Effect of Hydrographic Variability on the Distribution of Microbial Communities in Taiwan Strait in Winter
Abstract
1. Introduction
2. Material and Methods
2.1. Study Sites and Samplings
2.2. Microbial Counts
3. Results
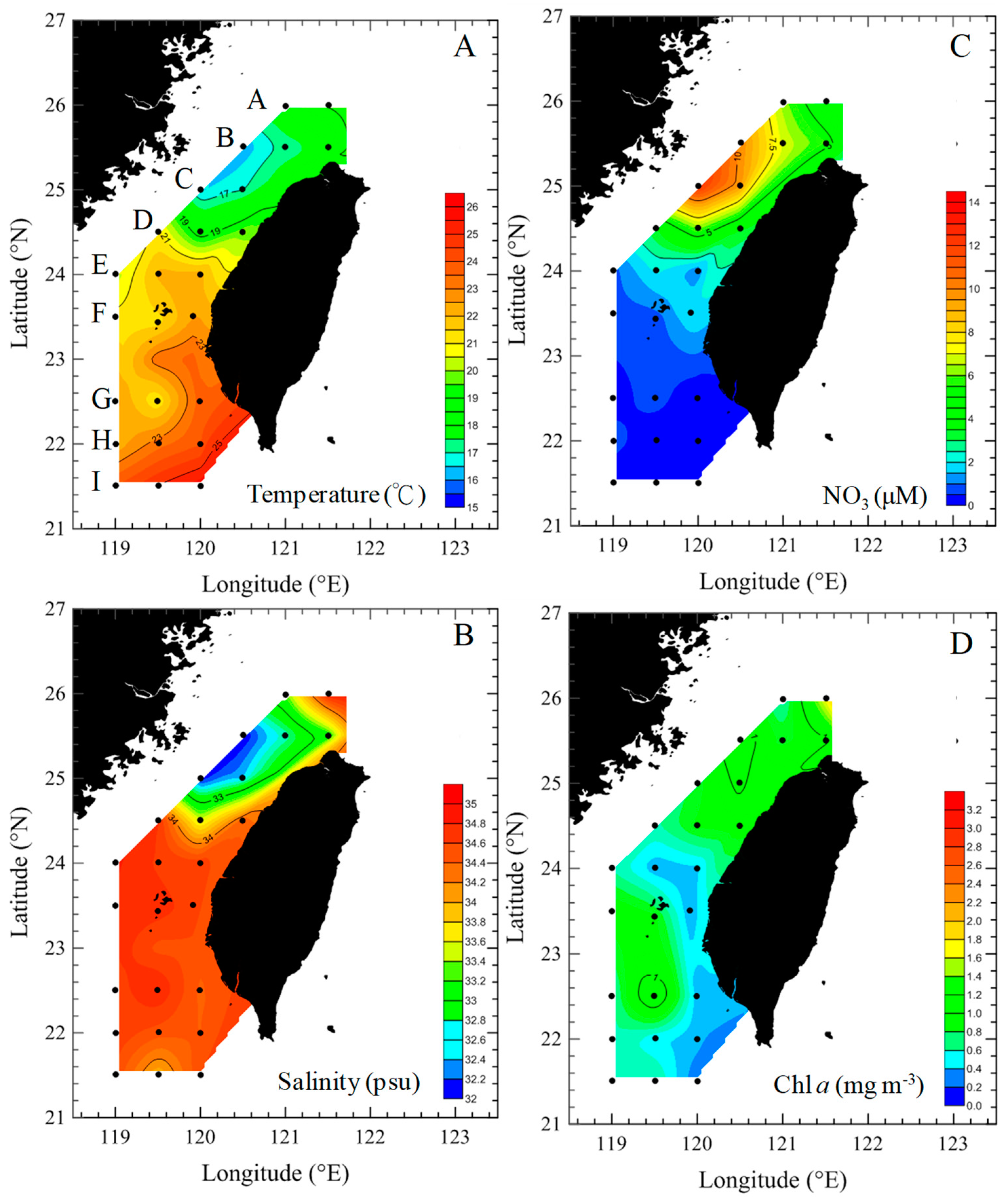
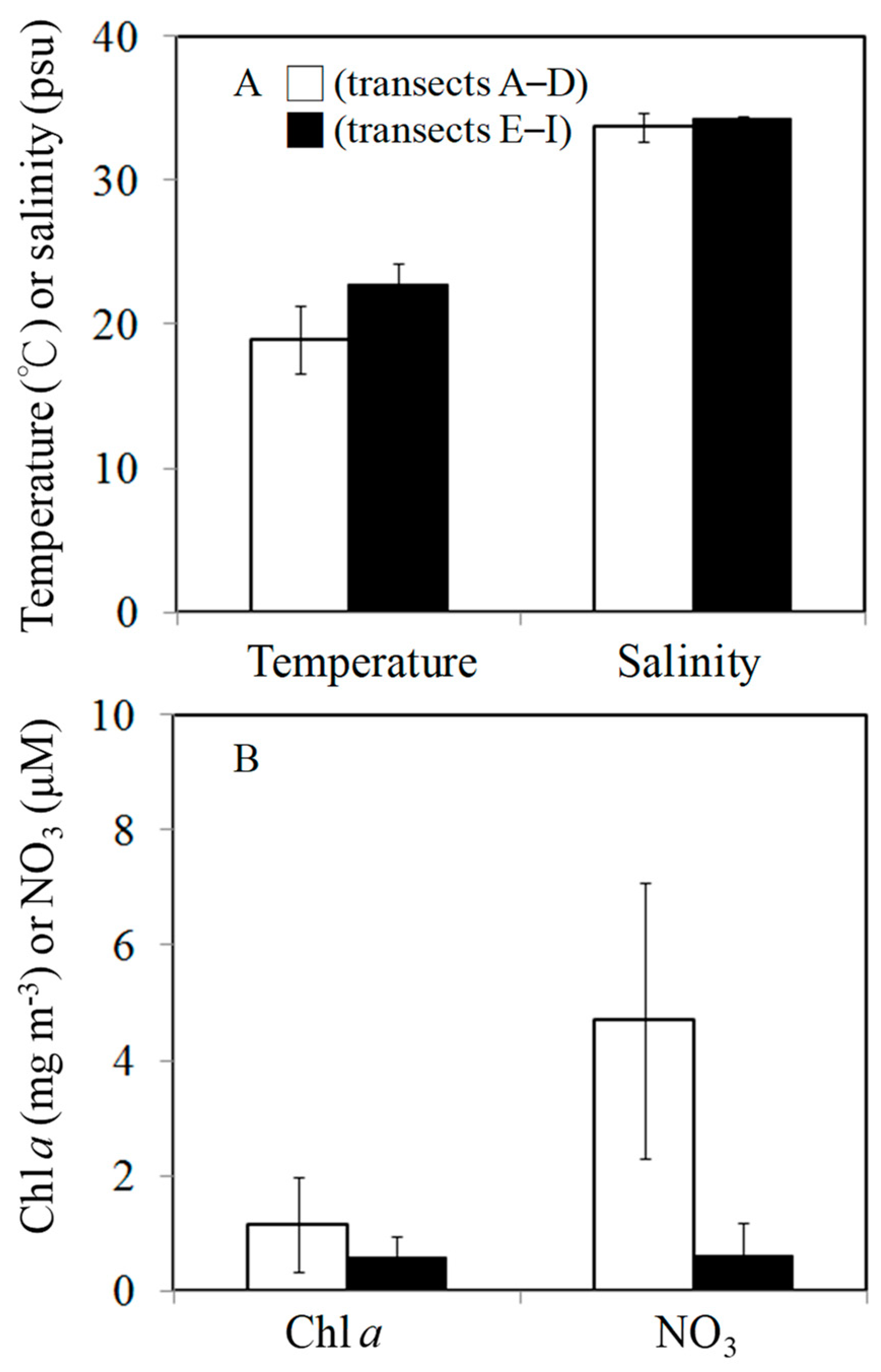
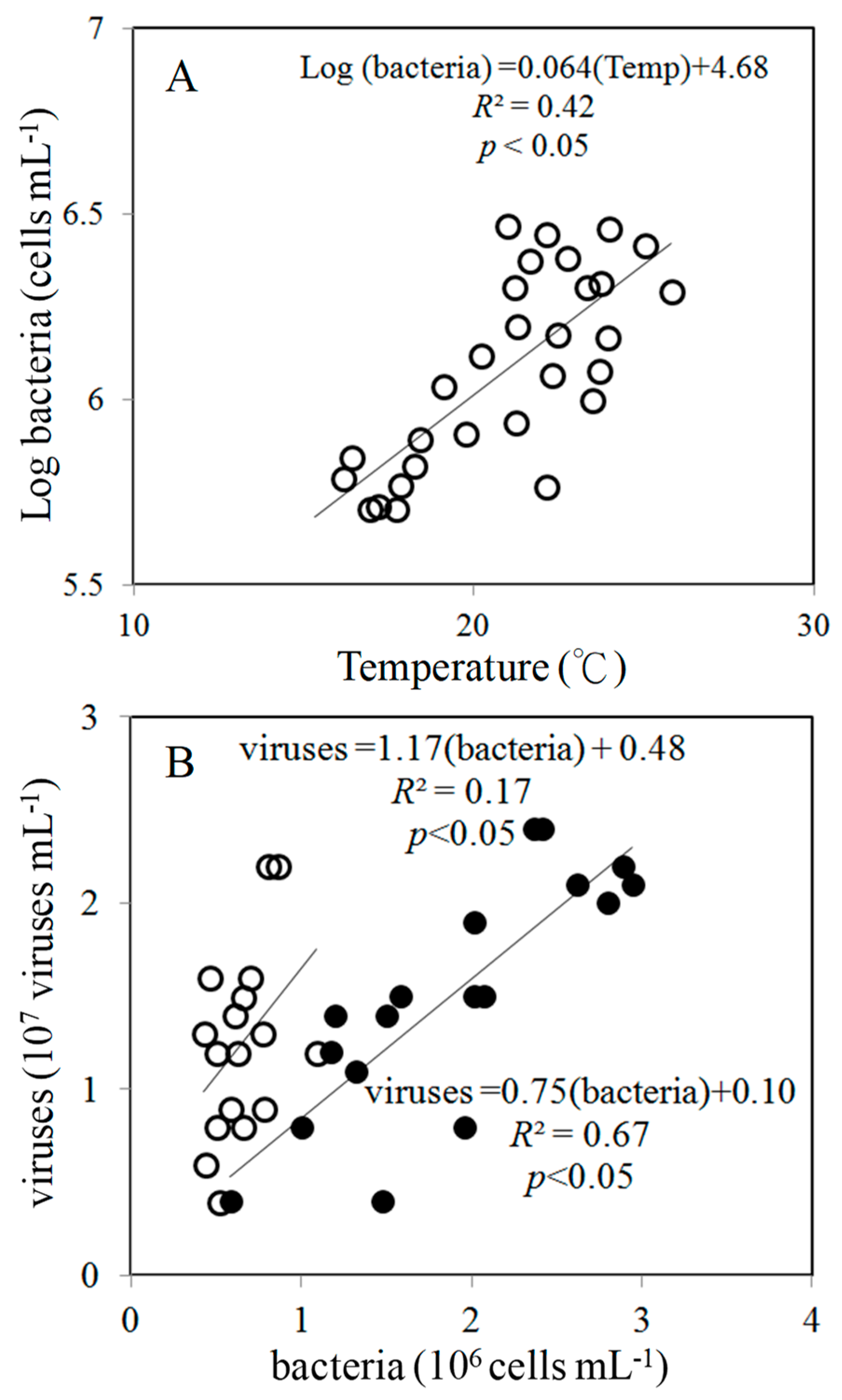
3.1. Environmental Conditions
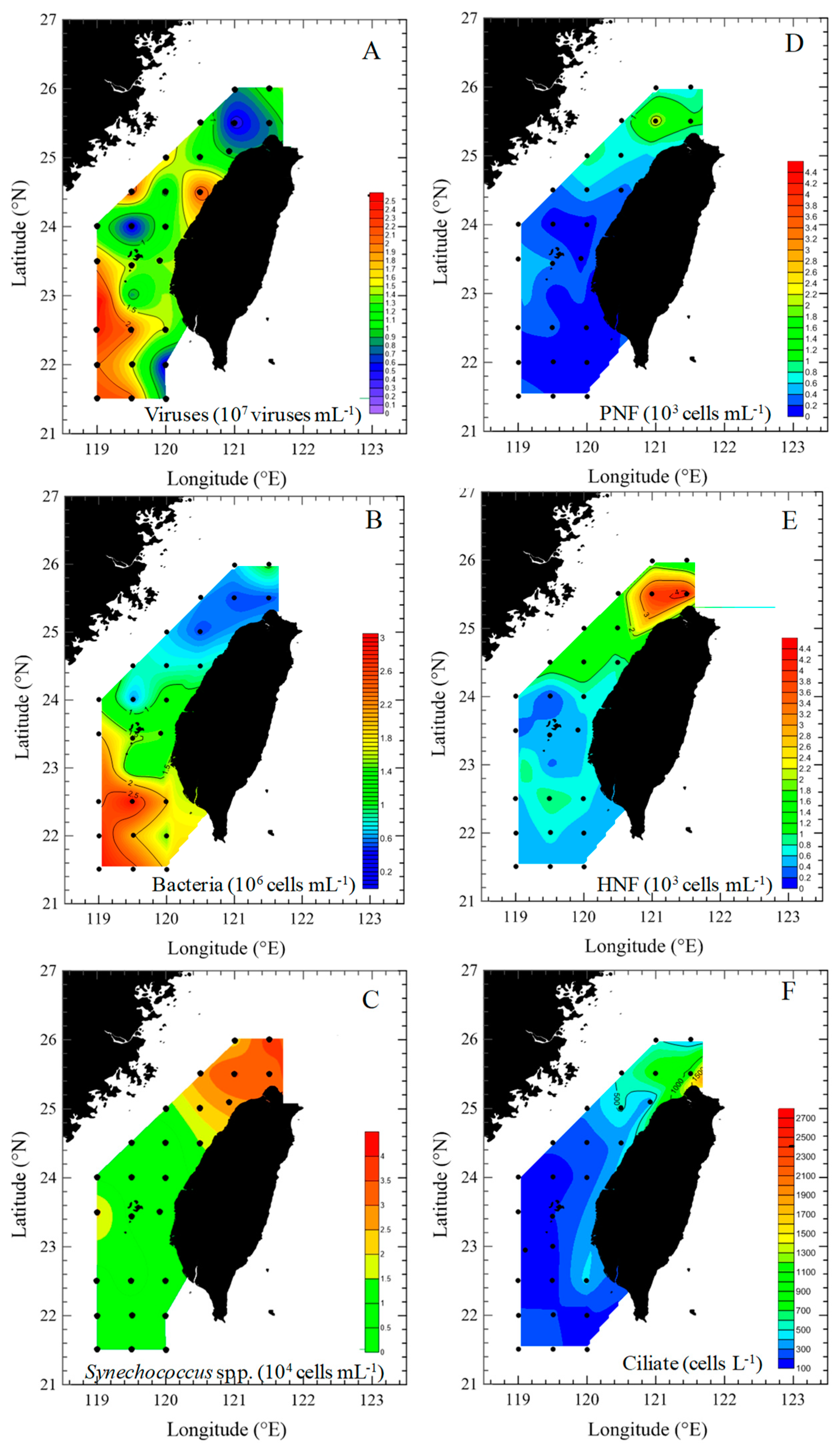
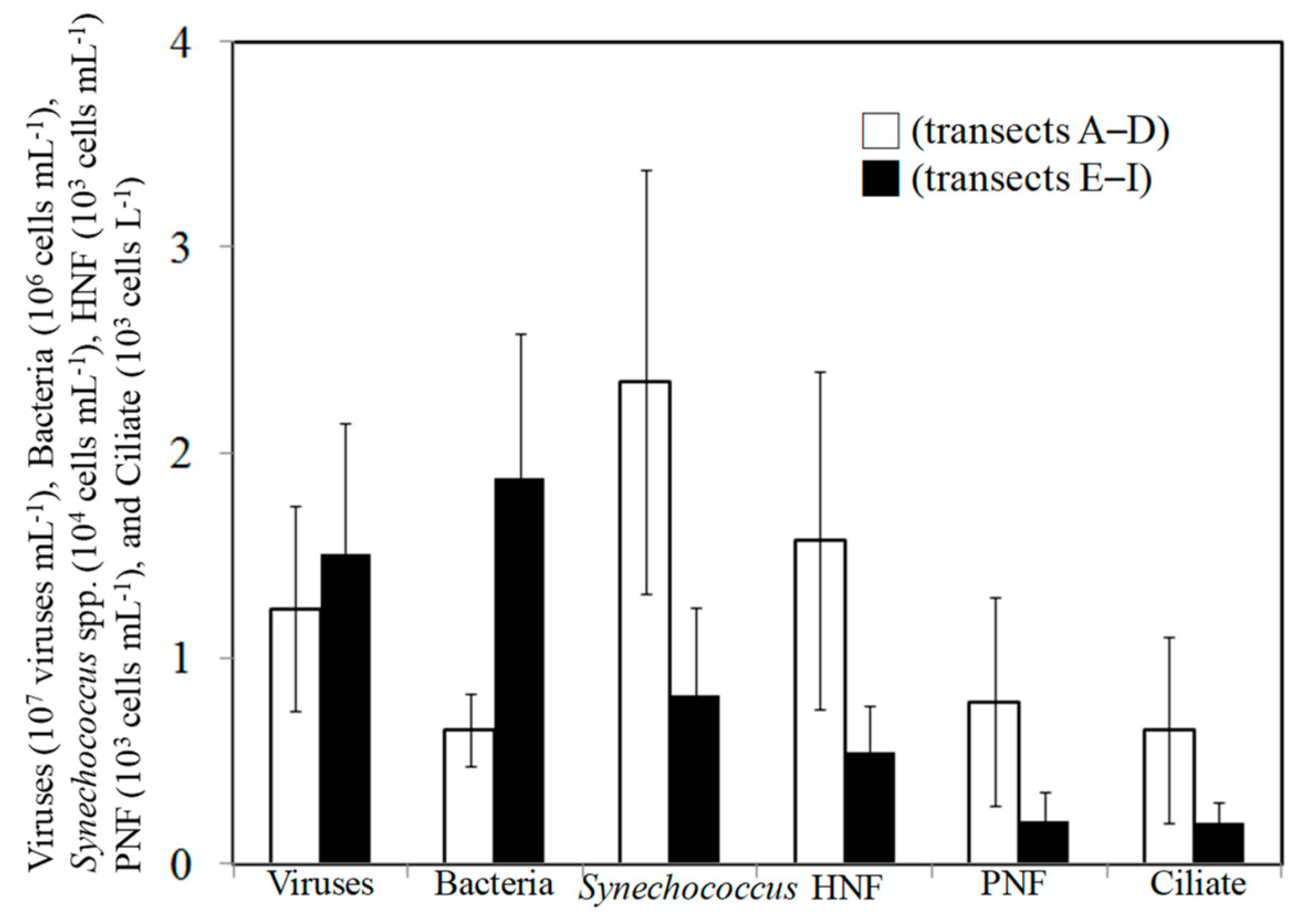
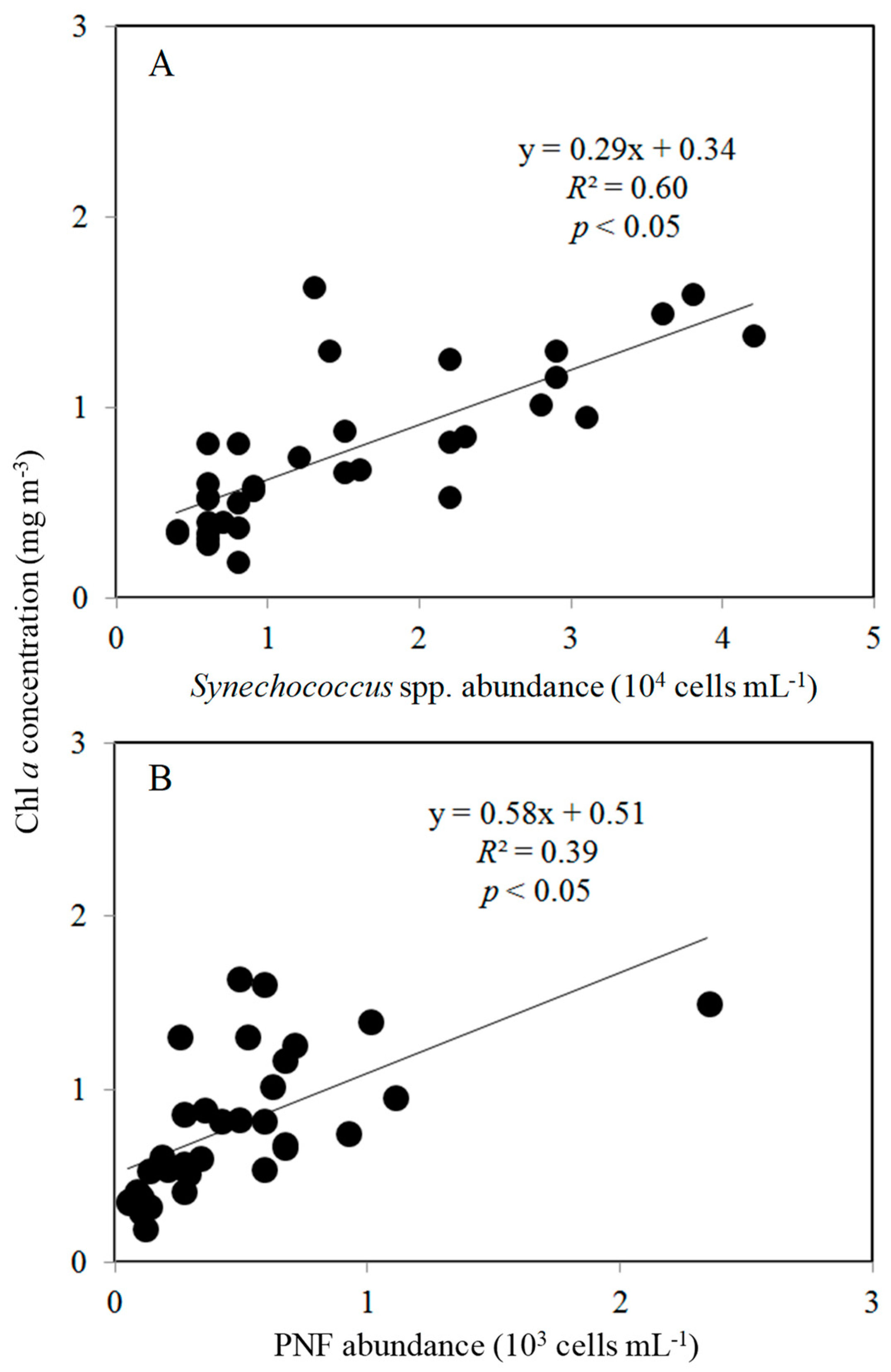
3.2. Spatial Changes in Microbial Community
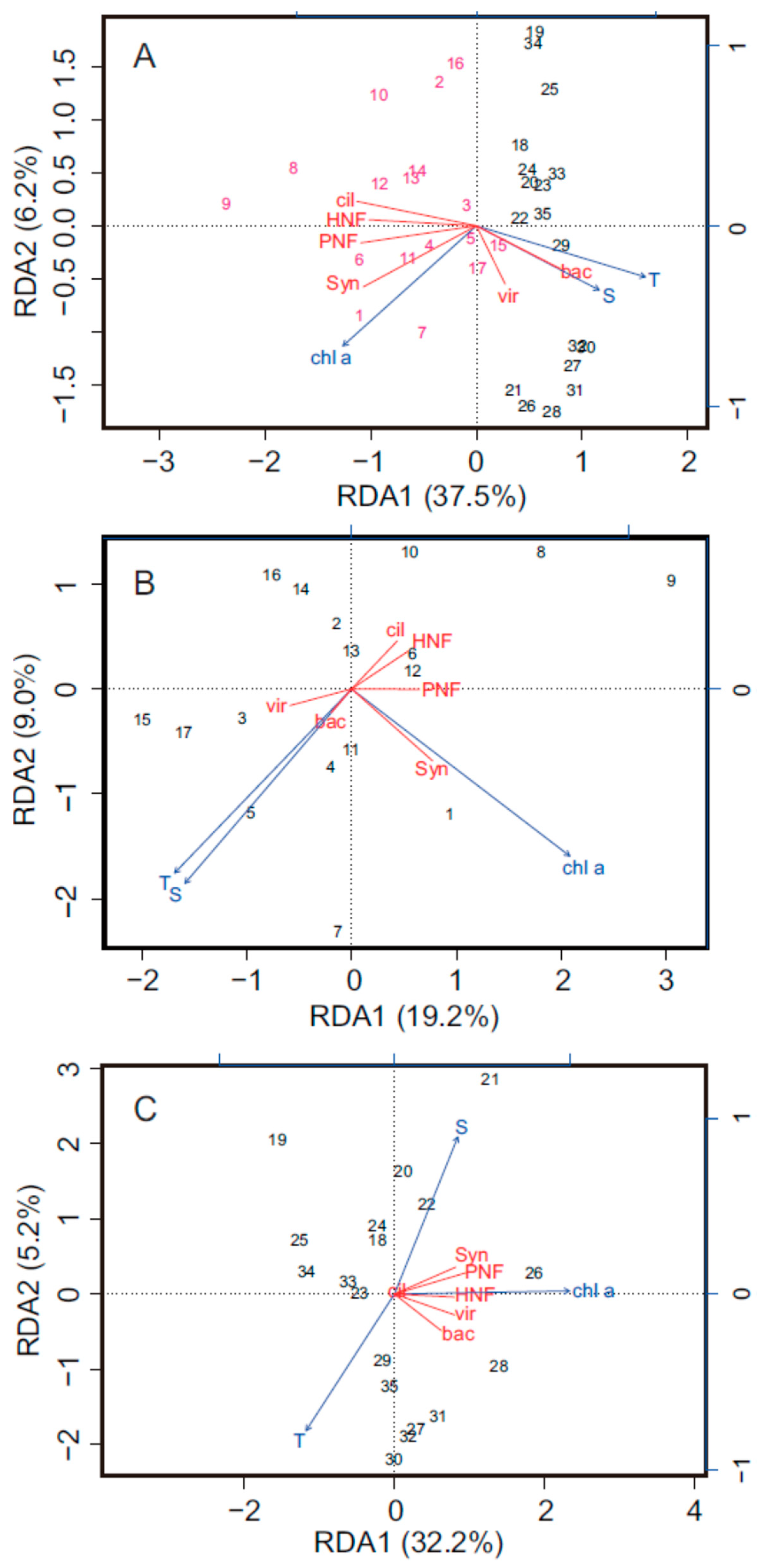
4. Discussion
4.1. Spatial Variations in Bacterial and Viral Abundance
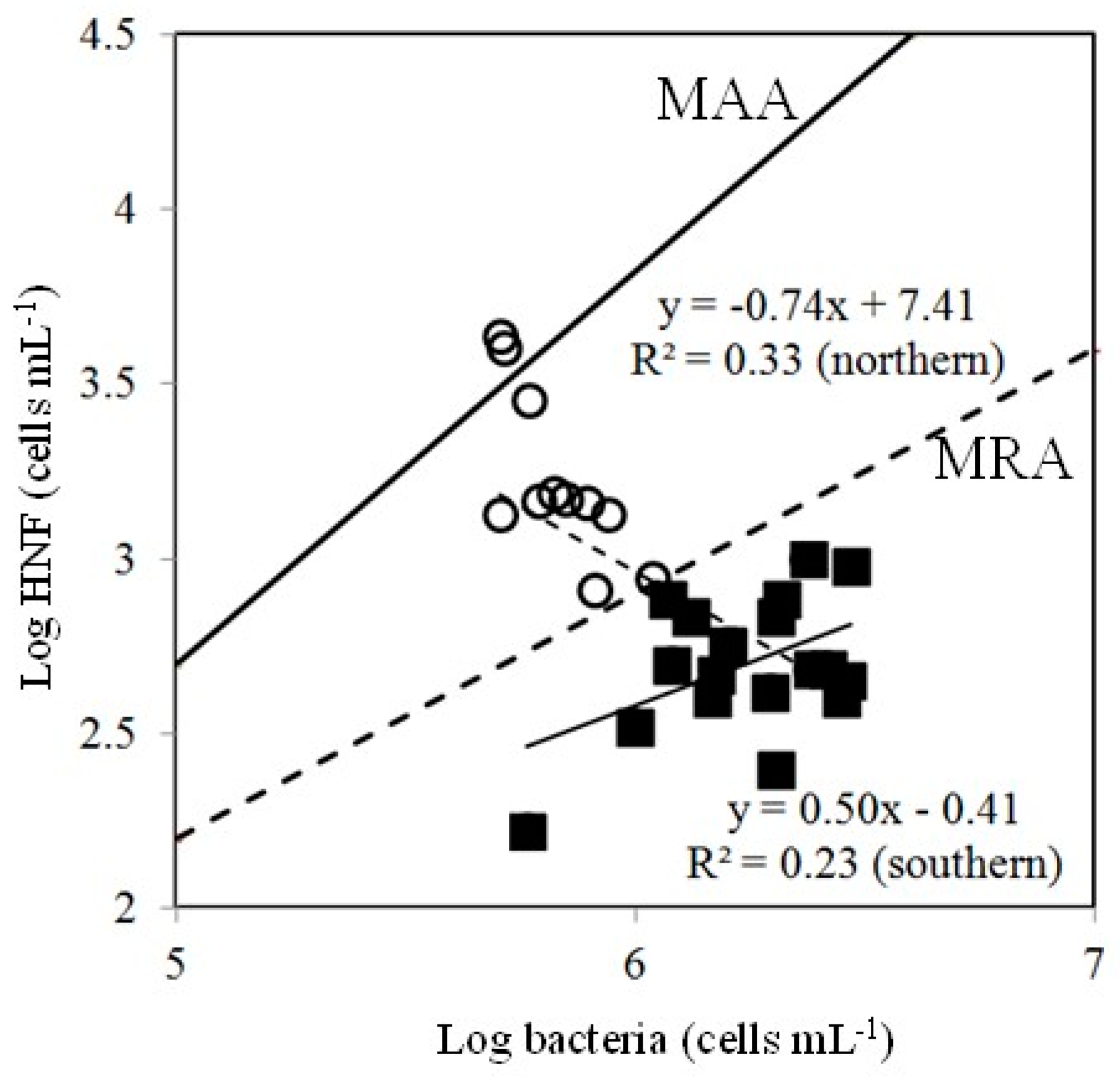
4.2. Effects of Bottom-Up and Top-Down Controls on HNFs
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pomeroy, L.R. The ocean’s food web: A changing paradigm. Bioscience 1974, 24, 499–504. [Google Scholar] [CrossRef]
- Christaki, U.; Giannakourou, A.; Van Wambeke, F.; Gregori, G. Nanoflagellate predation on auto-and heterotrophic picoplankton in the oligotrophic Mediterranean Sea. J. Plankton Res. 2001, 23, 1297–1310. [Google Scholar] [CrossRef]
- Tsai, A.Y.; Chiang, K.P.; Chang, J.; Gong, G.C. Seasonal diel variations of picoplankton and nanoplankton in a subtropical Western Pacific coastal ecosystem. Limnol. Oceanogr. 2005, 50, 1221–1231. [Google Scholar] [CrossRef]
- Unrein, F.; Massana, R.; Alonso-Saez, L.; Gasol, J.M. Significant year-round effect of small mixotrophic flagellates on bacterioplankton in an oligotrophic coastal system. Limnol. Oceanogr. 2007, 52, 456–469. [Google Scholar] [CrossRef]
- Tsai, A.Y.; Gong, G.C.; Hung, J. Seasonal variations of virus- and nanoflagellate-mediated mortality of heterotrophic bacteria in the coastal ecosystem of subtropical western Pacific. Biogeosciences 2013, 10, 3055–3065. [Google Scholar] [CrossRef]
- Schultz, G.E., Jr.; White, E.D., III; Ducklow, H.W. Bacterioplankton dynamics in the York River estuary: Primary influence of temperature and freshwater inputs. Aquat. Microb. Ecol. 2003, 30, 135–148. [Google Scholar] [CrossRef][Green Version]
- Iriarte, A.; Sarobe, A.; Orive, E. Seasonal variability in bacterial abundance, production and protistan bacterivory in the lower Urdaibai estuary, Bay of Biscay. Aquat. Microb. Ecol. 2008, 52, 273–282. [Google Scholar] [CrossRef]
- Tsai, A.Y.; Gong, G.C.; Sanders, R.W.; Chen, W.H.; Chao, C.F.; Chiang, K.P. Importance of bacterivory by pigmented and heterotrophic nanoflagellates during the warm season in a subtropical western Pacific coastal ecosystem. Aquat. Microb. Ecol. 2011, 63, 9–18. [Google Scholar] [CrossRef]
- Li, W.K.W. Annual average abundance of heterotrophic bacteria and Synechococcus in surface ocean waters. Limnol. Oceanogr. 1998, 43, 1746–1753. [Google Scholar] [CrossRef]
- Ochs, C.A.; Cole, J.J.; Liken, G.E. Population dynamics of bacterioplankton in an oligotrophic lake. J. Plankton Res. 1995, 17, 365–391. [Google Scholar] [CrossRef]
- Shiah, F.K.; Ducklow, H.W. Temperature regulation of heterotrophic bacterioplankton abundance, production, and specific growth rate in Chesapeake Bay. Limnol. Oceanogr. 1994, 39, 1243–1258. [Google Scholar] [CrossRef]
- Sanders, R.W.; Caron, D.A.; Berninger, U.G. Relationships between bacteria and heterotrophic nanoplankton in marine and fresh waters- an interecosystem comparison. Mar. Ecol. Peog. Ser. 1992, 86, 1–14. [Google Scholar] [CrossRef]
- Sherr, E.B.; Sherr, B.F. Significance of predation by protists in aquatic microbial food webs. Antonie Van Leeuwenhoek 2002, 81, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Gasol, J.M.; Simons, A.M.; Kalff, J. Patterns of top-down versus bottom-up regulation of heterotrophic nanoflagellates in temperate lakes. J. Plankton Res. 1995, 17, 1879–1903. [Google Scholar] [CrossRef]
- Gasol, J.M.; Vaque, D. Lack of coupling between heterotrophic nanoflagellates and bacteria: A general phenomenon across aquatic systems? Limnol. Oceanogr. 1993, 38, 657–665. [Google Scholar] [CrossRef]
- Gasol, J.M. A framework for the assessment of top-down vs. bottom-up control of heterotrophic nanoflagellate abundance. Mar. Ecol. Prog. Ser. 1994, 113, 291–300. [Google Scholar] [CrossRef]
- Kisand, V.; Zingel, P. Dominance of ciliate grazing on bacteria during spring in a shallow eutrophic lake. Aquat. Microb. Ecol. 2000, 22, 135–142. [Google Scholar] [CrossRef]
- Berglund, J.; Samuelsson, K.; Kull, T.; Muren, U.; Andersson, A. Relative strength of resource and predation limitation of heterotrophic nanoflagellates in a low-productive sea area. J. Plankton Res. 2005, 27, 923–935. [Google Scholar] [CrossRef]
- Samuelsson, K.; Berglund, J.; Andersson, A. Factors structuring the heterotrophic flagellate and ciliate community along a brackish water primary production gradient. J. Plankton Res. 2006, 28, 345–359. [Google Scholar] [CrossRef][Green Version]
- Carrias, J.F.; Amblard, C.; Quiblier-Lloberas, C.; Bourdier, G. Seasonal dynamics of free and attached heterotrophic nanoflagellates in an oligotrophic lake. Freshw. Biol. 1998, 39, 91–101. [Google Scholar] [CrossRef]
- Auer, B.; Arndt, H. Taxonomic composition and biomass of heterotrophic flagellates in relation to lake trophy and season. Freshw. Biol. 2001, 46, 959–972. [Google Scholar] [CrossRef]
- Kosolapova, N.G.; Kosolapov, D.B. The diversity and distribution of heterotrophic nanoflagellates in the eutrophic Lake Nero. Inland Water Biol. 2009, 2, 42–49. [Google Scholar] [CrossRef]
- Liang, W.D.; Tang, T.Y.; Yang, Y.J.; Ko, M.T.; Chung, W.S. Upper-ocean currents around Taiwan. Deep Sea Res. 2003, 50, 1085–1105. [Google Scholar] [CrossRef]
- Jan, S.; Wang, J.; Chern, C.S.; Chao, S.Y. Seasonal variation of the circulation in the Taiwan Strait. J. Mar. Syst. 2002, 35, 249–268. [Google Scholar] [CrossRef]
- Gong, G.C.; Shiah, F.-K.; Liu, K.-K.; Wen, Y.-H.; Liang, M.-H. Spatial and temporal variation of chlorophyll a, primary productivity and chemical hydrography in the southern East China Sea. Cont. Shelf Res. 2000, 20, 411–436. [Google Scholar] [CrossRef]
- Stoecker, D.K.; Taniguchi, A.; Michaels, A.E. Abundance of autotrophic, mixotrophic, and heterotrophic planktonic ciliates in shelf and slope waters. Mar. Ecol. Prog. Ser. 1989, 50, 241–254. [Google Scholar] [CrossRef]
- Tsai, A.Y.; Gong, G.C.; Liu, H. Seasonal variations in virioplankton and picoplankton in semi-enclosed and open coastal waters. Terr. Atoms. Ocean. Sci. 2018, 29, 465–472. [Google Scholar] [CrossRef]
- Porter, K.G.; Feig, Y.S. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 1980, 25, 943–948. [Google Scholar] [CrossRef]
- Brusaard, C.P.D. Optimization of procedures for counting viruses by flow cytometry. Appl. Environ. Microbial. 2004, 70, 1506–1513. [Google Scholar] [CrossRef]
- White, P.A.; Kalff, J.; Rasmussen, J.B.; Gasol, J.M. The effect of temperature and algal biomass on bacterial production and specific growth rate in freshwater and marine habitats. Microb. Ecol. 1991, 21, 99–118. [Google Scholar] [CrossRef]
- Coveney, M.F.; Wetzel, R.G. Effects of nutrients on specific growth rate of bacterioplankton in oligotrophic lake water cultures. Appl. Environ. Microbiol. 1992, 58, 150–156. [Google Scholar] [PubMed]
- Sala, M.M.; Peters, F.; Gasol, J.M.; Pedros-Alio, C.; Marrase, C.; Vaque, D. Seasonal and spatial variations in the nutrient limitation of bacterioplankton growth in the northwestern Mediterranean. Aquat. Microb. Ecol. 2002, 27, 47–56. [Google Scholar] [CrossRef]
- Rejas, D.; Muylaert, K.; Meester, L.D. Nutrient limitation of bacteria and sources of nutrients supporting nutrient-limited bacterial growth in an Amazonina floodplain lake. Aquat. Microb. Ecol. 2005, 39, 57–67. [Google Scholar] [CrossRef][Green Version]
- Hock, M.P.; Kirchman, D.L. Seasonal and inter-annual variability in bacterial production and biomass in a temperate estuary. Mar. Ecol. Prog. Ser. 1993, 98, 283–295. [Google Scholar] [CrossRef]
- Peduzzi, P.; Schiemer, F. Bacteria and viruses in the water column of tropical freshwater reservoirs. Environ. Microbiol. 2004, 6, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Wommack, K.E.; Colwell, R.R. Virioplankton: Viruses in aquatic ecosystems. Microbiol. Mol. Biol. Rev. 2000, 64, 69–114. [Google Scholar] [CrossRef]
- Bettarel, Y.; Sime-Ngando, T.; Amblard, C.; Dolan, J. Viral activity in two contrasting lake ecosystems. Appl. Environ. Microbiol. 2004, 70, 2941–2951. [Google Scholar] [CrossRef]
- Sarmento, H. New paradigms in tropical limnology: The importance of the microbial food web. Hydrobiologia 2012, 686, 1–14. [Google Scholar] [CrossRef]
- Arditi, R.; Ginzburg, L.R.; Akcakaya, H.R. Variation in plankton densities among lakes: A case for ratio-dependent predation models. Am. Nat. 1991, 138, 1287–1296. [Google Scholar] [CrossRef]
- Sherr, B.F.; Sherr, E.B.; Newell, S.Y. Abundance and productivity of heterotrophic nanoplankton in Georgia coastal waters. J. Plankton Res. 1984, 6, 195–202. [Google Scholar] [CrossRef]
- Wright, R.T.C.; Wright, R.T.; Ledo, M.E. Dynamics of planktonic bacteria and heterotrophic flagellates in Parker Estuary, northern Massachusetts. Cont. Shelf Res. 1987, 7, 1383–1387. [Google Scholar] [CrossRef]
- Wikner, J.; Hagstrom, A. Evidence of a tightly coupled nanoplankton predator-prey link regulating the bacterivores in the marine environment. Mar. Ecol. Prog. Ser. 1988, 50, 137–145. [Google Scholar] [CrossRef]
- Berninger, U.G.; Finlay, B.J.; Kuuppo-Leinikki, P. Protozoan control of bacterial abundances in freshwater. Limnol. Oceanogr. 1991, 36, 139–147. [Google Scholar] [CrossRef]
- Solic, M.; Krstulovic, N.; Vilibic, I.; Bojanic, N.; Kuspilic, G.; Sestanovic, S.; Santic, D.; Ordulj, M. Variability in the bottom-up and top-down controls of bacteria on trophic and temporal scales in the middle Adriatic Sea. Aquat. Microb. Ecol. 2009, 58, 15–29. [Google Scholar] [CrossRef]
- Paine, R.T. Food Webs: Linkage, interaction strength and community infrastructure. J. Anim. Ecol. 1980, 49, 667–685. [Google Scholar] [CrossRef]
- Suttle, C.A. The significance of viruses to mortality in aquatic microbial communities. Microb. Ecol. 1994, 28, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, J.A.; Noble, R.T. Viruses and protists cause similar bacterial mortality in coastal seawater. Limnol. Oceanogr. 1995, 40, 1236–1242. [Google Scholar] [CrossRef]
- Steward, F.C.; Smith, D.C.; Azam, F. Abundance and production of bacteria and viruses in the Bering and Chukchi Sea. Mar. Ecol. Prog. Ser. 1996, 131, 287–300. [Google Scholar] [CrossRef]
- Taira, Y.; Uchimiya, M.; Kudo, I. Simultaneous estimation of viral lysis and protozoan grazing on bacterial mortality using a modified virus-dilution method. Mar. Ecol. Prog. Ser. 2009, 379, 23–32. [Google Scholar] [CrossRef]
- Bratbak, G.; Heldal, M.; Thingstad, T.F.; Riemann, B.; Haslund, O.H. Incorporation of viruses into the budget of microbial C-transfer. A first approach. Mar. Ecol. Prog. Ser. 1992, 83, 273–280. [Google Scholar] [CrossRef]
- Weisse, T. The annual cycle of heterotrophic freshwater nanoflagellates: Role of bottom-up versus top-down control. J. Plankton Res. 1991, 13, 167–185. [Google Scholar] [CrossRef]
- Nakano, S.P.M.; Manage, M.; Nishibe, Y.; Kawabata, Z. Trophic linkage among heterotrophic nanoflagellates, ciliates and metazoan zooplankton in a hypereutrophic pond. Aquat. Microb. Ecol. 2001, 25, 259–270. [Google Scholar] [CrossRef]
- Suzuki, T.; Miyabe, C. Ecological balance between ciliate plankton and its prey candidates, pico- and nanoplankton, in the East China Sea. Hydrobiology 2007, 586, 403–410. [Google Scholar] [CrossRef]
- Kuosa, H. Picoplanktonic algae in the northern Baltic Sea-seasonal dynamics and flagellate grazing. Mar. Ecol. Prog. Ser. 1991, 73, 269–276. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, G.-C.; Yeh, H.-M.; Chen, Y.-K.; Hsieh, C.-h.; Ho, P.-C.; Tsai, A.-Y. Effect of Hydrographic Variability on the Distribution of Microbial Communities in Taiwan Strait in Winter. Diversity 2019, 11, 193. https://doi.org/10.3390/d11100193
Gong G-C, Yeh H-M, Chen Y-K, Hsieh C-h, Ho P-C, Tsai A-Y. Effect of Hydrographic Variability on the Distribution of Microbial Communities in Taiwan Strait in Winter. Diversity. 2019; 11(10):193. https://doi.org/10.3390/d11100193
Chicago/Turabian StyleGong, Gwo-Ching, Hsin-Ming Yeh, Yu-Kai Chen, Chih-hao Hsieh, Pei-Chi Ho, and An-Yi Tsai. 2019. "Effect of Hydrographic Variability on the Distribution of Microbial Communities in Taiwan Strait in Winter" Diversity 11, no. 10: 193. https://doi.org/10.3390/d11100193
APA StyleGong, G.-C., Yeh, H.-M., Chen, Y.-K., Hsieh, C.-h., Ho, P.-C., & Tsai, A.-Y. (2019). Effect of Hydrographic Variability on the Distribution of Microbial Communities in Taiwan Strait in Winter. Diversity, 11(10), 193. https://doi.org/10.3390/d11100193




