Tradeoffs with Growth and Behavior for Captive Box Turtles Head-Started with Environmental Enrichment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Species and Animal Husbandry
2.2. Growth
2.3. Behavioral Assays
2.3.1. General Information
2.3.2. Foraging Behavior
2.3.3. Antipredator Behavior
2.3.4. Shelter Emergence
3. Results
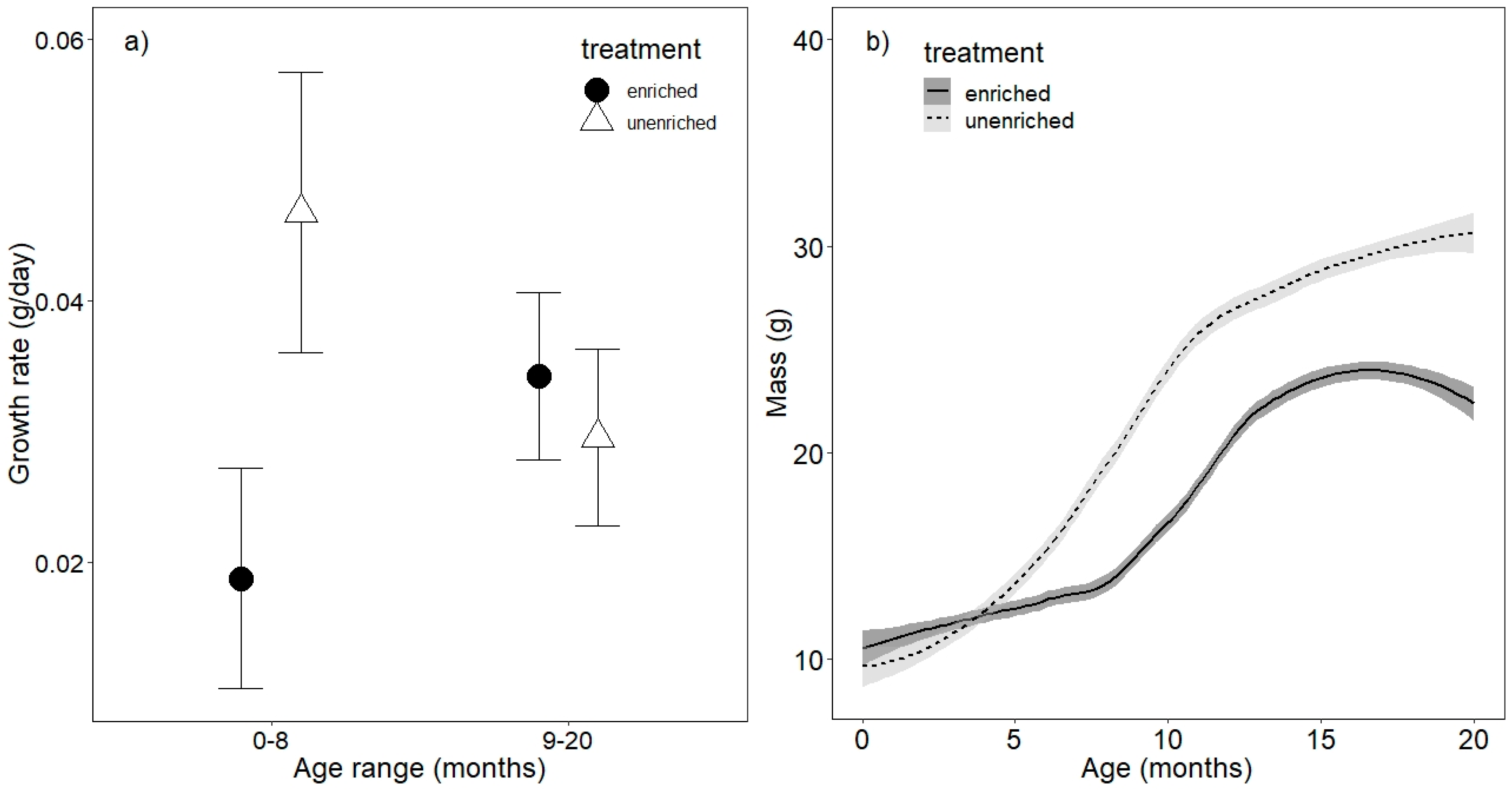
3.1. Growth
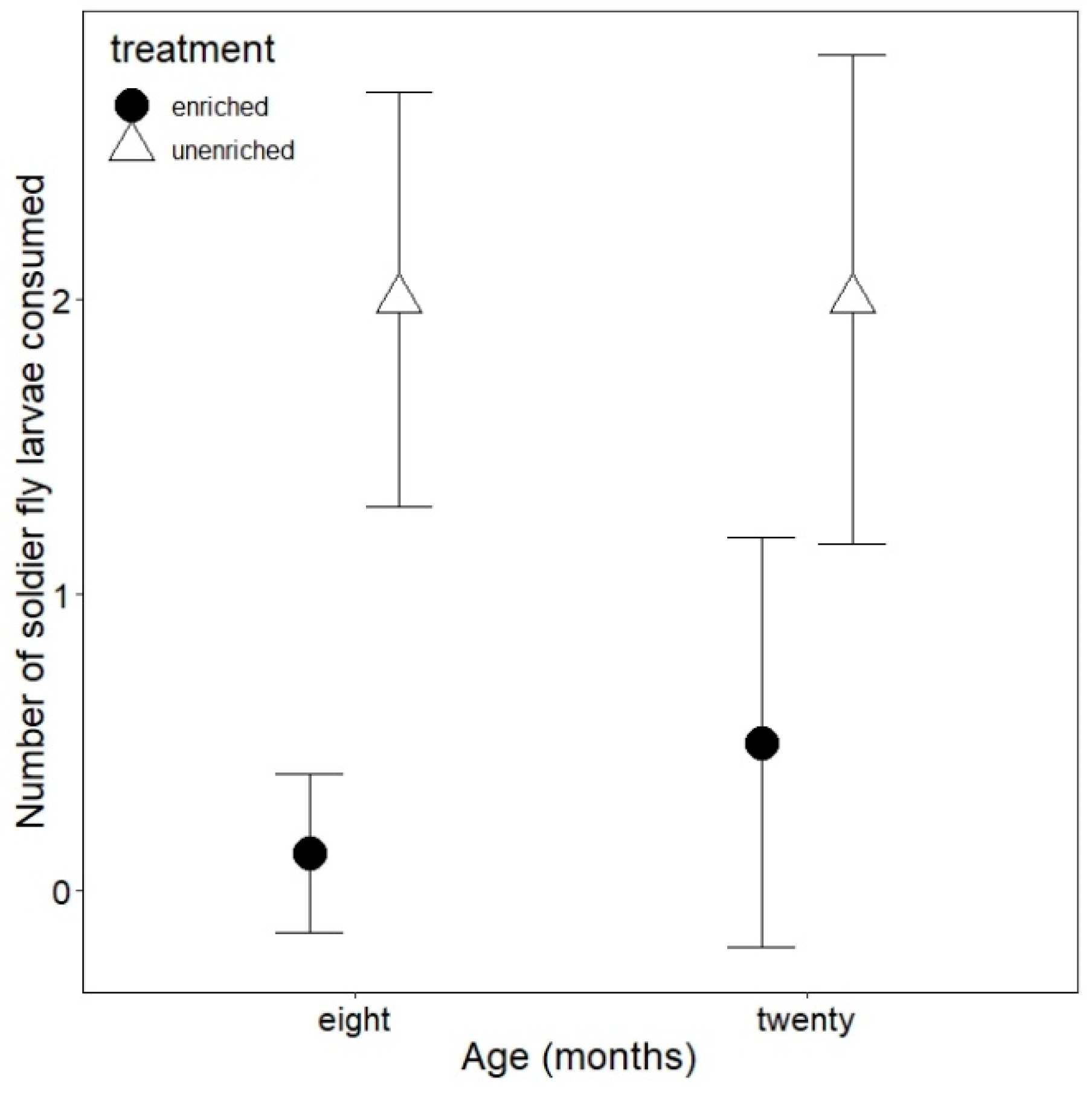
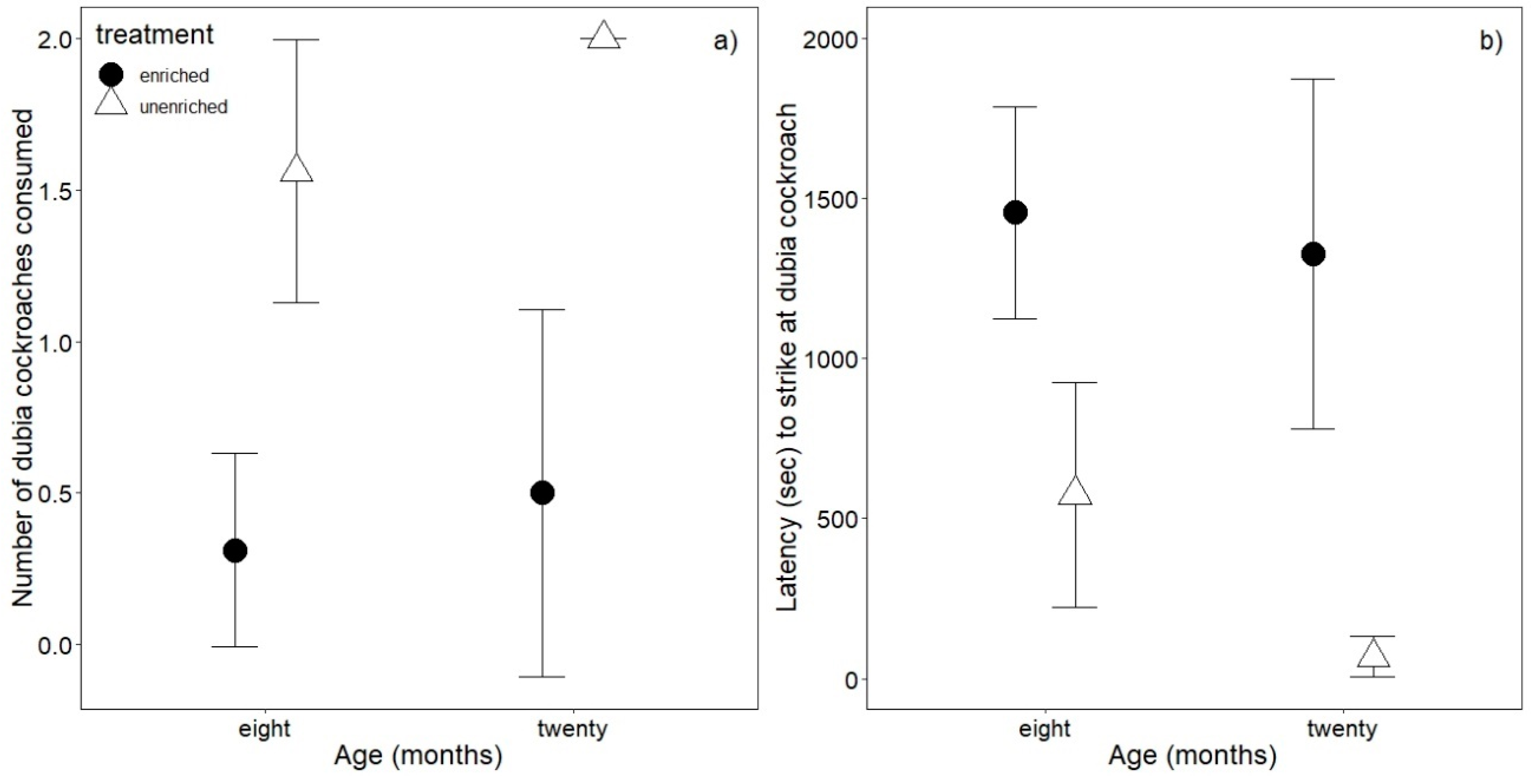
3.2. Foraging Behavior
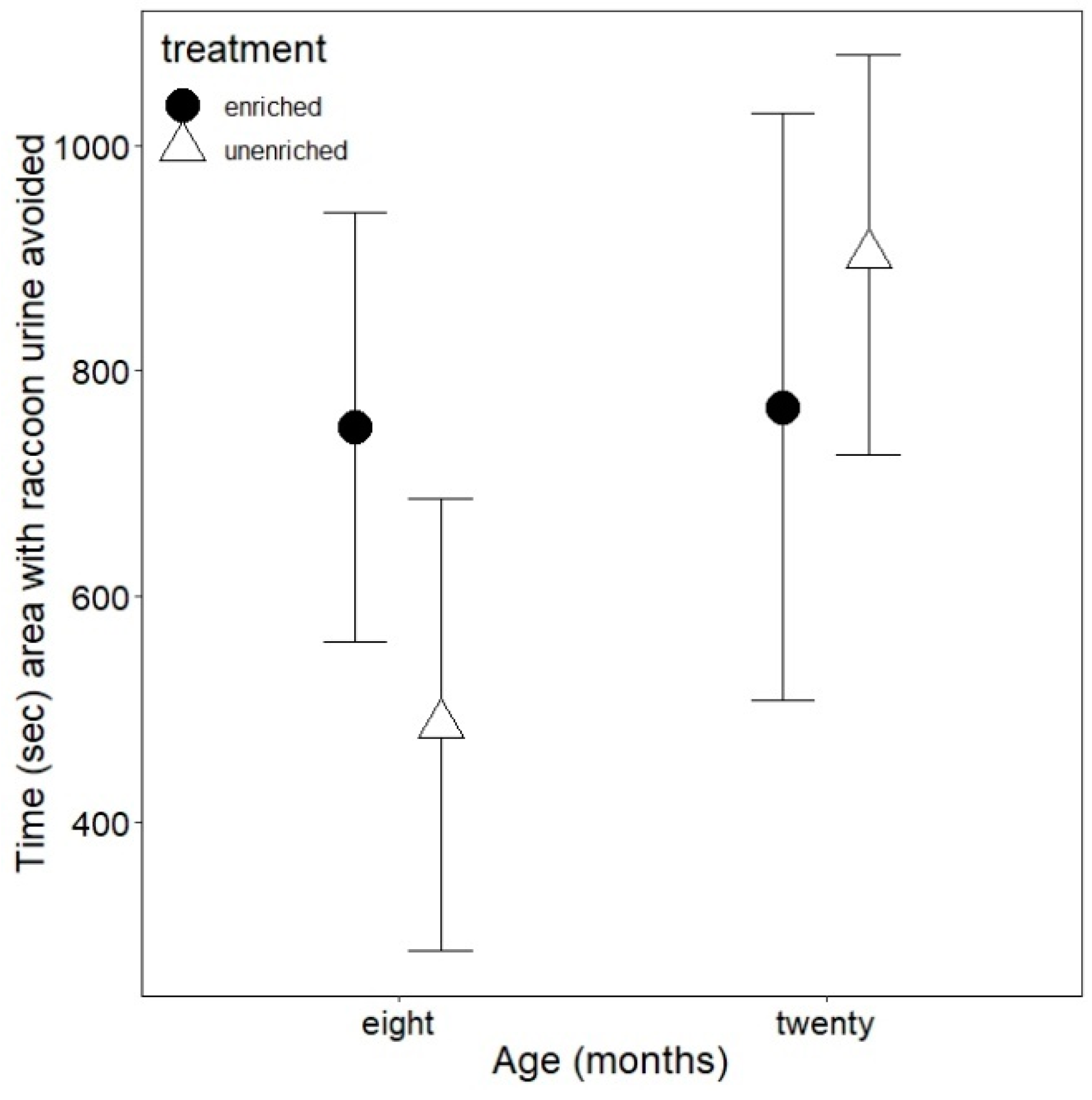
3.3. Antipredator Behavior
3.4. Shelter Emergence
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roe, J.H.; Frank, M.R.; Gibson, S.E.; Attum, O.; Kingsbury, B.A. No place like home: An experimental comparison of reintroduction strategies using snakes. J. Appl. Ecol. 2010, 47, 1253–1261. [Google Scholar] [CrossRef]
- Wines, M.P.; Johnson, V.M.; Lock, B.; Antonio, F.; Godwin, J.C.; Rush, E.M.; Guyer, C. Optimal husbandry of hatchling eastern indigo snakes (Drymarchon couperi) during a captive head-start program. Zoo Biol. 2015, 34, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Daly, J.A.; Buhlmann, K.A.; Todd, B.D.; Moore, C.T.; Peaden, J.M.; Tuberville, T.D. Comparing growth and body condition of indoor-reared, outdoor-reared, and direct-released juvenile Mojave desert tortoises. Herpetol. Conserv. Biol. 2018, 13, 622–633. [Google Scholar]
- DeGregorio, B.A.; Weatherhead, P.J.; Tuberville, T.D.; Sperry, J.H. Time in captivity affects foraging behavior of ratsnakes: Implications for translocation. Herpetol. Conserv. Biol. 2013, 8, 581–590. [Google Scholar]
- Swaisgood, R.R.; Martin-Wintle, M.S.; Owen, M.A.; Zhou, X.; Zhang, H. Developmental stability of foraging behavior: Evaluating suitability of captive giant pandas for translocation. Anim. Conserv. 2018, 21, 474–482. [Google Scholar] [CrossRef]
- King, R.B.; Stanford, K.M. Headstarting as a management tool: A case study of the plains gartersnake. Herpetologica 2006, 62, 282–292. [Google Scholar] [CrossRef]
- Jule, K.R.; Leaver, L.A.; Lea, S.E.G. The effects of captive experience on reintroduction survival in carnivores: A review and analysis. Biol. Conserv. 2008, 141, 355–363. [Google Scholar] [CrossRef]
- Swaisgood, R.R. Current status and future directions of applied behavioral research for animal welfare and conservation. Appl. Anim. Behav. Sci. 2007, 102, 139–162. [Google Scholar] [CrossRef]
- Swaisgood, R.R. The conservation-welfare nexus in reintroduction programmes: A role for sensory ecology. Anim. Welf. 2010, 19, 125–137. [Google Scholar]
- Burghardt, G.M. Environmental enrichment and cognitive complexity in reptiles and amphibians: Concepts, review, and implications for captive populations. Appl. Anim. Behav. Sci. 2013, 147, 286–298. [Google Scholar] [CrossRef]
- Reading, R.P.; Miller, B.; Shepherdson, D. The value of enrichment to reintroduction success. Zoo Biol. 2013, 32, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.; Anderson, S.H. Effects of experience and cage enrichment on predatory skills of black-footed ferrets (Mustela nigripes). J. Mammal. 1999, 80, 263–269. [Google Scholar] [CrossRef]
- Kenison, E.K.; Williams, R.N. Training for translocation: Predator conditioning induces behavioral plasticity and physiological changes in captive eastern hellbenders (Cryptobranchus alleganiensis alleganiensis) (Cryptobranchidae, Amphibia). Diversity 2018, 10, 13. [Google Scholar] [CrossRef]
- Krech, D.; Rosenzweig, M.R.; Bennett, E.L. Effects of environmental complexity and training on brain chemistry. J. Comp. Physiol. Psychol. 1960, 53, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Heppell, S.S. Application of life-history theory and population model analysis to turtle conservation. Copeia 1998, 1998, 367–375. [Google Scholar] [CrossRef]
- Dodd, C.K., Jr. North American Box Turtles: A Natural History; University of Oklahoma Press: Norman, OK, USA, 2002; 256 p. [Google Scholar]
- Pike, D.A.; Pizzatto, L.; Pike, B.A.; Shine, R. Estimating survival rates for uncatchable animals: The myth of high juvenile mortality in reptiles. Ecology 2008, 89, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Arsovski, D.; Olivier, A.; Bonnet, X.; Drilholle, S.; Tomović, L.; Béchet, A.; Golubović, A.; Besnard, A. Covariates streamline age-specific early life survival estimates of two chelonian species. J. Zool. 2018, 306, 223–234. [Google Scholar] [CrossRef]
- Congdon, J.D.; Nagle, R.D.; Kinney, O.M. Front-loading life histories: The enduring influence of juvenile growth on age, size, and reproduction of primiparous female freshwater turtles. Evol. Ecol. Res. 2018, 19, 353–364. [Google Scholar]
- Burke, R.L. Head-starting turtles: Learning from experience. Herpetol. Conserv. Biol. 2015, 10, 299–308. [Google Scholar]
- Buhlmann, K.A.; Koch, S.L.; Butler, B.O.; Tuberville, T.D.; Palermo, V.J.; Bastarache, B.A.; Cava, Z.A. Reintroduction and head-starting: Tools for Blanding’s turtle (Emydoidea blandingii) conservation. Herpetol. Conserv. Biol. 2015, 10, 436–454. [Google Scholar]
- Kiester, A.R.; Willey, L.L. Terrapene carolina (Linnaeus 1758)–Eastern box turtle, common box turtle. Chel. Res. Monogr. 2015, 5. [Google Scholar] [CrossRef]
- van Dijk, P.P. Terrapene carolina (errata version published in 2016). The IUCN Red List of Threatened Species, 2011. e.T21641A97428179. Available online: http://dx.doi.org/10.2305/IUCN.UK.2011-1.RLTS.T21641A9303747.en (accessed on 7 March 2019).
- Platt, S.G.; Hall, C.; Liu, H.; Borg, C.K. Wet-season food habits and intersexual diet overlap of Florida box turtles (Terrapene carolina bauri) on National Key Deer Refuge, Florida. Southeast. Nat. 2009, 8, 335–346. [Google Scholar] [CrossRef]
- Tetzlaff, S.J.; Schiltz, N.G. Terrapene carolina (Eastern Box Turtle) Scavenging. Herpetol. Rev. 2016, 47, 453–454. [Google Scholar]
- Tetzlaff, S.J.; Sperry, J.H.; DeGregorio, B.A. Captive-reared juvenile box turtles innately prefer naturalistic habitat: implications for translocation. Appl. Anim. Behav. Sci. 2018, 204, 128–133. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Chivers, D.P.; Wildly, E.L.; Kiesecker, J.M.; Blaustein, A.R. Avoidance response of juvenile pacific treefrogs to chemical cues of introduced predatory bullfrogs. J. Chem. Ecol. 2001, 27, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Stapley, J. Differential avoidance of snake odours by a lizard: Evidence for prioritized avoidance based on risk. Ethology 2003, 109, 785–796. [Google Scholar] [CrossRef]
- Sacerdote-Velat, A.B.; Earnhardt, J.M.; Mulkerin, D.; Boehm, D.; Glowacki, G. Evaluation of headstarting and release techniques for population augmentation and reintroduction of the smooth green snake. Anim. Conserv. 2014, 17, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Almli, L.M.; Burghardt, G.M. Environmental enrichment alters the behavioral profile of ratsnakes (Elaphe). J. Appl. Anim. Welf. Sci. 2006, 9, 85–109. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, A. The effects of past experience on the rat’s response to novelty. Can. J. Exp. Psychol. 1961, 15, 15–19. [Google Scholar] [CrossRef]
- Grandin, T. Effect of Rearing Environment and Environmental Enrichment on Behavior and Neural Development in Young Pigs. Ph.D. dissertation, University of Illinois at Urbana-Champaign, Champaign, IL, USA, 1989. [Google Scholar]
- Volkmar, F.R.; Greenough, W.T. Rearing complexity affects branching of dendrites in the visual cortex of the rat. Science 1972, 176, 1445–1447. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.L. Relocations, repatriations, and translocations of amphibians and reptiles: Taking a broader view. Herpetologica 1991, 47, 350–357. [Google Scholar]
- Lovich, J.E.; Ennen, J.R.; Agha, M.; Gibbons, J.W. Where have all the turtles gone, and why does it matter? Bioscience 2018, 68, 771–781. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tetzlaff, S.J.; Sperry, J.H.; DeGregorio, B.A. Tradeoffs with Growth and Behavior for Captive Box Turtles Head-Started with Environmental Enrichment. Diversity 2019, 11, 40. https://doi.org/10.3390/d11030040
Tetzlaff SJ, Sperry JH, DeGregorio BA. Tradeoffs with Growth and Behavior for Captive Box Turtles Head-Started with Environmental Enrichment. Diversity. 2019; 11(3):40. https://doi.org/10.3390/d11030040
Chicago/Turabian StyleTetzlaff, Sasha J., Jinelle H. Sperry, and Brett A. DeGregorio. 2019. "Tradeoffs with Growth and Behavior for Captive Box Turtles Head-Started with Environmental Enrichment" Diversity 11, no. 3: 40. https://doi.org/10.3390/d11030040
APA StyleTetzlaff, S. J., Sperry, J. H., & DeGregorio, B. A. (2019). Tradeoffs with Growth and Behavior for Captive Box Turtles Head-Started with Environmental Enrichment. Diversity, 11(3), 40. https://doi.org/10.3390/d11030040




