Do Local Environmental Factors and Lunar Cycle Influence Timing and Synchrony of Oviposition of a Turtle with Strict Nocturnal Nesting?
Abstract
1. Introduction
2. Materials and Methods
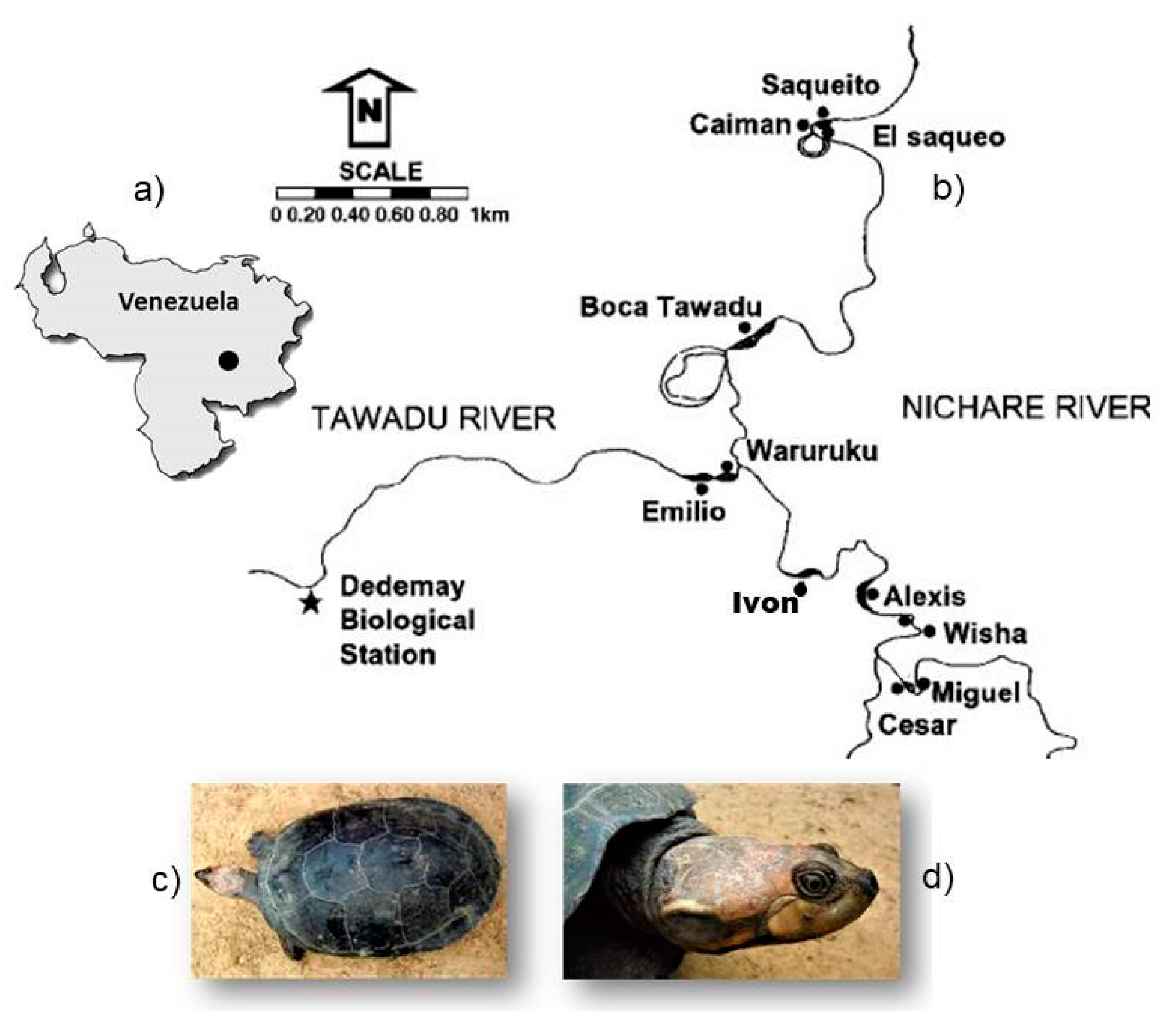
2.1. Study Area
2.2. Nesting Season
2.3. Timing of Nesting
2.4. Environmental Variables
2.5. Lunar Cycle
2.6. Data Analysis
3. Results
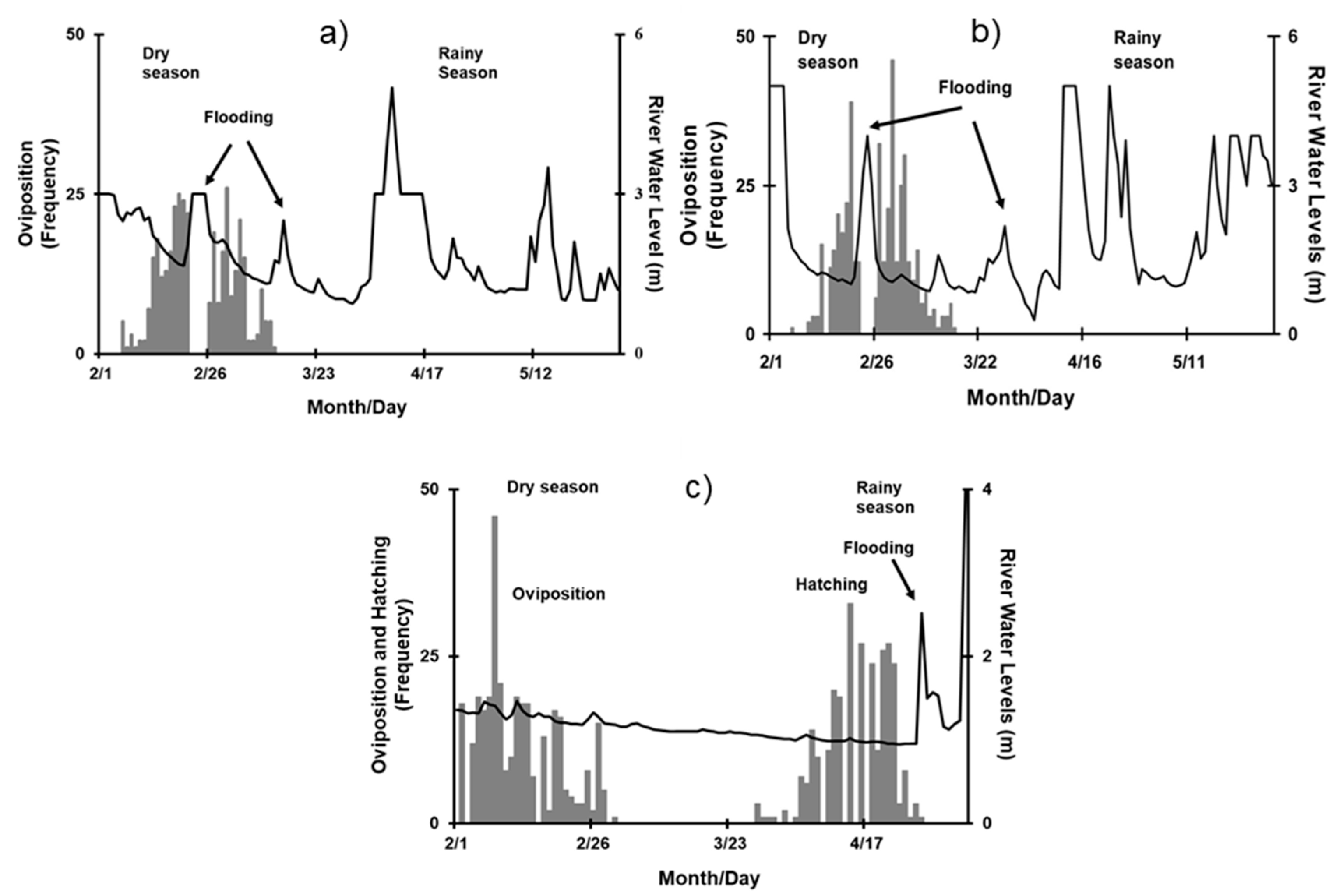
3.1. Nocturnal Nesting Behavior
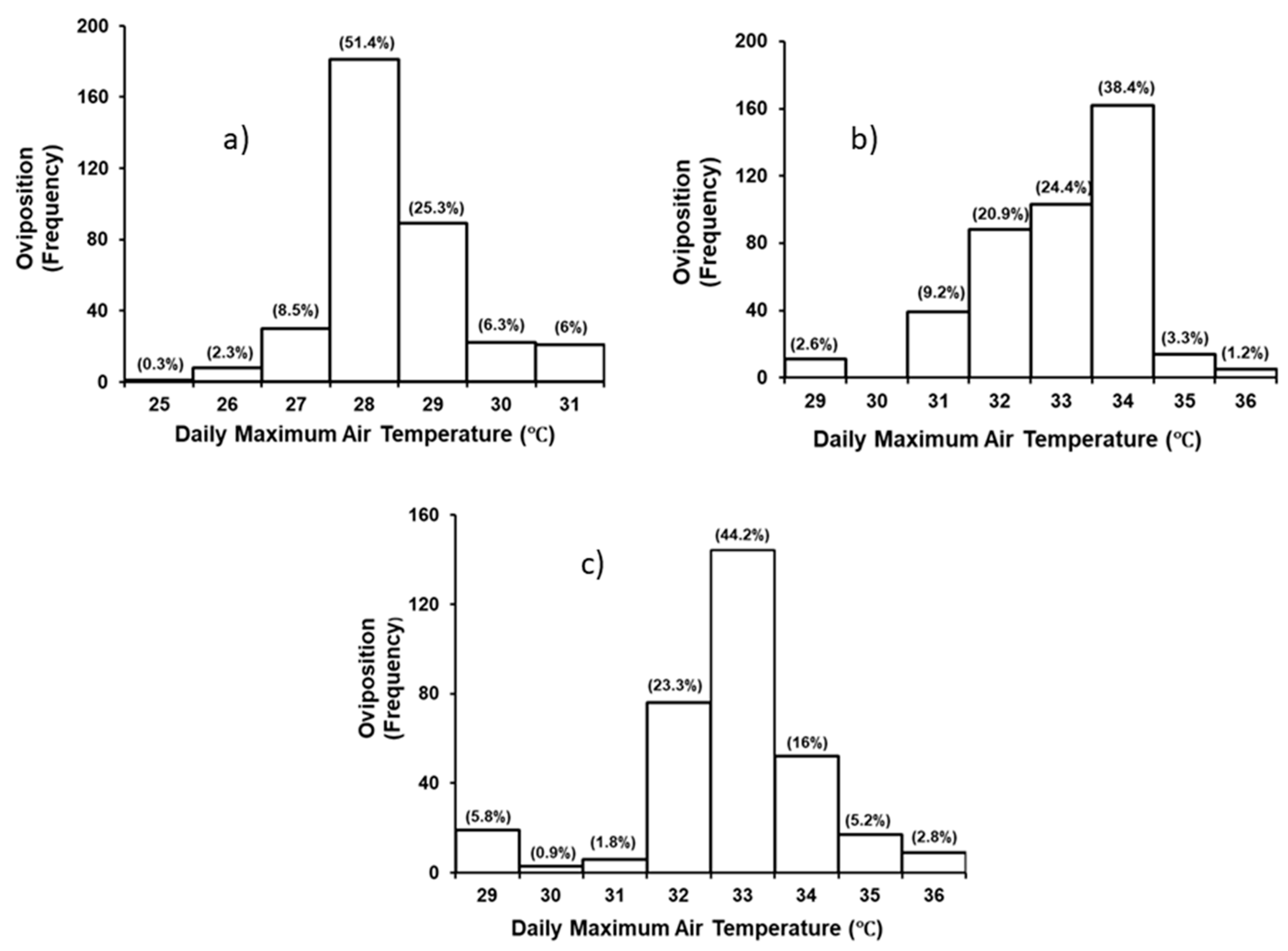
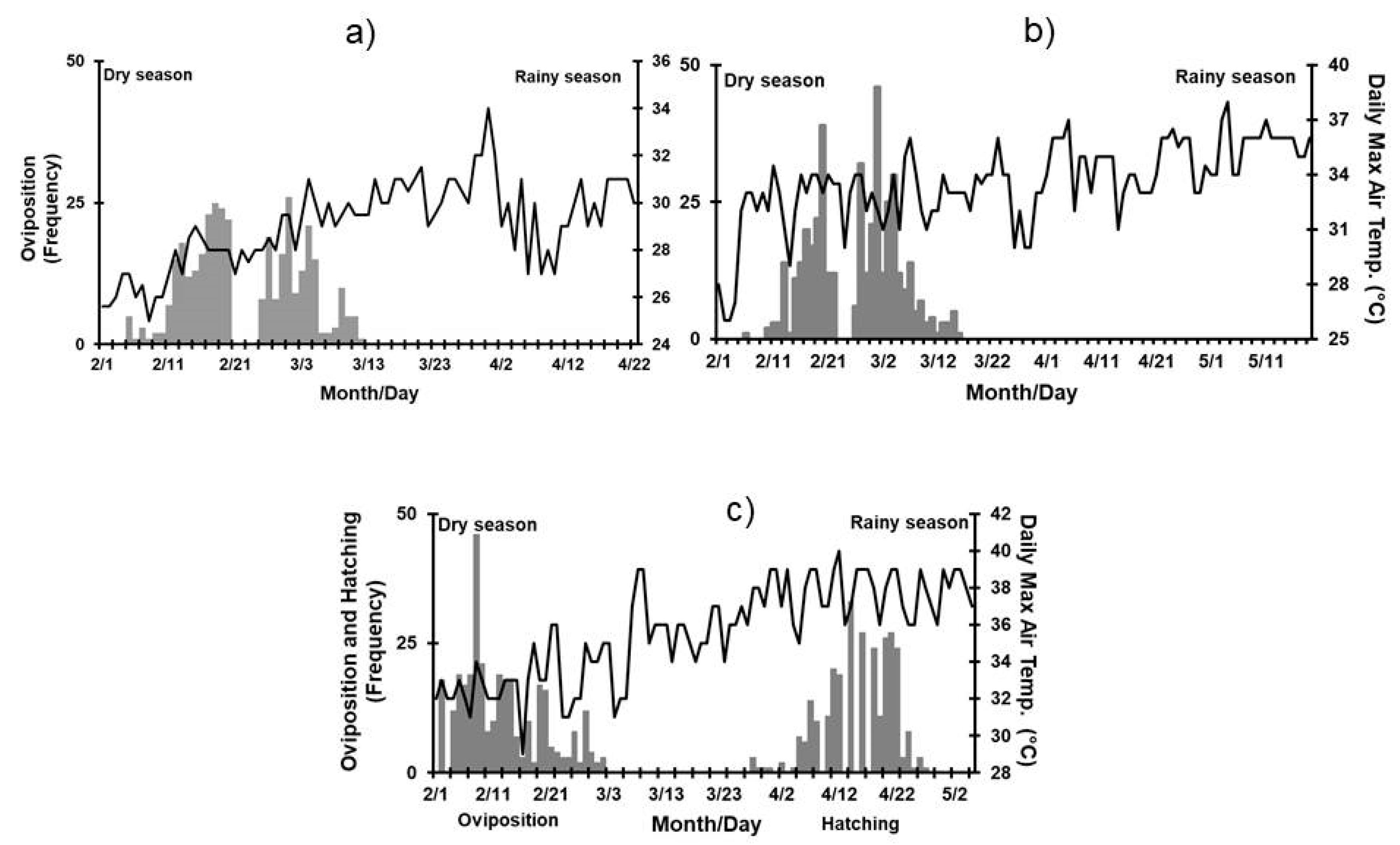
3.2. Effect of Environmental Variables on Nesting
3.3. Effect of the Lunar Cycle on Nesting
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stearns, S.C. The Evolution of Life Histories; Oxford University Press: Oxford, UK, 1992; p. 262. ISBN 978-0-198-57741-6. [Google Scholar]
- Roff, D.A. Evolution of Life Histories: Theory and Analysis; Chapman and Hall: New York, NY, USA, 1992; p. 548. ISBN 978-0-412-02391-0. [Google Scholar]
- Brommer, J.E.; Merilä, J.; Kokko, H. Reproductive timing and individual fitness. Ecol. Lett. 2002, 5, 802–810. [Google Scholar] [CrossRef]
- Brown, G.P.; Shine, R. Why do most tropical animals reproduce seasonally? Testing hypotheses on an Australian snake. Ecology 2006, 87, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Whelan, S.; Strickland, D.; Morand-Ferron, J.; Norris, D.R. Male experience buffers female laying date plasticity in a winter- breeding, food-storing passerine. Anim. Behav. 2016, 121, 61–70. [Google Scholar] [CrossRef]
- Olsson, M.; Shine, R. The seasonal timing of oviposition in sand lizards (Lacerta agilis): Why early clutches are better. J. Evol. Biol. 1997, 10, 369–381. [Google Scholar] [CrossRef]
- Spencer, R.J.; Thompson, M.B.; Banks, P.B. Hatch or wait? A dilemma in reptilian incubation. Oikos 2001, 93, 401–406. [Google Scholar] [CrossRef]
- Brown, G.P.; Shine, R.; Madsen, T. Responses of three sympatric snake species to tropical seasonality in northern Australia. J. Trop. Ecol. 2002, 18, 549–568. [Google Scholar] [CrossRef]
- Visser, M.E.; Holleman, L.J.M.; Caro, S.P. Temperature has a causal effect on avian timing of reproduction. Proc. R. Soc. Lond. B Biol. Sci. 2009, 276, 2323–2331. [Google Scholar] [CrossRef]
- Pike, D.A. Environmental correlates of nesting in Loggerhead turtles, Caretta caretta. Anim. Behav. 2008, 76, 603–610. [Google Scholar] [CrossRef]
- Wheeler, C.A.; Lind, A.J.; Welsh, H.W., Jr.; Cummings, A.K. Factors that influence the timing of calling and oviposition of a lotic frog in Northwestern California. J. Herpetol. 2018, 52, 289–298. [Google Scholar] [CrossRef]
- Ngo, B.V.; Ngo, C.D.; Hou, P.C. Reproductive ecology of Quasipaa verrucospinosa (Bourret, 1937): Living in the tropical rain forests of central Vietnam. J. Herpetol. 2013, 47, 138–147. [Google Scholar] [CrossRef]
- Vitt, L.J.; Lacher, T. Behavior, habitat, diet and reproduction of the iguanid lizard Polychrus acutirostris in the caatinga of northeastern Brazil. Herpetologica 1981, 37, 53–63. [Google Scholar] [CrossRef]
- De Fries, L.; da Rosa, G.; da Silva, J.P.; Vilella, F.S.; Becker, F.G. Reproduction of two loricariid species in a confined river and implications for environmental impacts of dams. Neotrop. Ichthyol. 2018, 16, e170163. [Google Scholar] [CrossRef]
- Christens, E.; Bider, J.R. Nesting activity and hatching success of the painted turtle (Chrysemys picta marginata) in southwestern Quebec. Herpetologica 1987, 43, 55–65. [Google Scholar]
- Iverson, J.B.; Smith, G.R. Reproductive ecology of the painted turtle (Chrysemys picta) in the Nebraska Sandhills and across its range. Copeia 1993, 1, 1–21. [Google Scholar] [CrossRef]
- Bowen, K.D.; Spencer, R.J.; Janzen, F.J.A. Comparative study of environmental factors that affect nesting in Australian and North American freshwater turtles. J. Zool. 2005, 267, 397–404. [Google Scholar] [CrossRef]
- Congdon, J.D.; Tinkle, D.W.; Breitenbach, G.L.; van Loben Sels, R.C. Nesting ecology and hatching success in the turtle Emydoidea blandingii. Herpetologica 1983, 39, 417–429. [Google Scholar] [CrossRef]
- Obbard, M.E.; Brooks, R.J. Prediction of the onset of the annual nesting season of the common snapping turtle, Chelydra serpentine. Herpetologica 1987, 43, 324–328. [Google Scholar]
- Booth, D.T. The natural history of nesting in two Australian freshwater turtles. Aust. Zool. 2010, 35, 198–203. [Google Scholar] [CrossRef]
- Espinoza, T.; Connell, M.; Marshall, S.; Beukeboom, R.; McDougal, A. Nesting behaviour of the endangered Mary River turtle: Monitoring and modelling to inform e-flow strategies. Aust. J. Zool. 2018, 66, 15–26. [Google Scholar] [CrossRef]
- Tucker, J.K. Natural History Notes on Nesting, Nests, and Hatchling Emergence in the Red-Eared Slider Turtle, Trachemys Scripta Elegans, in West-Central Illinois; Illinois Natural History Survey Biological Notes 140: Champaign, IL, USA, 1997; p. 13. ISSN 0073-490X. [Google Scholar]
- Ferreira, P.D., Jr.; Castro, P.T.A. Nest placement of the giant Amazon River turtle, Podocnemis expansa, in the Araguaia River, Goiás state, Brazil. AMBIO 2005, 34, 212–217. [Google Scholar] [CrossRef]
- Thorbjarnarson, J.B.; Perez, N.; Escalona, T. Nesting of Podocnemis unifilis in the Capanaparo River, Venezuela. J. Herpetol. 1993, 27, 344–347. [Google Scholar] [CrossRef]
- Polisar, J. Reproductive biology of a flood-season nesting freshwater turtle of the northern neotropics: Dermatemys mawii in Belize. Chelonian Conserv. Biol. 1996, 2, 13–25. [Google Scholar]
- Ojasti, J. Utilización de la Fauna Silvestre de América Latina: Situación y Perspectivas Para un Manejo Sostenible; FAO Conservation Guide 25; Food and Agriculture Organization of the United Nations: Roma, Italy, 1993; p. 248. ISBN 978-9-253-03316-4. [Google Scholar]
- Escalona, T.; Fa, J.E. Survival of nests of the terecay turtle (Podocnemis unifilis) in the Nichare-Tawadu rivers, Venezuela. J. Zool. Lond. 1998, 244, 303–312. [Google Scholar] [CrossRef]
- Janzen, F.J.A. Climate change and temperature-dependent sex determination in reptiles. Proc. Natl. Acad. Sci. USA 1994, 91, 7487–7490. [Google Scholar] [CrossRef]
- Chiew, F.H.S.; McMahon, T.A. Modelling the impacts of climate change on Australian streamflow. Hydrol. Process. 2002, 16, 1235–1245. [Google Scholar] [CrossRef]
- Eisemberg, C.C.; Machado, R.A.; Famelli, S.; Pereira, F.F.; Bernardes, V.C.; Vogt, R.C. Vulnerability of giant South American turtle (Podocnemis expansa) nesting habitat to climate-change-induced alterations to fluvial cycles. Trop. Conserv. Sci. 2016, 1–12. [Google Scholar] [CrossRef]
- McDowall, R.M. Lunar rhythms in aquatic animals: A general review. Tuatara J. Biol. Soc. 1969, 17, 133–143. [Google Scholar]
- Bentley, M.G.; Olive, P.J.; Last, K.S. Sexual satellites, moonlight and the nuptial dances of worms: The influence of the moon on the reproduction of marine animals. Earth Moon Planets 1999, 85–86, 67–84. [Google Scholar] [CrossRef]
- Takemura, A.; Susilo, E.S.; Rahman, S.M.D.; Morita, M. Perception and possible utilization of moonlight intensity for reproductive activities in a lunar-synchronized spawner, the golden rabbitfish. J. Exp. Zool. 2004, 301A, 844–851. [Google Scholar] [CrossRef]
- Grant, R.A.; Chadwick, E.A.; Halliday, T. The lunar cycle: A cue for amphibian reproductive phenology? Anim. Behav. 2009, 78, 349–357. [Google Scholar] [CrossRef]
- Weaver, R. Effects of simulated moonlight on activity in the desert nightsnake (Hypsiglena chlorophaea). Northwest Sci. 2011, 85, 497–500. [Google Scholar] [CrossRef]
- Lillywhite, H.B.; Brischoux, F. Is it better in the moonlight? Nocturnal activity of insular cottonmouth snakes increases with lunar light levels. J. Zool. 2012, 286, 194–199. [Google Scholar] [CrossRef]
- Longland, W.S.; Price, M.V. Direct observations of owls and heteromyd rodents: Can predation risk explain microhabitat use? Ecology 1991, 72, 2261–2273. [Google Scholar] [CrossRef]
- Kolter, B.P.; Brown, J.S.; Hasson, O. Factors affecting gerbil foraging behavior and rates of owl predation. Ecology 1991, 72, 2249–2260. [Google Scholar] [CrossRef]
- Pinou, T.; Pacete, K.J.; De Niz, A.P.; Gall, L.; Lazo-Wasem, E. Lunar illumination and sea turtle nesting. Herpetol. Rev. 2009, 40, 409–410. [Google Scholar]
- Medem, F. Morphologie, oekologie und verbreitung der schildkröte, Podocnemis unifilis in Kolumbiem. Senckenb. Biol. 1964, 45, 353–368. [Google Scholar]
- Zwink, W.; Young, P. Desova e eclosão de Podocnemis expansa (Schweigger, 1812) (Chelonia: Pelomedusidae) no Río Trombetas, Parú, Brasil. In Anais FOREST ‘90, Proceedings of the first Simpósio Internacional de Estudos Ambientais em Florestas Tropicais Úmidas, Manaus, Brasil, 7–13 October 1990; Biosfera: Rio de Janeiro, Brasil, 1990; pp. 34–35. [Google Scholar]
- Escalona, T.; Valenzuela, N.; Adams, C.D. Nesting ecology in the freshwater turtle Podocnemis unifilis: Spatiotemporal patterns and inferred explanations. Fun. Ecol. 2009, 23, 826–835. [Google Scholar] [CrossRef]
- Escalona, T.; Adams, C.D.; Valenzuela, N. A lengthy solution to the optimal propagule size problem in the large-bodied South American freshwater turtle, Podocnemis unifilis. Evol. Ecol. 2017, 32, 29–41. [Google Scholar] [CrossRef]
- Soini, P. Ecología reproductiva de la taricaya (Podocnemis unifilis) en el Río Pacaya, Perú. Folía Amazon. 1994, 6, 105–124. [Google Scholar] [CrossRef]
- Caputo, F.P.; Canestrelli, D.; Boitani, L. Conserving the terecay (Podocnemis unifilis, Testudines: Pelomedusidae) through a community-based sustainable harvest of its eggs. Biol. Conserv. 2005, 126, 84–92. [Google Scholar] [CrossRef]
- Alho, C.J.R.; Pádua, L.F.M. Reproductive parameters and nesting behavior of the Amazon turtle Podocnemis expansa (Testudinata: Pelomedusidae) in Brazil. Can. J. Zool. 1982, 60, 97–103. [Google Scholar] [CrossRef]
- Astronomical Applications Department of the U.S. Naval Observatory Home Page. Available online: https://aa.usno.navy.mil/index.php (accessed on 14 May 2019).
- Baker, G.; Dekker, W.R.J. Lunar synchrony in the reproduction of the Moluccan Megapode Megapodius wallacei. Ibis 2000, 142, 382–388. [Google Scholar] [CrossRef]
- Delignette-Muller, M.L.; Dutang, C. fitdistrplus: An R Package for Fitting Distributions. J. Stat. Softw. 2015, 64, 1–34. [Google Scholar] [CrossRef]
- Feng, C.; Wang, H.; Lu, N.; Chen, T.; He, H.; Lu, Y.; Tu, X.M. Log-transformation and its implications for data analysis. Shanghai Arch. Psychiatry 2014, 26, 105–109. [Google Scholar] [CrossRef]
- JMP®, Version 13.0.1.; SAS Institute Inc.: Cary, NC, USA, 2016.
- Souza, R.R.; Vogt, R.C. Incubation Temperature Influences Sex and Hatchling Size in the Neotropical Turtle Podocnemis unifilis. J. Herpetol. 1994, 28, 453–464. [Google Scholar] [CrossRef]
- Paéz, V.P.; Bock, B. Nesting ecology of the yellow-spotted river turtle in the Colombian Amazon. In Conservation, Restoration, and Management of Tortoises and Turtles, Proceedings of the 1993 International Conference, New York, NY, USA, 11–16 July 1993; New York Turtle and Tortoise Society and the WCS Turtle Recovery Program: New York, NY, USA, 1997; pp. 219–224. ISBN 0-9659050-0-4. [Google Scholar]
- Vanzolini, P.E. Notes on the nesting behaviour of Podocnemis expansa in the Amazon valley (Testudines, Pelomedusidae). Pap. Avul. Zool. 1967, 20, 191–215. [Google Scholar]
- Mittermeier, R. South America’s river turtles: Saving them by use. Oryx 1978, 14, 222–230. [Google Scholar] [CrossRef]
- Rowe, J.W.; Coval, K.A.; Campbell, K.C. Reproductive characteristics of female midland painted turtles (Chrysemys picta marginata) from a population on Beaver Island, Michigan. Copeia 2003, 2, 326–336. [Google Scholar] [CrossRef]
- Fachín-Terán, A.; Von Mülhen, E.M. Reproducción de la taricaya Podocnemis unifilis Troschel 1848 (Testudines: Podocnemididae) en la várzea del medio Solimões, Amazonas, Brasil. Ecol. Appl. 2003, 2, 125–132. [Google Scholar] [CrossRef]
- Fachín-Terán, A.; Vogt, R.C.; Thorbjarnarson, J.B. Seasonal movements of Podocnemis sextuberculata (Testudines: Podocnemididae) in the Mamirauá Sustainable Development Reserve, Amazonas, Brazil. Chelonian Conserv. Biol. 2006, 5, 18–24. [Google Scholar] [CrossRef]
- Plummer, M.V. Some aspects of nesting success in the turtle, Trionyx muticus. Herpetologica 1976, 32, 353–359. [Google Scholar]
- Lenhart, C.; Naber, J.R.; Nieber, J.L. Impacts of hydrologic change on sandbar nesting availability for riverine turtles in eastern Minnesota, USA. Water 2013, 5, 1243–1261. [Google Scholar] [CrossRef]
- Frye, A.B.; Hardy, K.; Hedrick, A.; Iverson, J.B. Factors Affecting nesting times in the painted turtle Chrysemys picta in Nebraska. Chelonian Conserv. Biol. 2017, 16, 44–51. [Google Scholar] [CrossRef]
- Schwanz, L.E.; Janzen, F.J. Climate change and temperature-dependent sex determination: Can individual plasticity in nesting phenology prevent extreme sex ratios? Physiol. Biochem. Zool. 2008, 81, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.B.; Blount, J.D.; Godley, B.J.; Witt, M.J.; Broderick, A.C. Rate of egg maturation in marine turtles exhibits ‘universal temperature dependence. J. Anim. Ecol. 2011, 80, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Mougeot, F.; Bretagnolle, V. Predation risk and moonlight avoidance in nocturnal seabirds. J. Avian Biol. 2000, 31, 376–386. [Google Scholar] [CrossRef]
- von Humboldt, A. The route of Humboldt—Venezuela and Colombia; Villegas Editores: Bogotá, CO, USA, 1994; Volume I, p. 350. ISBN 978-9-589-13896-0. [Google Scholar]
- Kress, S.W. Using animal behavior for conservation: Case studies in seabird restoration from Maine coast, USA. J. Yamashina Inst. Ornithol. 1997, 29, 1–26. [Google Scholar] [CrossRef]
- Golden Gate Weather Services (2017) El Niño and La Niña Years and Intensities Home Page. Available online: https://ggweather.com/enso/oni.htm (accessed on 14 May 2019).
- NOAA Climate.gov Home Page. Available online: https://www.climate.gov/news-features/blogs/enso/la-niña-did-you-orchestrate (accessed on 14 May 2019).



| Year | Rainfall (mm) Mean ± SD | F-value | df | p-value | R2 | Slope | |
|---|---|---|---|---|---|---|---|
| 1999 | 3.79 ± 8.68 | 0.70 | 1,30 | >0.05, ns | 0.02 | −0.20 | |
| 2000 | 1.85 ± 3.63 | 0.80 | 1,35 | >0.05, ns | 0.02 | 0.21 | |
| 2001 | 0.26 ± 0.58 | 0.005 | 1,27 | >0.05, ns | 0.0002 | 0.03 | |
| Year | River Level (m) Mean ± SD | F-value | df | p-value | R2 | Slope |
|---|---|---|---|---|---|---|
| 1999 | 2.21 ± 0.84 | 0.81 | 1,30 | >0.05, ns | 0.03 | −2.71 |
| 2000 | 1.39 ± 0.68 | 4.06 | 1,35 | >0.05, ns | 0.10 | −2.38 |
| 2001 | 1.29 ± 0.08 | 14.15 | 1,27 | <0.001 | 0.34 | 8.14 |
| Year | Full Moon Mean ± SD | New Moon Mean ± SD | Qs | Qc | T-K p-value |
|---|---|---|---|---|---|
| 1999 | 15.43 ± 6.40 | 18.71 ± 5.35 | 1.69 | 3.86 | >0.05, ns |
| 2000 | 20.33 ± 10.01 | 7.36 ± 8.99 | 3.98 | 3.82 | 0.039 |
| 2001 | 20 ± 12.46 | 5.86 ± 4.88 | 4.77 | 3.89 | 0.012 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escalona, T.; Valenzuela, N.; Adams, D.C. Do Local Environmental Factors and Lunar Cycle Influence Timing and Synchrony of Oviposition of a Turtle with Strict Nocturnal Nesting? Diversity 2019, 11, 78. https://doi.org/10.3390/d11050078
Escalona T, Valenzuela N, Adams DC. Do Local Environmental Factors and Lunar Cycle Influence Timing and Synchrony of Oviposition of a Turtle with Strict Nocturnal Nesting? Diversity. 2019; 11(5):78. https://doi.org/10.3390/d11050078
Chicago/Turabian StyleEscalona, Tibisay, Nicole Valenzuela, and Dean C. Adams. 2019. "Do Local Environmental Factors and Lunar Cycle Influence Timing and Synchrony of Oviposition of a Turtle with Strict Nocturnal Nesting?" Diversity 11, no. 5: 78. https://doi.org/10.3390/d11050078
APA StyleEscalona, T., Valenzuela, N., & Adams, D. C. (2019). Do Local Environmental Factors and Lunar Cycle Influence Timing and Synchrony of Oviposition of a Turtle with Strict Nocturnal Nesting? Diversity, 11(5), 78. https://doi.org/10.3390/d11050078





