Predicting Leaf Trait Variability as a Functional Descriptor of the Effect of Climate Change in Three Perennial Grasses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Approach to Field Study & Sample Collection
2.2. Watering Regimes & Morphological Measurements
2.3. Statistical Analysis
3. Results
3.1. Species-specific Variability
3.2. Drought Impact
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Hooke, J.; Sandercock, P. (Eds.) Combating Desertification and Land Degradation; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Shinoda, M.; Nachinshonhor, G.U.; Nemoto, M. Impact of drought on vegetation dynamics of the Mongolian steppe: A field experiment. J. Arid Environ. 2010, 74, 63–69. [Google Scholar] [CrossRef]
- Wellstein, C.; Poschlod, P.; Gohlke, A.; Chelli, S.; Campetella, G.; Rosbakh, S.; Canullo, R.; Kreyling, J.; Jentsch, A.; Beierkuhnlein, C. Effects of extreme drought on specific leaf area of grassland species: A meta-analysis of experimental studies in temperate and sub-Mediterranean systems. Glob. Chang. Biol. 2017, 23, 2473–2481. [Google Scholar] [CrossRef] [PubMed]
- Morin, X.; Lechowicz, M.J.; Augspurger, C.; O’keefe, J.; Viner, D.; Chuine, I. Leaf phenology in 22 North American tree species during the 21st century. Glob. Chang. Biol. 2009, 15, 961–975. [Google Scholar] [CrossRef]
- Tucker, S.S.; Craine, J.M.; Nippert, J.B. Physiological drought tolerance and the structuring of tallgrass prairie assemblages. Ecosphere 2011, 2, 1–19. [Google Scholar] [CrossRef]
- McGlone, C.M.; Sieg, C.H.; Kolb, T.E.; Nietupsky, T. Established native perennial grasses out-compete an invasive annual grass regardless of soil water and nutrient availability. Plant Ecol. 2012, 213, 445–457. [Google Scholar] [CrossRef]
- Norton, M.R.; Malinowski, D.P.; Volaire, F. Plant drought survival under climate change and strategies to improve perennial grasses. A review. Agron. Sustain. Dev. 2016, 36, 29. [Google Scholar] [CrossRef] [Green Version]
- Fazlioglu, F.; Bonser, S.P. Phenotypic plasticity and specialization in clonal versus non-clonal plants: A data synthesis. Acta Oecologica 2016, 77, 193–200. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; Ter Steege, H.; Morgan, H.D.; Van Der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef] [Green Version]
- Dengler, N.G.; Taylor, W.C. Developmental Aspects of C4 Photosynthesis; Springer: Dordrecht, The Netherlands, 2000; pp. 471–495. [Google Scholar]
- Petit, J.R.; Mourner, L.; Jouzel, J.; Korotkevich, Y.S.; Kotlyakov, V.I.; Lorius, C. Palaeoclimatological and chronological implications of the Vostok core dust record. Nature 1990, 343, 56–58. [Google Scholar] [CrossRef]
- Benaouda, Z.; Mehdadi, Z.; Bouchaour, I. Influence pédoclimatique sur l’évolution des formations forestières en zone semi-aride (cas de la forêt de Tenira, Ouest algérien). Sécheresse 2005, 16, 115–120. [Google Scholar]
- Le Houérou, H. La végétation de la Tunisie steppique: Structure, écologie, sociologie, répartition, évolution, utilisation, biomasse, productivité (avec référence aux végétations analogues d’Algérie, de Libye et du Maroc). Ann. Inst. Nat. Rech. Agro. Tu. 1969, 42, 622. [Google Scholar]
- Krichen, K.; Ben Mariem, H.; Chaieb, M. Ecophysiological requirements on seed germination of a Mediterranean perennial grass (Stipa tenacissima L.) under controlled temperatures and water stress. S. Afr. J. Bot. 2014, 94, 210–217. [Google Scholar] [CrossRef] [Green Version]
- Krichen, K.; Vilagrosa, A.; Chaieb, M. Environmental factors that limit Stipa tenacissima L. germination and establishment in Mediterranean arid ecosystems in a climate variability context. Acta Physiol. Plant. 2017, 39. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, A.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 167–234. [Google Scholar] [CrossRef]
- Vile, D.; Garnier, E.; Shipley, B.; Laurent, G.; Navas, M.-L.; Roumet, C.; Lavorel, S.; Díaz, S.; Hodgson, J.G.; Lloret, F.; et al. Specific leaf area and dry matter content estimate thickness in laminar leaves. Ann. Bot. 2005, 96, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Hiziroglu, S. Dimensional Changes in Wood; Oklahoma State University: Stillwater, OK, USA, 2007. [Google Scholar] [CrossRef]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant Ecological Strategies: Some Leading Dimensions of Variation between Species. Annu. Rev. Ecol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef] [Green Version]
- Nawazish, S.; Hameed, M.; Naurin, S. Leaf anatomical adaptations of Cenchrus ciliaris L. from the salt range, Pakistan against drought stress. Pakistan J. Bot. 2006, 38, 1723–1730. [Google Scholar]
- Jorge, M.; Wouw, M.; Hanson, J. Characterisation of a collection of buffel grass. Trop. Grassl. 2008, 42, 27–39. [Google Scholar]
- Boughalleb, F.; Abdellaoui, R.; Hadded, Z.; Neffati, M. Anatomical adaptations of the desert species Stipa lagascae against drought stress. Biologia 2015, 70, 1042–1052. [Google Scholar] [CrossRef]
- Abdellaoui, R.; Boughalleb, F.; Hadded, Z. Etude de quelques critères adaptatifs de Stipa lagascae vis-à-vis du déficit hydrique. J. New Sci. 2016, 15, 1350–1359. [Google Scholar]
- Du, N.; Guo, W.; Zhang, X.; Wang, R. Morphological and physiological responses of Vitex negundo L. var. heterophylla (Franch.) Rehd. to drought stress. Acta Physiol. Plant. 2010, 32, 839–848. [Google Scholar] [CrossRef]
- Xu, B.; Li, F.; Shan, L.; Ma, Y.; Ichizen, N.; Huang, J. Gas exchange, biomass partition, and water relationships of three grass seedlings under water stress. Weed Biol. Manag. 2006, 6, 79–88. [Google Scholar] [CrossRef]
- Bloom, A.J.; Chapin, F.S.; Mooney, H.A. Resource Limitation in Plants-An Economic Analogy. Annu. Rev. Ecol. Syst. 1985, 16, 363–392. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and Metabolic Limitations to Photosynthesis under Drought and Salinity in C3 Plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Rucker, K.S.; Kvien, C.K.; Holbrook, C.C.; Hook, J.E. Identification of Peanut Genotypes with Improved Drought Avoidance Traits. Peanut Sci. 1995, 22, 14–18. [Google Scholar] [CrossRef] [Green Version]
- Krichen, K.; Vilagrosa, A.; Chaieb, M. Divergence of functional traits at early stages of development in Stipa tenacissima populations distributed along an environmental gradient of the Mediterranean. Plant Ecol. 2019, 220, 995–1008. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Monson, R.K. Evolutionary and Ecological Aspects of Photosynthetic Pathway Variation. Annu. Rev. Ecol. Syst. 1993, 24, 411–439. [Google Scholar] [CrossRef]
- Osborne, C.P.; Sack, L. Evolution of C4 plants: A new hypothesis for an interaction of CO2 and water relations mediated by plant hydraulics. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 583–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Xu, W.; Yu, X.; Shen, H.; Li, H.; Cheng, D.; Liu, A.; Liu, J.; Liu, C.; Zhao, S.; et al. Cuticular Wax Accumulation Is Associated with Drought Tolerance in Wheat Near-Isogenic Lines. Front. Plant Sci. 2016, 7, 1809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oppenheimer, H.R. Adaptation to drought: Xerophytism. In Plant Water Relationships in Arid and Semi-Arid Conditions; UNESCO: Paris, France, 1960; p. 105. [Google Scholar]
- Clarke, J.M. Effect of leaf rolling on leaf water loss in Triticum spp. Can. J. Plant Sci. 1986, 66, 885–891. [Google Scholar] [CrossRef] [Green Version]
- Kaur, V.; Pulivendula, P.; Kumari, A. Excised leaf water loss in wheat (Triticum aestivum L.) as affected by short periods of heat and water-deficit treatment followed by recovery. Wheat Inf. Serv. 2016, 122, 1–6. [Google Scholar]
- Akita, S.; Moss, D.N. Differential Stomatal Response between C3 and C4 Species to Atmospheric CO2 Concentration and Light. Crop Sci. 2010, 12, 789. [Google Scholar] [CrossRef]
- Pirasteh-Anosheh, H.; Saed-Moucheshi, A.; Pakniyat, H.; Pessarakli, M. Stomatal responses to drought stress. In Water Stress and Crop Plants; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 24–40. [Google Scholar]
- Rahbarian, R.; Khavari-Nejad, R.; Ganjeali, A.; Bagheri, A.; Najafi, F. Drought Stress Effects on Photosynthesis, Chlorophyll Fluorescence and Water Relations in Tolerant and Susceptible Chickpea (Cicer arietinum L.) Genotypes. Acta Biol. Cracoviensia Ser. Bot. 2011, 53, 47–56. [Google Scholar] [CrossRef]
- Kalapos, T. Leaf water potential-leaf water deficit relationship for ten species of a semiarid grassland community. Plant Soil 1994, 160, 105–112. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Yang, S.; Sun, X.; Yin, X.; Zhao, Y.; Yang, Y. Comparative proteomics analyses of intraspecific differences in the response of Stipa purpurea to drought. Plant Divers. 2016, 38, 101–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, Z.S.; Shahid, H.; Cho, J.-I.; Park, S.-H.; Ryu, T.-H.; Park, S.-C. Physiological responses of two halophytic grass species under drought stress environment. Acta Bot. Croat. 2016, 75, 31–38. [Google Scholar] [CrossRef]
- Schonfeld, M.A.; Johnson, R.C.; Carver, B.F.; Mornhinweg, D.W. Water Relations in Winter Wheat as Drought Resistance Indicators. Crop Sci. 1988, 28, 526. [Google Scholar] [CrossRef]
- Merah, O. Potential importance of water status traits for durum wheat improvement under Mediterranean conditions. J. Agric. Sci. 2001, 137, 139–145. [Google Scholar] [CrossRef]
- Siddiqui, Z.S.; Cho, J.-I.; Kwon, T.-R.; Ahn, B.-O.; Lee, K.-S.; Jeong, M.-J.; Ryu, T.-H.; Lee, S.-K.; Park, S.-C.; Park, S.-H. Physiological mechanism of drought tolerance in transgenic rice plants expressing Capsicum annuum methionine sulfoxide reductase B2 (CaMsrB2) gene. Acta Physiol. Plant. 2014, 36, 1143–1153. [Google Scholar] [CrossRef]
- Vendramini, F.; Díaz, S.; Gurvich, D.E.; Wilson, P.J.; Thompson, K.; Hodgson, J.G. Leaf traits as indicators of resource-use strategy in floras with succulent species. New Phytol. 2002, 154, 147–157. [Google Scholar] [CrossRef]
- Bao, L.; Liu, Y.H. Comparison of leaf functional traits in different forest communities in Mt. Dongling of Beijing. Acta Ecol. Sin. 2009, 29, 3692–3703. [Google Scholar]
- Garnier, E.; Laurent, G. Leaf anatomy, specific mass and water content in congeneric annual and perennial grass species. New Phytol. 1994, 128, 725–736. [Google Scholar] [CrossRef]
- Zhong, M.; Wang, J.; Liu, K.; Wu, R.; Liu, Y.; Wei, X.; Pan, D.; Shao, X. Leaf Morphology Shift of Three Dominant Species along Altitudinal Gradient in an Alpine Meadow of the Qinghai-Tibetan Plateau. Pol. J. Ecol. 2014, 62, 639–648. [Google Scholar] [CrossRef]
- Cunningham, S.A.; Summerhayes, B.; Westoby, M. Evolutionary Divergences in Leaf Structure and Chemistry, Comparing Rainfall and Soil Nutrient Gradients. Ecol. Monogr. 1999, 69, 569. [Google Scholar] [CrossRef]
- Canavar, Ö.; Götz, K.-P.; Ellmer, F.; Chmielewski, F.-M.; Kaynak, M.A. Determination of the relationship between water use efficiency, carbon isotope discrimination and proline in sunflower genotypes under drought stress. Aust. J. Crop Sci. 2014, 8, 232–242. [Google Scholar]
- Zhang, H.; Ma, J.; Sun, W.; Chen, F. Altitudinal variation in functional traits of Picea schrenkiana var. tianschanica and their relationship to soil factors in Tianshan Mountains, Northwest China. Acta Ecol. Sin. 2010, 30, 5747–5758, (In Chinese, English summary). [Google Scholar]
- Hong, T.; Wu, C.Z.; Chen, C. Elevational resource niche of SLA of 5 dominant trees in Fujian coastal hilly region. J. Fujian Coll. For. 2013, 8–11. [Google Scholar]
- Essaghi, S.; Hachmi, M.; Yessef, M.; Dehhaoui, M. Leaf shrinkage: A predictive indicator of the potential variation of the surface area-to-volume ratio according to the leaf moisture content. SpringerPlus 2016, 5, 1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, A.-C.; Boyer, J.S. Leaf shrinkage decreases porosity at low water potentials in sunflower. Funct. Plant Biol. 2007, 34, 24. [Google Scholar] [CrossRef]
- Kadioglu, A.; Terzi, R. A Dehydration Avoidance Mechanism: Leaf Rolling. Bot. Rev. 2007, 73, 290–302. [Google Scholar] [CrossRef]
- Pugnaire, F.I.; Haase, P. Comparative physiology and growth of two perennial tussock grass species in a semi-arid environment. Ann. Bot. 1996, 77, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Pugnaire, F.I.; Haase, P.; Incoll, L.; Clark, S.C. Response of the tussock grass Stipa tenacissima to watering in a semi-arid environment. Funct. Ecol. 1996, 10, 265–274. [Google Scholar] [CrossRef]
- Castro-Díez, P.; Puyravaud, J.P.; Cornelissen, J.H.C. Leaf structure and anatomy as related to leaf mass per area variation in seedlings of a wide range of woody plant species and types. Oecologia 2000, 124, 476–486. [Google Scholar] [CrossRef] [PubMed]
| Species | Cenchrus ciliaris L. | Stipa parviflora Desf. | Stipa lagascae R. & Sch. | |
|---|---|---|---|---|
| Harvesting medium | Bou Hedma national park | Béja | Djerba island | |
| GPS Coordinates | Latitude | 34°28′46,60° | 38°0′55.094″ | 33,53′26° |
| Longitude | 9°40′04,47° | 7°51′45.832″ | 10°47′22″ | |
| Climate (1) | Aride | Semi-arid | Arid | |
| Temperature (°C) (1) | T min | 18.8 | 9.3 | 11.3 |
| T Average | 17.9 | 18 | 19.9 | |
| Annual | ||||
| T max | 23 | 27.3 | 27.9 | |
| Precipitation (mm) (1) | 223 | 662 | 200 | |
| Treatments | Number of Pots/Treatment | Water Irrigation Quantities | Annual Irrigation Quantities |
|---|---|---|---|
| (mm/Month) | (mm/Year) | ||
| Treatment 1 | 11 pots | 15 | 180 |
| Treatment 2 | 11 pots | 8.33 | 100 |
| Treatment 3 | 11 pots | 4.16 | 50 |
| Treatment 4 | 11 pots | At dry | At dry |
| Abbreviations | Explanation | Units |
|---|---|---|
| LL | Leaf Length | Cm |
| LW | Leaf Width | Cm |
| FW | Fresh Weight | G |
| DW | Dry Weight | G |
| RWC | Relative Water Content RWC = ((FW − DW)/(MFW − DW)) × 100 | % |
| FA | Fresh Area | cm2 |
| SLA | Specific Leaf Area | cm2/g−1 |
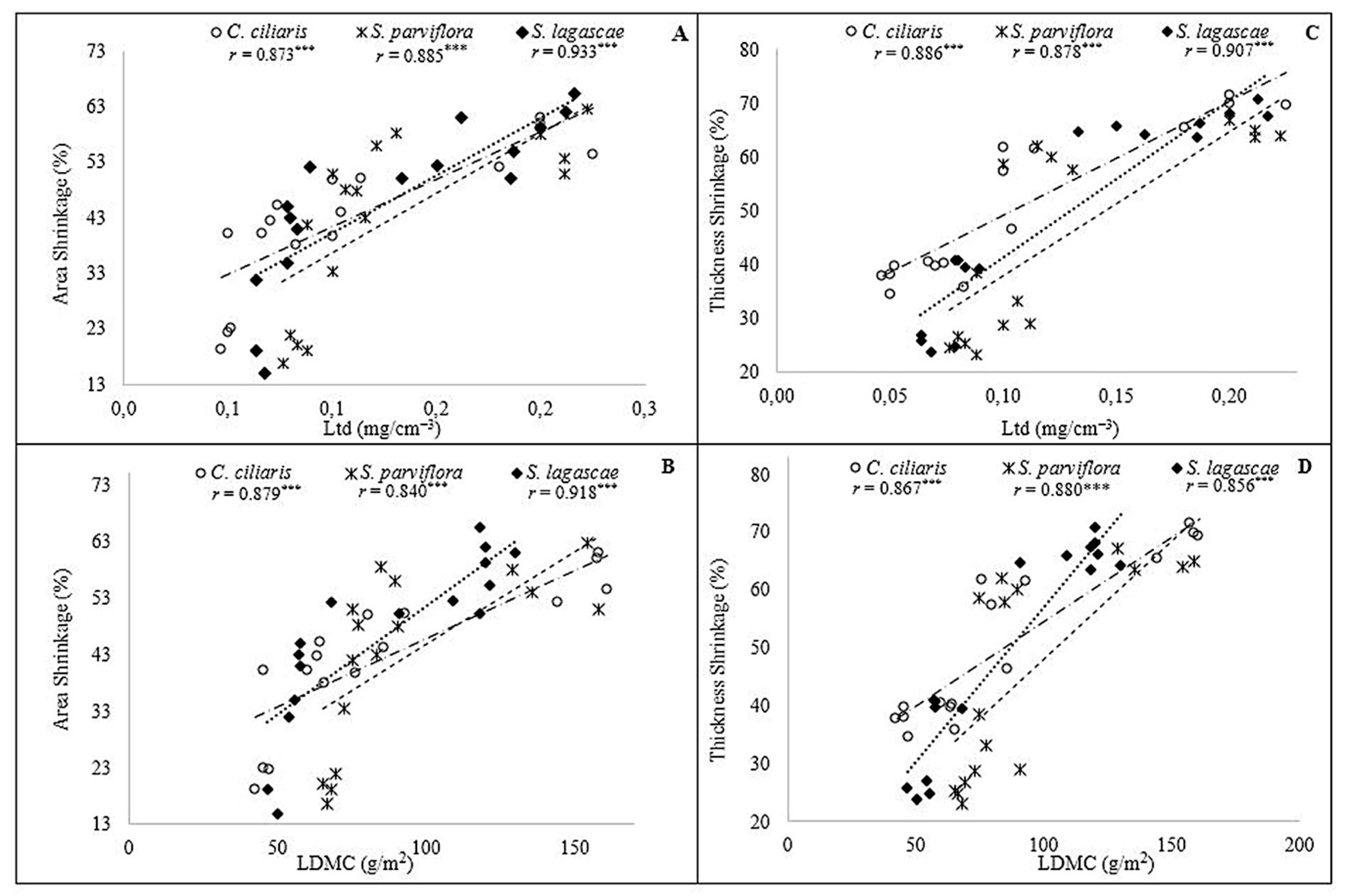
| LDMC | Leaf Dry Mater Content | g/m2 |
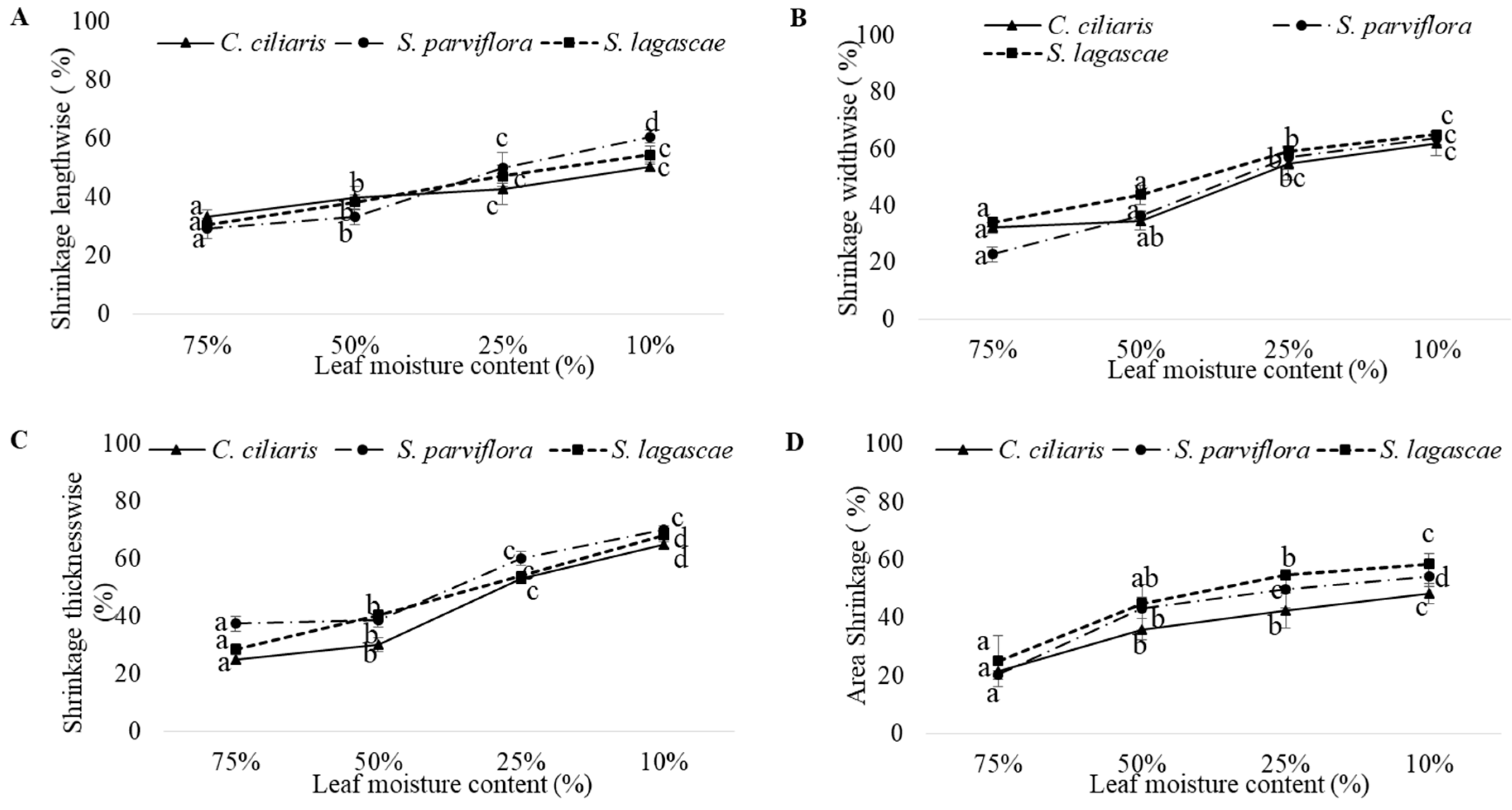
| Dimensional Shrinkage | Dimensional Shrinkage = ((Size difference/initial size) × 100) [18] | % |
| Lth | Leaf Thickness Index | µm |
| Ltd | Leaf Tissue Density Leaf Mass Per Area [19] | mg/cm−3 mg/cm−2 |
| NGL/T | Number of Green leaves/Tiller | |
| NGL/S | Number of Green leaves/Seedling | |
| NSL/T | Number of Senescent leaves/Tiller | |
| NSL/S | Number of Senescent leaves/Seedling | |
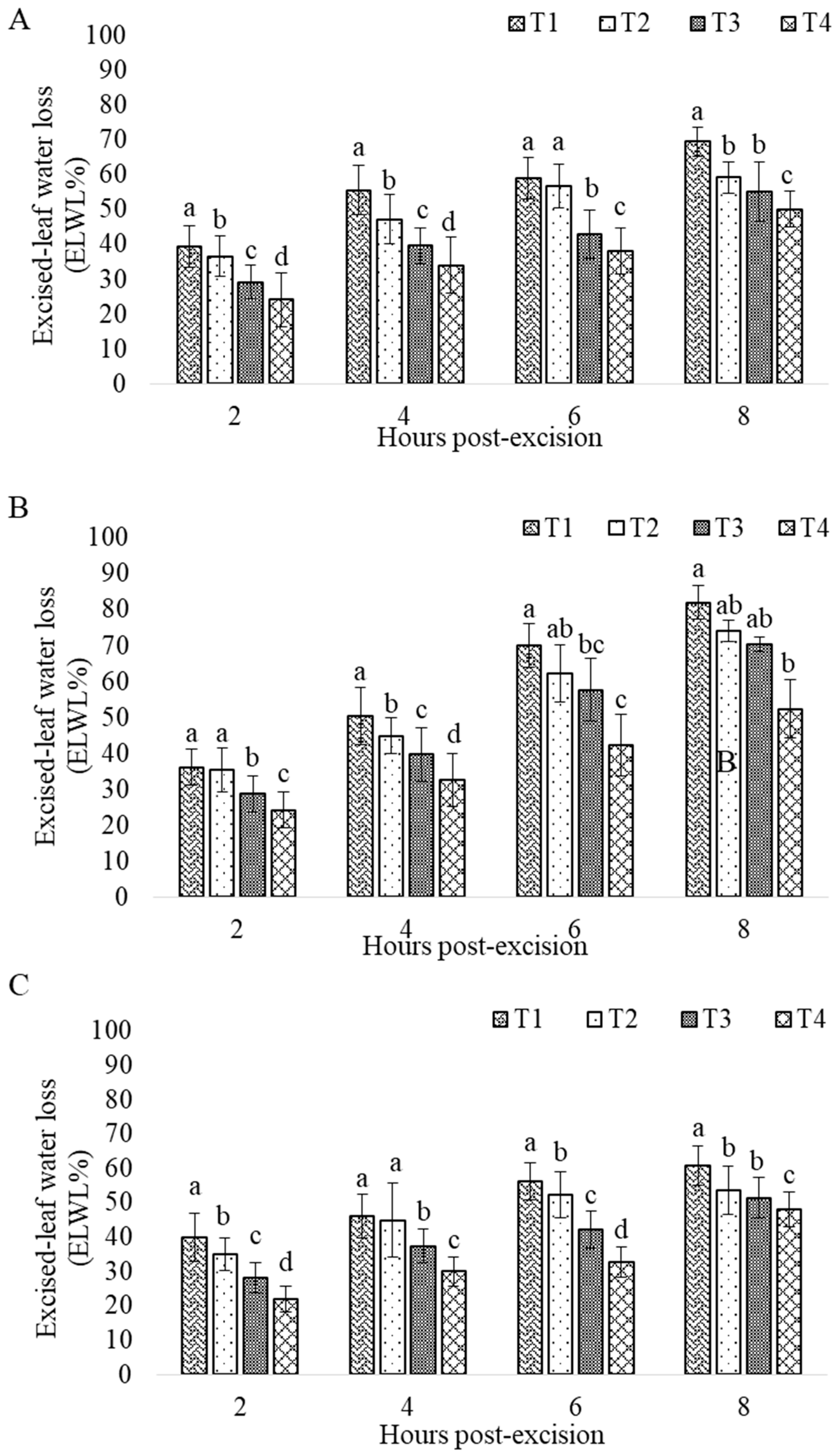
| ELWL | Excised Leaf Water Loss W1: Initial Weight W2: weight after water loss after x time X interval: 2 to 8 | % h |
| Species | T | LL | LW | RWC | SLA | LDMC | Lth | Ltd | NGL/T | NGL/S | NSL/TL | NSL/S |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C. ciliaris L. | T1 | 6.00 ± 0.60 a | 0.45 ± 0.05 a | 90.00 ± 2.00 a | 187.00 ± 6.00 a | 45.00 ± 1.90 d | 107.00 ± 2.90 c | 0.04 ± 0.002 d | 32.00 ± 1.00 a | 139.00 ± 11.00 a | 9.00 ± 1.00 c | 15.00 ± 1.00 c |
| T2 | 5.60 ± 0.80 a | 0.42 ± 0.05 a | 85.70 ± 3.00 ab | 96.00 ± 7.00 b | 63.00 ± 9.30 c | 141.00 ± 21.00 b | 0.07 ± 0.01 c | 21.00 ± 5.00 b | 120.00 ± 10.00 a | 13.00 ± 2.00 b | 22.00 ± 3.00 b | |
| T3 | 5.30 ± 0.80 a | 0.40 ± 0.08 a | 78.00 ± 2.00 bc | 62.00 ± 3.00 c | 83.00 ± 7.20 b | 153.00 ± 11.50 bc | 0.10 ± 0.006 b | 13.00 ± 1.00 c | 81.00 ± 5.00 b | 22.00 ± 1.00 a | 24.00 ± 1.20 b | |
| T4 | 3.20 ± 0.90 b | 0.30 ± 0.07 a | 73.10 ± 4.00 c | 30.00 ± 4.00 d | 155.00 ± 7.50 a | 164.00 ± 7.50 a | 0.25 ± 0.01 a | 10.00 ± 0.80 d | 37.00 ± 4.00 c | 27.00 ± 1.00 a | 34.00 ± 2.00 a | |
| F-value | 9.20 | 1.33 | 13.02 | 284.41 | 327.50 | 27.31 | 254.21 | 139.04 | 185.63 | 74.63 | 99.49 | |
| p-value | 0.002 | 0.31 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| S. parviflora Desf. | T1 | 5.00 ± 0.20 a | 0.28 ± 0.05 a | 79.50 ± 8.00 a | 130.00 ± 9.00 a | 67.00 ± 2.9 c | 115.00 ± 4.30 b | 0.06 ± 0.006 d | 20.00 ± 2.00 a | 71.00 ± 9.00 a | 4.00 ± 1.20 c | 10.00 ± 1.00 c |
| T2 | 3.00 ± 0.20 b | 0.20 ± 0.06 a | 75.00 ± 9.60 a | 93.00 ± 18.00 b | 78.00 ± 3.30 b | 127.00 ± 7.10 ab | 0.08 ± 0.009 c | 15.00 ± 3.00 ab | 50.00 ± 13.00 b | 7.00 ± 2.00 b | 12.00 ± 2.00 b | |
| T3 | 2.40 ± 0.40 c | 0.17 ± 0.02 ab | 68.00 ± 4.90 b | 60.00 ± 12.00 c | 83.00 ± 6.05 bc | 146.00 ± 19.90 ab | 0.11 ± 0.013 b | 10.00 ± 3.00 bc | 33.00 ± 5.00 c | 11.00 ± 2.00 bc | 16.00 ± 2.00 bc | |
| T4 | 1.30 ± 0.10 d | 0.11 ± 0.05 b | 62.00 ± 6.70 c | 27.00 ± 1.00 d | 144.00 ± 14.20 a | 152.00 ± 7.10 a | 0.21 ± 0.008 a | 7.00 ± 1.70 c | 20.00 ± 4.00 d | 15.00 ± 2.90 a | 20.00 ± 1.00 a | |
| F-value | 89.73 | 8.10 | 28.08 | 64.88 | 57.40 | 3.72 | 107.60 | 17.43 | 50.31 | 17.91 | 25.65 | |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| S. lagascae R.& Sch. | T1 | 5.00 ± 0.80 a | 0.40 ± 0.10 a | 74.00 ± 6.60 a | 134.00 ± 13.00 a | 51.00 ± 3.90 b | 109.00 ± 5.00 b | 0.06 ± 0.007 c | 18.00 ± 3.00 a | 65.00 ± 3.00 a | 3.00 ± 0.90 c | 10.00 ± 1.00 c |
| T2 | 3.80 ± 0.5 ab | 0.30 ± 0.09 ab | 70.00 ± 5.60 a | 100.00 ± 22.00 b | 60.00 ± 4.70 b | 121.00 ± 10.20 b | 0.08 ± 0.009 c | 13.00 ± 4.30 a | 46.00 ± 10.00 b | 6.00 ± 1.70 c | 14.00 ± 2.00 b | |
| T3 | 3.20 ± 0.20 b | 0.30 ± 0.07 ab | 67.00 ± 5.40 ab | 51.00 ± 9.00 c | 112.00 ± 14.40 a | 130.00 ± 8.70 a | 0.15 ± 0.019 b | 9.00 ± 1.80 b | 32.00 ± 3.00 c | 10.00 ± 1.70 b | 17.00 ± 1.00 b | |
| T4 | 2.00 ± 0.40 c | 0.20 ± 0.04 b | 53.00 ± 4.10 b | 22.00 ± 1.00 c | 119.00 ± 8.50 a | 139.00 ± 9.90 a | 0.20 ± 0.01 a | 6.00 ± 0.90 b | 19.00 ± 3.00 d | 14.00 ± 1.20 a | 21.00 ± 1.00 a | |
| F-value | 20.15 | 4.75 | 6.48 | 80.98 | 91.92 | 61.37 | 116.73 | 28.09 | 85.08 | 33.03 | 43.35 | |
| p-value | <0.0001 | 0.02 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Parameters | SS | DF | MS | F-Values | p-Values | |
|---|---|---|---|---|---|---|
| LL | Treatment | 5.77 | 3 | 1.925 | 72.36 | <0.0001 |
| Species | 2.78 | 2 | 1.391 | 52.31 | <0.0001 | |
| Treatment X Species | 0.55 | 6 | 0.092 | 3.440 | <0.0001 | |
| LW | Treatment | 2.32 | 3 | 0.774 | 13.93 | <0.0001 |
| Species | 5.23 | 2 | 2.617 | 47.11 | <0.0001 | |
| Treatment X Species | 0.57 | 6 | 0.096 | 1.730 | <0.0001 | |
| RWC | Treatment | 4.39 | 3 | 1.466 | 34.05 | <0.0001 |
| Species | 13.89 | 2 | 6.949 | 31.88 | <0.0001 | |
| Treatment X Species | 4.50 | 6 | 0.750 | 1.21 | <0.0001 | |
| SLA | Treatment | 19.46 | 3 | 6.488 | 337.04 | <0.0001 |
| Species | 0.34 | 2 | 0.174 | 7.03 | <0.0001 | |
| Treatment X Species | 0.31 | 6 | 0.052 | 4.00 | <0.0001 | |
| LDMC | Treatment | 6.08 | 3 | 2.028 | 323.50 | <0.0001 |
| Species | 0.16 | 2 | 0.082 | 13.00 | <0.0001 | |
| Treatment X Species | 0.70 | 6 | 0.117 | 18.650 | <0.0001 | |
| Lth | Treatment | 2.04 | 3 | 0.682 | 25.79 | <0.0001 |
| Species | 0.08 | 2 | 0.043 | 11.47 | <0.0001 | |
| Treatment X Species | 0.39 | 6 | 0.066 | 4.51 | <0.0001 | |
| Ltd | Treatment | 8.90 | 3 | 2.969 | 426.33 | <0.0001 |
| Species | 0.57 | 2 | 0.289 | 44.32 | <0.0001 | |
| Treatment X Species | 0.51 | 6 | 0.085 | 11.00 | <0.0001 | |
| NGL/T | Treatment | 7.93 | 3 | 2.646 | 98.85 | <0.0001 |
| Species | 1.69 | 2 | 0.848 | 31.68 | <0.0001 | |
| Treatment X Species | 0.07 | 6 | 0.012 | 0.44 | <0.0001 | |
| NGL/S | Treatment | 10.89 | 3 | 3.631 | 250.33 | <0.0001 |
| Species | 6.79 | 2 | 3.398 | 234.23 | <0.0001 | |
| Treatment X Species | 0.16 | 6 | 0.027 | 1.85 | <0.0001 | |
| NSL/TL | Treatment | 13.15 | 3 | 4.386 | 78.38 | <0.0001 |
| Species | 6.71 | 2 | 3.357 | 59.98 | <0.0001 | |
| Treatment X Species | 0.36 | 6 | 0.061 | 1.08 | <0.0001 | |
| NSL/S | Treatment | 4.17 | 3 | 1.392 | 121.61 | <0.0001 |
| Species | 2.22 | 2 | 1.112 | 97.16 | <0.0001 | |
| Treatment X Species | 0.04 | 6 | 0.007 | 0.57 | <0.0001 | |
| Traits | C. ciliaris | S. parviflora | S. lagascae | |||
|---|---|---|---|---|---|---|
| r | p-Values | r | p-Values | r | p-Values | |
| SLA | ||||||
| RWC | 0.87 | <0.0001 | 0.806 | <0.0001 | 0.885 | <0.0001 |
| LDMC | −0.990 | <0.0001 | −0.914 | <0.0001 | −0.920 | <0.0001 |
| Ltd | 0.990 | <0.0001 | 0.943 | <0.0001 | 0.981 | <0.0001 |
| LDMC | ||||||
| RWC | −0.868 | <0.0001 | −0.709 | 0.002 | −0.868 | 0.001 |
| Ltd | −0.996 | <0.0001 | −0.948 | <0.0001 | −0.910 | <0.0001 |
| Lth | ||||||
| RWC | −0.987 | <0.0001 | −0.717 | 0.002 | −0.781 | 0.001 |
| Ltd | −0.837 | <0.0001 | −0.837 | <0.0001 | −0.763 | 0.001 |
| Parameters | SS | DF | MS | F-Values | p-Values |
|---|---|---|---|---|---|
| Species | 2195.64 | 2 | 1097.82 | 7.21 | <0.0001 |
| Treatment | 46,804.52 | 3 | 15,601.51 | 102.51 | <0.0001 |
| Hours | 95,350.50 | 3 | 31,783.50 | 208.83 | <0.0001 |
| Species X Treatment | 1319.95 | 6 | 219.99 | 1.45 | 0.1 |
| Species X Hours | 3066.84 | 6 | 511.14 | 3.36 | 0.003 |
| Treatment X Hours | 3303.47 | 9 | 367.05 | 2.41 | 0.01 |
| Species X Treatment X Hours | 4575.52 | 18 | 254.20 | 1.67 | 0.03 |
| Parameters | SS | DF | MS | F-Values | p-Values | |
|---|---|---|---|---|---|---|
| Shrinkage lengthwise | Species | 89.774 | 2 | 44.887 | 0.632 | 0.534 |
| LMC | 21,827.088 | 3 | 7275.696 | 102.403 | <0.0001 | |
| Species X LMC | 128.025 | 6 | 21.338 | 0.300 | 0.935 | |
| Shrinkage widthwise | Species | 497.367 | 2 | 248.683 | 2717 | 0.071 |
| LMC | 22,297.775 | 3 | 7432.592 | 81.208 | <0.0001 | |
| Species X LMC | 465.642 | 6 | 77.607 | 0.848 | 0.536 | |
| Shrinkage thicknesswise | Species | 1036.792 | 2 | 518.396 | 43.274 | <0.0001 |
| LMC | 31,127.459 | 3 | 10,375.820 | 866.137 | <0.0001 | |
| Species X LMC | 969.729 | 6 | 161.621 | 13.492 | <0.0001 | |
| Area Shrinkage | Species | 89.774 | 2 | 44.887 | 0.632 | 0.534 |
| LMC | 21,827.088 | 3 | 7275.696 | 102.403 | <0.0001 | |
| Species X LMC | 128.025 | 6 | 21.338 | 0.300 | 0.935 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamdani, M.; Krichen, K.; Chaieb, M. Predicting Leaf Trait Variability as a Functional Descriptor of the Effect of Climate Change in Three Perennial Grasses. Diversity 2019, 11, 233. https://doi.org/10.3390/d11120233
Hamdani M, Krichen K, Chaieb M. Predicting Leaf Trait Variability as a Functional Descriptor of the Effect of Climate Change in Three Perennial Grasses. Diversity. 2019; 11(12):233. https://doi.org/10.3390/d11120233
Chicago/Turabian StyleHamdani, Marwa, Khouloud Krichen, and Mohamed Chaieb. 2019. "Predicting Leaf Trait Variability as a Functional Descriptor of the Effect of Climate Change in Three Perennial Grasses" Diversity 11, no. 12: 233. https://doi.org/10.3390/d11120233
APA StyleHamdani, M., Krichen, K., & Chaieb, M. (2019). Predicting Leaf Trait Variability as a Functional Descriptor of the Effect of Climate Change in Three Perennial Grasses. Diversity, 11(12), 233. https://doi.org/10.3390/d11120233





