Seasonal Diversity of Endophytic Bacteria Associated with Cinnamomum camphora (L.) Presl.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location of the Study
2.2. Sample Collection
2.3. Laboratory Analyses
2.3.1. Sample Preparation for Analysis
2.3.2. Extraction of DNA and PCR Amplification
2.3.3. 16.S rDNA Library Construction and High-Throughput Sequencing
2.4. Data Preprocessing and Bioinformatics
3. Results
3.1. Extraction and Quantitative Detection of Total DNA from Samples
3.2. Processing of Sequence Information
3.3. Alpha Diversity of Bacterial Communities in Cinnamomum camphora Leaves
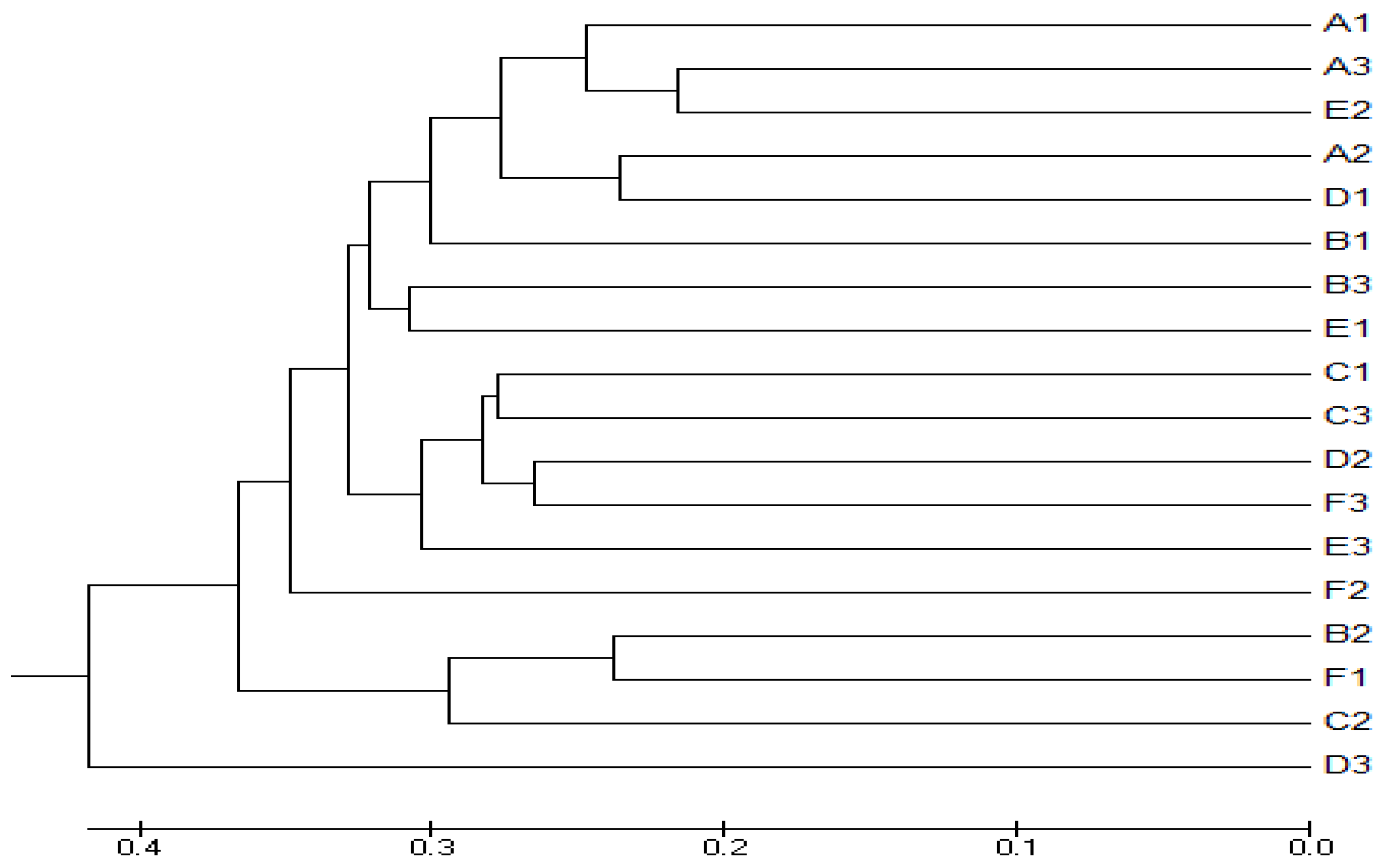
3.4. Beta Diversity Analysis
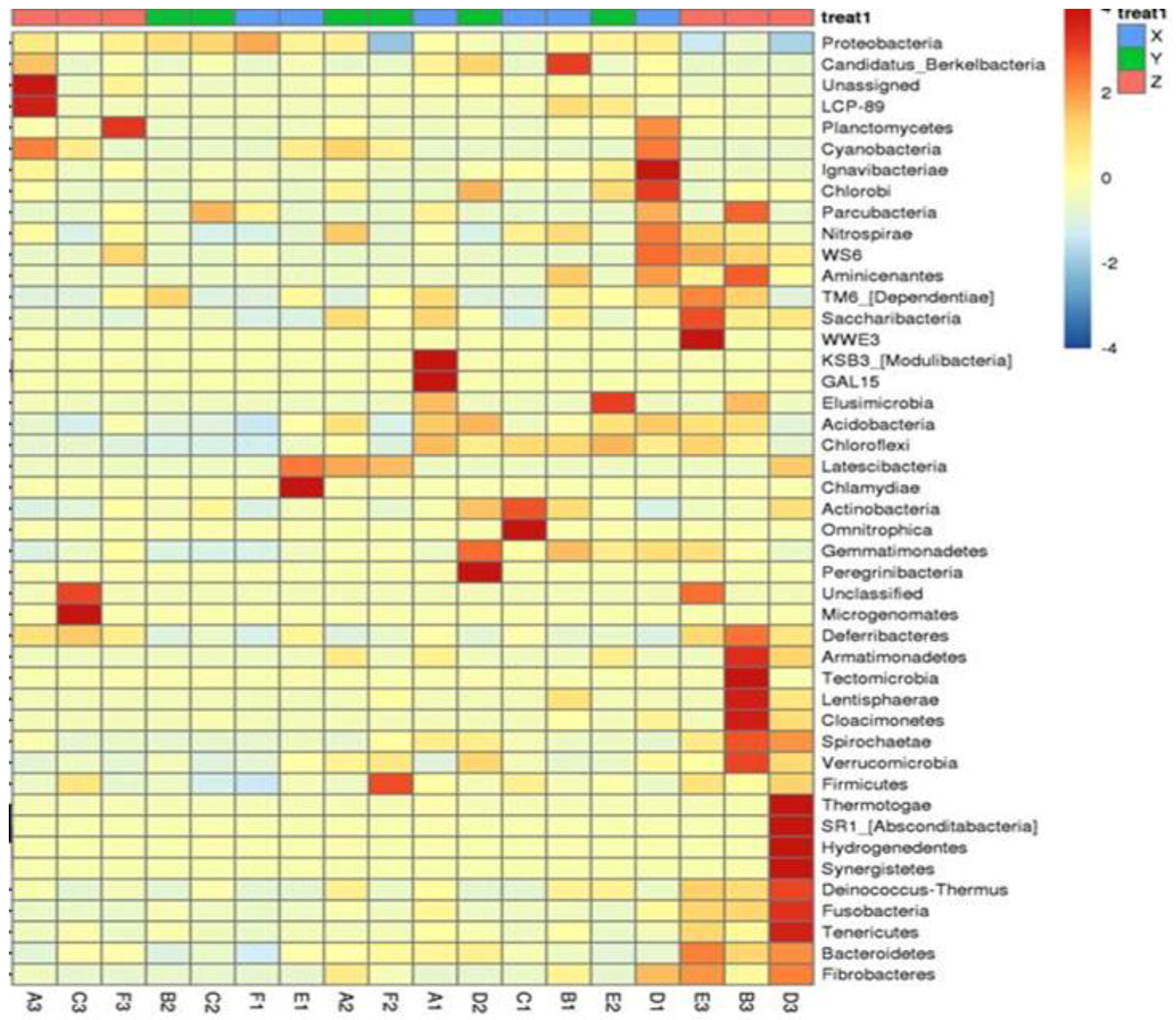
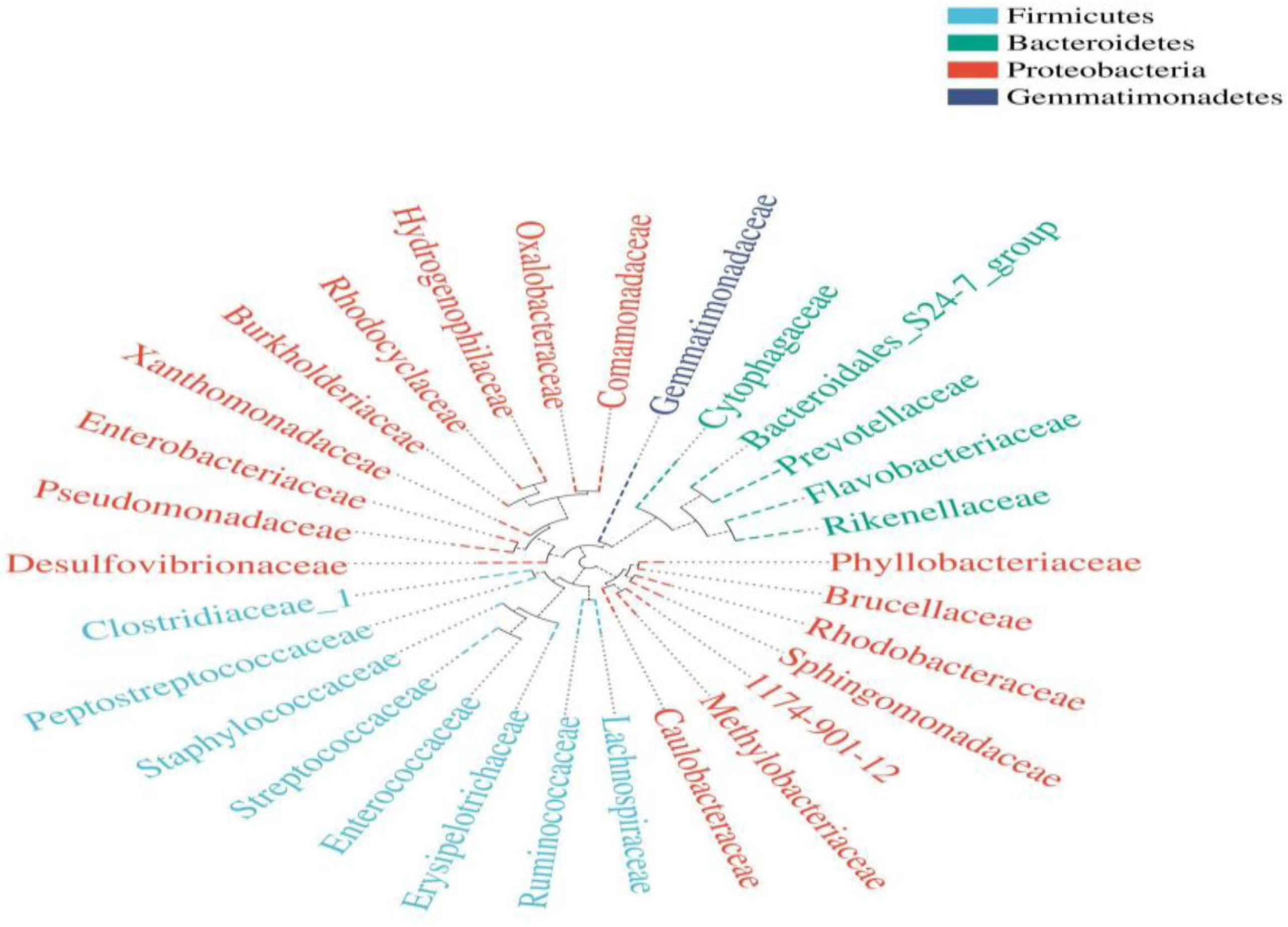
Composition of the Structural Diversity of Bacteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rosenblueth, M.; Martínez-Romero, E. Bacterial endophytes and their interactions with hosts. Mol. Plant Microbe Interact. 2006, 19, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Fesel, P.H.; Zuccaro, A. Dissecting Endophytic Lifestyle Along the Parasitism/Mutualism Continuum in Arabidopsis. Curr. Opin. Microbiol. 2016, 32, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Hardoim, P.R.; Van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A.J.M.; Reviews, M.B. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Potshangbam, M.; Devi, S.I.; Sahoo, D.; Strobel, G.A. Functional characterization of endophytic fungal community associated with Oryza sativa L. and Zea mays L. Front. Microbiol. 2017, 8, 325. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted interactions between endophytes and plant: Developments and prospects. Front. Microbiol. 2018, 9, 2732. [Google Scholar] [CrossRef] [PubMed]
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.-S.; Patra, J.K. Endophytes: A treasure house of bioactive compounds of medicinal importance. Front. Microbiol. 2016, 7, 1538. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Mitter, B.; Colli-Mull, J.G.; Gangl, H.; Sessitsch, A. Endophytes of grapevine flowers, berries, and seeds: Identification of cultivable bacteria, comparison with other plant parts, and visualization of niches of colonization. Microb. Ecol. 2011, 62, 188–197. [Google Scholar] [CrossRef]
- Hassan, S.E.-D. Plant growth-promoting activities for bacterial and fungal endophytes isolated from medicinal plant of Teucrium polium L. J. Adv. Res. 2017, 8, 687–695. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, A.; Singh, R.; Pandey, K.D. Endophytic bacteria: A new source of bioactive compounds. 3 Biotech 2017, 7, 315. [Google Scholar] [CrossRef]
- Khan, M.; Khatun, A.; Islam, T. Promotion of plant growth by phytohormone producing bacteria. In Microbes in Action; Nova Science Publishers: New York, NY, USA, 2016. [Google Scholar]
- Frizzo, C.D.; Santos, A.C.; Paroul, N.; Serafini, L.A.; Dellacassa, E.; Lorenzo, D.; Moyna, P. Essential oils of camphor tree (cinnamomum camphora nees & eberm) cultivated in southern brazil. Braz. Arch. Biol. Technol. 2000, 43, 313–316. [Google Scholar]
- Wang, S.; Xie, Y. China Species Red List; Higher Education Press: Beijing, China, 2004; Volume 1. [Google Scholar]
- Edwards, V.H. The Aromatherapy Companion: Medicinal Uses/Ayurvedic Healing/Body-Care Blends/Perfumes & Scents/Emotional Health & Well-Being; Storey Publishing: North Adams, MA, USA, 2015. [Google Scholar]
- Strobel, G.; Daisy, B.; Castillo, U.; Harper, J. Natural products from endophytic microorganisms. J. Nat. Prod. 2004, 67, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Arora, J.; Ramawat, K. An introduction to endophytes. In Endophytes: Biology and Biotechnology; Springer: New York, NY, USA, 2017; pp. 1–23. [Google Scholar]
- Zhan, W.; Zeng, L.-T.; Fang, Z.; Shi, S.; Zhang, C.-B.; Weng, Q.-B.; School of Life Sciences, Guizhou Normal University. Isolation of the Endophytic Bacteria from Cinnamomum Camphora and Evaluation of Antioxidant Activity of the Fermentation Product. Sci. Technol. Food Ind. 2016, 75, 71–75. [Google Scholar]
- Roose-Amsaleg, C.; Garnier-Sillam, E.; Harry, M. Extraction and purification of microbial DNA from soil and sediment samples. Appl. Soil Ecol. 2001, 18, 47–60. [Google Scholar] [CrossRef]
- Chowdhury, M.E.K.; Jeon, J.; Rim, S.O.; Park, Y.-H.; Lee, S.K.; Bae, H. Composition, diversity and bioactivity of culturable bacterial endophytes in mountain-cultivated ginseng in korea. Sci. Rep. 2017, 7, 10098. [Google Scholar] [CrossRef] [PubMed]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16s ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than blast. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I. Qiime allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Amato, K.R.; Yeoman, C.J.; Kent, A.; Righini, N.; Carbonero, F.; Estrada, A.; Gaskins, H.R.; Stumpf, R.M.; Yildirim, S.; Torralba, M. Habitat degradation impacts black howler monkey (alouatta pigra) gastrointestinal microbiomes. ISME J. 2013, 7, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.; Ryan, P. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Achari, G.A.; Ramesh, R. Diversity, biocontrol, and plant growth promoting abilities of xylem residing bacteria from solanaceous crops. Int. J. Microbiol. 2014, 2014, 296521. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, L.; Harvey, I. An association of a lolium endophyte with ryegrass staggers. N. Z. Vet. J. 1981, 29, 185–186. [Google Scholar] [CrossRef] [PubMed]
- Quecine, M.C.; Araújo, W.L.; Rossetto, P.B.; Ferreira, A.; Tsui, S.; Lacava, P.T.; Mondin, M.; Azevedo, J.L.; Pizzirani-Kleiner, A.A. Sugarcane growth promotion by the endophytic bacterium pantoea agglomerans 33.1. Appl. Environ. Microbiol. 2012, 78, 7511–7518. [Google Scholar] [CrossRef] [PubMed]
- Suman, A.; Yadav, A.N.; Verma, P. Endophytic microbes in crops: Diversity and beneficial impact for sustainable agriculture. In Microbial Inoculants in Sustainable Agricultural Productivity; Springer: New York, NY, USA, 2016; pp. 117–143. [Google Scholar]
- Dalmasso, A.; Civera, T.; Pattono, D.; Cardazzo, B.; Bottero, M.T. Characterization of microbiota in plaisentif cheese by high-throughput sequencing. LWT Food Sci. Technol. 2016, 69, 490–496. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S. Bacterial community diversity on in-shell walnut surfaces from six representative provinces in china. Sci. Rep. 2017, 7, 10054. [Google Scholar] [CrossRef]
- Deng, Z.-S.; Zhang, B.-C.; Qi, X.-Y.; Sun, Z.-H.; He, X.-L.; Liu, Y.-Z.; Li, J.; Chen, K.-K.; Lin, Z.-X. Root-associated endophytic bacterial community composition of pennisetum sinese from four representative provinces in china. Microorganisms 2019, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Beckers, B.; De Beeck, M.O.; Weyens, N.; Boerjan, W.; Vangronsveld, J. Structural variability and niche differentiation in the rhizosphere and endosphere bacterial microbiome of field-grown poplar trees. Microbiome 2017, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Yang, X.-Y.; Yan, Z.-Q.; Liu, Q.; Li, X.-Z.; Chen, J.-X.; Zhang, D.-H.; Zeng, L.-M.; Qin, B. Characterization of rhizosphere and endophytic bacterial communities from leaves, stems and roots of medicinal Stellera chamaejasme L. Syst. Appl. Microbiol. 2014, 37, 376–385. [Google Scholar] [CrossRef]
- Reiter, B.; Sessitsch, A. Bacterial endophytes of the wildflower crocus albiflorus analyzed by characterization of isolates and by a cultivation-independent approach. Can. J. Microbiol. 2006, 52, 140–149. [Google Scholar] [CrossRef]
- Seo, W.T.; Lim, W.J.; Kim, E.J.; Yun, H.D.; Lee, Y.H.; Cho, K.M. Endophytic bacterial diversity in the young radish and their antimicrobial activity against pathogens. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 493–503. [Google Scholar] [CrossRef]
- Bodenhausen, N.; Horton, M.W.; Bergelson, J. Bacterial communities associated with the leaves and the roots of arabidopsis thaliana. PLoS ONE 2013, 8, e56329. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Liu, S.L.; Liu, M.; Kong, B.H.; Liu, L.; Li, Y.H. A primary assessment of the endophytic bacterial community in a xerophilous moss (grimmia montana) using molecular method and cultivated isolates. Braz. J. Microbiol. 2014, 45, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.; Berg, C.; Landa, B.B.; Auerbach, A.; Moissl-Eichinger, C.; Berg, G. Plant genotype-specific archaeal and bacterial endophytes but similar bacillus antagonists colonize mediterranean olive trees. Front. Microbiol. 2015, 6, 138. [Google Scholar]
- Dong, L.; Cheng, R.; Xiao, L.; Wei, F.; Wei, G.; Xu, J.; Wang, Y.; Guo, X.; Chen, Z.; Chen, S. Diversity and composition of bacterial endophytes among plant parts of panax notoginseng. Chin. Med. 2018, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Muresu, R.; Tondello, A.; Polone, E.; Sulas, L.; Baldan, B.; Squartini, A. Antioxidant treatments counteract the non-culturability of bacterial endophytes isolated from legume nodules. Arch. Microbiol. 2013, 195, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.N.; Padmavathy, S. Impact of endophytic microorganisms on plants, environment and humans. Sci. World J. 2014, 2014, 250693. [Google Scholar] [CrossRef] [PubMed]
- Hardoim, P.R.; van Overbeek, L.S.; van Elsas, J.D. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 2008, 16, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Mahnert, A.; Moissl-Eichinger, C. Beneficial effects of plant-associated microbes on indoor microbiomes and human health? Front. Microbiol. 2014, 5, 15. [Google Scholar] [CrossRef]
- Rout, M.E.; Southworth, D. The root microbiome influences scales from molecules to ecosystems: The unseen majority1. Am. J. Bot. 2013, 100, 1689–1691. [Google Scholar] [CrossRef]
- Diouf, D.; Samba-Mbaye, R.; Lesueur, D.; Ba, A.T.; Dreyfus, B.; De Lajudie, P.; Neyra, M. Genetic diversity of acacia seyal del. Rhizobial populations indigenous to senegalese soils in relation to salinity and ph of the sampling sites. Microb. Ecol. 2007, 54, 553–566. [Google Scholar] [CrossRef]
- Moulin, L.; Munive, A.; Dreyfus, B.; Boivin-Masson, C. Nodulation of legumes by members of the β-subclass of proteobacteria. Nature 2001, 411, 948–950. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, K.; Wemheuer, B.; Korolkow, V.; Wemheuer, F.; Nacke, H.; Schöning, I.; Schrumpf, M.; Daniel, R. Driving forces of soil bacterial community structure, diversity, and function in temperate grasslands and forests. Sci. Rep. 2016, 6, 33696. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, X.; Wu, Y.; Zhang, L.; Cheng, J.; Wei, G.; Lin, Y. Natural revegetation of a semiarid habitat alters taxonomic and functional diversity of soil microbial communities. Sci. Total Environ. 2018, 635, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Zgadzaj, R.; James, E.K.; Kelly, S.; Kawaharada, Y.; de Jonge, N.; Jensen, D.B.; Madsen, L.H.; Radutoiu, S. A legume genetic framework controls infection of nodules by symbiotic and endophytic bacteria. PLoS Genet. 2015, 11, e1005280. [Google Scholar] [CrossRef]
- Ferrando, L.; Mañay, J.F.; Scavino, A.F. Molecular and culture-dependent analyses revealed similarities in the endophytic bacterial community composition of leaves from three rice (oryza sativa) varieties. FEMS Microbiol. Ecol. 2012, 80, 696–708. [Google Scholar] [CrossRef] [PubMed]
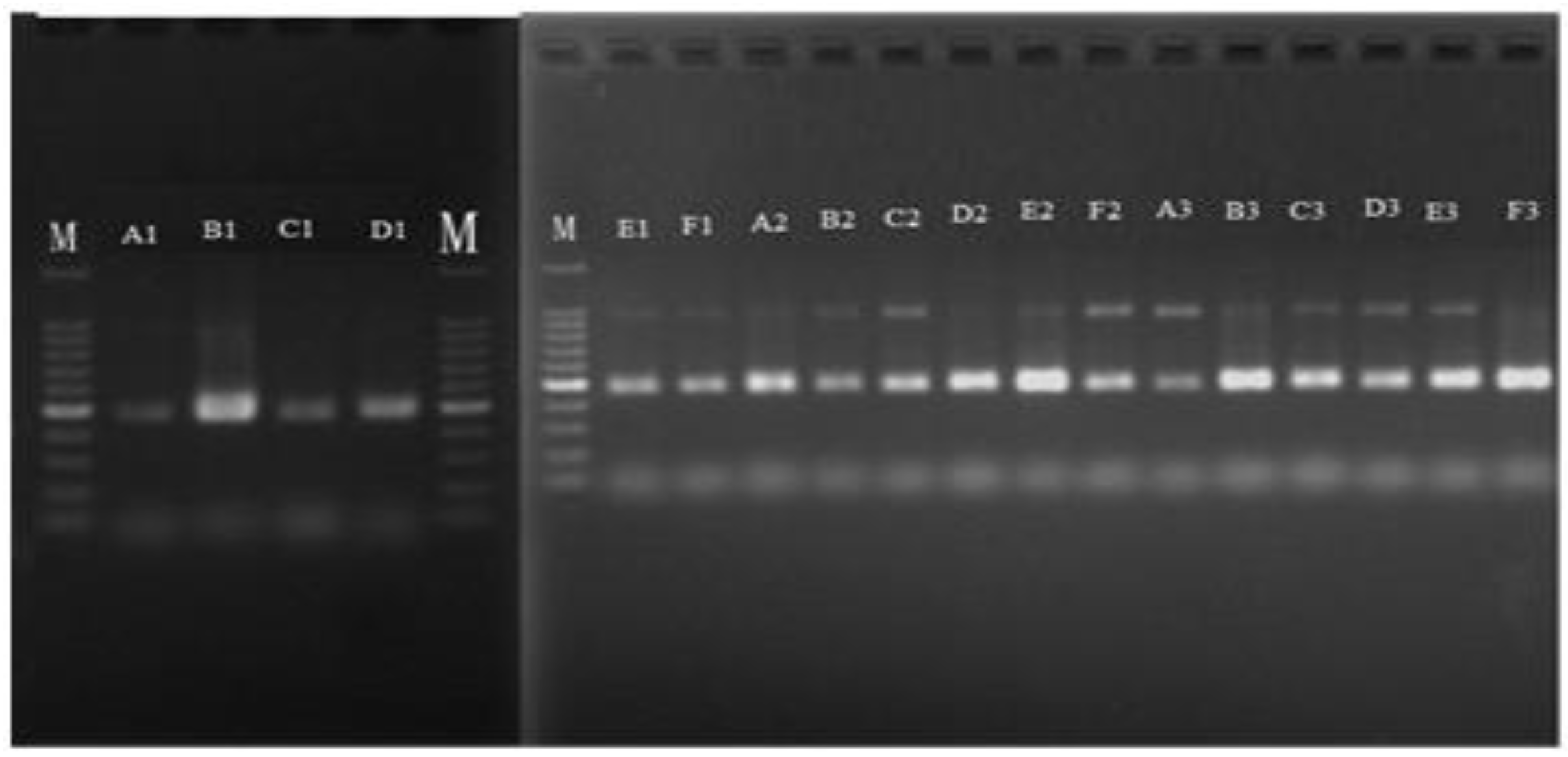
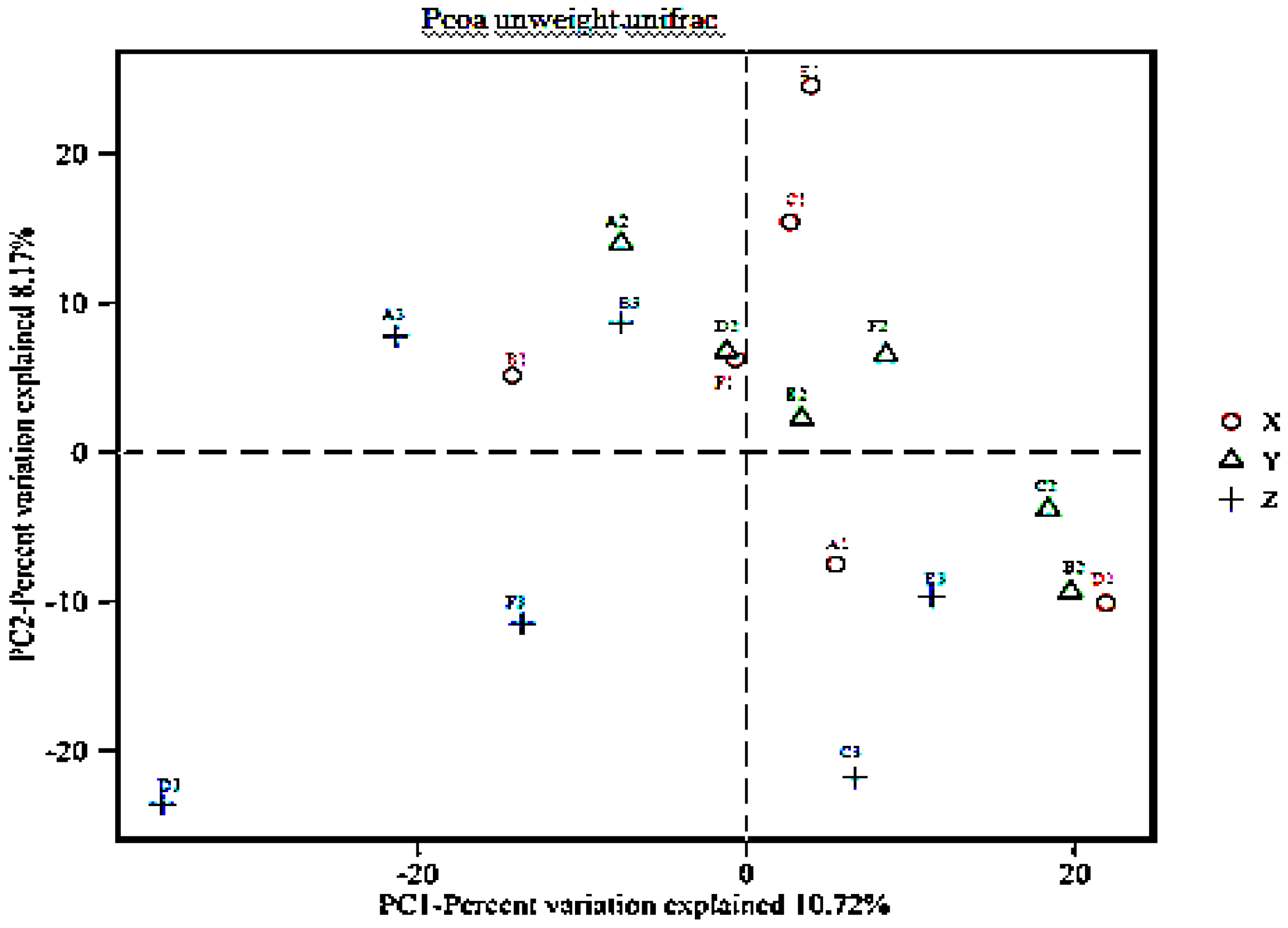

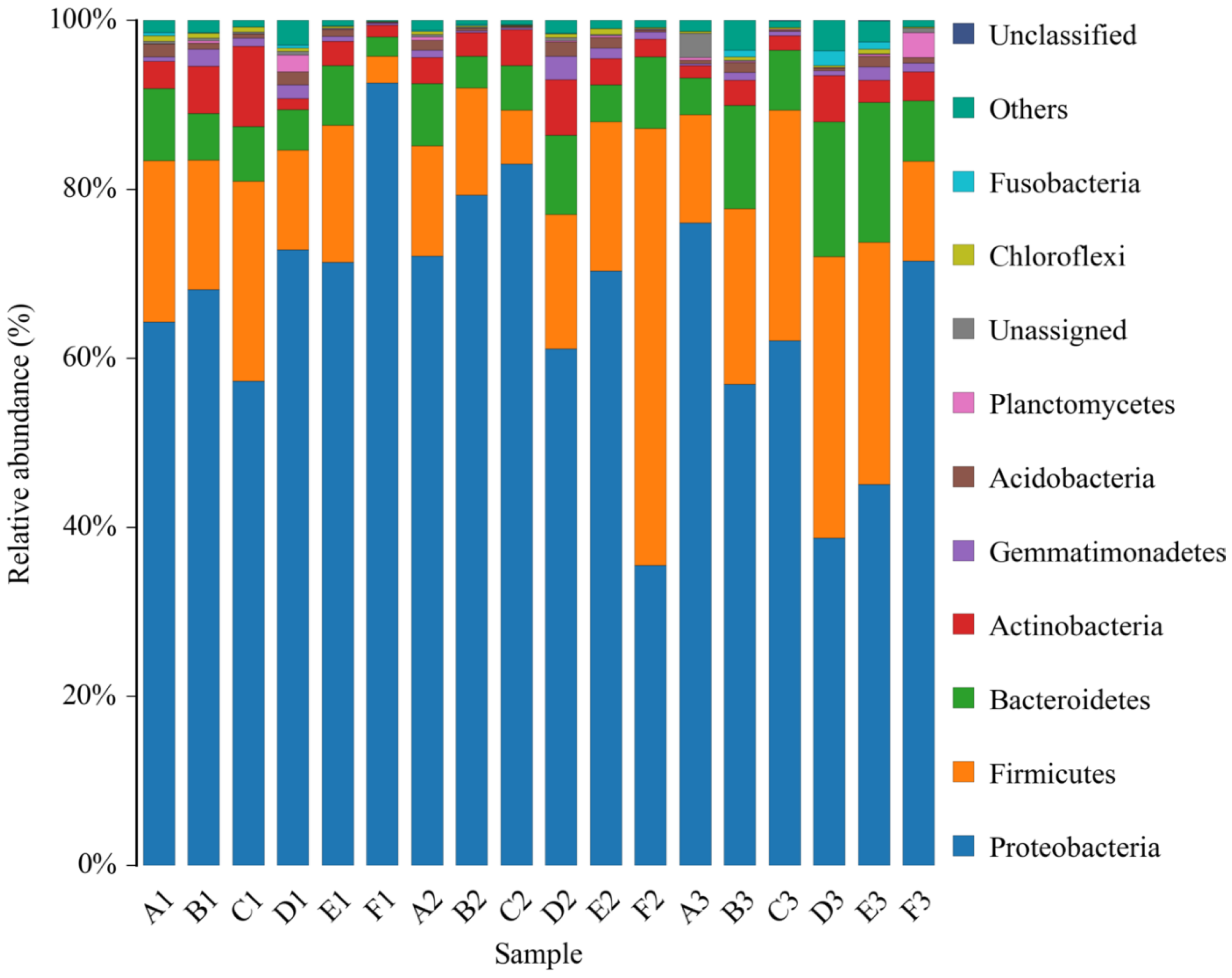
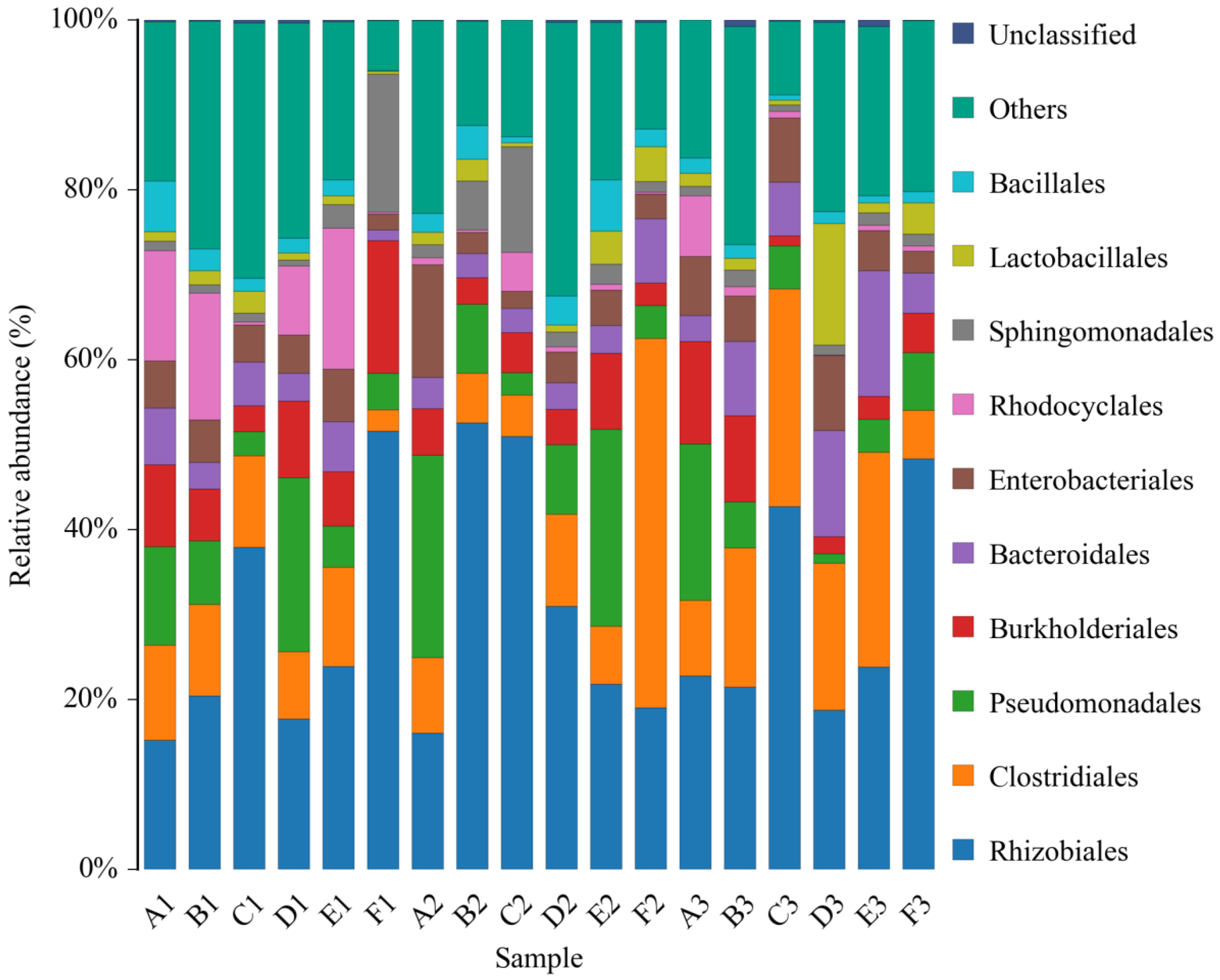
| Time | Sample ID | |||||
|---|---|---|---|---|---|---|
| NP 209 | NP PC-1 | NP 195 | NP MDA-1 | NP 187 | FU | |
| April (X) | A1 | B1 | C1 | D1 | E1 | F1 |
| July (Y) | A2 | B2 | C2 | D2 | E2 | F2 |
| October (Z) | A3 | B3 | C3 | D3 | E3 | F3 |
| Sample ID | PE Reads | Raw Tags | Clean Tags | Effective Tags | Avg Length (bp) |
|---|---|---|---|---|---|
| A1 | 635,983 | 596,365 | 574,312 | 553,476 | 444 |
| A2 | 612,984 | 578,687 | 559,132 | 545,451 | 445 |
| A3 | 437,572 | 404,908 | 386,289 | 379,373 | 442 |
| B1 | 629,137 | 588,063 | 566,411 | 543,775 | 442 |
| B2 | 598,480 | 564,240 | 549,649 | 534,123 | 436 |
| B3 | 615,982 | 584,748 | 566,126 | 556,061 | 441 |
| C1 | 621,682 | 583,501 | 563,848 | 557,754 | 439 |
| C2 | 989,097 | 936,449 | 912,801 | 898,117 | 435 |
| C3 | 431,426 | 409,194 | 398,148 | 393,178 | 436 |
| D1 | 682,742 | 640,843 | 616,566 | 607,505 | 445 |
| D2 | 547,166 | 517,633 | 502,378 | 492,925 | 440 |
| D3 | 1,096,559 | 1,077,600 | 1,052,125 | 1,025,234 | 442 |
| E1 | 753,426 | 709,316 | 683,623 | 657,209 | 442 |
| E2 | 487,276 | 460,700 | 444,107 | 434,587 | 444 |
| E3 | 691,461 | 659,694 | 641,167 | 632,130 | 439 |
| F1 | 745,999 | 708,816 | 690,867 | 675,521 | 436 |
| F2 | 803,207 | 763,307 | 742,869 | 732,976 | 437 |
| F3 | 436,209 | 405,940 | 393,706 | 387,708 | 437 |
| Sample ID | OTU | ACE | Chao1 | Simpson | Shannon | Coverage |
|---|---|---|---|---|---|---|
| A1 | 1086 | 1139.84 | 1174.50 | 0.037 | 4.9111 | 0.9998 |
| A2 | 883 | 1009.85 | 1042.75 | 0.0719 | 4.4501 | 0.9998 |
| A3 | 747 | 1764.72 | 1117.56 | 0.0806 | 3.9148 | 0.9992 |
| B1 | 892 | 930.18 | 950.24 | 0.0409 | 4.8375 | 0.9999 |
| B2 | 530 | 684.305 | 684.071 | 0.0556 | 3.9892 | 0.9998 |
| B3 | 935 | 1077.09 | 1017.22 | 0.0317 | 5.3337 | 0.9998 |
| C1 | 742 | 1645.06 | 1040.47 | 0.0628 | 4.3294 | 0.9996 |
| C2 | 509 | 741.62 | 602.84 | 0.0953 | 3.637 | 0.9999 |
| C3 | 523 | 1115.03 | 900.00 | 0.1655 | 3.6222 | 0.9995 |
| D1 | 704 | 732.51 | 755.67 | 0.0678 | 4.2485 | 0.9999 |
| D2 | 773 | 924.20 | 873.50 | 0.0628 | 4.8507 | 0.9998 |
| D3 | 1034 | 1054.59 | 1050.16 | 0.0463 | 5.0395 | 1.0 |
| E1 | 874 | 1220.39 | 1065.29 | 0.0485 | 4.4676 | 0.9998 |
| E2 | 688 | 756.54 | 731.05 | 0.0566 | 4.3287 | 0.9998 |
| E3 | 589 | 1154.67 | 1233.10 | 0.0458 | 5.1862 | 0.9998 |
| F1 | 573 | 617.72 | 675.08 | 0.0574 | 3.7455 | 0.9999 |
| F2 | 1038 | 1139.78 | 1095.03 | 0.0242 | 5.2494 | 0.9999 |
| F3 | 549 | 818.44.32 | 645.46 | 0.0913 | 4.0069 | 0.9997 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmagzob, A.A.H.; Ibrahim, M.M.; Zhang, G.-F. Seasonal Diversity of Endophytic Bacteria Associated with Cinnamomum camphora (L.) Presl. Diversity 2019, 11, 112. https://doi.org/10.3390/d11070112
Elmagzob AAH, Ibrahim MM, Zhang G-F. Seasonal Diversity of Endophytic Bacteria Associated with Cinnamomum camphora (L.) Presl. Diversity. 2019; 11(7):112. https://doi.org/10.3390/d11070112
Chicago/Turabian StyleElmagzob, Abubakr Abdelwhab Hamd, Muhammed Mustapha Ibrahim, and Guo-Fang Zhang. 2019. "Seasonal Diversity of Endophytic Bacteria Associated with Cinnamomum camphora (L.) Presl." Diversity 11, no. 7: 112. https://doi.org/10.3390/d11070112
APA StyleElmagzob, A. A. H., Ibrahim, M. M., & Zhang, G.-F. (2019). Seasonal Diversity of Endophytic Bacteria Associated with Cinnamomum camphora (L.) Presl. Diversity, 11(7), 112. https://doi.org/10.3390/d11070112





