Species Diversity of Epilithon Diatoms and the Quality of the Waters of the Donuzlav Gulf Ecosystem (Crimea, the Black Sea)
Abstract
:1. Introduction
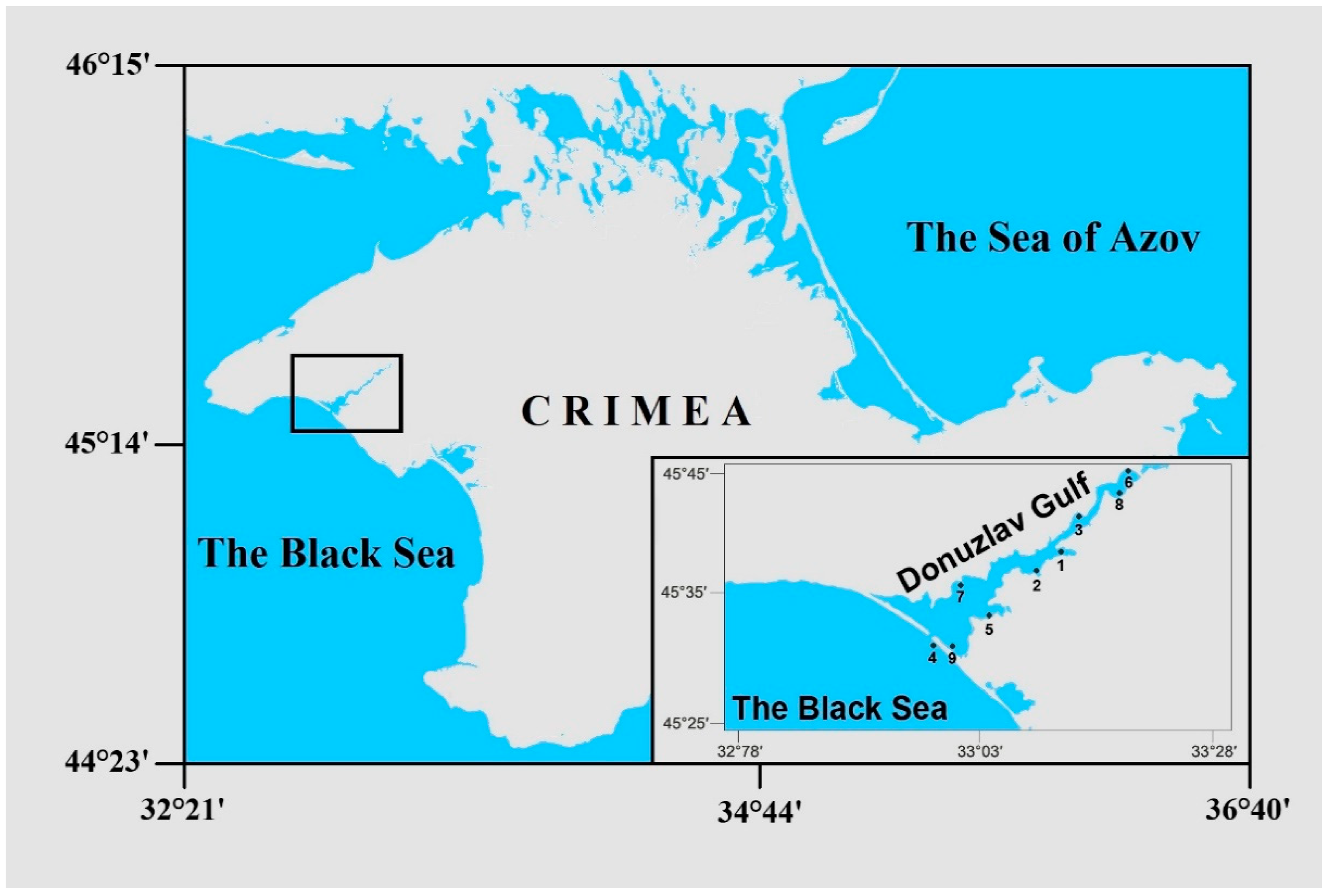
2. Materials and Methods
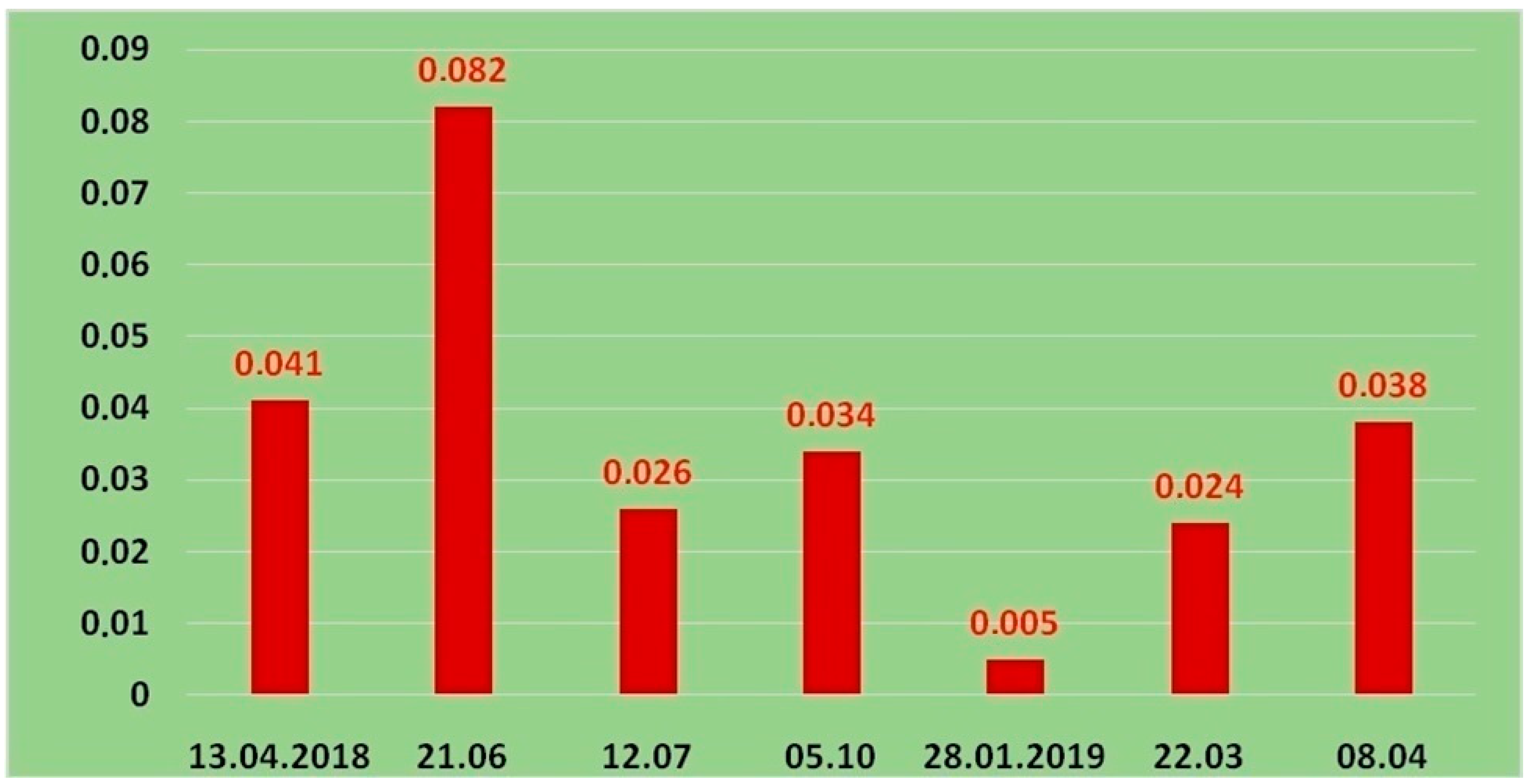
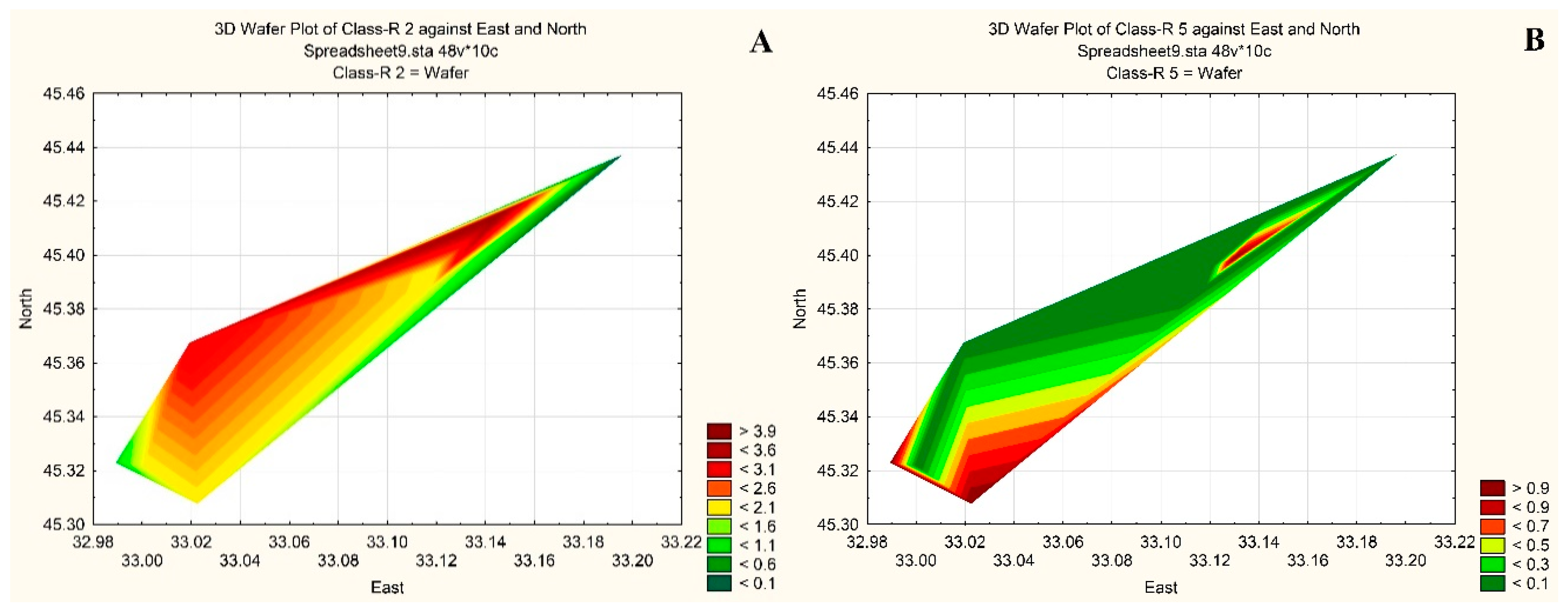
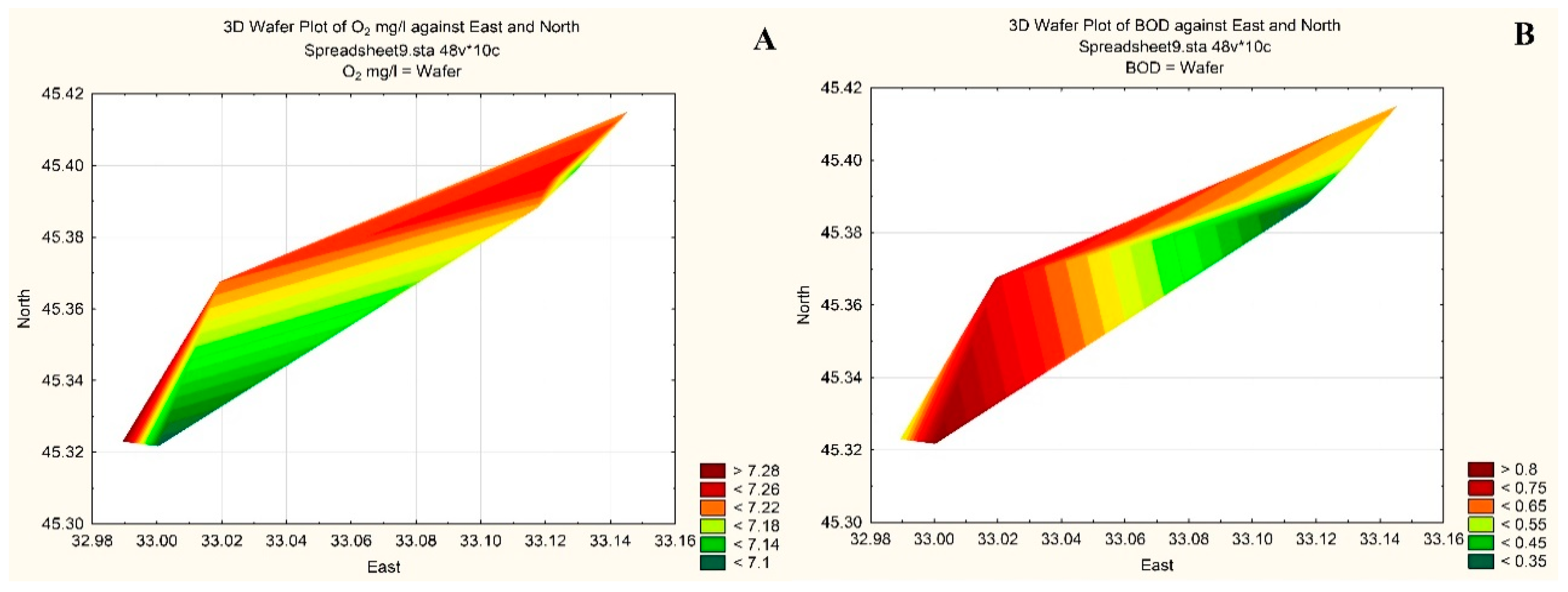
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kovrigina, N.P.; Nemirovsky, M.S. Hydrochemical characteristics of waters near Lake Donuzlav according to 1990–1997. Ecol. Morya 1999, 48, 10–15. (In Russian) [Google Scholar]
- Samyshev, E.Z.; Senichkina, L.G.; Sergeeva, N.G.; Mikhailova, T.V.; Pankratova, T.M. Structure and functioning of the plankton and benthic communities of oz. Donuzlav in the conditions of anthropogenic pollution and assessment of prospects of its fishery use. In Systems Control Environment: Proceeding Sci./NAS of Ukraine; IGI Publisher: Sevastopol, Russia, 2001; pp. 301–325. [Google Scholar]
- Zhugaylo, S.S.; Avdeeva, T.M.; Pugach, M.N.; Adzhiumerov, E.N. Current state of water quality and bottom sediments in Lake Donuzlav in modern times. Aquat. Bioresour. Environ. 2018, 1, 32–38. [Google Scholar]
- Barinova, S.S.; Bilous, O.P.; Tsarenko, P.M. Algal Indication of Water Bodies in Ukraine: Methods and Prospects; Publishing House of Haifa University: Haifa, Israel; Kyiv, Ukraine, 2019; 367p. (In Russian) [Google Scholar]
- Barinova, S.S.; Medvedeva, L.A.; Anisimova, O.V. Diversity of Algal Indicators in the Environmental Assessment; Pilies Studio Publisher: Tel’Aviv, Israel, 2006; 498p. (In Russian) [Google Scholar]
- Harding, W.R.; Taylor, J.C. The South African Diatom Index (SADI)—A Preliminary Index for Indicating Water Quality in Rivers and Streams in Southern Africa; WRC Report; Water Research Commission: Pretoria, South Africa, 2011; pp. 1–11. [Google Scholar]
- Harding, W.R.; Taylor, J.C. Diatoms as Indicators of Historical Water Quality: A Comparison of Samples Taken in the Wemmershoek Catchment (Western Province, South Africa) in 1960 and 2008; Water Research Commission: Pretoria, South Africa, 2014; pp. 601–606. [Google Scholar]
- Potapova, M.; Charles, D.F. Diatom metrics for monitoring eutrophication in river of the United States. Ecol. Indic. 2007, 7, 48–70. [Google Scholar] [CrossRef]
- Prygiel, J. Management of the diatom monitoring network in France. J. App. Phycol. 2002, 14, 19–26. [Google Scholar] [CrossRef]
- Szczepocka, E.; Żelazna-Wieczorek, J. Diatom biomonitoring—Scientific foundations, commonly discussed issues and frequently made errors. Oceanol. Hydrobiol. Stud. 2018, 47, 314–325. [Google Scholar] [CrossRef]
- Barinova, S.S.; Bondarenko, A.V.; Ryabushko, L.I.; Kapranov, S.V. Microphytobenthos as indicator of water quality and organic pollution in the Western coastal zone of the Sea of Azov. Oceanol. Hydrobiol. Stud. 2019, 48, 21–35. [Google Scholar] [CrossRef]
- Begun, A.A. Of the state of the marine environment on diatoms of epiphytonmacrophytes (Peter the Great Bay, Japan Sea). Izv. Tinro 2012, 169, 1–17. [Google Scholar]
- Ryabushko, L.I.; Begun, A.A. Diatoms of Microphytobenthos of the Sea of Japan; Book 1; N. Orianda Publishers: Sevastopol-Simferopol, Russia, 2015; 288p. (In Russian) [Google Scholar]
- Zalat, A.A. Distribution and origin of diatoms in the bottom sediments of the Suez canal lakes and adjacent areas, Egypt. Diat. Res. 2002, 17, 243–266. [Google Scholar] [CrossRef]
- Desrosiers, C.; Leflaivea, J.; Eulinb, A.; Ten-Hagea, L. Bioindicators in marine waters: Benthic diatoms as a tool to assess water quality from eutrophic to oligotrophic coastal ecosystems. Ecol. Indic. 2013, 32, 25–34. [Google Scholar] [CrossRef]
- Ryabushko, L.I.; Bondarenko, A.V. The Qualitative and Quantitative Characteristics of the Benthic Diatoms near Kazantip Cape of the Sea of Azov. J. Black Sea Mediterr. Environ. 2016, 22, 237–249. (In Russian) [Google Scholar]
- Bondarenko, A.V.; Ryabushko, L.I.; Sadogurskaya, S.A. Microalgae of Benthos and Plankton in the Coastal Waters of the Nature Reserve “Kazantipskiy” (the Sea of Azov, Crimea). In Biodiversity and Environment of Protected Areas 2018, 4, 25–48. (In Russian) [Google Scholar]
- Ryabushko, L.I. Microphytobenthos of the Black Sea; Gaevskay, A.V., Ed.; ECOSI-Gidrofizica: Sevastopol, Russia, 2013; 416p. (In Russian) [Google Scholar]
- Ryabushko, L.I.; Balycheva, D.S.; Ryabushko, V.I. Microphytobenthos Diatoms of the Black Sea: Biodiversity and Ecology. Ecol. Montenegrina 2017, 10, 1–15. [Google Scholar]
- Ryabushko, L.I.; Balycheva, D.S.; Strizhak, A.V. Diatoms of epiphyton some species of green algae-macrophytes and periphyton of anthropogenic substrates of the Crimean coastal waters of the Black Sea. Algologia 2013, 23, 419–437. (In Russian) [Google Scholar] [CrossRef]
- Ryabushko, L.I.; Pospelova, N.V.; Balycheva, D.S.; Kovrigina, N.P.; Troshchenko, O.A.; Kapranov, S.V. Epizoon microalgae of the cultivated mollusk Mytilus galloprovincialis Lam. 1819, phytoplankton, hydrological and hydrochemical characteristics in the mussel-and-oyster farm area (Sevastopol, the Black Sea). Mar. Biol. J. 2017, 2, 67–83. (In Russian) [Google Scholar] [CrossRef]
- Ryabushko, L.I.; Lishaev, D.N.; Shiroyan, A.G. The species, ecological and geographical diversity of microphytobenthos microalgae in the Crimean coast of the Black Sea. In Proceedings of the 2nd International UNIDOCAP the Blacк Sea Symposium on Biodiversity, Samsun, Turkey, 28–30 November 2018; Progr. & Abstract. Book: Samsun, Turkey, 2018; p. 33. (In Russian). [Google Scholar]
- Graham, A.A.; McCaughan, D.J.; McKee, F.S. Measurement of surface area of stones. Hydrobiologia 1988, 157, 85–87. [Google Scholar] [CrossRef]
- Oksiyuk, O.P.; Yurchenko, V.V. About the weight diatoms. Hydrobiol. J. 1971, 7, 116. (In Russian) [Google Scholar]
- Grasshoff, K. (Ed.) Methods of Seawater Analysis; Verlag Chemie: Weinheim, Germany; New York, NY, USA, 1976; 317p. [Google Scholar]
- Methods of Hydrochemical Studies of Basic Hydrochemical Elements; VNIRO: Moscow, Russia, 1988; 120p. (In Russian)
- Sladeček, V. Diatoms as indicators of organic pollution. Acta Hydrochem. Hydrobiol. 1986, 14, 555–566. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. Available online: http://www.algaebase.org (accessed on 20 December 2018).
- Guslyakov, N.E.; Zakordonets, O.A.; Gerasimyuk, V.P. Atlas of Diatoms of the Benthos in the North-Western Part of the Black Sea and Adjacent Reservoirs; Nauk. Dumka: Kyiv, Ukraine, 1992; 112p. (In Russian) [Google Scholar]
- Proshkina-Lavrenko, A.I. Diatoms of the Black Sea Benthos; Nauka Publishers: Leningrad, Moscow, 1963; 244p. (In Russian) [Google Scholar]
- Ryabushko, L.I.; Begun, A.A. Diatoms of the Microphytobenthos of the Sea of Japan (Synopsis and Atlas); Book 2; PK «KIA» Publishers: Sevastopol, Russia, 2016; 324p. (In Russian) [Google Scholar]
- Smith, W.F. A Synopsis of the British Diatomaceae; John Van Voorst: London, UK, 1853; Volume 1, 89p. [Google Scholar]
- Barinova, S. Ecological Mapping in Application to Aquatic Ecosystems BioIndication: Problems and Methods. Int. J. Environ. Sci. Nat. Resour. 2017, 3, 1–7. [Google Scholar] [CrossRef]


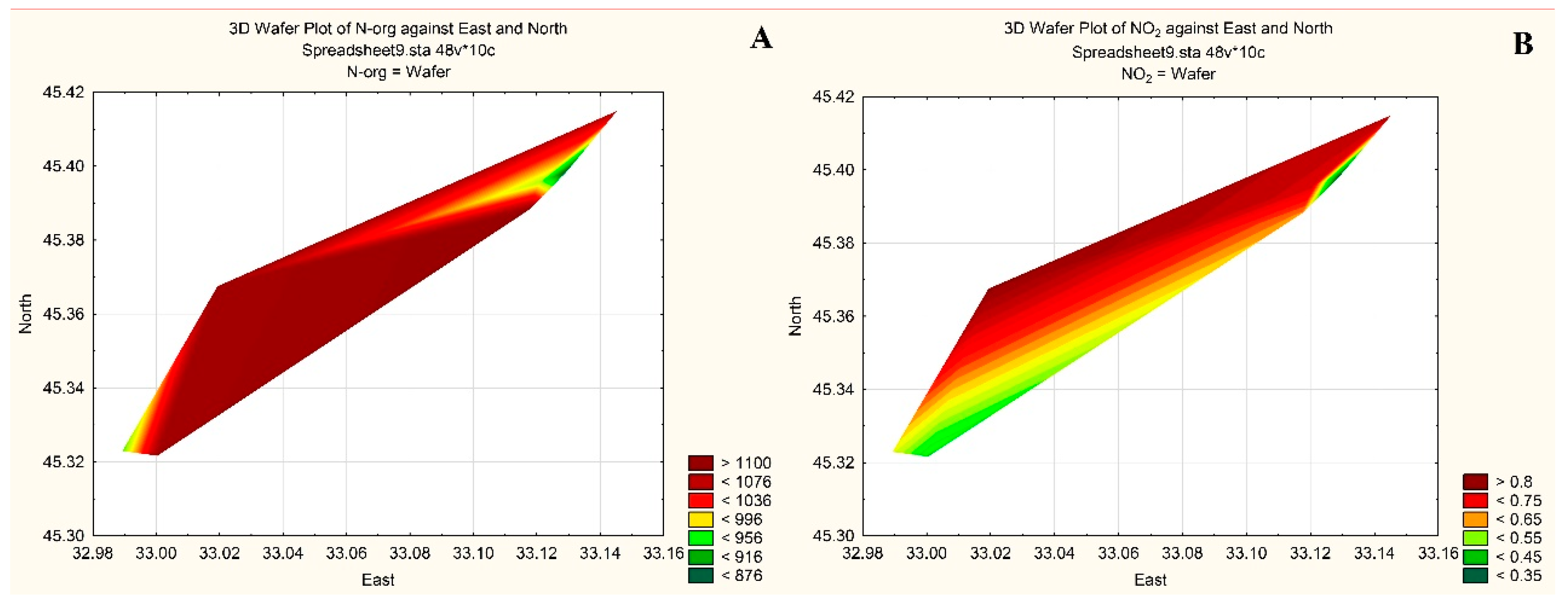
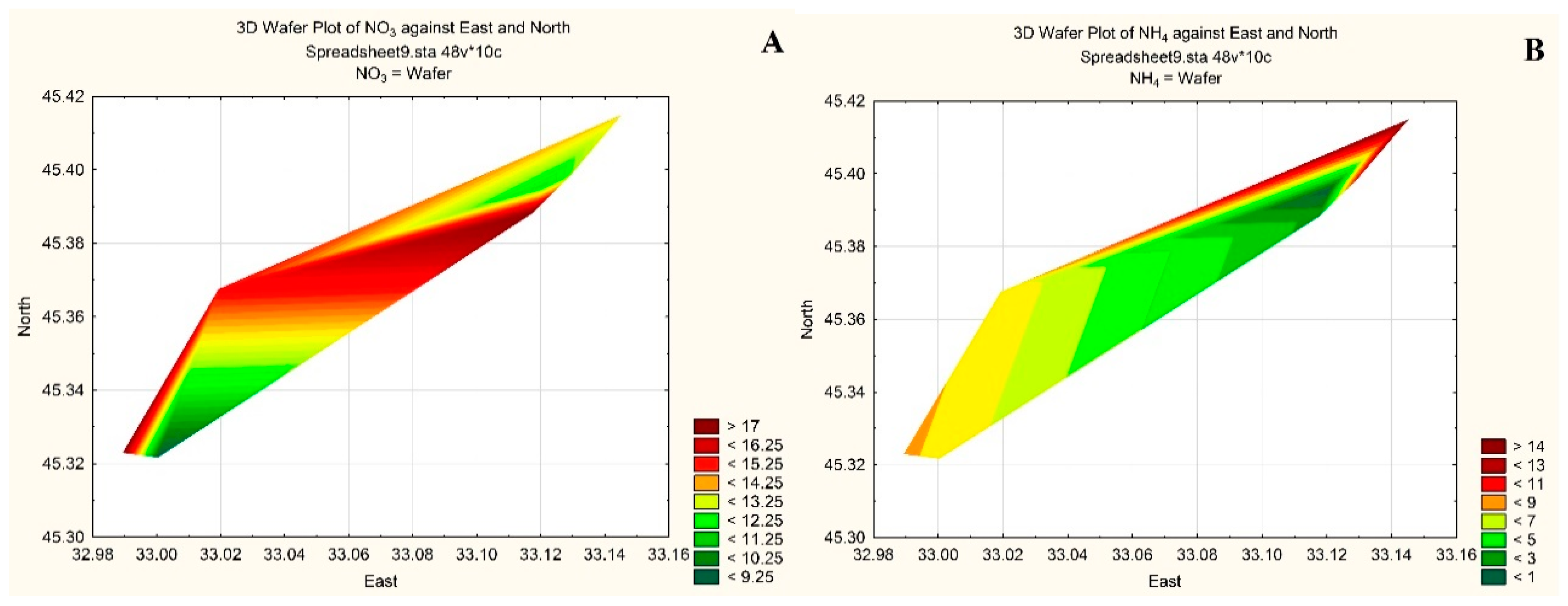

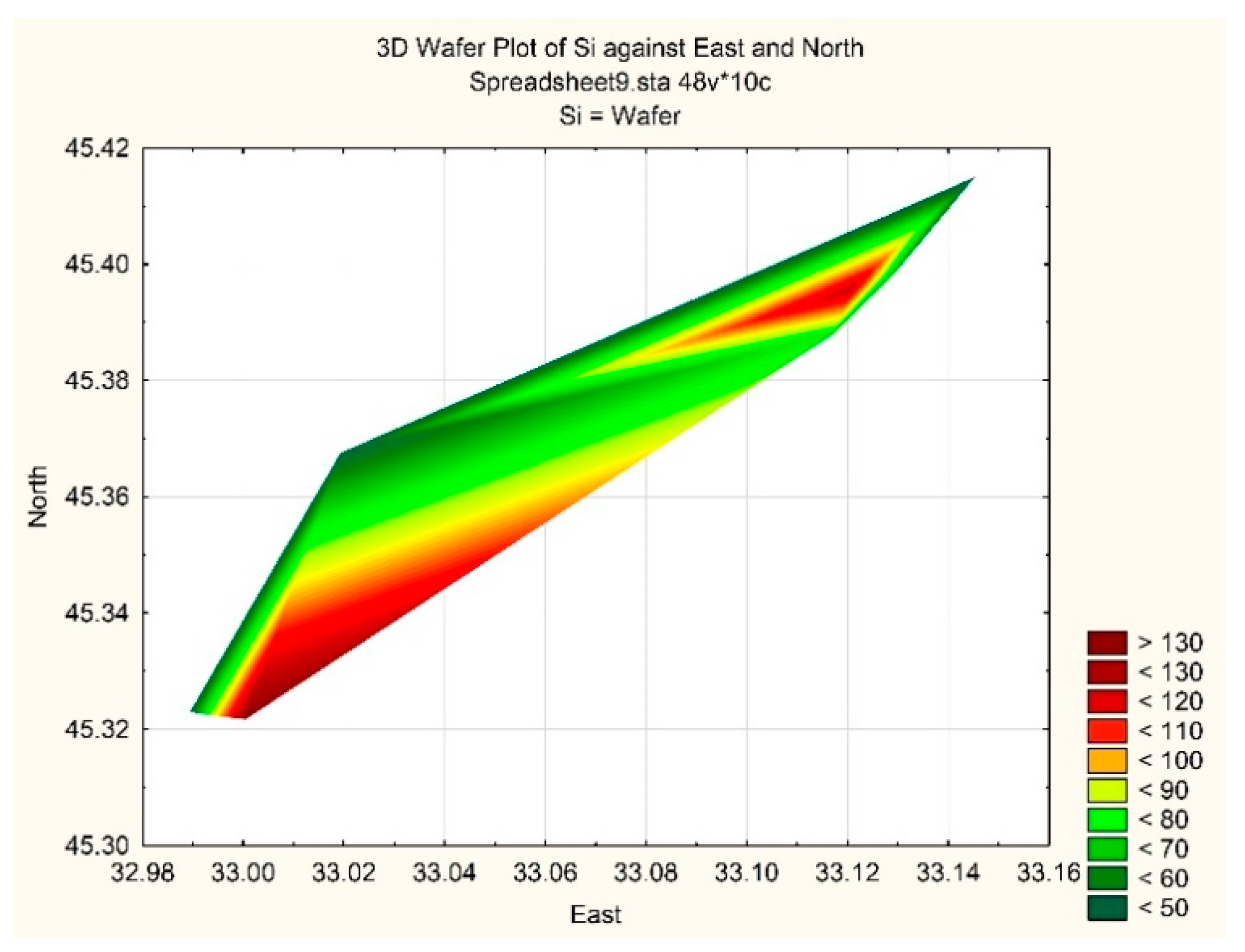
| Taxa | ECD | PhG | |
|---|---|---|---|
| S | RS | ||
| Achnanthes brevipes C.A. Agardh 1824 | β | BM | K |
| Achnanthes longipes C.A. Agardh 1824 | β | M | ABT |
| Amphora angusta W. Gregory 1857 | – | BM | K |
| Amphora arcus W. Gregory 1857 | – | M | AB |
| Amphora ovalis (Kützing) Kützing 1844 | o-β | B | K |
| Amphora proteus W. Gregory 1857 | α-β | M | K |
| Ardissonea crystallina (C.A. Agardh) Grunow 1880 | β | BM | BT |
| Bacillaria paxillifera (O.F. Müller) T. Marsson 1901 | o-α | BM | K |
| Berkeleya micans (Lyngbye) Grunow 1880 | o | BM | B not |
| Berkeleya rutilans (Trentepohl ex Roth) Grunow 1880 | – | BM | AB not |
| Caloneis liber (W. Smith) P. Cleve 1894 | – | M | K |
| Carinasigma rectum (Donkin) G. Reid 2012 | – | M | BT not |
| Cocconeis costata W. Gregory1855 | – | M | K |
| Cocconeis scutellum Ehrenberg 1838 | β | BM | K |
| Cylindrotheca closterium (Ehrenberg) Reimann et Lewin 1964 | β | M | K |
| Diploneis bombus (Ehrenberg) Ehrenberg 1894 | – | M | ABT |
| Diploneis chersonensis (Grunow) P. Cleve 1894 | – | M | ABT |
| Diploneis smithii (Brébisson) P. Cleve 1894 | – | BM | K |
| Entomonei spaludosa (W. Smith) Reimer 1975 | – | BM | AB not |
| Falcula media var. subsalina Proschkina-Lavrenko 1963 | o | M | B |
| Grammatophora marina (Lyngbye) Kützing 1844 | β | M | K |
| Gyrosigma prolongatum (W. Smith) Griffith et Henfrey 1856 | – | M | ABT |
| Halamphora coffeiformis (C.A. Agardh) Levkov 2009 | – | BM | ABT |
| Halamphora costata (W. Smith) Levkov 2009 | – | M | BT |
| Halamphora hyalina (Kützing) Rimet et R. Jahn 2018 | β | M | ABT not |
| Haslea ostrearia (Gaillon) Simonsen 1974 | – | M | B |
| Licmophora abbreviata C.A. Agardh 1831 | β | M | K |
| Licmophora dalmatica (Kützing) Grunow 1867 | – | M | B |
| Licmophora flabellata (Greville) C.A. Agardh 1831 | β | M | BTnot |
| Licmophora gracilis (Ehrenberg) Grunow 1867 | – | M | ABT |
| Licmophora hastata Mereschkowsky 1901 | – | M | B |
| Lyrella clavata (W. Gregory) 1990 | – | M | BT |
| Melosira lineata (Dillwyn) C.A. Agardh 1824 | α | BM | ABT |
| Melosira moniliformis (O.F. Müller) C.A. Agardh 1824 | ο-β | BM | K |
| Navicula cancellata Donkin 1873 | – | M | K |
| Navicula directa (W. Smith) Ralfs ex Pritchard 1861 | – | M | K |
| Navicula distans (W. Smith) Ralfs ex Pritchard 1861 | – | M | B |
| Navicula pennata var. pontica Mereschkowsky 1902 | – | BM | BT |
| Navicula perrhombus Hustedt ex Simonsen 1962 | – | M | BT |
| Navicula salinarum Grunow 1880 | β-o | B | AB not |
| Nitzschia hybrida f. hyalina Proschkina-Lavrenko 1963 | β | BM | B |
| Nitzschia lanceolata W. Smith 1853 | – | B | BT not |
| Nitzschia sigma (Kützing) W. Smith 1853 | – | B | ABT |
| Nitzschia tenuirostris Mereschkowsky 1902 | – | B | B |
| Parlibellus delognei (Van Heurck) E.J. Cox 1988 | – | M | ABT |
| Petroneis monilifer (Cleve) A.J. Stickle et D.G. Mann 1990* | – | M | AB |
| Plagiotropis lepidoptera (W. Gregory) Kuntze 1898 | o | M | ABT |
| Pleurosigma elongatum (W. Smith) 1852 | – | BM | K |
| Psammodictyon panduriforme (W. Gregory) D.G. Mann 1990 | – | M | BT not |
| Rhaphoneis amphiceros (Ehrenberg) Ehrenberg 1844 | – | BM | ABT |
| Seminavis ventricosa (W. Gregory) M. Garsia-Baptista 1993 | β | M | K |
| Striatella delicatula (Kützing) Grunow ex Van Heurck 1885 | – | BM | ABT |
| Striatella unipunctata (Lyngbye) C.A. Agardh 1832 | – | M | BT |
| Tabularia fasciculata (C.A. Agardh) D.M. Williams et Round 1986 | x-o | BM | K |
| Tabularia parva (Kützing) D.M. Williams et Round 1990 | α | BM | ABT |
| Tabularia tabulata (C.A. Agardh) Snoeijs 1992 | β-α | BM | K |
| Thalassiosira eccentrica (Ehrenberg) P. Cleve 1904 | – | M | K |
| Trachyneis aspera (Ehrenberg) P. Cleve 1894 | β | M | ABT not |
| Tryblionella coarctata (Grunow) D.G. Mann 1990 | – | BM | B |
| Undatella lineolata (Ehrenberg) L.I. Ryabushko 2006 | – | BM | ABT |
| № st. | O2 | BOD5, mg O2 L−1 | NO2 | NO3 | NH4 | Norg | PO4 | Porg | Si | Oxid., mg O2 L−1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mg L−1 | % | µg L−1 | |||||||||
| 1 | 10.22 | 103.3 | 0.55 | 0.3 | 12.4 | 10.6 | 875 | 0.6 | 7.6 | 66.6 | 2.98 |
| 2 | 10.42 | 105.1 | 0.56 | 0.6 | 17.6 | 8.3 | 955 | 1.4 | 6.1 | 52.3 | 3.37 |
| 3 | 10.32 | 103.8 | 0.61 | 0.8 | 13.7 | 14.2 | 1072 | 0.9 | 6.9 | 49.7 | 2.68 |
| 5 | 10.30 | 103.6 | 0.34 | 0.6 | 17.8 | 2.7 | 1104 | 1.0 | 7.8 | 75.1 | 2.69 |
| 6 | 10.35 | 104.1 | 0.57 | 0.8 | 11.6 | 0.6 | 972 | 1.7 | 6.7 | 124.4 | 3.40 |
| 7 | 10.32 | 103.8 | 0.71 | 0.9 | 15.4 | 7.7 | 1093 | 1.1 | 8.1 | 51.7 | 3.57 |
| 8 | 10.14 | 106.7 | 0.82 | 0.5 | 9.2 | 7.7 | 1094 | 1.1 | 9.5 | 137.4 | 3.67 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryabushko, L.I.; Lishaev, D.N.; Kovrigina, N.P. Species Diversity of Epilithon Diatoms and the Quality of the Waters of the Donuzlav Gulf Ecosystem (Crimea, the Black Sea). Diversity 2019, 11, 114. https://doi.org/10.3390/d11070114
Ryabushko LI, Lishaev DN, Kovrigina NP. Species Diversity of Epilithon Diatoms and the Quality of the Waters of the Donuzlav Gulf Ecosystem (Crimea, the Black Sea). Diversity. 2019; 11(7):114. https://doi.org/10.3390/d11070114
Chicago/Turabian StyleRyabushko, Larisa I., Denis N. Lishaev, and Nelya P. Kovrigina. 2019. "Species Diversity of Epilithon Diatoms and the Quality of the Waters of the Donuzlav Gulf Ecosystem (Crimea, the Black Sea)" Diversity 11, no. 7: 114. https://doi.org/10.3390/d11070114
APA StyleRyabushko, L. I., Lishaev, D. N., & Kovrigina, N. P. (2019). Species Diversity of Epilithon Diatoms and the Quality of the Waters of the Donuzlav Gulf Ecosystem (Crimea, the Black Sea). Diversity, 11(7), 114. https://doi.org/10.3390/d11070114





