An Anomalous Phylogenetic Position for Deraiotrema platacis Machida, 1982 (Lepocreadiidae) from Platax pinnatus on the Great Barrier Reef
Abstract
:1. Introduction
2. Materials and Methods
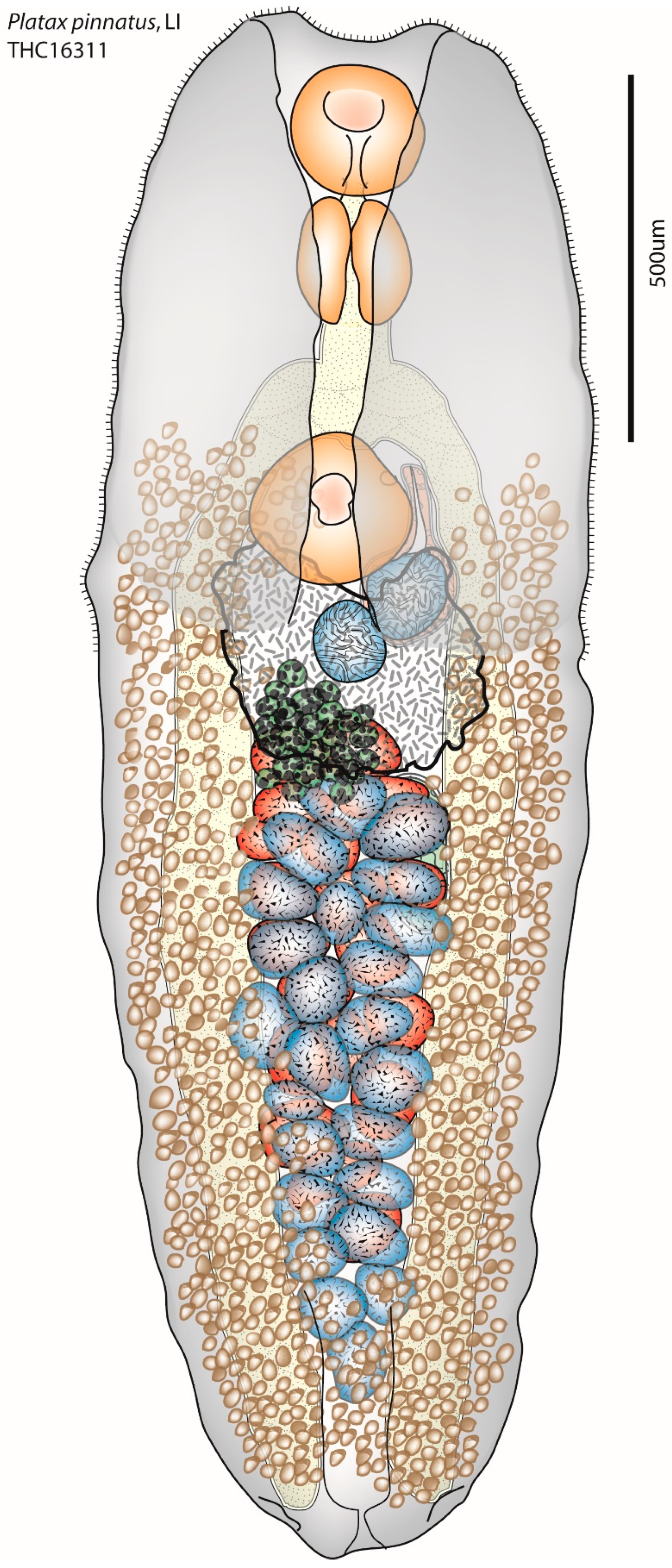
2.1. Specimen Collection and Morphological Analysis
2.2. Molecular Sequencing and Phylogenetic Analysis
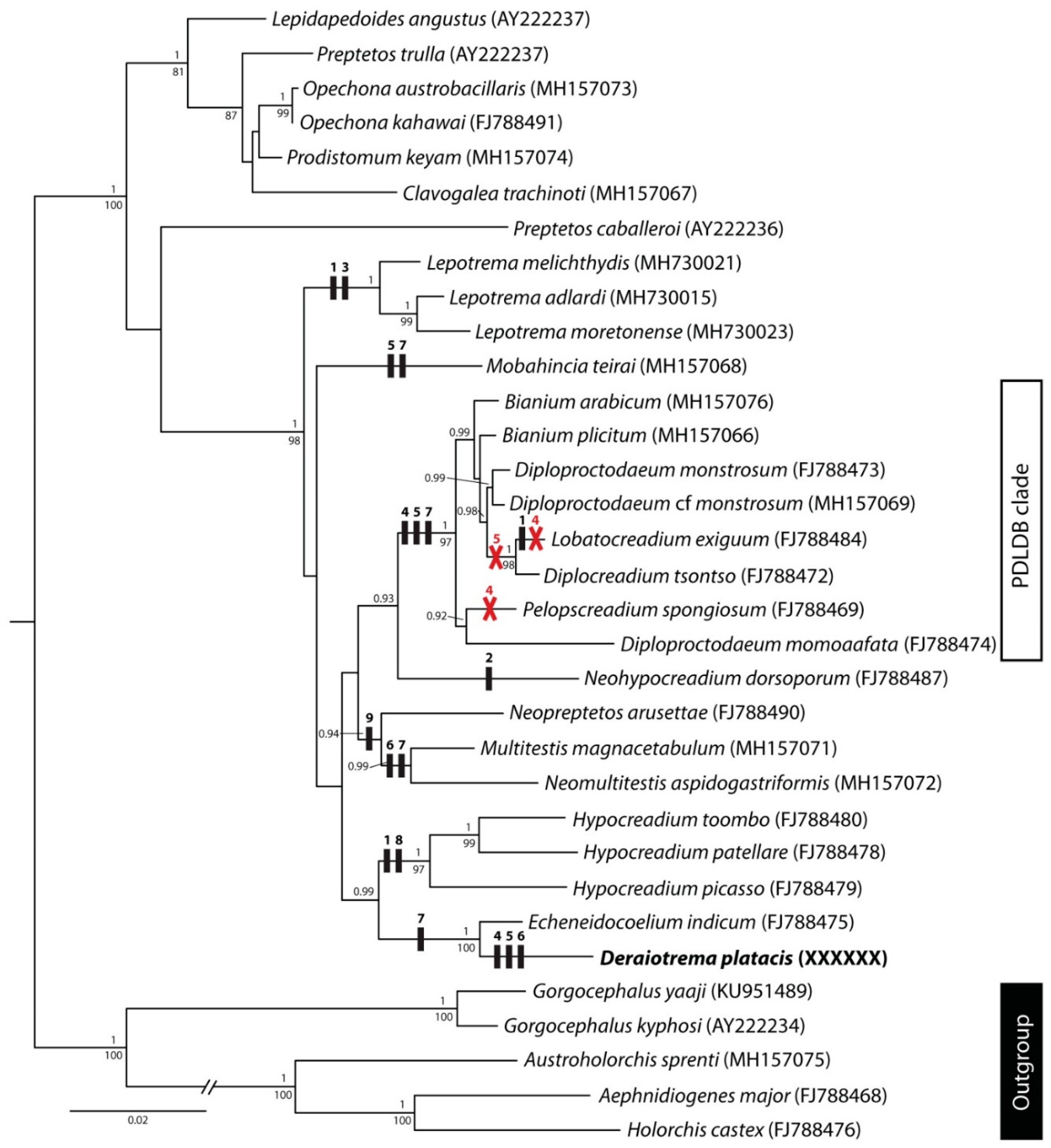
3. Molecular Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, R.A.; Waeschenbach, A.; Cribb, T.H.; Weedall, G.D.; Dyal, P.; Littlewood, D.T.J. The phylogeny of the Lepocreadioidea (Platyhelminthes: Digenea) inferred from nuclear and mitochondrial genes: Implications for their systematics and evolution. Acta Parasitol. 2009, 54, 310–329. [Google Scholar] [CrossRef]
- Bray, R.A.; Cribb, T.H. Reorganisation of the superfamily Lepocreadioidea Odhner, 1905 based on an inferred molecular phylogeny. Syst. Parasitol. 2012, 83, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Bray, R.A.; Cribb, T.H.; Cutmore, S.C. Lepocreadiidae Odhner, 1905 and Aephnidiogenidae Yamaguti, 1934 (Digenea: Lepocreadioidea) of fishes from Moreton Bay, with the erection of a new family and genus. Syst. Parasitol. 2018, 95, 479–498. [Google Scholar] [CrossRef] [PubMed]
- Cribb, T.H.; Bray, R.A. Gut wash, body soak, blender and heat-fixation: Approaches to the effective collection, fixation and preservation of trematodes of fishes. Syst. Parasitol. 2010, 76, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cutmore, S.C.; Diggles, B.K.; Cribb, T.H. Transversotrema Witenberg, 1944 (Trematoda: Transversotrematidae) from inshore fishes of Australia: Description of a new species and significant range extensions for three congeners. Syst. Parasitol. 2016, 93, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Wee, N.Q.X.; Cribb, T.H.; Bray, R.A.; Cutmore, S.C. Two known and one new species of Proctoeces from Australian teleosts: Variable host-specificity for closely related species identified through multi-locus molecular data. Parasitol. Int. 2017, 66, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.A.T.; Blair, D. Nuclear rDNA ITS sequence variation in the trematode genus Echinostoma: An aid to establishing relationships within the 37-collar-spine group. Parasitology 1995, 111, 609–615. [Google Scholar] [CrossRef]
- Cribb, T.H.; Anderson, G.R.; Adlard, R.D.; Bray, R.A. A DNA-based demonstration of a three-host life-cycle for the Bivesiculidae (Platyhelminthes: Digenea). Int. J. Parasitol. 1998, 28, 1791–1795. [Google Scholar] [CrossRef]
- Littlewood, D.T.J. Molecular phylogenetics of cupped oysters based on partial 28S ribosomal RNA gene sequences. Mol. Phylogenet. Evol. 1994, 3, 221–229. [Google Scholar] [CrossRef]
- Littlewood, D.T.J.; Curini-Galletti, M.; Herniou, E.A. The interrelationships of Proseriata (Platyhelminthes: Seriata) tested with molecules and morphology. Mol. Phylogenet. Evol. 2000, 16, 449–466. [Google Scholar] [CrossRef]
- Littlewood, D.T.J.; Rohde, K.; Clough, K.A. Parasite speciation within or between host species?—Phylogenetic evidence from site-specific polystome monogeneans. Int. J. Parasitol. 1997, 27, 1289–1297. [Google Scholar] [CrossRef]
- Snyder, S.D.; Tkach, V.V. Phylogenetic and biogeographical relationships among some holarctic frog lung flukes (Digenea: Haematoloechidae). J. Parasitol. 2001, 87, 1433–1440. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Schleicher, T.; Schultz, J.; Muller, T.; Dandekar, T.; Wolf, M. 5.8S-28S rRNA interaction and HMM-based ITS2 annotation. Gene 2009, 430, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Ankenbrand, M.J.; Keller, A.; Wolf, M.; Schultz, J.; Forster, F. ITS2 Database V: Twice as much. Mol. Biol. Evol. 2015, 32, 3030–3032. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiler, E.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis, Version 3.2. 2017. 2018. Available online: http://mesquiteproject.wikispaces.com/installation (accessed on 10 January 2109).
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A tool for Phylogenetic Analysis and Post-analysis of Large Phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Bray, R.A.; Cutmore, S.C.; Cribb, T.H. Lepotrema Ozaki, 1932 (Lepocreadiidae: Digenea) from Indo-Pacific fishes, with the description of eight new species, characterised by morphometric and molecular features. Syst. Parasitol. 2018, 95, 693–741. [Google Scholar] [CrossRef]
- Olson, P.D.; Cribb, T.H.; Tkach, V.V.; Bray, R.A.; Littlewood, D.T.J. Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). Int. J. Parasitol. 2003, 33, 733–755. [Google Scholar] [CrossRef]
- Huston, D.C.; Cutmore, S.C.; Cribb, T.H. The life-cycle of Gorgocephalus yaaji Bray & Cribb, 2005 (Digenea: Gorgocephalidae) with a review of the first intermediate hosts for the superfamily Lepocreadioidea Odhner, 1905. Syst. Parasitol. 2016, 93, 653–665. [Google Scholar] [PubMed]
- Machida, M. Lepocreadiid trematodes from marine fishes of Palau. Proc. Jap. Soc. Syst. Zool. 1982, 23, 1–11. [Google Scholar]
- Bray, R.A. Family Lepocreadiidae Odhner, 1905. In Keys to the Trematoda. Volume 2; Jones, A., Bray, R.A., Gibson, D.I., Eds.; CABI Publishing and the Natural History Museum: Wallingford, UK, 2005; pp. 545–602. [Google Scholar]
- Dronen, N.O.; Blend, C.K.; Khalifa, R.M.A.; Mohamadain, H.S.; Karar, Y.F.M. Pelopscreadium aegyptense n. gen., n. sp. and Pelopscreadium spongiosum (Bray & Cribb, 1998) n. comb., (Digenea: Lepocreadiidae), each from disjunct populations of the Yellow boxfish, Ostracion cubicus Linnaeus (Ostraciidae). Zootaxa 2016, 4127, 567–578. [Google Scholar] [PubMed]
- Machida, M. Redescriptions of Mamaev’s two digenean trematodes Diploproctia drepanei (Lepocreadiidae) and Beluesca plectorhynchi (Cryptogonimidae). Bull. Nat. Mus. Nat. Sci. Ser. A 2014, 40, 161–165. [Google Scholar]
- Mamaev, Y.L. Helminths of some commercial fishes in the Gulf of Tong King. In Helminths of Animals of South-East Asia; Oshmarin, P.G., Mamaev, Y.L., Lebedev, B.I., Eds.; Izdatel’stvo Nauka: Moscow, Russia, 1970; pp. 127–190. (In Russian) [Google Scholar]
- Manter, H.W. Some digenetic trematodes of marine fishes of Beaufort, North Carolina. Parasitology 1931, 23, 396–411. [Google Scholar] [CrossRef]
- Linton, E. Trematodes from fishes mainly from the Woods Hole region, Massachusetts. Proc. U. S. Natl. Mus. 1940, 88, 1–172. [Google Scholar] [CrossRef]
- Ramadan, M.M. A review of the trematode genus Rhagorchis Manter 1931 (Lepocreadiidae), with a description of Rhagorchis manteri sp. nov., an intestinal parasite of a scarid fish from the Red Sea. Z. Parasitenkd. 1982, 67, 273–277. [Google Scholar] [CrossRef]
- Yamaguti, S. Synopsis of Digenetic Trematodes of Vertebrates (Vol. 1 & 2); Keigaku: Tokyo, Japan, 1971; Vol. I, p.1074; Vol. II, p. 1349. [Google Scholar]
- Manter, H.W. The digenetic trematodes of marine fishes of Tortugas, Florida. Am. Midl. Nat. 1947, 38, 257–416. [Google Scholar] [CrossRef]
- Sogandares-Bernal, F. Digenetic trematodes of marine fishes from the Gulf of Panama and Bimini, British West Indies. Tulane Stud. Zool. 1959, 7, 69–117. [Google Scholar]
- Nahhas, F.M.; Cable, R.M. Digenetic and aspidogastrid trematodes from marine fishes of Curaçao and Jamaica. Tulane Stud. Zool. 1964, 11, 169–228. [Google Scholar] [CrossRef]
- Dyer, W.G.; Williams, E.H.; Williams, L.B. Digenetic trematodes of marine fishes of the western and southwestern coasts of Puerto Rico. Proc. Helminthol. Soc. Wash. 1985, 52, 85–94. [Google Scholar]
- Bunkley-Williams, L.; Dyer, W.G.; Williams, E.H., Jr. Some aspidogastrid and digenean trematodes of Puerto Rican marine fishes. J. Aquat. Anim. Health 1996, 8, 87–92. [Google Scholar] [CrossRef]
- Nagaty, H.F. Trematodes of fishes from the Red Sea. Part 8. Five species in the families Schistorchidae, Acanthocolpidae, and Heterophyidae. J. Parasitol. 1957, 43, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Blend, C.K.; Karar, Y.F.M.; Dronen, N.O. Revision of the Megaperidae Manter, 1934 n. comb. [sic] (Syn. Apocreadiidae Skrjabin, 1942) including a reorganization of the Schistorchiinae Yamaguti, 1942. Zootaxa 2017, 4358, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Saoud, M.F.A.; Ramadan, M.M.; Al Kawari, K.S.R. Helminth parasites of fishes from the Arabian Gulf. 1. Preliminary general survey of fishes mainly from Qatari waters. Qatar Univ. Sci. Bull. 1986, 6, 199–229. [Google Scholar]
- Simha, S.S.; Pershad, R.S. Echeneidocoelium indicum n. gen. n. sp. (Trematoda) from the sucker fish, Echeneis remora, from Vishakapatnam (A.P.) India. Rivista di Parassitol. 1964, 25, 21–24. [Google Scholar]
- Madhavi, R. Redescription and systematic position of Echeneidocoelium indicum Simha and Pershad, 1964 (Trematoda: Digenea) from the sucker fish Echeneis naucrates. J. Parasitol. 1970, 56, 317–320. [Google Scholar] [CrossRef]
- Bray, R.A.; Cribb, T.H. Lepocreadiidae (Digenea) of Australian coastal fishes: New species of Opechona Looss, 1907, Lepotrema Ozaki, 1932 and Bianium Stunkard, 1930 and comments on other species reported for the first time or poorly known in Australian waters. Syst. Parasitol. 1998, 41, 123–148. [Google Scholar] [CrossRef]
- Manter, H.W. Studies on digenetic trematodes of fishes of Fiji. II. Families Lepocreadiidae, Opistholebetidae, and Opecoelidae. J. Parasitol. 1963, 49, 99–113. [Google Scholar] [CrossRef]
- Yamaguti, S. Digenetic Trematodes of Hawaiian Fishes; Keigaku: Tokyo, Japan, 1970; p. 436. [Google Scholar]
- Bray, R.A.; Cribb, T.H. The Australian species of Lobatocreadium Madhavi, 1972, Hypocreadium Ozaki, 1936 and Dermadena Manter, 1945 (Digenea: Lepocreadiidae), parasites of marine tetraodontiform fishes. Syst. Parasitol. 1996, 35, 217–236. [Google Scholar] [CrossRef]
- Smith, J.W. Superfamily Schistosomatoidea Stiles & Hassall, 1898. In Keys to the Trematode Parasites of Vertebrates. Volume 1; Gibson, D.I., Jones, A., Bray, R.A., Eds.; CAB International: Wallingford, UK, 2002; pp. 416–417. [Google Scholar]
- Kanev, I.; Radev, V.; Fried, B. Family Typhlocoelidae Harrah, 1922. In Keys to the Trematoda. Volume 1; Gibson, D.I., Jones, A., Bray, R.A., Eds.; CABI Publishing and the Natural History Museum: Wallingford, UK, 2002; pp. 153–158. [Google Scholar]
- Gibson, D.I. Family Syncoeliidae Looss, 1899. In Keys to the Trematoda. Volume 1; Gibson, D.I., Jones, A., Bray, R.A., Eds.; CABI Publishing and the Natural History Museum: Wallingford, UK, 2002; pp. 409–413. [Google Scholar]
- Blair, D.; Barton, D.P. Family Orchipedidae Skrjabin, 1913. In Keys to the Trematoda. Volume 3; Bray, R.A., Gibson, D.I., Jones, A., Eds.; CABI Publishing and the Natural History Museum: Wallingford, UK, 2008; pp. 265–269. [Google Scholar]
- Bray, R.A. Family Acanthocolpidae Lühe, 1906. In Keys to the Trematoda. Volume 2; Jones, A., Bray, R.A., Gibson, D.I., Eds.; CABI Publishing and the Natural History Museum: Wallingford, UK, 2005; pp. 603–619. [Google Scholar]
- Cribb, T.H. Family Opecoelidae Ozaki, 1925. In Keys to the Trematoda. Volume 2; Jones, A., Bray, R.A., Gibson, D.I., Eds.; CABI Publishing and the Natural History Museum: Wallingford, UK, 2005; pp. 443–531. [Google Scholar]
- Miller, T.L.; Cribb, T.H. Family Cryptogonimidae Ward, 1917. In Keys to the Trematoda. Volume 3; Bray, R.A., Gibson, D.I., Jones, A., Eds.; CABI Publishing and the Natural History Museum: Wallingford, UK, 2008; pp. 51–112. [Google Scholar]
- Madhavi, R. Family Monorchiidae Odhner, 1911. In Keys to the Trematoda. Volume 3; Bray, R.A., Gibson, D.I., Jones, A., Eds.; CABI Publishing and The Natural History Museum: Wallingford, UK, 2008; pp. 145–175. [Google Scholar]
- Campbell, R.A. Family Gorgoderidae Looss, 1899. In Keys to the Trematoda. Volume 3; Bray, R.A., Gibson, D.I., Jones, A., Eds.; CABI Publishing and the Natural History Museum: Wallingford, UK, 2008; pp. 191–213. [Google Scholar]
- Miller, T.L.; Cribb, T.H. Dramatic phenotypic plasticity within species of Siphomutabilus n. g. (Digenea: Cryptogonimidae) from Indo-Pacific caesionines (Perciformes: Lutjanidae). Syst. Parasitol. 2013, 86, 101–112. [Google Scholar] [CrossRef] [PubMed]
| Species | Host Species | GenBank Accession # | Reference |
|---|---|---|---|
| Lepocreadiidae Odhner, 1905 | |||
| Bianium arabicum Sey, 1996 | Lagocephalus lunaris (Bloch & Schneider) | MH157076 | [3]) |
| Bianium plicitum (Linton, 1928) Stunkard, 1931 | Torquigener pleurogramma (Regan) | MH157066 | [3] |
| Clavogalea trachinoti (Fischthal & Thomas, 1968) Bray & Gibson, 1990 | Trachinotus coppingeri Günther | MH157067 | [3] |
| Deraiotrema platacis Machida, 1982 | Platax pinnatus (Linnaeus) | XXXXXX | Current study |
| Diplocreadium tsontso Bray, Cribb & Barker, 1996 | Balistoides conspicillum (Bloch & Schneider) | FJ788472 | [1] |
| Diploproctodaeum momoaafata Bray, Cribb & Barker, 1996 | Ostracion cubicus Linnaeus | FJ788474 | [1] |
| Diploproctodaeum monstrosum Bray, Cribb & Justine, 2010 | Arothron stellatus (Anonymous) | FJ788473 | [1] |
| Diploproctodaeum cf monstrosum | Arothron hispidus (Linnaeus) | MH157069 | [3] |
| Echeneidocoelium indicum Simha & Pershad, 1964 | Echeneis naucrates Linnaeus | FJ788475 | [1] |
| Hypocreadium patellare Yamaguti, 1938 | Balistoides viridescens (Bloch & Schneider) | FJ788478 | [1] |
| Hypocreadium picasso Bray, Cribb & Justine, 2009 | Rhinecanthus aculeatus (Linnaeus) | FJ788479 | [1] |
| Hypocreadium toombo Bray & Justine, 2006 | Pseudobalistes fuscus (Bloch & Schneider) | FJ788480 | [1] |
| Lepidapedoides angustus Bray, Cribb & Barker, 1996 | Epinephelus cyanopodus (Richardson) | FJ788482 | [1] |
| Lepotrema adlardi (Bray, Cribb & Barker, 1993) Bray & Cribb, 1996 | Abudefduf bengalensis (Bloch) | MH730015 | [22] |
| Lepotrema melichthydis Bray, Cutmore & Cribb, 2018 | Melichthys vidua (Richardson) | MH730021 | [22] |
| Lepotrema moretonense Bray, Cutmore & Cribb, 2018 | Prionurus microlepidotus Lacépède | MH730023 | [22] |
| Lobatocreadium exiguum (Manter, 1963) | Pseudobalistes fuscus | FJ788484 | [1] |
| Mobahincia teirai Bray, Cribb & Cutmore, 2018 | Platax teira (Forsskål) | MH157068 | [3] |
| Multitestis magnacetabulum Mamaev, 1970 | Platax teira | MH157071 | [3] |
| Neohypocreadium dorsoporum Machida & Uchida, 1987 | Chaetodon flavirostris Günther | FJ788487 | [1] |
| Neomultitestis aspidogastriformis Bray & Cribb, 2003 | Platax teira | MH157072 | [3] |
| Neopreptetos arusettae Machida, 1982 | Pomacanthus sexstriatus (Cuvier) | FJ788490 | [1] |
| Opechona austrobacillaris Bray & Cribb, 1998 | Pomatomus saltatrix Linnaeus | MH157073 | [3] |
| Opechona kahawai Bray & Cribb, 2003 | Arripis trutta (Forster) | FJ788491 | [1] |
| Pelopscreadium spongiosum (Bray & Cribb, 1998) Dronen, Blend, Khalifa, Mohamadain & Karer, 2016 | Ostracion cubicus | FJ788469 | [1] |
| Preptetos caballeroi Pritchard, 1960 | Naso vlamingii (Valenciennes) | AY222236 | [23] |
| Preptetos trulla (Linton, 1907) Bray & Cribb, 1996 | Ocyurus chrysurus (Bloch) | AY222237 | [23] |
| Prodistomum keyam Bray & Cribb, 1996 | Monodactylus argenteus (Linnaeus) | MH157074 | [3] |
| Outgroup taxa | |||
| Aephnidiogenidae Yamaguti, 1934 | |||
| Aephnidiogenes major Yamaguti, 1934 | Diagramma pictum labiosum (Macleay) | FJ788468 | [1] |
| Austroholorchis sprenti (Gibson, 1987) Bray & Cribb, 1997 | Sillago maculata Quoy & Gaimard | MH157075 | [3] |
| Holorchis castex Bray & Justine, 2007 | Diagramma pictum pictum (Thunberg) | FJ788476 | [1] |
| Gorgocephalidae Manter, 1966 | |||
| Gorgocephalus kyphosi Manter, 1966 | Kyphosus vaigiensis (Quoy & Gaimard) | AY222234 | [23] |
| Gorgocephalus yaaji Bray & Cribb, 2005 | Kyphosus cinerascens (Forsskål) | KU951489 | [24] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bray, R.A.; Cutmore, S.C.; Cribb, T.H. An Anomalous Phylogenetic Position for Deraiotrema platacis Machida, 1982 (Lepocreadiidae) from Platax pinnatus on the Great Barrier Reef. Diversity 2019, 11, 104. https://doi.org/10.3390/d11070104
Bray RA, Cutmore SC, Cribb TH. An Anomalous Phylogenetic Position for Deraiotrema platacis Machida, 1982 (Lepocreadiidae) from Platax pinnatus on the Great Barrier Reef. Diversity. 2019; 11(7):104. https://doi.org/10.3390/d11070104
Chicago/Turabian StyleBray, Rodney A., Scott C. Cutmore, and Thomas H. Cribb. 2019. "An Anomalous Phylogenetic Position for Deraiotrema platacis Machida, 1982 (Lepocreadiidae) from Platax pinnatus on the Great Barrier Reef" Diversity 11, no. 7: 104. https://doi.org/10.3390/d11070104
APA StyleBray, R. A., Cutmore, S. C., & Cribb, T. H. (2019). An Anomalous Phylogenetic Position for Deraiotrema platacis Machida, 1982 (Lepocreadiidae) from Platax pinnatus on the Great Barrier Reef. Diversity, 11(7), 104. https://doi.org/10.3390/d11070104





