Evaluation of Gonadal Tissue to Validate Size at Reproductive Maturity in Kemp’s Ridley Sea Turtles Found Stranded in Texas, USA
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Collection
2.2. Evaluation of Female Gonads
2.3. Evaluation of Male Gonads
2.4. Histology
2.5. Statistical Analysis
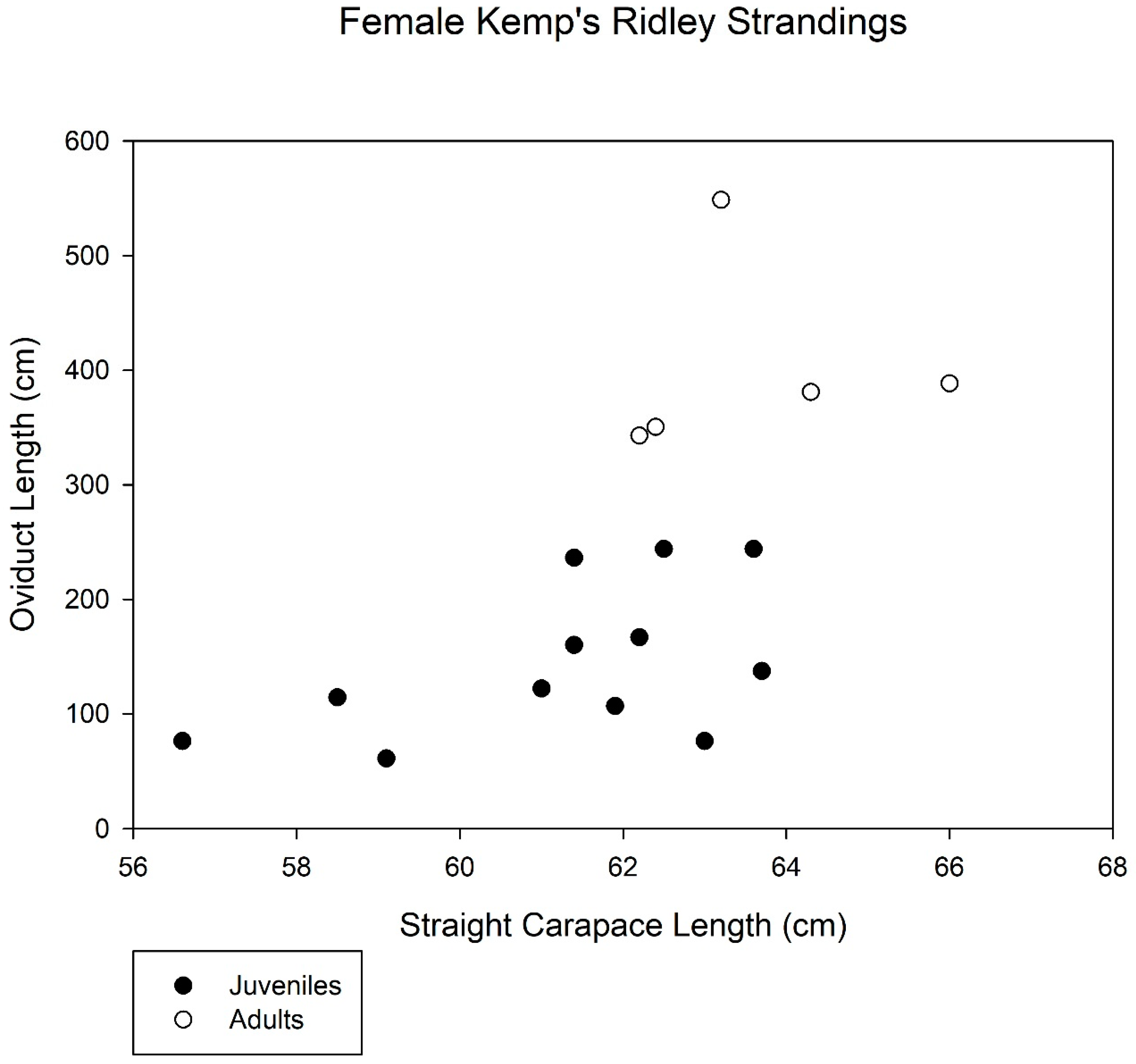
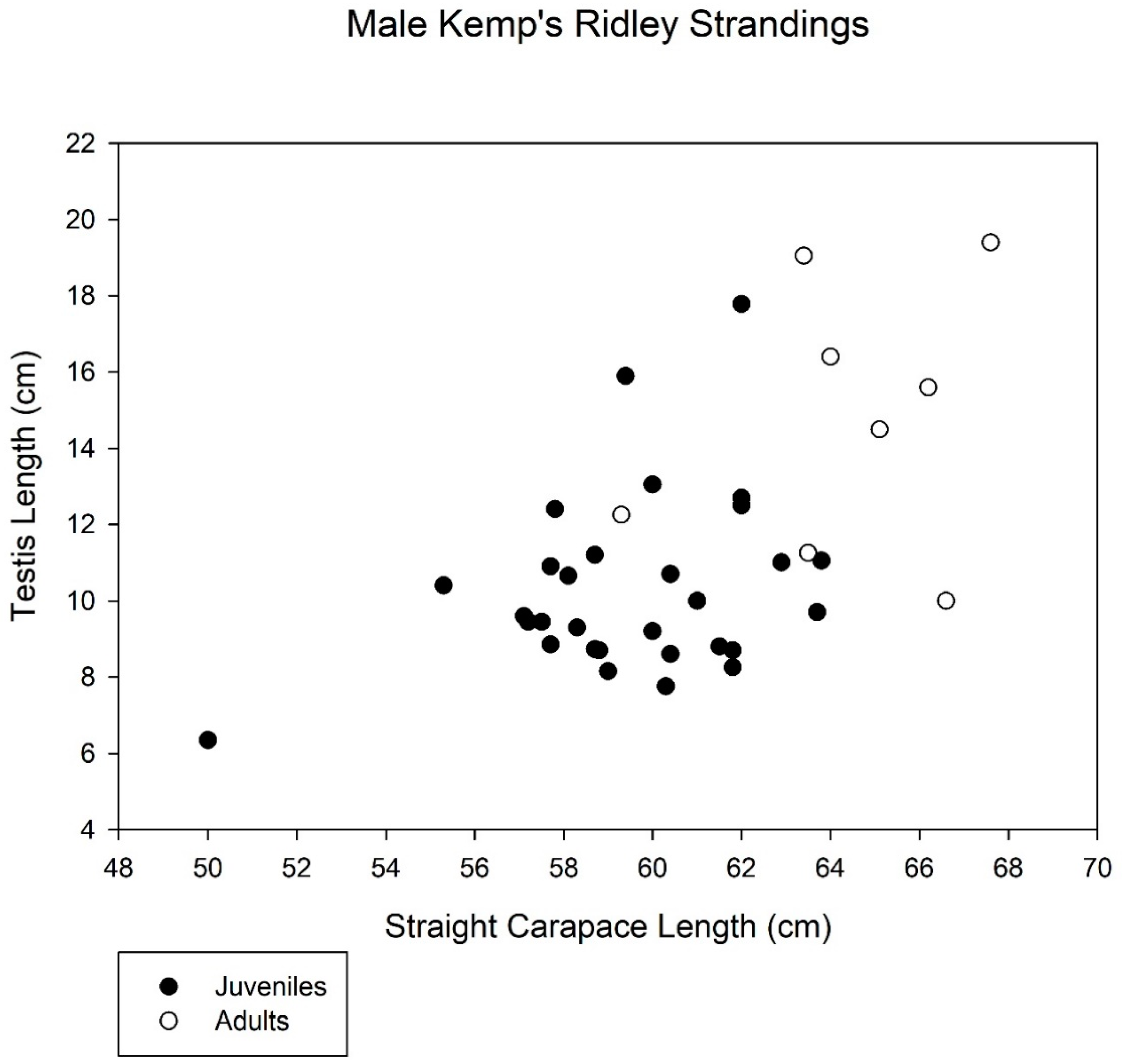
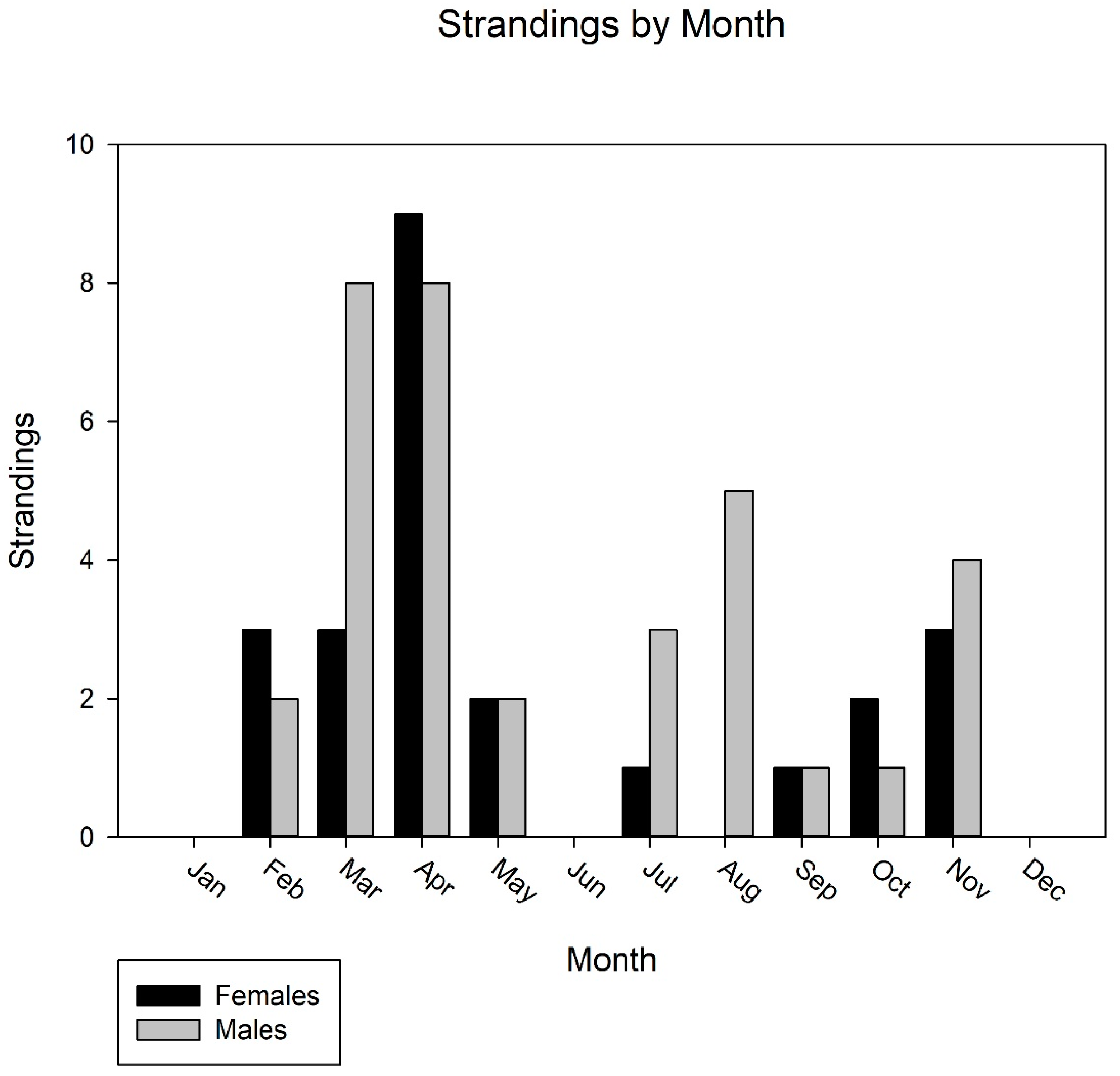
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IUCN. The IUCN Red List of Threatened Species. Version 2019.1. Available online: http://www.iucnredlist.org (accessed on 22 March 2019).
- Shaver, D.J.; Schroeder, B.A.; Byles, R.A.; Burchfield, P.M.; Pena, J.; Marquez, R.; Martinez, H.J. Movements and home ranges of adult male Kemp’s ridley sea turtles (Lepidochelys kempii) in the Gulf of Mexico investigated by satellite telemetry. Chelonian Conserv. Biol. 2005, 4, 817–827. [Google Scholar]
- Shaver, D.J.; Hart, K.M.; Fujisaki, I.; Rubio, C.; Sartain-Iverson, A.R.; Pena, J.; Gomez Gamez, D.; de Jesus Gonzales Diaz Miron, R.; Burchfield, P.M.; Martinez, H.J.; et al. Migratory corridors of adult female Kemp’s ridley turtles in the Gulf of Mexico. Biol. Conserv. 2016, 194, 158–167. [Google Scholar] [CrossRef]
- Bevan, E.; Wibbles, T.; Najera, B.M.Z.; Sarti, L.; Martinez, F.I.; Cuevas, J.M.; Gallaway, B.J.; Pena, L.J.; Burchfield, P.M. Estimating the historic size and current status of the Kemp’s ridley sea turtle (Lepidochelys kempii) population. Ecosphere 2016, 7, e01244. [Google Scholar] [CrossRef]
- Heppell, S.S.; Burchfield, P.M.; Pena, L.J. Kemp’s ridley recovery: How far we have come, and where are we headed? In Biology and Conservation of Ridley Sea Turtles; Plotkin, P.T., Ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2007; pp. 325–335. [Google Scholar]
- NMFS. Bi-national Recovery Plan for the Kemp’s ridley Sea Turtle (Lepidochelys kempii); United States Fish and Wildlife Service: Silver Spring, MD, USA, 2011.
- Caillouet, C.W., Jr.; Shaver, D.J.; Teas, W.G.; Nance, J.M.; Revera, D.B.; Cannon, A.C. Relationship between sea turtle strand rates and shrimp fishing intensities in the northwestern Gulf of Mexico: 1986–1989 versus 1990–1993. Oceanogr. Lit. Rev. 1996, 11, 1168–1169. [Google Scholar]
- Shaver, D.J. Sea turtle strandings in Texas. In Report of the Sea Turtle Health Assessment Workshop, 2–3 February 1998, Part I: Background and Information Needs, Charleston, South Carolina; Fair, P.A., Hansen, L.J., Eds.; NOAA Technical Memorandum: Charleston, SC, USA, 1998; pp. 75–80. [Google Scholar]
- Lewison, B.; Crowder, L.; Shaver, D.J. The impact of Turtle Excluder Devices and fisheries closures on loggerhead and Kemp’s ridley strandings in the western Gulf of Mexico. Conserv. Biol. 2003, 17, 1089–1097. [Google Scholar] [CrossRef]
- Frazier, J.; Arauz, R.; Chevalier JFormia, A.; Fretey, J.; Godfrey, M.H.; Marquz, M.R.; Pandav, B.; Shanker, K. Human turtle interactions at sea. In Biology and Conservation of Ridley Sea Turtles; Plotkin, P.T., Ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2007; pp. 253–295. [Google Scholar]
- Caillouet, C.W., Jr.; Putman, N.F.; Shaver, D.J.; Valverde, R.A.; Seney, E.E.; Lohmann, K.J.; Mansfield, K.L.; Gallaway, B.J.; Flanagan, J.P.; Godfrey, M.H. A call for evaluation of the contribution made by rescue, resuscitation, rehabilitation, and release translocations to Kemp’s ridley sea turtle (Lepidochelys kempii) population recovery. Herpetol. Conserv. Biol. 2016, 11, 486–496. [Google Scholar]
- Gallaway, B.J.; Gazey, W.J.; Caillouet, C.W., Jr.; Plotkin, P.T.; Grobois, A.A.; Amos, A.F.; Burchfield, P.M.; Carthy, R.R.; Castro Martínez, M.A.; Cole, J.G.; et al. Development of a Kemp’s ridley sea turtle stock assessment model. Gulf Mex. Sci. 2016, 33, 138–157. [Google Scholar] [CrossRef][Green Version]
- Gallaway, B.J.; Gazey, W.J.; Wibbels, T.; Bevan, E. Evaluation of the status of the Kemp’s ridley sea turtle following the 2010 Deepwater Horizon oil spill. Gulf Mex. Sci. 2016, 33, 192–202. [Google Scholar]
- Caillouet, C.W., Jr.; Shaver, D.J.; Landry, A.M., Jr.; Owens, D.W.; Pritchard, P.C. 2011. Kemp’s ridley sea turtle (Lepidochelys kempii) age at first nesting. Chelonian Conserv. Biol. 2011, 10, 288–293. [Google Scholar] [CrossRef]
- Crowder, L.B.; Hopkins-Murphy, S.R.; Royle, J.A. Effects of turtle excluder devices (TEDs) on loggerhead sea turtle strandings with implications for conservation. Copeia 1995, 1995, 773–779. [Google Scholar] [CrossRef]
- Magnuson, J.J.; Bjorndal, K.A.; DuPaul, W.D.; Graham, G.L.; Owens, D.W.; Peterson, C.H.; Pritchard, P.C.H.; Richardson, J.I.; Saul, G.E.; West, C.W. Decline of the Sea Turtles: Causes and Prevention; National Research Council, National Academy Science Press: Washington, DC, USA, 1990. [Google Scholar]
- Shaver, D.J.; (Padre Island National Seashore, Corpus Christi, TX, USA). Personal communication, 2019.
- National Marine Fisheries Service (NMFS) and US Fish; Wildlife Service (USFWS). Kemp’s Ridley Sea Turtle (Lepidochelys kempii) 5-Year Review: Summary and Evaluation; NMFS and USFWS: Silver Spring, MD, USA, 2015.
- Crowder, L.B.; Crouse, D.T.; Heppell, S.S.; Martin, T.H. Predicting the impact of turtle excluder devices on loggerhead sea turtle populations. Ecol. Appl. 1994, 4, 437–445. [Google Scholar] [CrossRef]
- Heppell, S.S.; Crowder, L.B.; Crouse, D.T. Models to evaluate headstarting as a management tool for long-lived turtles. Ecol. Appl. 1996, 6, 556–565. [Google Scholar] [CrossRef]
- Scott, R.; Marsh, R.; Hays, G.C. Life in the really slow lane: Loggerhead sea turtles mature late relative to other reptiles. Funct. Ecol. 2012, 26, 227–235. [Google Scholar] [CrossRef]
- Avens, L.; Snover, M.L. Age and age estimation in sea turtles. In The Biology of Sea Turtles; Wyneken, J., Lohmann, K.J., Musick, J.A., Eds.; CRC Press: Boca Raton, FL, USA, 2013; Volume 3, pp. 97–133. [Google Scholar]
- Avens, L.; Goshe, L.R.; Coggins, L.; Shaver, D.J.; Higgins, B.; Landry, A.M.; Bailey, R. Variability in age and size at maturation, reproductive longevity, and long-term growth dynamics for Kemp’s ridley sea turtles in the Gulf of Mexico. PLoS ONE 2017, 12, e0173999. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E. Applied Multivariate Methods for Data Analysis; Brooks/Cole Publishing: Pacific Grove, CA, USA, 1998. [Google Scholar]
- Xue, J.; Titterington, D.M. Do unbalanced data have a negative effect on LDA? Pattern Recognit. 2007, 41, 1558–1571. [Google Scholar] [CrossRef]
- Hintze, J. NCSS; NCSS, LLC.: Kaysville, UT, USA, 2007. [Google Scholar]
- Hamann, M.; Limpus, C.J.; Owens, D.W. Reproductive cycles of males and females. In The Biology of Sea Turtles; Lutz, P.L., Musick, J.A., Wyneken, J., Eds.; CRC Press: Boca Raton, FL, USA, 2003; Volume 2, pp. 135–161. [Google Scholar]
- Rostal, D.C. Seasonal Reproductive Biology of the Kemp’s ridley sea turtle (Lepidochelys kempii): Comparison of captive and wild populatons. Chelonian Conserv. Biol. 2004, 4, 786–798. [Google Scholar]
- Wyneken, J.; Epperly, S.P.; Crowder, L.B.; Vaughan, J.; Esper, K.B. Determining sex in posthatchling loggerhead sea turtles using multiple gonadal and accessory duct characteristics. Herpetologica 2007, 63, 19–30. [Google Scholar] [CrossRef]
- Lazar, B.; Lackovic, G.; Casale, P.; Freggi, D.; Tvrtkovic, N. Histological validation of gonad gross morphology to sex juvenile loggerhead sea turtles (Caretta caretta). Herpetol. J. 2008, 18, 137–140. [Google Scholar]
- Ball, G.F.; Wade, J. The Value of Comparative Approaches to our Understanding of Puberty as Illustrated by Investigations in Birds and Reptiles. Horm. Behav. 2013, 64, 211–214. [Google Scholar] [CrossRef][Green Version]
- Marquez-M, R. Synopsis of the Biological Data on the Kemp’s Ridley Turtle, Lepidochelys Kempii (Garman, 1880); NOAA Technical Memo NMFS-SEFSC 343; US Dept of Commerce: Miami, FL, USA, 1994.
- Bjorndal, K.A.; Parsons, J.; Mustin, W.; Bolton, A.B. Variation in age and size at sexual maturity in Kemp’s ridley sea turtles. Endanger. Species Res. 2014, 25, 57–67. [Google Scholar] [CrossRef]
- Caillouet, C.W. Interruption of the Kemp’s ridley population’s pre-2010 exponential growth in the Gulf of Mexico and its aftermath: One hypothesis. Mar. Turt. Newsl. 2014, 143, 1–7. [Google Scholar]
- Snover, M.L.; Hohn, A.A.; Crowder, L.B.; Heppell, S.S. Age and growth in Kemp’s ridley sea turtles: Evidence from mark recapture and skeletochronology. In Biology and Conservation of Ridley Sea Turtles; Plotkin, P.T., Ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2007; pp. 89–105. [Google Scholar]
- Owens, D.W. Hormones in the life history of sea turtles. In The Biology of Sea Turtles; Lutz, P.L., Musick, J.A., Eds.; CRC Press: Boca Raton, FL, USA, 1997; pp. 315–341. [Google Scholar]
- Miller, J.D.; Limpus, C.J. Ontogeny of Marine Turtle Gonads. In The Biology of Sea Turtles; Lutz, P.L., Musick, J.A., Wyneken, J., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 199–224. [Google Scholar]
- Limpus, C.J. Puberty and first breeding in Caretta caretta. In Proceedings of the Tenth Annual Workshop on Sea Turtle Biology and Conservation; Technical Memorandum NMFS-SEFC-278; Richardson, T.H., Richardson, J.I., Donnelley, M., Eds.; NOAA: Miami, FL, USA, 1990; pp. 81–83. [Google Scholar]
- Caillouet, C.W., Jr.; Raborn, S.W.; Shaver, D.J.; Putman, N.F.; Gallaway, B.J.; Mansfield, K.L. Did Declining Carrying Capacity for the Kemp’s Ridley Sea Turtle Population within the Gulf of Mexico Contribute to the Nesting Setback in 2010−2017? Chelonian Conserv. Biol. 2018, 17, 123–133. [Google Scholar] [CrossRef]
- Kocmoud, A.R.; Hsiao-Hsuan, W.; Grant, W.E.; Gallaway, B.J. Population dynamics of the endangered Kemp’s ridley sea turtle following the 2010 oil spill in the Gulf of Mexico: Simulation of potential cause-effect relationships. Ecol. Model. 2019, 392, 159–178. [Google Scholar] [CrossRef]
- Shaver, D.J.; Hart, K.M.; Fujisaki, I.; Bucklin, D.; Iverson, A.R.; Rubio, C.; Backof, T.F. Foraging area fidelity for Kemp’s ridleys in the Gulf of Mexico. Ecol. Evol. 2013, 3, 2002–2012. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, H.H. A historical view of sea turtle populations in the western Gulf of Mexico. In Biology and Conservation of Sea Turtles; Bjorndal, K., Ed.; Smithsonian Institution Press: Washington, DC, USA, 1981; pp. 447–454. [Google Scholar]
- Shaver, D.J. Feeding ecology of wild and head-started Kemp’s ridley sea turtles in south Texas waters. J. Herpetol. 1991, 25, 327–334. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Craven, K.S.; Hodgson, J.Y.S.; Shaver, D.J.; Walker, J.S.; Villalba-Guerra, M.R.; Owens, D.W. Evaluation of Gonadal Tissue to Validate Size at Reproductive Maturity in Kemp’s Ridley Sea Turtles Found Stranded in Texas, USA. Diversity 2019, 11, 76. https://doi.org/10.3390/d11050076
Craven KS, Hodgson JYS, Shaver DJ, Walker JS, Villalba-Guerra MR, Owens DW. Evaluation of Gonadal Tissue to Validate Size at Reproductive Maturity in Kemp’s Ridley Sea Turtles Found Stranded in Texas, USA. Diversity. 2019; 11(5):76. https://doi.org/10.3390/d11050076
Chicago/Turabian StyleCraven, Kathryn Stephenson, Jay York Seabright Hodgson, Donna Jill Shaver, Jennifer Shelby Walker, Martha Ramona Villalba-Guerra, and David William Owens. 2019. "Evaluation of Gonadal Tissue to Validate Size at Reproductive Maturity in Kemp’s Ridley Sea Turtles Found Stranded in Texas, USA" Diversity 11, no. 5: 76. https://doi.org/10.3390/d11050076
APA StyleCraven, K. S., Hodgson, J. Y. S., Shaver, D. J., Walker, J. S., Villalba-Guerra, M. R., & Owens, D. W. (2019). Evaluation of Gonadal Tissue to Validate Size at Reproductive Maturity in Kemp’s Ridley Sea Turtles Found Stranded in Texas, USA. Diversity, 11(5), 76. https://doi.org/10.3390/d11050076




