization
3. Results
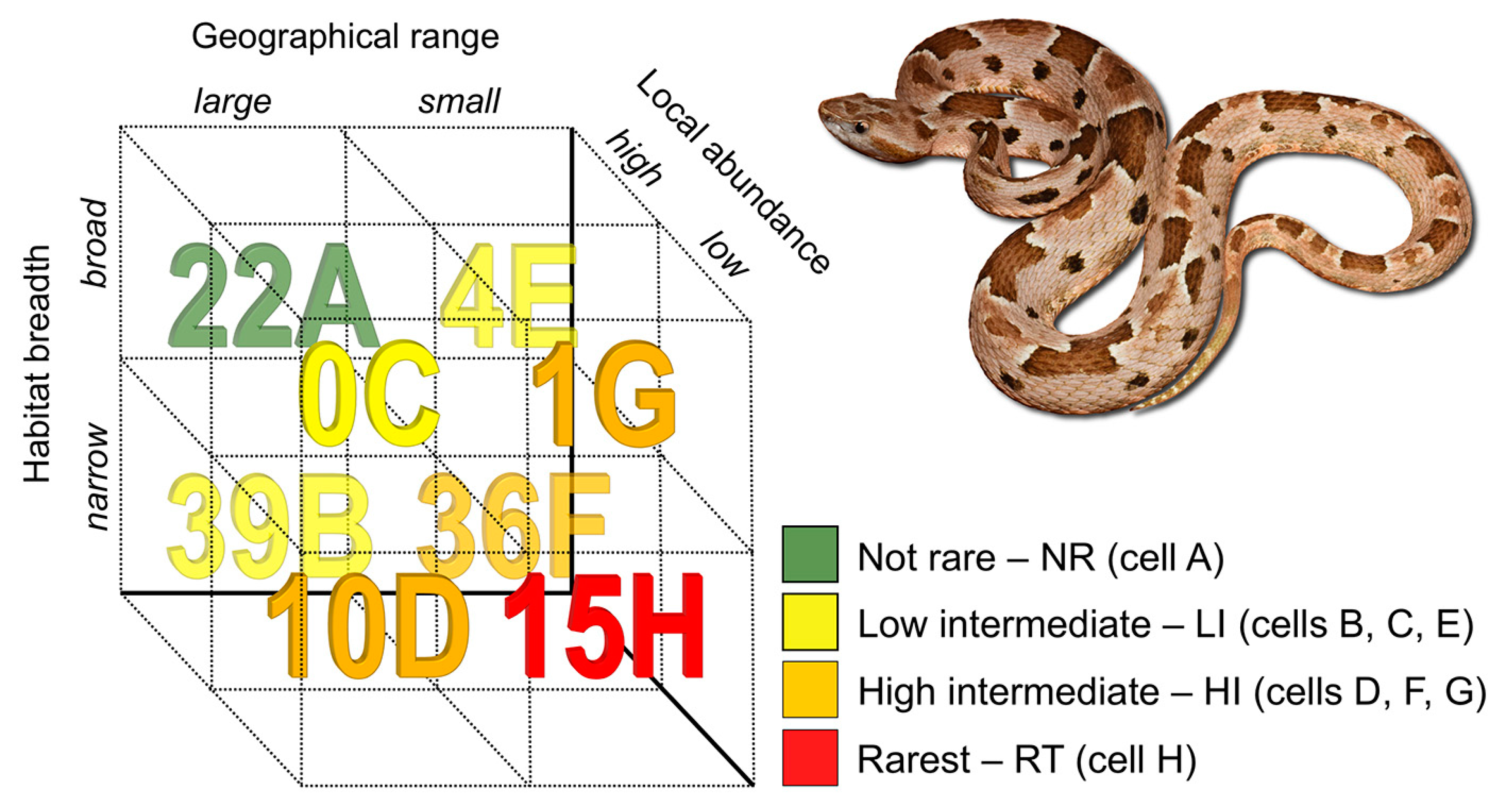
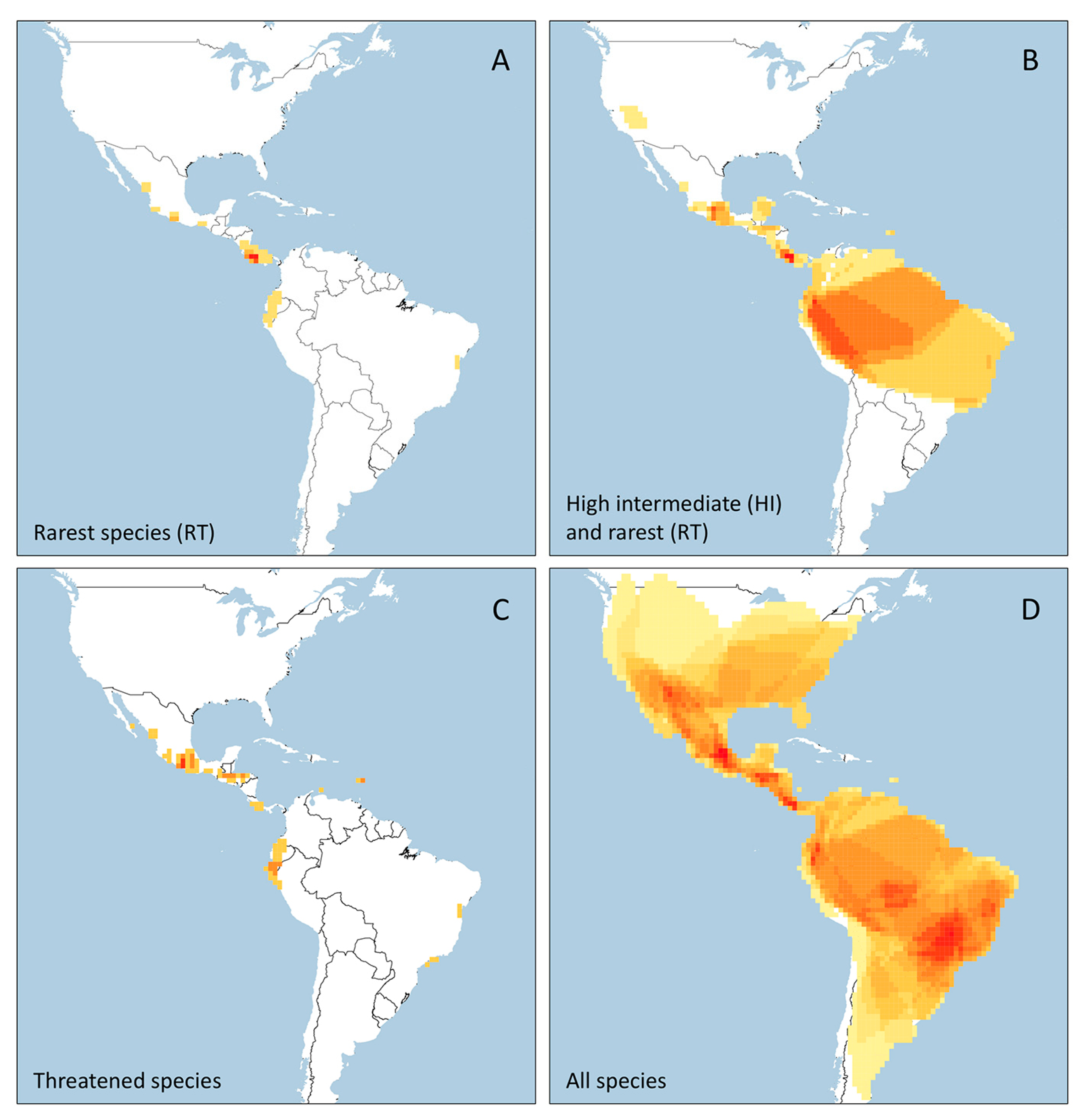
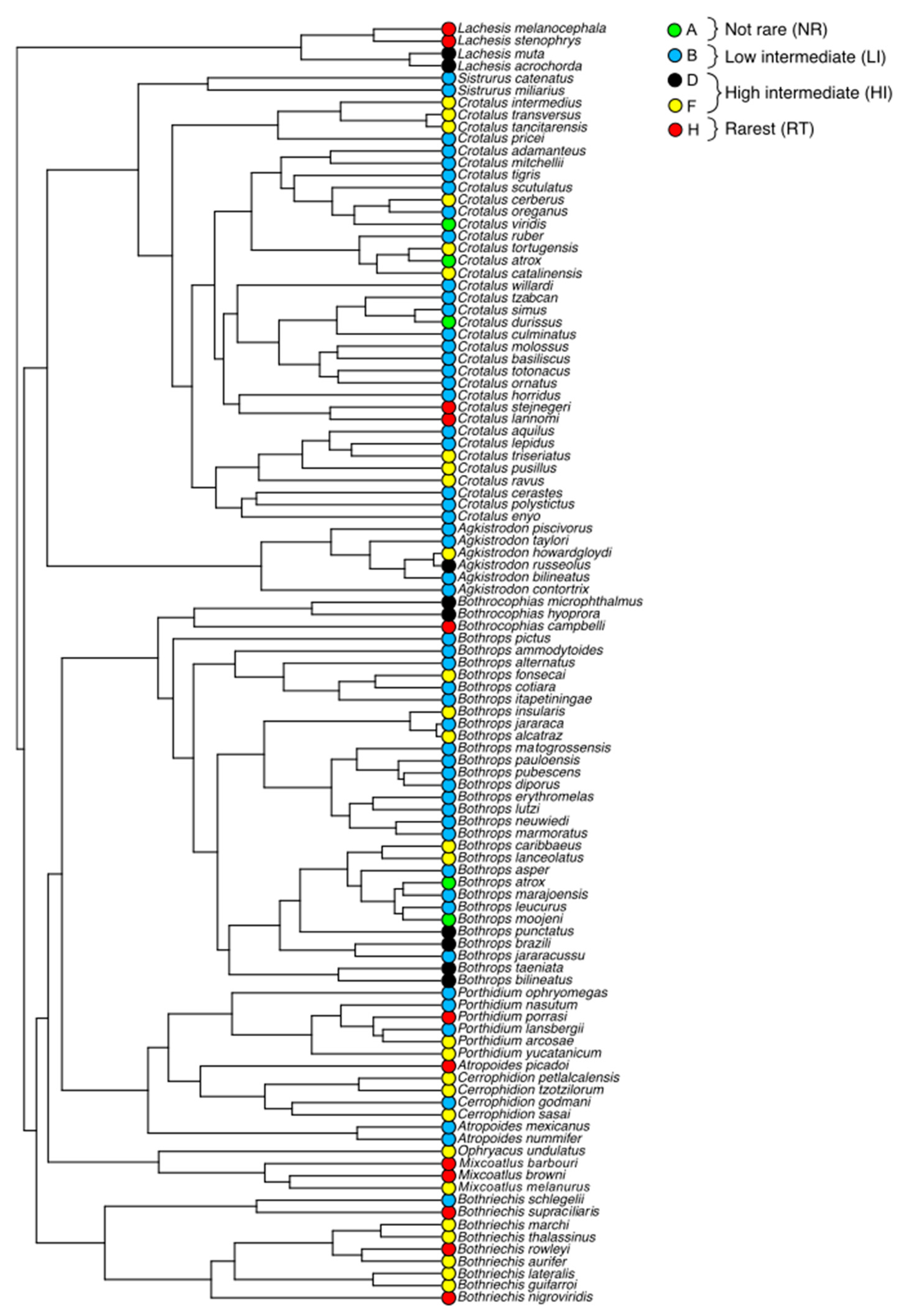
3.1. Rarity Patterns
3.2. Predicting Rarity
3.3. Rarity and Other Assessments of Extinction Risk or Prioritization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fisher, R.A.; Corbet, A.S.; Williams, C.B. The relation between the number of species and the number of individuals in a random sample from an animal population. J. Anim. Ecol. 1943, 12, 42–58. [Google Scholar] [CrossRef]
- Hubbell, S.P. A Unified Theory of Biodiversity and Biogeography; Princeton University Press: Princeton, NJ, USA, 2001; pp. 1–392. [Google Scholar]
- Raphael, M.G.; Marcot, B.G. Introduction. In Conservation of Rare or Little-Known Species: Biological, Social and Economic Considerations; Raphael, M.T., Moline, R., Eds.; Island Press: Washington, DC, USA, 2007; pp. 1–16. [Google Scholar]
- Pimm, S.L.; Jones, H.L.; Diamond, J. On the risk of extinction. Am. Nat. 1988, 132, 757–785. [Google Scholar] [CrossRef]
- Johnson, C.N. Species extinction and the relationship between distribution and abundance. Nature 1998, 394, 272–274. [Google Scholar] [CrossRef]
- Flather, C.H.; Sieg, C.H. Species Rarity: Definition, Causes, and Classification. In Conservation of Rare or Little-Known Species: Biological, Social and Economic Considerations; Raphael, M.T., Moline, R., Eds.; Island Press: Washington, DC, USA, 2007; pp. 40–66. [Google Scholar]
- Gaston, K.J. Rarity; Chapman and Hall Press: London, UK, 1994; pp. 1–205. [Google Scholar]
- Gaston, K.J. What is rarity? In The Biology of Rarity; Kunin, W.E., Gaston, K.J., Eds.; Population and Community Biology Series; Springer: Dordrecht, The Netherlands, 1997; Volume 17, pp. 1–21. [Google Scholar]
- Mace, G.M.; Collar, N.J.; Gaston, K.J.; Hilton-Taylor, C.; Akçakaya, R.; Leader-Williams, N.; Milner-Gulland, E.J.; Stuart, S.N. Quantification of extinction risk: IUCN’s System for Classifying Threatened Species. Conserv. Biol. 2008, 22, 1424–1442. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, D. Seven Forms of Rarity. In The Biological Aspects of Rare Plant Conservation; Synge, H., Ed.; John Wiley & Sons: Chichester, UK, 1981; pp. 205–217. [Google Scholar]
- Gaston, K.J.; Blackburn, T.M. Evolutionary age and risk of extinction in the global avifauna. Evol. Ecol. 1997, 11, 557–565. [Google Scholar] [CrossRef]
- Pitman, N.C.A.; Terborgh, J.; Silman, M.R.; Nuñez, P.V. Tree species distributions in an upper Amazonian forest. Ecology 1999, 80, 2651–2661. [Google Scholar] [CrossRef]
- Ricklefs, R.E. Rarity and diversity in Amazonian forest trees. Trends Ecol. Evol. 2000, 15, 83–84. [Google Scholar] [CrossRef]
- Yu, J.; Dobson, F.S. Seven forms of rarity in mammals. J. Biogeogr. 2000, 27, 131–139. [Google Scholar] [CrossRef]
- Casazza, G.; Barberis, G.; Minuto, L. Ecological characteristics and rarity of endemic plants of the Italian Maritime Alps. Biol. Conserv. 2005, 123, 361–371. [Google Scholar] [CrossRef]
- Esparza-Olguín, L.; Valverde, T.; Mandujano, M.C. Comparative demographic analysis of three Neobuxbaumia species (Cactaceae) with differing degree of rarity. Popul. Ecol. 2005, 47, 229–245. [Google Scholar] [CrossRef]
- Söderström, L.; Séneca, A.; Santos, M. Rarity patterns in members of the Lophoziaceae/Scapaniaceae complex occurring North of the Tropics—Implications for conservation. Biol. Conserv. 2007, 135, 352–359. [Google Scholar] [CrossRef]
- Espeland, E.K.; Emam, T.M. The value of structuring rarity: The seven types and links to reproductive ecology. Biodivers. Conserv. 2011, 20, 963–985. [Google Scholar] [CrossRef]
- Harnik, P.G.; Simpson, C.; Payne, J.L. Long-term differences in extinction risk among the seven forms of rarity. Proc. R. Soc. Lond B Biol. Sci. 2012, 279, 4969–4976. [Google Scholar] [CrossRef] [PubMed]
- Anacker, B.L.; Gogol-Prokurat, M.; Leidholm, K.; Schoenig, S. Climate change vulnerability assessment of rare plants in California. Madroño 2013, 60, 193–211. [Google Scholar] [CrossRef]
- Bennett, J.; Vellend, M.; Lilley, P.; Cornwell, W.; Arcese, P. Abundance, rarity and invasion debt among exotic species in a patchy ecosystem. Biol. Invasions 2013, 15, 707–716. [Google Scholar] [CrossRef]
- Toledo, L.F.; Becker, C.G.; Haddad, C.F.B.; Zamudio, K.R. Rarity as indicator of endangerment in neotropical frogs. Biol. Conserv. 2014, 179, 54–62. [Google Scholar] [CrossRef]
- Andelman, S.J.; Groves, C.; Regan, H.M. A review of protocols for selecting species at risk in the context of US Forest Service viability assessments. Acta Oecol. 2004, 26, 75–83. [Google Scholar] [CrossRef]
- Hanski, I. Dynamics of regional distribution: The core and satellite species hypothesis. Oikos 1982, 38, 210–221. [Google Scholar] [CrossRef]
- Giam, X.; Olden, J.D. Drivers and interrelationships among multiple dimensions of rarity for freshwater fishes. Ecography 2018, 41, 331–344. [Google Scholar] [CrossRef]
- Loza, M.I.; Jiménez, I.; Jorgenses, P.M.; Arellano, G.; Macía, M.J.; Torrez, V.W.; Ricklefs, R.E. Phylogenetic patterns of rarity in a regional species pool of tropical woody plants. Glob. Ecol. Biogeogr. 2017, 26, 1043–1054. [Google Scholar] [CrossRef]
- Cofre, H.L.; Böhning-Gaese, K.; Marquet, P.A. Rarity in Chilean forest birds: Which ecological and life-history traits matter? Divers. Distrib. 2007, 13, 203–212. [Google Scholar] [CrossRef]
- Uetz, P. (Ed.) The Reptile Database. Available online: http://www.reptile-database.org (accessed on 1 September 2015).
- Campbell, J.A.; Lamar, W.W. The Venomous Reptiles of the Western Hemisphere; Cornell University Press: Ithaca, NY, USA, 2004; pp. 1–528. [Google Scholar]
- Wüster, W.; Peppin, L.; Pook, C.E.; Walker, D.E. A nesting of vipers: Phylogeny and historical biogeography of the Viperidae (Squamata: Serpentes). Mol. Phylogenetics Evol. 2008, 49, 445–459. [Google Scholar] [CrossRef]
- Alencar, L.R.V.; Quental, T.B.; Grazziotin, F.G.; Alfaro, M.L.; Martins, M.; Venzon, M.; Zaher, H. Diversification in vipers: Phylogenetic relationships, time of divergence and shifts in speciation rates. Mol. Phylogenetics Evol. 2016, 105, 50–62. [Google Scholar] [CrossRef]
- Alencar, L.R.V.; Martins, M.; Greene, H.W. Evolutionary History of Vipers. In Encyclopedia of Life Sciences; John Wiley & Sons Ltd.: Chichester, UK, 2018. [Google Scholar]
- Castoe, T.A.; Parkinson, C.L. Bayesian mixed models and the phylogeny of pitvipers (Viperidae: Serpentes). Mol. Phylogenetics Evol. 2006, 39, 91–110. [Google Scholar] [CrossRef]
- Swaroop, S.; Grab, B. Snakebite mortality in the world. Bull. World Health Organ. 1954, 10, 35–76. [Google Scholar]
- Chippaux, J.P. Snake-bites: Appraisal of the global situation. Bull. World Health Organ. 1998, 75, 515–524. [Google Scholar]
- Galán, J.A.; Sánches, E.E.; Rodríguez-Acosta, A.; Pérez, J.C. Neutralization of venoms from two Southern Pacific Rattlesnakes (Crotalus helleri) with commercial antivenoms and endothermic animal sera. Toxicon 2004, 43, 791–799. [Google Scholar] [CrossRef]
- McCue, M.D. Cost of producing venom in three North American Pitvipers Species. Copeia 2006, 2006, 818–825. [Google Scholar] [CrossRef]
- Gibbs, H.L.; Mackessy, S.P. Functional basis of a molecular adaptation: Prey-specific toxic effects of venom from Sistrurus rattlesnakes. Toxicon 2009, 53, 672–679. [Google Scholar] [CrossRef]
- Klauber, L.M. Rattlesnakes: Their Habits, Life Histories, and Influence on Mankind, 2nd ed.; University of California Press: Berkeley, CA, USA, 1972; pp. 1–1533. [Google Scholar]
- Martins, M.; Marques, M.; Marques, O.; Sazima, I. Ecological and phylogenetic correlates of feeding habits in neotropical pitvipers of the genus Bothrops. In Biology of the Vipers; Schuett, G.W., Höggren, M., Douglas, M.E., Greene, H.W., Eds.; Eagle Mountain Publishing: Eagle Mountain, UT, USA, 2002; pp. 307–328. [Google Scholar]
- Böhm, M.; Collen, B.; Baillie, J.E.M.; Bowles, P.; Chanson, J.; Cox, N.; Hammerson, G.; Hoffmann, M.; Livingstone, S.R.; Rama, M.; et al. The conservation status of the world’s reptiles. Biol. Conserv. 2013, 157, 372–385. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2017-2. Available online: https://www.iucnredlist.org/ (accessed on 1 July 2017).
- Maritz, B.; Penner, J.; Martins, M.; Crnobrnja-Isailović, J.; Spear, S.; Alencar, L.R.V.; Sigala-Rodriguez, J.; Messenger, K.; Clark, R.; Jenkins, C.; et al. Identifying global priorities for the conservation of vipers. Biol. Conserv. 2016, 204, 94–102. [Google Scholar] [CrossRef]
- Grismer, L.L. An evolutionary classification of reptiles on islands in the Gulf of California, Mexico. Herpetologica 1999, 55, 446–469. [Google Scholar]
- Constable, H.; Guralnick, R.; Wieczorek, J.; Spencer, C.; Peterson, A.T. VertNet Steering Committee. VertNet: A New Model for Biodiversity Data Sharing. PLoS Biol. 2010, 8, e1000309. [Google Scholar] [CrossRef]
- Nogueira, C.C. Atlas of Brazilian Snakes. Manuscript in preparation.
- IUCN. IUCN Red List Categories and Criteria: Version 3.1, 2nd ed.; IUCN Species Survival Commission: Gland, Switzerland; IUCN: Cambridge, UK, 2012; pp. 1–32. [Google Scholar]
- QGIS Development Team. QGIS Geographic Information System. Version 2.8. Open Source Geospatial Foundation Project. Available online: http://www.qgis.org/ (accessed on 1 March 2016).
- Crisp, D.J.; Southward, A.J. The distribution of intertidal organisms along the coasts of the English Channel. J. Mar. Biol. Assoc. UK 1958, 37, 157–203. [Google Scholar] [CrossRef]
- Dobson, A.J.; Barnett, A. An Introduction to Generalized Linear Models; Chapman and Hall/CRC Press: Boca Raton, FL, USA, 2008; pp. 1–320. [Google Scholar]
- Agresti, A.; Kateri, M. Categorical data analysis. In International Encyclopedia of Statistical Science: 206–208; Lovric, M., Ed.; Springer: Berlin, Germany, 2011. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; pp. 206–208. [Google Scholar]
- Bonner, J.T. Why Size Matters: From Bacteria to Blue Whales; Princeton University Press: Princeton, NJ, USA, 2011; pp. 1–176. [Google Scholar]
- White, E.P.; Ernest, S.M.; Kerkhoff, A.J.; Enquist, B.J. Relationships between body size and abundance in ecology. Trends Ecol. Evol. 2007, 22, 323–330. [Google Scholar] [CrossRef]
- Whitaker, P.B.; Shine, R. A radiotelemetric study of movements and shelter-site selection by free-randing brownsnakes (Pseudonaja textilis, Elapidae). Herpetol. Monogr. 2003, 17, 130–144. [Google Scholar] [CrossRef]
- Roth, E.D. Spatial ecology of a cottonmouth (Agkistrodon piscivorus) population in East Texas. J. Herpetol. 2005, 39, 308–313. [Google Scholar] [CrossRef]
- Reed, R.N. Interspecific patterns of species richness, geographic range size, and body size among New World venomous snakes. Ecography 2003, 26, 107–117. [Google Scholar] [CrossRef]
- Greene, H.W. Snakes: The Evolution of Mystery in Nature; University of California Press: Berkeley, CA, USA, 1997; pp. 1–366. [Google Scholar]
- Feldman, A.; Sabath, N.; Pyron, A.R.; Mayrose, I.; Meiri, S. Body sizes and diversification rates of lizards, snakes, amphisbaenians and the tuatara. Glob. Ecol. Biogeogr. 2016, 25, 187–197. [Google Scholar] [CrossRef]
- Rapoport, E.H. Areography: Geographical Strategies of Species; Pergamon Press: Oxford, UK, 1982; pp. 1–286. [Google Scholar]
- Stevens, G.C. The latitudinal gradient in geographical range: How so many species coexist in the tropics. Am. Nat. 1989, 133, 240–256. [Google Scholar] [CrossRef]
- Meliadou, A.; Troumbis, A.Y. Aspects of heterogeneity in the distribution of diversity of the European herpetofauna. Acta Oecol. 1997, 18, 393–412. [Google Scholar] [CrossRef]
- Arita, H.T.; Rodríguez, P.; Vázquez-Domínguez, E. Continental and regional ranges of North American mammals: Rapoport’s rule in real and null worlds. J. Biogeogr. 2005, 32, 961–971. [Google Scholar] [CrossRef]
- Whitton, F.J.S.; Purvis, A.; Orme, C.D.L.; Olalla-Tarraga, M.A. Understanding global patterns in amphibian geographic range size: Does Rapoport rule? Glob. Ecol. Biogeogr. 2012, 21, 179–190. [Google Scholar] [CrossRef]
- Dobzhansky, T. Evolution in the tropics. Am. Sci. 1950, 38, 209–221. [Google Scholar]
- Pagel, M.D.; May, R.M.; Collie, A.R. Ecological aspects of the geographical distribution and diversity of mammalian species. Am. Nat. 1991, 137, 791–815. [Google Scholar] [CrossRef]
- Eeley, H.A.C.; Foley, R.A. Species richness, species range size and ecological specialisation among African primates: Geographical patterns and conservation implications. Biodivers. Conserv. 1999, 8, 1033–1056. [Google Scholar] [CrossRef]
- Johnson, C.N. Rarity in the tropics: Latitudinal gradients in the distribution and abundance in Australian mammals. J. Anim. Ecol. 1998, 67, 689–698. [Google Scholar] [CrossRef]
- Symonds, M.R.R.; Christidis, L.; Johnson, C.N. Latitudinal gradients in abundance, and the causes of rarity in the tropics: A test using Australian honeyeaters (Aves: Meliphagidae). Oecologia 2006, 149, 406–417. [Google Scholar] [CrossRef]
- Hadfield, J.D. MCMC Methods for Multi-Response Generalized Linear Mixed Models: The MCMCglmm R Package. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef]
- Verde Arregoitia, L.D.; Leach, K.; Reid, N.; Fisher, D.O. Diversity, extinction, and threat status in Lagomorphs. Ecography 2015, 38, 1155–1165. [Google Scholar] [CrossRef]
- Heidelberger, P.; Welch, P.D. Simulation run length control in the presence of an initial transient. Oper. Res. 1983, 31, 1109–1144. [Google Scholar] [CrossRef]
- WCS/CIESIN (Wildlife Conservation Society/Center for International Earth Science Information Network, Columbia University). Last of the Wild Project, Version 2, 2005 (LWP-2): Global Human Influence Index (HII) Dataset (Geographic); NASA Socioeconomic Data and Applications Center (SEDAC): Palisades, NY, USA, 2005. Available online: http://sedac.ciesin.columbia.edu/data/set/wildareas-v2-human-footprint-geographic (accessed on 2 February 2014).
- Médail, F.; Verlaque, R. Ecological characteristics and rarity of endemic plants from southeast France and Corsica: Implication for biodiversity conservation. Biol. Conserv. 1997, 80, 269–281. [Google Scholar] [CrossRef]
- Wilson, L.D.; McCranie, J.R. The conservation status of the herpetofauna of Honduras. Amphib. Reptil. Conserv. 2004, 3, 6–33. [Google Scholar]
- Johnson, J.D.; Mata-Silva, V.; Wilson, L.D. A conservation reassessment of the Central American herpetofauna based on the EVS measure. Amphib. Reptil. Conserv. 2015, 9, 1–94. [Google Scholar]
- Wilson, L.D.; Mata-Silva, V.; Johnson, J.D. A conservation reassessment of the reptiles of Mexico based on the EVS measure. Amphib. Reptil. Conserv. 2013, 7, 1–47. [Google Scholar]
- SEMARNAT (Secretaría de Medio Ambiente y Recursos Naturales). Norma Oficial Mexicana NOM-059-SEMARNAT-2010-Protección Ambiental-Especies Nativas de México de Flora y Fauna Silvestres-Categorías de Riesgo y Especificaciones Para su Inclusión, Exclusión o Cambio-Lista de Especies en Riesgo. Diario Oficial de la Federación, 30 December 2010; 1–78. [Google Scholar]
- Foufopoulos, J.; Ives, A.R. Reptiles extinctions on Land-Bridge Islands: Life-history atributes and vulnerability to extinction. Am. Nat. 1999, 153, 1–25. [Google Scholar] [CrossRef]
- Reed, R.N.; Shine, R. Lying in wait for extinction: Ecological correlates of conservation status among Australian Elapid Snakes. Conserv. Biol. 2002, 16, 451–461. [Google Scholar] [CrossRef]
- Luiselli, L. Testing hypotheses on the ecological patterns of rarity using a novel model of study: Snake communities worldwide. Ecology 2006, 6, 44–58. [Google Scholar] [CrossRef]
- Dunn, E.R. Relative abundance of some Panamanian snakes. Ecology 1949, 30, 39–57. [Google Scholar] [CrossRef]
- Duellman, W.E. The Biology of An Equatorial Herpetofauna in Amazonian Ecuador. Misc. Publ. Mus. Nat. Hist. Univ. Kansas 1978, 65, 1–352. [Google Scholar]
- Martins, M.; Oliveira, M.E. Natural history of snakes in forests of the Manaus region, Central Amazonia, Brazil. Herpetol. Nat. Hist. 1998, 6, 78–150. [Google Scholar]
- Fitch, H.S. A Kansas Snake Community: Composition and Changes Over 50 Years; Krieger Publishing Company: Malabar, FL, USA, 1999; pp. 1–165. [Google Scholar]
- Ford, B.N.; Lancaster, D.L. The species-abundance distribution of snakes in a bottomland hardwood forest of the Southern United States. J. Herpetol. 2007, 41, 385–394. [Google Scholar] [CrossRef]
- Sawaya, R.J.; Marques, O.A.V.; Martins, M. Composition and natural history of a Cerrado snake assemblage at Itirapina, São Paulo state, southeastern Brazil. Biota Neotrop. 2008, 8, 129–151. [Google Scholar] [CrossRef]
- Böhm, M.; Kemp, R.; Williams, R.; Davidson, A.D.; Garcia, A.; McMillan, K.M.; Bramhall, H.R.; Collen, B. Rapoport’s rule and determinants of species range size in snakes. Divers. Distrib. 2017, 23, 1472–1481. [Google Scholar] [CrossRef]
- Slatyer, R.A.; Hirst, M.; Sexton, J.P. Niche breadth predicts geographical range size: A general ecological pattern. Ecol. Lett. 2013, 16, 1104–1114. [Google Scholar] [CrossRef]
- Laube, I.; Korntheuer, H.; Schwager, M.; Trautmann, S.; Rahbek, C.; Böhning-Gaese, K. Towards a more mechanistic understanding of traits and range sizes. Glob. Ecol. Biogeogr. 2013, 22, 233–241. [Google Scholar] [CrossRef]
- Estrada, A.; Meireles, C.; Morales-Castilla, I.; Poschlod, P.; Vieites, D.; Araújo, M.B.; Early, R. Species’ intrinsic traits inform their range limitations and vulnerability under environmental change. Glob. Ecol. Biogeogr. 2015, 24, 849–858. [Google Scholar] [CrossRef]
- Holt, R.D. Rarity and evolution: Some theoretical considerations. In The Biology of Rarity; Kunin, W.E., Gaston, K.J., Eds.; Population and Community Biology Series; Springer: Dordrecht, The Netherlands, 1997; Volume 17, pp. 209–234. [Google Scholar]
- Harcourt, A.H. Rarity in the tropics: Biogeography and macroecology of the primates. J. Biogeogr. 2006, 33, 2077–2087. [Google Scholar] [CrossRef]
- Hawkins, B.A.; Diniz-Filho, J.A.F. ‘Latitude’ and geographical patterns in species richness. Ecography 2004, 27, 268–272. [Google Scholar] [CrossRef]
- Letcher, A.J.; Harvey, P.H. Variation in Geographical Range Size Among Mammals of the Palearctic. Am. Nat. 1994, 144, 30–42. [Google Scholar] [CrossRef]
- Collen, B.; Dulvy, N.K.; Gaston, K.J.; Gärdenfors, U.; Keith, D.A.; Punt, A.E.; Regan, H.M.; Böhm, M.; Hedges, S.; Seddon, M.; et al. Clarifying misconceptions of extinction risk assessment with the IUCN Red List. Biol. Lett. 2016, 12, 20150843. [Google Scholar] [CrossRef]
- Martins, M.; (University of São Paulo, São Paulo, São Paulo, Brazil). Personal communication, 2018.
| IUCN Categories | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Rarity Category | EX | EW | CR | EN | VU | NT | LC | DD | Total |
| Not Rare | 0 | 0 | 0 | 0 | 0 | 0 | 22 | 0 | 22 |
| Low Intermediate | 0 | 0 | 0 | 2 | 0 | 2 | 38 | 1 | 43 |
| High Intermediate | 0 | 0 | 6 | 2 | 4 | 3 | 29 | 3 | 47 |
| Rarest | 0 | 0 | 1 | 3 | 4 | 1 | 4 | 2 | 15 |
| Total | 0 | 0 | 7 | 7 | 8 | 6 | 93 | 6 | 127 |
| Rarest Species in this Study (Category RT) | IUCN | Mexican Red List | EVS Mexico | EVS Central America | Among top 30 Threat Index in Maritz et al. [43] |
|---|---|---|---|---|---|
| Bothriechis thalassinus | CR | H | no | ||
| Bothrocophias campbelli | EN | no | |||
| Bothrops ayerbei | DD | no | |||
| Bothrops venezuelensis | DD | no | |||
| Cerrophidion sasai | LC | H | no | ||
| Cerrophidion tzotzilorum | LC | yes | |||
| Cerrphidion wilsoni | EN | H | no | ||
| Crotalus caliginis | EN | no | |||
| Crotalus catalinensis | VU | A | H | no | |
| Crotalus intermedius | VU | A | H | no | |
| Crotalus muertensis | VU | H | no | ||
| Crotalus stejnegeri | LC | A | H | no | |
| Crotalus transversus | LC | P | H | yes | |
| Mixcoatlus browni | VU | H | no | ||
| Porthidium arcosae | NT | no |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birskis-Barros, I.; R. V. Alencar, L.; I. Prado, P.; Böhm, M.; Martins, M. Ecological and Conservation Correlates of Rarity in New World Pitvipers. Diversity 2019, 11, 147. https://doi.org/10.3390/d11090147
Birskis-Barros I, R. V. Alencar L, I. Prado P, Böhm M, Martins M. Ecological and Conservation Correlates of Rarity in New World Pitvipers. Diversity. 2019; 11(9):147. https://doi.org/10.3390/d11090147
Chicago/Turabian StyleBirskis-Barros, Irina, Laura R. V. Alencar, Paulo I. Prado, Monika Böhm, and Marcio Martins. 2019. "Ecological and Conservation Correlates of Rarity in New World Pitvipers" Diversity 11, no. 9: 147. https://doi.org/10.3390/d11090147
APA StyleBirskis-Barros, I., R. V. Alencar, L., I. Prado, P., Böhm, M., & Martins, M. (2019). Ecological and Conservation Correlates of Rarity in New World Pitvipers. Diversity, 11(9), 147. https://doi.org/10.3390/d11090147