Abstract
High-mountain ecosystems are spots of plant diversity in which species composition and traits depict a long evolutionary history of species adaptation to steep environmental gradients. We investigated the main trends in plant species composition and reproductive and dispersal traits (pollen vector, diaspore appendages, dispersal of diaspores and fruit type) in central Mediterranean summits in relation to environmental factors (altitude, aspect, debris cover and slope). Based on 114 plots, with floristic and environmental data collected in the year 2016 on alpine calcareous grasslands in the central Apennines, we explored how species composition varies in relation to environmental factors using CCA (canonical correspondence analysis). Then, we analyzed the relationships among species presence, the occurrence of reproductive and dispersal traits and environmental variables. We used for this analysis the fourth-corner model approach. Our results highlight a consistent response of floristic composition and of structural and ecological characteristics to environmental gradients, with elevation and debris cover being the most important ones. The environmental characteristics of the analyzed ecosystems (e.g., steep slopes and harsh environments) combined with the persistence of perennial plant species already present in each stand, the high precision of pollination and the prevalence of short-distance dissemination strategies should allow the calcareous endemic plant communities of the analyzed Mediterranean summits to be conserved at least for a mid-term period slowing down the expansion of the warm-adapted species, less adapted to the local environmental constrains.
1. Introduction
Mountain environments throughout the world host highly specialized flora and fauna [1]. In Europe, mountain habitats contain approximately 20% of the native flora [2], most of which are endemics and rare [3] and constitute hotspots of plant diversity [1,4,5]. Such diversity is in part due to the particular climatic conditions that rapidly vary over very short distances along altitudinal gradients. In addition, high topographic diversity results in great habitat diversity, which in turn promotes high levels of specialization and species richness. In the Mediterranean area, high-elevation habitats are represented by few isolated peaks hosting a high number of endemic and rare plants of great biogeographic interest [3]. Such mountain environments [6,7,8], are particularly threatened by global warming because of their pronounced orographic discontinuity, which make them worryingly vulnerable to biodiversity loss [9].
In the mountains of the temperate biome, many species have shifted their upper distribution limit upslope in conjunction with recent climate change, leading to growing species richness values in mountain summits [10]. Simultaneously with the upward shift of species, a decline in cold-adapted species is occurring [11] mainly due to the sensitivity of cold adapted species to warmer temperatures [12,13] and to their weak competitive ability compared with species from lower altitudinal belts [14].
The ability of plants to respond to warmer conditions (i.e., by colonizing new areas or changing local abundance) depends on both the reproductive/dispersal traits of the species that support different colonization capacities and on the local ecological conditions, which act as filters of plant establishment, selecting those species for which the local conditions of the site are suitable [15]. This is particularly evident in mountain ecosystems, where reproduction and dispersal strategies play a key role in ensuring species persistence. High mountains can be considered cold deserts characterized by a scarcity of soils suitable for germination and a short vegetative period in which seeds can develop and disperse.
In the context of climate change, the identification of the plant traits of newly appearing or disappearing species can help to understand the ongoing selection processes, which are important for predicting future species assemblages [8,16,17,18,19]. Moreover, regarding the distribution pattern of reproductive traits, the pollination spectra could be processed in the same way for various areas, allowing the expression of the availability and effectiveness of the various pollination agents in relation to the climate and the fauna [20].
In this framework the effects of altitude and aspect on the composition and dispersal traits (pollen vector, diaspore morphology, dispersal of diaspores, fruit type) of vascular plant species can provide invaluable information about the adaptive value of plant characteristics during climate change [21]. Indeed, in high elevation ecosystems, as altitude increases, climatic conditions become more severe for plant growth due to lower mean temperatures, higher solar radiation, shorter vegetative periods, etc. [22]. These climatic constraints play a key role in the selection and evolution of plants [23,24]. Plant species show a series of characteristics (or traits) that enable them to live and reproduce in these extreme environments. For example, as altitude increases, some growth forms tend to be more abundant [17,25]. Concerning the dispersal traits, recent research has affirmed that the alpine flora tends to have a short-distance seed dispersal and it is likely to be threatened by a migration lag as the local climate undergoes rapid changes [11,21,26]. Reproductive strategies in particular tend to differentiate along altitudinal (and climatic) gradients [26,27]. In addition to altitude, small-scale topography and geomorphological processes (nivation niches, long lasting snow cover, slope instability etc.) also play an important role in creating a great variety of microhabitats that differ significantly in species composition over short spatial scales [28,29,30]. For example, the thermal regimes that affect plant growth differ among aspects, leading to high species richness in the southeastern exposition of north-temperate orobiome [22]. On the other hand, the microhabitat diversity may allow the cold-adapted species to maintain a refugium along valley slopes following local temperature gradients and within topographic/geomorphological traps [24]. There is a growing body of scientific literature that addresses the distribution of plant traits in alpine environments; still, most of these studies are focused on only a few environmental variables (e.g., [18]) or describe limited areas (e.g., [21,31]). In this context, analyzing the relationships between plant traits and the environment in other ranges would be very useful to gain a more global understanding of plant adaptations and a better awareness of the assembly rules and composition of plant communities [25]. In this context, the present study explores the main trends in plant species composition and reproductive and dispersal traits in central Mediterranean summits in relation to environmental factors. Specifically, we organized our analysis into two steps: we first explored how species composition varies in relation to environmental factors (altitude, aspect, debris cover and slope), and we then analyzed whether these patterns are mirrored in reproductive and dispersal plant traits (pollen vector, diaspore appendages, dispersal of diaspores and fruit type). In this way, we should be able to formulate a hypothesis about the ability of species to maintain their position and/or colonize new areas under warmer conditions.
In relation to the environmental variability that characterizes Mediterranean mountain summits and specifically the central Apennines [32], we should expect a consistent response in floristic composition and plant traits (e.g., [22,28]). Moreover, as in most of the terrestrial ecosystems and specifically in stressful environments, reproductive traits should be particularly effective in ensuring that blossoms produce an abundance of fertile seeds with a minimum investment of energy, for instance, through highly precise pollination mechanisms [33]. Furthermore, in isolated mountain summits, diaspore traits should ensure that seeds remain inside suitable habitats [34] and allow them to avoid dispersing to lower altitudinal belts characterized by different unfavorable environmental conditions.
The identification of floristic gradients in response to environmental variability should offer sound bases for better interpretation of the effects of climate and land use change on high-mountain vegetation and should allow the responses of different species to changes in the extreme conditions of mountain summits to be understood [28].
2. Materials and Methods
2.1. Study Area
The study area is representative of Mediterranean high mountains [35] includes the widest high-mountain zone with alpine vegetation of the Apennines and comprises the higher sectors (from ~2400 to 2790 m a.s.l.) of Majella National Park (Figure 1). This area is characterized by a large limestone ridge that reaches 2793 m a.s.l. (Mt. Amaro) and lies along a north-south axis between the latitudes 42°00’14” N (Guado di Coccia) and 42°9’33” N (Majelletta). The highest part of the massif is characterized by a wide plateau summit, bordered by steep inclinations, incised by deep valleys [36]. We specifically analyzed the vegetation of the ridges (sensu [7]) characterized by Silene acaulis subsp. acaulis and Viola magellensis plant communities.
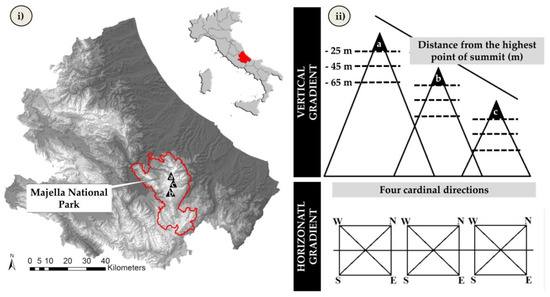
Figure 1.
(i) Study area (triangles represent the analyzed mountain summits of the Majella massif: (a) Mt. Mammoccio, (b) Mt Macellaro, (c) Mt. Femmina Morta). (ii) scheme representing the GLORIA multi-summit approach [36] followed for collecting data in the additional plots [37].
2.2. Data Collection
Floristic and environmental data were collected in 144 plots (1 m2) in summer 2015 on three mountain summits of the Majella massif (Mt. Femmina Morta, 2405 m a.s.l.; Mt. Macellaro, 2635 m a.s.l.; and Mt. Mammoccio, 2727 m a.s.l.). The sampling design followed the multi-summit approach of the GLORIA project [37]; see Figure 1, involving data collection in clusters of four grid plots located at 25, 45 and 65 m downslope of the highest point of each mountain summit and placed in each of the four cardinal directions. In short, we sampled the four cardinal directions of three summits at three distances from the summit point, for a total of 36 clusters of 4 plots, i.e., 144 plots. For each plot, we compiled a complete list of the vascular plant species and a set of environmental variables (elevation, slope, debris cover and aspect: northness and eastness).
Nomenclature of plant species conforms with Bartolucci et al. 2018 [38].
2.3. Plant Traits
To address our aims, relevant plant dispersal traits were selected and gathered from the literature. This approach was chosen because it would have been impossible to assess and measure all of the traits in the field, given the considerable number of plots and species. The traits represented the following main plant reproduction and dispersal functions: pollen vector, dispersal of diaspores, fruit type and diaspore appendages.
The data were collected from different sources, including databases (LEDA traitbase: A database of life-history traits of Northwest European flora and VIOLA: the VegetatIOn database of the centraL Apennines [39,40]) and the literature [27,41,42,43,44,45]. When possible, missing values were extrapolated from closely related species. Traits with many multiple states were simplified by grouping similar states together. The traits and states that were analyzed are listed in Table 1. For a complete list of species along with the relative families and dispersal traits see Table A1.

Table 1.
Groups of traits considered along with references of sources and states.
2.4. Data Analysis
To explore vegetation patterns in relation to environmental gradients (elevation, debris cover, slope, aspect: northness and eastness), we performed a direct gradient analysis using CCA (canonical correspondence analysis). First, we checked for correlation among environmental variables performing a Pearson correlation analysis. None of all variables exceeded the chosen 0.7 threshold (Figure A1), thus we retained them for subsequent analysis. We analyzed a matrix of 144 plots × 80 species, and we considered only the species with more than two presences. Monte Carlo tests (999 permutations) of constrained ordination scores against environmental variables were performed to assess the significance of the correlations. CCA analysis was performed using the vegan package in R [47].
Then, we analyzed the relationship between species presence, traits occurrence and environmental variables using the fourth-corner model approach [48,49]. Briefly, we explored trait responses to environmental gradients by the simultaneous analysis of the information contained in three tables: L (species distribution across samples), R (environmental characteristics of samples), and Q (species traits) [50]. We adopted the model-based solution to the fourth-corner model in which generalized linear models (GLMs) are fitted for species presence (L) as a function of a matrix of traits (Q) and a matrix of environmental predictors (R) and their 2-way interactions [49,51]. The interactions between species traits and environmental variables are the fourth-corner terms that allow quantification of how the environmental response of species changes as traits change. We used the traitglm function in the mvabund R package [51] to fit such model. Generalized linear models were fit using a Lasso penalty [52], which automatically performs model selection by setting to zero any interaction coefficients that do not reduce the Bayesian Information Criterion (BIC). The statistical significance of the fourth-corner terms was assessed by an analysis of deviance using 999 re-samples [47]. We graphically represented the fourth-corner coefficients using the levelplot function from the lattice package in R [53].
3. Results
The CCA (Figure 2) highlights the presence of clear environmental gradients mainly due to altitude, slope and debris cover. The constrained explained variance is high at approximately 27%, which indicates that the abiotic factors significantly contributed in disentangling the vegetation composition pattern. The first two axes accounted for 72% of the constrained variance, with the first axis explaining 55% (F = 0.530, p < 0.001). The other axes were not significant. The first axis was mainly correlated with debris cover (biplot score = −0.82) and elevation (biplot score = −0.77). The second axis was mainly correlated with slope (biplot score = 0.96) and, to a lesser extent, with elevation (biplot score = −0.49) and eastness (biplot score = −0.33). In correspondence with the first axis, two groups of species that occupy sites with similar environmental characteristics were also evident. One group, on the left (negative scores on the first (CCA1) axis—Table 2), is composed of cryophilous species such as Crepis pygmaea, Alyssum cuneifolium, Saxifraga exarata subsp. ampullacea, Veronica aphylla and Galium magellensis that tend to occur at higher altitudes in areas rich in debris. A second group, on the right, encompasses thermophilic species such as Poa molinerii, Saxifraga tridactylites, Polygala alpestris subsp. alpestris, Oxytropis campestris and Sempervivum arachnoideum that occur at lower altitudes. In correspondence with the second axis, two groups of species were also evident. One group, in the upper sector (positive scores on the second (CCA2) axis—Table 2), is composed of species adapted to steep slopes, such as Veronica aphylla and Euphrasia minima subsp. minima. A second group, on the lower sector (negative scores in the second (CCA2) axis), encompasses species such as Trifolium pratensis subsp. semipurpureum and Ranunculus brevifolius that occur on moderate slopes.
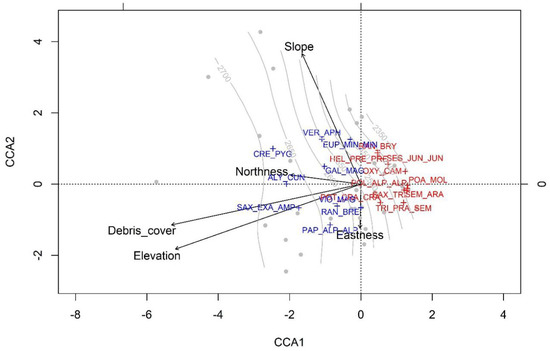
Figure 2.
Biplot of the canonical correspondence analysis (CCA). Only the 20% of the species with the best fits are shown. Gray dots indicate the sites, isolines, site altitudes, and arrows the environmental variables. The species acronyms reported in two colors (blue: cryophilous; red: termophilic) are defined in Table 2.

Table 2.
Species scores on the first two CCA axes. Only the 20% of the species with the best fits are shown. For each species, the complete name, the acronym and the pollination and dispersal traits are reported. pv = pollen vector, dd = dispersal of diaspores, ft =fruit type, sa = diaspore appendages. The species acronyms are reported in two colors, blue: cryophilous and red: termophilic.
Concerning the different strategies of pollination, we observed a clear dominance of entomophilous species, with 67% of the analyzed flora pollinated by insects (Table 3). Moreover, entomophilous species are present in 62% of the plots. On the other hand, ~20% of the species have a wind pollination dispersion strategy, and this trait is present in ~30% of the plots. Self-pollination occurs in few species, and this character is present but rare in the sampled plots (~9% of the plots).

Table 3.
Abundance of the different traits in the analyzed flora and in the sampled plots. N: number of sampled species; N%: percentage of the sampled species; Plots: number of plots in which each trait is present; Plot%: percentage of plots in which each trait is present.
Analyzing the dispersal of diaspores (Table 3), we found that autochory, which accounts for 48.28% of the analyzed flora, is the most widespread dispersal mode, followed by meteochory (41.38%). The zoochory dispersion mechanism plays a less important role in this environment, with only 10% of species being dispersed by animals.
Regarding the presence and characteristics of seed appendages (Table 3), ~60% of the species have nude seeds, and the other 40% have awns, a pappus or wings.
Concerning fruit types (Table 2), the species with achenes and capsules totaled 46.5% and 36.21% of the whole species set, respectively, whereas the other fruit types reached ~17%.
According with the fourth-corner analysis (Figure 3), the species traits explain a significant amount of the variation in species distribution along the environmental variables (deviance = 134.2, p = 0.001). Indeed, each plant trait presented a specific behavior, but a general trend could be observed with stronger correlations with altitude and slope, weak correlations with debris cover and very weak relationships with aspect (as shown by the coefficient values).
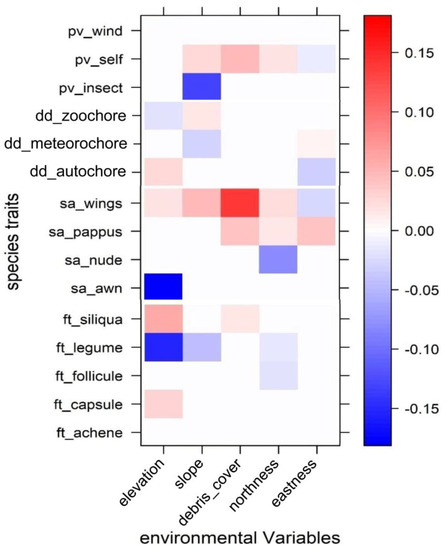
Figure 3.
Results of the fourth-corner model. Traits are colored according to their fourth-corner coefficients: red indicates a significant positive trait-variable association, and blue indicates a significant negative trait-variable association. Color depth indicates the strength of the trait-variable association. pv: pollen vector, dd: dispersal of diaspores, sa: seed appendages, ft: fruit type.
The fourth-corner analysis of pollination strategy (Figure 3) revealed that species pollinated by insects have a negative association with slope, while self-pollinated species tend to prefer environments characterized by north-facing, steep slopes with high debris cover. Concerning the dispersal of diaspores, the results do not show any significant trends; only slight preferences of autochorous species for higher altitudes and of zoochorous species for lower altitudes were observed. However, the fourth-corner model suggests that meteochorous species avoid steep slopes and prefer northern aspects (high value of northness).
Concerning the diaspores appendages (Figure 3), most of the species have nude diaspores (Table 2), and these species are widely distributed across the different environmental gradients with a weak negative relationship with northness. The species with winged seeds show a clear preference for extreme conditions and are positively correlated with altitude, slope, debris cover and a cold aspect (northness). In contrast, the species with awned seeds have a clear preference for lower elevations.
The distribution of fruit type is mainly related to the altitudinal gradient, with the species with siliques and capsules preferentially present at higher elevations and with legumes present at lower areas (Figure 3). There is also some evidence that legumes decrease at higher slopes and northness values.
4. Discussion
Our results revealed that differences in species composition and trait distributions are related to abiotic factors and that elevation and debris cover in particular seem to play the most important roles in determining such differences.
In particular, there was evidence of a pronounced change in species composition along the altitudinal gradient, with a clear assemblage of species adapted to harsh environmental conditions with poor soils, high debris cover and low temperatures at higher elevations (e.g., Alyssum cuneifolium, Viola magellensis, Galium magellensis) and a group of species living in milder conditions in the lower sectors (Oxytropis campestris, Sesleria juncifolia subsp. juncifolia, Helictochloa praetutiana subsp. praetutiana). Our results correspond to a common trend in high mountains in which elevation is related to both decreasing temperatures and increasing rock and debris cover [28]. Indeed, in high-mountain environments, a slight increase in altitude often corresponds to a significant increase in stressful conditions [18,22].
On the other hand, in the central Apennines, as in other mountain systems [17,21,28,54,55,56,57], aspect plays a less-pronounced role than altitude does in explaining species and trait distribution patterns. Despite the subordinate role of aspect relative to elevation in shaping plant trait distributions [17,28,58,59], recent research has found that in a global change scenario, warmer aspects of alpine summits support higher numbers of colonization events and thus higher richness values [60]. In this context, aspect should play a key role in determining the pace of climate change-induced migration processes [22].
Regarding pollination, an entomophilous strategy is the most common one in the analyzed area. This strategy, quite common in terrestrial ecosystems, has the evolutionary advantage of high pollination precision [33] which ensures that the blossoms will produce many fertile seeds. In pollination ecology, ensuring pollen dispersal precision is crucial for the persistence of many species, and the evolution of pollination mechanisms in entomophilous blossoms is, on the whole, evolution towards an increasingly higher precision [61]. Indeed, in ecosystems characterized by sparse vegetation, the role of entomophilous pollination should be decisive in ensuring reproductive success. Even though some authors argue that the activity of insects in high mountains is limited [62,63], a study in the Alps demonstrated that the blossom visitation rates in high-mountain and foothill vegetation are comparable [64]. The other pollination strategies (e.g., wind and self-pollination) are less represented in the analyzed summits. Wind pollination is a good strategy in windy areas but has the drawback of requiring the production of high amounts of pollen to fertilize few blossoms. Such low fertilization precision should represent a further limit for small, isolated populations of anemophilous species that have no chance or reduced chances of cross-pollination [33]. On the other hand, even if self-pollination were an adequate functional behavior in environments in which pollen cannot easily reach mature blossoms, autogamy would still lead to negative genetic effects by increasing homozygosity and could promote the splitting off of microspecies [33].
Pollination traits (entomophily, anemophily, autogamy) heterogeneously vary across the different environmental gradients. For example, we found a negative relationship between the insect pollination strategy and steep slopes, and such a relationship should be related to the preference of insects for flat areas with less wind stress [65]. On the other hand, the self-pollination strategy tends to occur only in extreme environments (debris-rich, steep, north-exposed slopes) because, in such harsh conditions, self-pollination may be the only strategy allowing the species’ persistence.
Concerning the fruit types, the dominance of achenes and capsules is evident, while the other fruit types (legume, follicule and siliqua) together only represent 15% of the total species. The distribution of fruit types is similar to the patterns observed by Pellissier et al. [25] the Alps, with achenes and capsules being dominant in the high mountains. As observed in the Alps, species with capsules and siliqua in the central Apennines also tend to prefer the higher sectors [25]. On the other hand, species with achenes are widely distributed along the entire altitudinal gradient. Matteodo et al. [17] observed an increase in species with achenes in the higher vegetation belts of the Alps and related the pattern to such species’ good colonization ability. Similarly, the wide distribution of species with achenes in central Apennine summits is most likely related to their colonization ability, which could be considered a pre-adaptation to climate warming [17]. As observed by Pellissier et al. [25] in the Alps, also in the Apennines species with legume tend to only rarely occur at higher elevations.
In the analyzed summits the most common strategies for the dispersal of diaspores are meteochore and autochore, which together account for 90% of the total sampled flora. Meteochore species are widely spread throughout the world but are especially prominent in open habitats as summits and high mountain slopes, steppes, prairies, garrigue, screes and deserts [66,67]. Seed dispersal is often regulated by climatic conditions, and specifically, the local meteorological variability significantly impacts seed dispersal distances [33]. In alpine habitats, the presence of strong winds [68] and perhaps the low mammal density should favor meteochore dispersion [25]. Moreover, the observed abundance of species with autonomous seed dispersal in high-altitude environments, has been previously found in other open habitats and arid environments where competition for space is absent [28,30,33]. Indeed, in alpine stressful habitats, most plants show low specialized dissemination mechanism that may be more related to phylogeny than to function [29]. Here selection pressure on dispersal mechanisms is mostly random because of various environmental constraints (e.g., difficulties in setting seeds, low chance of seedling or juvenile establishment and intense slope dynamics). On the other hand, the low seed-dispersal distance might favor germination and survival around the parent plants [69] which in turn may serve as an additional facilitating factor during the establishment of new individuals. As suggested by [24,70], the resource limitation at higher elevations in alpine debris slopes should affect changes on inter-specific interactions that shift from competition to facilitation mechanisms.
Zoochore which is a common strategy for the dispersal of diasporas at lower altitudes, in disturbed habitats and in grazed vegetation types [71], is present but rare in the analyzed summits. The modest presence of this strategy is most likely related to the scarcity of epizoohorous dispersion [71] at higher altitudes [72].
As regards diaspore morphology, most of the analyzed flora have nude seeds, a characteristic that strongly differentiates the analyzed summits from several terrestrial ecosystems in which the presence of diaspore appendages is very common [73]. Such results could be explained by the tendency of alpine vegetation to adopt local-scale dispersal strategies (e.g., [74]). Indeed, local dispersal ensures that seeds settle close to the mother plant and prevents the seeds from dispersing to unsuitable habitats on lower mountains or in the foothills [34,75]. Regardless, when considering the species with seed appendages, an elevation gradient emerges. Similar to what was observed in the Swiss Alps [25] species with winged seeds tend to preferentially occur at higher altitudes, while species with awns tend to be more frequent at lower altitudes. Such variation was also described by Navarro et al. [76], who affirmed that winged seeds tend to occur in the highly stressed environments of mountain summits because the presence of wings provides a sort of lift to diaspores that allows them to be transported by winds. Concerning the presence of seeds with awns, it is important to note that when such a seed lies on the ground, the orientation of the awns allows the seed to lie obliquely on the ground, which is the optimum position for seed burial. Seeds with a pappus are scarce but distributed along the entire gradient, with a slight preference for sites with high debris cover. The distance that a plumed diaspore can travel depends not only on wind velocity but also on air humidity. In a dry atmosphere, the hairs of the plumes often spread out, thereby facilitating abscission and increasing buoyancy. In general, the low air humidity in high altitude environments should therefore aid in the dispersal of plumed diaspores [21].
It is interesting to interpret our results and compare them with recent findings in the context of the effects of climate change on high-mountain ecosystems. For instance, Matteodo et al. [17] and Ninot et al. [31] hypothesized a decrease in species with nude seeds, and at the same time they postulated an increase in species with winged seeds and species with a pappus in mountain summits. At the same time, Wipf et al. [77], in a multitemporal vegetation analysis of the Alps, demonstrated an increase in species with awns, such as grasses. Because many species with awned seeds, mainly graminoids, are thermophilic and frequent at lower elevations (subalpine belt) [25], we should expect an upward shift of such species. Similar processes have been observed in the Alps by recent resampling analyses in which, in addition the expansion of thermophilic species, a role of warmer conditions in the colonization and dispersal processes was also postulated [17,21]. Anyway, in the analyzed Mediterranean summits, the long persistence of species and the presence of several taxa characterized by nude seeds with a short dissemination distance that feed the local seed bank [30] should ensure the persistence of the alpine grasslands for a mid-term period. The persistence of species together with the observed process of species filling caused by the increase in abundance of the species already present in alpine and subalpine grasslands [19,78], suggest that the pattern of dispersal strategies of summit vegetation in central Apennines will change slightly in the next decades.
5. Conclusions
This study offers a preliminary picture of the composition of plant dispersal traits across environmental gradients on central Mediterranean high mountains. The study of central Appennines uncovered an important influence of elevation and debris cover gradients on floristic species composition patterns and structural and ecological characteristics of alpine plant communities. We observed a steep floristic gradient that should offer sound bases for better interpreting the effects of climate change on high-mountain vegetation and that should allow the response of different species to changes in the extreme conditions of mountain summits to be understood [28]. In this context, considering the altitudinal gradient as a surrogate for temperature, we should expect an increase over time in the more thermophilic species (e.g., Oxytropis campestris, Sesleria juncifolia subsp. juncifolia, Helictochloa praetutiana subsp. praetutiana) in response to the ongoing increase in temperatures and global warming processes. In a similar way, concerning the debris cover and soil development gradient, the ongoing rise in soil nutrients in alpine environments due to higher rates of organic degradation and nitrogen deposition [79] should promote the slow expansion more mesic species, such as Oxytropis campestris, Poa molinerii, and Sempervivum arachnoideum.
The observed relationships among altitudes and the analyzed traits highlight an important role of temperature in shaping the distribution of dispersal strategies in alpine calcareous grasslands. Moreover, the concurrent increase at higher elevations in the cover of calcareous debris seems to restrict the establishment and survival of plants on the upper summits to a few cold-adapted species that are able to live and grow on dry and poor soils. We also observed that several of the alpine and subalpine species occurring in the analyzed area have nude diaspores and a short-distance dissemination strategy, which provides support for the hypothesis of the evolutionary convergence of species under harsh and isolated ecosystems. Short range dispersal allows some seeds to leave the overcrowded maternal site, but still to remain within the neighborhood where the probability of site suitability may be higher than random. Short distance dispersal provides an efficient way of filling space. When a seed is established near its mother, the mother site will be refilled the next year and available space will be filled concentrically and solidly around the population founders [33]. To conclude, it is worth noting that the environmental characteristics of Apennines high-elevation summits (e.g., steep slopes and harsh environments) combined with the persistence of plant species already present in each stand, the high precision of pollination and the prevalence of short-distance dissemination strategies should allow the calcareous endemic plant communities at high elevations to persist for a mid-term period slowing down the expansion of the warm-adapted species, less adapted to the local environmental constrains that are expected to become more severe.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, M.D.M.; M.L.C., L.F., A.S.; Methodology, M.D.M.; M.L.C., L.F., V.D.C., L.D.M., A.R.F., A.S.; Software, M.D.M.; L.F., Validation, M.D.M.; M.L.C., L.F., V.D.C., L.D.M., A.R.F., A.S.; Formal Analysis, M.D.M.; M.L.C., L.F., A.S.; Investigation, M.D.M.; M.L.C., L.F., V.D.C., L.D.M., A.R.F., A.S.; Resources, V.D.C., L.D.M., A.R.F., A.S.; Data Curation, M.D.M.; L.F., V.D.C., L.D.M., A.R.F., A.S.; Writing-Original Draft Preparation, M.D.M.; M.L.C., L.F., V.D.C., L.D.M., A.R.F., A.S.; Writing-Review & Editing, M.D.M.; M.L.C., L.F., A.S.; Visualization, M.D.M.; M.L.C., L.F.; Supervision, A.R.F., A.S.; Project Administration, L.D.M., A.R.F., A.S.; Funding Acquisition, L.D.M., A.R.F., A.S.
Funding
This study was partially supported by the Italian project of strategic interest NEXTDATA, in the context of ‘Data-LTER-Mountain’ action, http://www.nextdataproject.it/?q=en.
Acknowledgments
We thank Alberto Evangelista, Adriano Stinca, Andrea Scolastri and Giovanni Pelino for their support during the fieldwork activities and the State Forest Service for their logistic support.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Complete list of species along with the corresponding taxonomic family and the analyzed dispersal traits. pv: pollen vector, dd: dispersal of diaspores, sa: seed appendages, ft: fruit type.
Table A1.
Complete list of species along with the corresponding taxonomic family and the analyzed dispersal traits. pv: pollen vector, dd: dispersal of diaspores, sa: seed appendages, ft: fruit type.
| Species Name | Taxonomic Family | pv | dd | ft | sa |
|---|---|---|---|---|---|
| Achillea barrelieri subsp. barrelieri | Asteraceae | insect | autochory | achene | wings |
| Adonis distorta | Ranunculaceae | insect | zoochory | achene | awn |
| Alyssum cuneifolium | Brassicaceae | insect | autochory | siliqua | wings |
| Androsace villosa subsp. villosa | Primulaceae | insect | autochory | capsule | nude |
| Anthyllis montana subsp. montana | Fabaceae | insect | meteochory | legume | nude |
| Anthyllis vulneraria subsp. pulchella | Fabaceae | insect | meteochory | legume | nude |
| Arabis alpina subsp. caucasica | Brassicaceae | insect | autochory | siliqua | nude |
| Arenaria grandiflora subsp. grandiflora | Caryophyllaceae | insect | autochory | capsule | nude |
| Armeria gracilis | Plumbaginaceae | insect | meteochory | achene | wings |
| Aster alpinus subsp. alpinus | Asteraceae | insect | meteochory | achene | wings |
| Bellis perennis | Asteraceae | insect | autochory | achene | nude |
| Bistorta vivipara | Polygonaceae | insect | autochory | achene | nude |
| Bunium petraeum | Apiaceae | insect | zoochory | achene | nude |
| Campanula cochleariifolia | Campanulaceae | insect | autochory | capsule | nude |
| Campanula scheuchzeri subsp. scheuchzeri | Campanulaceae | insect | autochory | capsule | nude |
| Carduus chrysacanthus | Asteraceae | insect | meteochory | achene | pappus |
| Carex humilis | Cyperaceae | wind | meteochory | achene | nude |
| Carex kitaibeliana | Cyperaceae | wind | zoochory | achene | nude |
| Carex myosuroides | Juncaceae | wind | meteochory | achene | awn |
| Carum heldreichii | Apiaceae | insect | zoochory | achene | nude |
| Cerastium thomasii | Caryophyllaceae | wind | autochory | capsule | nude |
| Cerastium tomentosum | Caryophyllaceae | self | autochory | capsule | nude |
| Clinopodium alpinum subsp. alpinum | Lamiaceae | insect | zoochory | achene | nude |
| Crepis aurea subsp. glabrescens | Asteraceae | insect | meteochory | achene | pappus |
| Crepis magellensis | Asteraceae | insect | meteochory | achene | pappus |
| Crepis pygmaea | Asteraceae | self | meteochory | achene | pappus |
| Doronicum columnae | Asteraceae | insect | meteochory | achene | pappus |
| Draba aizoides subsp. aizoides | Brassicaceae | insect | autochory | siliqua | nude |
| Edraianthus graminifolius subsp. graminifolius | Campanulaceae | insect | autochory | capsule | nude |
| Erigeron epiroticus | Asteraceae | insect | meteochory | achene | pappus |
| Erysimum majellense | Brassicaceae | insect | autochory | siliqua | nude |
| Euphrasia minima subsp. minima | Scrophulariaceae | self | autochory | capsule | nude |
| Festuca violacea subsp. italica | Poaceae | wind | meteochory | achene | awn |
| Galium magellense | Rubiaceae | wind | zoochory | achene | nude |
| Gentiana nivalis | Gentianaceae | self | autochory | capsule | nude |
| Gentiana orbicularis | Gentianaceae | insect | autochory | capsule | nude |
| Gentiana verna subsp. verna | Gentianaceae | insect | autochory | capsule | nude |
| Helianthemum oelandicum subsp. alpestre | Cistaceae | insect | zoochory | capsule | nude |
| Helictochloa praetutiana subsp. praetutiana | Poaceae | wind | meteochory | achene | awn |
| Iberis saxatilis subsp. saxatilis | Brassicaceae | insect | meteochory | siliqua | nude |
| Leontopodium nivale | Asteraceae | insect | meteochory | achene | pappus |
| Leucanthemum tridactylites | Asteraceae | insect | autochory | achene | nude |
| Linaria alpina | Scrophulariaceae | insect | autochory | capsule | nude |
| Luzula spicata subsp. italica | Juncaceae | wind | autochory | capsule | nude |
| Myosotis graui | Boraginaceae | insect | autochory | achene | nude |
| Omalotheca diminuta | Asteraceae | wind | meteochory | achene | pappus |
| Oreojuncus monanthos | Cyperaceae | wind | zoochory | capsule | nude |
| Oxytropis campestris | Fabaceae | insect | autochory | legume | nude |
| Papaver alpinum subsp. alpinum | Caryophyllaceae | insect | meteochory | capsule | nude |
| Paronychia kapela subsp. kapela | Papaveraceae | insect | autochory | achene | nude |
| Pedicularis elegans | Scrophulariaceae | insect | autochory | capsule | nude |
| Phyteuma orbiculare | Campanulaceae | insect | autochory | capsule | nude |
| Pilosella lactucella | Asteraceae | self | meteochory | achene | pappus |
| Plantago atrata subsp. atrata | Plantaginaceae | wind | zoochory | capsule | nude |
| Poa alpina subsp. alpina | Poaceae | wind | meteochory | achene | awn |
| Poa molinerii | Poaceae | wind | meteochory | achene | awn |
| Polygala alpestris subsp. alpestris | Polygalaceae | insect | meteochory | capsule | awn |
| Potentilla crantzii subsp. crantzii | Rosaceae | insect | autochory | achene | awn |
| Pulsatilla alpina subsp. alpina | Ranunculaceae | insect | meteochory | achene | awn |
| Ranunculus brevifolius | Ranunculaceae | insect | meteochory | achene | awn |
| Ranunculus breyninus | Ranunculaceae | insect | meteochory | achene | awn |
| Sabulina verna subsp. verna | Caryophyllaceae | wind | autochory | capsule | nude |
| Salix retusa | Salicaceae | wind | meteochory | capsule | awn |
| Saxifraga adscendens subsp. adscendens | Saxifragaceae | self | autochory | capsule | nude |
| Saxifraga exarata subsp. ampullacea | Saxifragaceae | self | autochory | capsule | nude |
| Saxifraga oppositifolia subsp. oppositifolia | Saxifragaceae | self | autochory | capsule | nude |
| Saxifraga paniculata | Saxifragaceae | self | autochory | capsule | nude |
| Saxifraga tridactylites | Saxifragaceae | self | autochory | capsule | nude |
| Scorzoneroides montana subsp. montana | Asteraceae | insect | meteochory | achene | pappus |
| Sedum atratum | Crassulaceae | insect | autochory | follicule | nude |
| Sempervivum arachnoideum | Crassulaceae | insect | autochory | follicule | nude |
| Senecio squalidus subsp. squalidus | Asteraceae | insect | meteochory | achene | pappus |
| Sesleria juncifolia subsp. juncifolia | Poaceae | wind | meteochory | achene | awn |
| Silene acaulis subsp. acaulis | Caryophyllaceae | insect | autochory | capsule | nude |
| Taraxacum apenninum | Asteraceae | insect | meteochory | achene | pappus |
| Thymus praecox subsp. polytrichus | Lamiaceae | insect | autochory | legume | nude |
| Trifolium pratense subsp. semipurpureum | Fabaceae | insect | zoochory | legume | nude |
| Trifolium thalii | Fabaceae | insect | meteochory | legume | nude |
| Trinia dalechampii | Apiaceae | insect | autochory | achene | nude |
| Valeriana saliunca | Valerianaceae | insect | meteochory | achene | pappus |
| Veronica aphylla | Scrophulariaceae | insect | autochory | capsule | nude |
| Viola eugeniae subsp. eugeniae | Violaceae | insect | zoochory | capsule | nude |
| Viola magellensis | Violaceae | insect | autochory | capsule | nude |
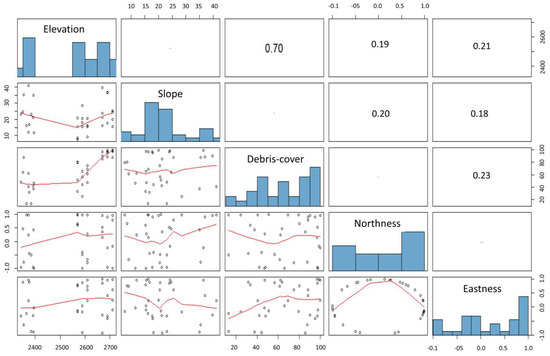
Figure A1.
Pearson correlation matrix among environmental variables. None of the variables exceeded the chosen 0.7 threshold thus we retained all of them for subsequent analysis.
References
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Väre, H.; Lampinen, R.; Humphries, C.; Williams, P. Taxonomic diversity of vascular plants in the European alpine areas. In Alpine Biodiversity in Europe; Springer: Berlin, Heidelberg, Germany, 2003; pp. 133–148. [Google Scholar]
- Pauli, H.; Gottfried, M.; Grabherr, G. Effects of climate change on the alpine and nival vegetation of the Alps. J. Mt. Ecol. 2003, 7, 9–12. [Google Scholar]
- Barthlott, W.; Lauer, W.; Placke, A. Global distribution of species diversity in vascular plants: Towards a world map of phytodiversity (globale verteilung der artenvielfalt höherer pflanzen: Vorarbeiten zu einer weltkarte der phytodiversität). Erdkunde 1996, 50, 317–327. [Google Scholar] [CrossRef]
- Pauli, H.; Gottfried, M.; Reiter, K.; Klettner, C.; Grabherr, G. Signals of range expansions and contractions of vascular plants in the high Alps: Observations (1994–2004) at the Gloria master site schrankogel, Tyrol, Austria. Glob. Chang. Biol. 2007, 13, 147–156. [Google Scholar] [CrossRef]
- Kazakis, G.; Ghosn, D.; Vogiatzakis, I.; Papanastasis, V. Vascular plant diversity and climate change in the alpine zone of the Lefka Ori, Crete. Biodivers. Conserv. 2007, 16, 1603–1615. [Google Scholar] [CrossRef]
- Stanisci, A.; Carranza, M.L.; Pelino, G.; Chiarucci, A. Assessing the diversity pattern of cryophilous plant species in high elevation habitats. Plant Ecol. 2011, 212, 595–600. [Google Scholar] [CrossRef]
- Gutiérrez-Girón, A.; Gavilán, R. Plant functional strategies and environmental constraints in mediterranean high mountain grasslands in central Spain. Plant Ecol. Divers. 2013, 6, 435–446. [Google Scholar] [CrossRef]
- Nogués-Bravo, D.; Araújo, M.; Romdal, T.; Rahbek, C. Scale effects and human impact on the elevational species richness gradients. Nature 2008, 453, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Pauli, H.; Gottfried, M.; Dullinger, S.; Abdaladze, O.; Akhalkatsi, M.; Alonso, J.L.B.; Coldea, G.; Dick, J.; Erschbamer, B.; Calzado, R.F.; et al. Recent plant diversity changes on Europe’s mountain summits. Science 2012, 336, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Dullinger, S.; Gattringer, A.; Thuiller, W.; Moser, D.; Zimmerman, N.; Guisan, A. Climate warming, dispersal limitation and extinction debt of European mountain plants. Nat. Clim. Chang. 2012, 2, 619–622. [Google Scholar] [CrossRef]
- Becker, A.B. Predicting Global Change Impacts on Mountain Hydrology and Ecology: Integrated Catchment Hydrology/Altitudinal Gradient Studies Workshop Report: Documentation Resulting from an International Workshop, Kathmandu, Nepal 30 March–2 April 1996; International Geosphere Biosphere Programme: Stockholm, Swedish, 1997. [Google Scholar]
- Beniston, M. Mountain Environments in Changing Climates; Routledge: London, UK, 2002; ISBN 9780415102247. [Google Scholar]
- Chapin, F., III; Körner, C. Patterns, causes, changes, and consequences of biodiversity in arctic and alpine ecosystems. In Arctic and Alpine Biodiversity: Patterns, Causes and Ecosystem Consequences; Springer: Berlin, Heidelberg, Germany, 2013; Volume 113, pp. 313–320. [Google Scholar]
- Guisan, A.; Rahbek, C. Sesam—A new framework integrating macroecological and species distribution models for predicting spatio-temporal patterns of species assemblages. J. Biogeogr. 2011, 38, 1433–1444. [Google Scholar] [CrossRef]
- Byers, D.L.; Chang, S.M. Studying plant–pollinator interactions facing climate change and changing environments. Appl. Plant Sci. 2017, 5, 1700052. [Google Scholar] [CrossRef] [PubMed]
- Matteodo, M.; Wipf, S.; Rixen, C.; Vittoz, P. Elevation gradient of successful plant traits for colonizing alpine summits under climate change. Environ. Res. Lett. 2013, 8, 024043. [Google Scholar] [CrossRef]
- Pescador, D.S.; de Bello, F.; Valladares, F.; Escudero, A. Plant trait variation along an altitudinal gradient in mediterranean high mountain grasslands: Controlling the species turnover effect. PLoS ONE 2015, 10, e0118876. [Google Scholar] [CrossRef] [PubMed]
- Frate, L.; Carranza, M.L.; Evangelista, A.; Stinca, A.; Schaminée, J.H.; Stanisci, A. Climate and land use change impacts on mediterranean high-mountain vegetation in the Apennines since the 1950s. Plant Ecol. Divers. 2018, 1–13. [Google Scholar] [CrossRef]
- Kugler, H. Die verbreitung anemogamer arten in Europa. Berichte Deutschen Botanischen Gesellschaft 1975, 88, 441–450. [Google Scholar] [CrossRef]
- Vittoz, P.; Dussex, N.; Wassef, J.; Guisan, A. Diaspore traits discriminate good from weak colonisers on high-elevation summits. Basic Appl. Ecol. 2009, 10, 508–515. [Google Scholar] [CrossRef]
- Winkler, M.; Lamprecht, A.; Steinbauer, K.; Hülber, K.; Theurillat, J.P.; Breiner, F.; Choler, P.; Ertl, S.; Gutiérrez Girón, A.; Rossi, G.; et al. The rich sides of mountain summits—A pan-European view on aspect preferences of alpine plants. J. Biogeogr. 2016, 43, 2261–2273. [Google Scholar] [CrossRef]
- Jump, A.S.; Penuelas, J. Running to stand still: Adaptation and the response of plants to rapid climate change. Ecol. Lett. 2005, 8, 1010–1020. [Google Scholar] [CrossRef]
- Gentili, R.; Bacchetta, G.; Fenu, G.; Cogoni, D.; Abeli, T.; Rossi, G.; Salvatore, M.C.; Baroni, C.; Citterio, S. From cold to warm-stage refugia for boreo-alpine plants in southern European and Mediterranean mountains: The last chance to survive or an opportunity for speciation? Biodiversity 2015, 16, 247–261. [Google Scholar] [CrossRef]
- Pellissier, L.; Fournier, B.; Guisan, A.; Vittoz, P. Plant traits co-vary with altitude in grasslands and forests in the European Alps. Plant Ecol. 2010, 211, 351–365. [Google Scholar] [CrossRef]
- Weppler, T.; Stoll, P.; Stöcklin, J. The relative importance of sexual and clonal reproduction for population growth in the long-lived alpine plant Geum reptans. J. Ecol. 2006, 94, 869–879. [Google Scholar] [CrossRef]
- Kühn, I.; Bierman, S.M.; Durka, W.; Klotz, S. Relating geographical variation in pollination types to environmental and spatial factors using novel statistical methods. New Phytol. 2006, 172, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Körner, C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems; With 47 Tables; Springer: Berlin, Heidelberg, Germany, 2003; ISBN 978-3-642-18970-8. [Google Scholar]
- Matteodo, M.; Ammann, K.; Verrecchia, E.P.; Vittoz, P. Snowbeds are more affected than other subalpine–alpine plant communities by climate change in the Swiss Alps. Ecol. Evolut. 2016, 6, 6969–6982. [Google Scholar] [CrossRef] [PubMed]
- Illa, E.; Carrillo, E.; Ninot, J.M. Patterns of plant traits in Pyrenean alpine vegetation. Flora Morphol. Distrib. Funct. Ecol. Plants 2006, 201, 528–546. [Google Scholar] [CrossRef]
- Ninot, J.M.; Grau, O.; Carrillo, E.; Guàrdia, R.; Lluent, A.; Illa, E. Functional plant traits and species assemblage in Pyrenean snowbeds. Folia Geobot. 2013, 48, 23–38. [Google Scholar] [CrossRef]
- Stanisci, A.; Pelino, G.; Blasi, C. Vascular plant diversity and climate change in the alpine belt of the central Apennines (Italy). Biodivers. Conserv. 2005, 14, 1301–1318. [Google Scholar] [CrossRef]
- Faegri, K.; Van Der Pijl, L. Principles of Pollination Ecology; Pergamon Press: Oxford, UK, 1979; ISBN 9781483293035. [Google Scholar]
- Kinlan, B.P.; Gaines, S.D. Propagule dispersal in marine and terrestrial environments: A community perspective. Ecology 2003, 84, 2007–2020. [Google Scholar] [CrossRef]
- Van Gils, H.; Conti, F.; Ciaschetti, G.; Westinga, E. Fine resolution distribution modelling of endemics in Majella National Park, central Italy. Plant Biosyst. 2012, 146, 276–287. [Google Scholar] [CrossRef]
- Giraudi, C. Middle to late holocene glacial variations, periglacial processes and alluvial sedimentation on the higher Apennine massifs (Italy). Quat. Res. 2005, 64, 176–184. [Google Scholar] [CrossRef]
- Pauli, H.; Gottfried, M.; Lamprecht, A.; Niessner, S.; Rumpf, S.; Winkler, M.; Steinbauer, K.; Grabherr, G. The GLORIA Field Manual–Standard Multi-Summit Approach, Supplementary Methods and Extra Approaches; Global Observation Research Initiative in Alpine Environment; GLORIA-Coordination, Austrian Academy of Sciences & University Of Natural Resources and Life Sciences: Vienna, Austria, 2015; Volume 5, ISBN 978-92-79-45694-7. [Google Scholar]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.; Ardenghi, N.; Astuti, G.; Bacchetta, G.; Ballelli, S.; Banfi, E. An updated checklist of the vascular flora native to Italy. Plant Biosyst. 2018, 152, 179–303. [Google Scholar] [CrossRef]
- Kleyer, M.; Bekker, R.; Knevel, I.; Bakker, J.; Thompson, K.; Sonnenschein, M.; Poschlod, P.; Van Groenendael, J.; Klimeš, L.; Klimešová, J. The LEDA traitbase: A database of life-history traits of the northwest European flora. J. Ecol. 2008, 96, 1266–1274. [Google Scholar] [CrossRef]
- Evangelista, A.; Frate, L.; Stinca, A.; Carranza, M.; Stanisci, A. VIOLA-the vegetation database of the central Apennines: Structure, current status and usefulness for monitoring Annex I EU habitats (92/43/EEC). Plant Sociol. 2016, 53, 47–58. [Google Scholar] [CrossRef]
- Pignatti, S. Flora D’Italia; Edagricole: Bologna, Italy, 1982; ISBN 8850624499. [Google Scholar]
- Pignatti, S. Valori di Bioindicazione Delle Piante Vascolari Della Flora D’Italia; Dipartimento di Botanica ed Ecologia dell’Università Camerino: Camerino, Italy, 2005. [Google Scholar]
- Landolt, E.; Bäumler, B.; Erhardt, A.; Hegg, O.; Klötzli, F.; Lämmler, W.; Nobis, M.; Rudmann-Maurer, K.; Schweingruber, F.; Theurillat, J. Flora Indicativa–Ecological Indicator Values and Biological Attributes of the Flora of Switzerland and the Alps. Haupt Verlag: Berne, Switzerlad, 2010; ISBN 978-3-258-07461-0. [Google Scholar]
- Gottfried, M.; Pauli, H.; Futschik, A.; Akhalkatsi, M.; Barančok, P.; Alonso, J.L.B.; Coldea, G.; Dick, J.; Erschbamer, B.; Kazakis, G.; et al. Continent-wide response of mountain vegetation to climate change. Nat. Clim. Chang. 2012, 2, 111–115. [Google Scholar] [CrossRef]
- Aeschimann, D.; Lauber, K.; Moser, D.M.; Theurillat, J.-P. Flora Alpina: Ein Atlas Sämtlicher 4500 Gefässpflanzen Der Alpen; Haupt Verlag: Berne, Switzerland, 2004; ISBN 978-8808071590. [Google Scholar]
- Klotz, S.; Kühn, I.; Durka, W.; Briemle, G. Biolflor: Eine Datenbank mit Biologisch-Ökologischen Merkmalen zur Flora von Deutschland; Bundesamt für naturschutz: Bonn, Germany, 2002; Volume 38, ISBN 978-3784335087. [Google Scholar]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M. The vegan package. Community Ecol. Package 2007, 10, 631–637. [Google Scholar]
- Dray, S.; Choler, P.; Doledec, S.; Peres-Neto, P.R.; Thuiller, W.; Pavoine, S.; ter Braak, C.J.F. Combining the fourth-corner and the RLQ methods for assessing trait responses to environmental variation. Ecology 2014, 95, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.M.; Warton, D.I.; Andrew, N.R.; Binns, M.; Cassis, G.; Gibb, H. The fourth-corner solution–using predictive models to understand how species traits interact with the environment. Methods Ecol. Evolut. 2014, 5, 344–352. [Google Scholar] [CrossRef]
- Dolédec, S.; Chessel, D.; Ter Braak, C.; Champely, S. Matching species traits to environmental variables: A new three-table ordination method. Environ. Ecol. Stat. 1996, 3, 143–166. [Google Scholar] [CrossRef]
- Wang, Y.; Naumann, U.; Wright, S.T.; Warton, D.I. Mvabund—An R package for model-based analysis of multivariate abundance data. Methods Ecol. Evolut. 2012, 3, 471–474. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. Unsupervised learning. In The Elements of Statistical Learning; Springer: Berlin, Heidelberg, Germany, 2009; pp. 485–585. ISBN 978-0-387-84858-7. [Google Scholar]
- Sarkar, D. Lattice: Multivariate Data Visualization with R; Springer Science & Business Media: New York, NY, USA, 2008; ISBN 978-0-387-75969-2. [Google Scholar]
- Beniston, M. Climatic change in mountain regions: A review of possible impacts. Clim. Chang. 2003, 59, pp. 5–31. [CrossRef]
- Gottfried, M.; Pauli, H.; Reiter, K.; Grabherr, G. Potential effects of climate change on alpine and nival plants in the Alps. In Mountain Biodiversity, a Global Assessment; Parthenon: Boca Raton, FL, USA, 2002; pp. 213–223. ISBN 0-884736-02-5. [Google Scholar]
- Pauli, H.; Gottfried, M.; Reiter, K.; Grabherr, G. High mountain summits as sensitive indicators of climate change effects on vegetation patterns: The “multi summit-approach” of GLORIA (GLobal Observation Research Initiative in Alpine environments). In Global Change and Protected Areas; Advances in Global Change Research; Visconti, G., Beniston, M., Iannorelli, E.D., Barba, D., Eds.; Springer: Dordrecht, 2001; volume 9. [Google Scholar]
- Jiménez-Alfaro, B.; Marcenó, C.; Bueno, Á.; Gavilán, R.; Obeso, J.R. Biogeographic deconstruction of alpine plant communities along altitudinal and topographic gradients. J. Veg. Sci. 2014, 25, 160–171. [Google Scholar] [CrossRef]
- Choler, P.; Michalet, R.; Callaway, R.M. Facilitation and competition on gradients in alpine plant communities. Ecology 2001, 82, 3295–3308. [Google Scholar] [CrossRef]
- Mark, A.F.; Dickinson, K.J.; Allen, J.; Smith, R.; West, C.J. Vegetation patterns, plant distribution and life forms across the alpine zone in southern tierra del fuego, argentina. Austral Ecol. 2001, 26, 423–440. [Google Scholar] [CrossRef]
- Pauli, H.; Gottfried, M.; Grabherr, G. Vascular plant distribution patterns at the low-temperature limits of plant life-the alpine-nival ecotone of mount schrankogel (Tyrol, Austria). Phytocoenologia 1999, 29, 297–325. [Google Scholar] [CrossRef]
- Proctor, M.; Yeo, P.; Lack, A. The Natural History of Pollination; Harper Collins Publishers: London, UK, 1996; ISBN 000219905X. [Google Scholar]
- Arroyo, M.T.K.; Primack, R.; Armesto, J. Community studies in pollination ecology in the high temperate andes of central Chile I. Pollination mechanisms and altitudinal variation. Am. J. Bot. 1982, 69, 82–97. [Google Scholar] [CrossRef]
- Berry, P.E.; Calvo, R.N. Wind pollination, self-incompatibility, and altitudinal shifts in pollination systems in the high Andean genus Espeletia (Asteraceae). Am. J. Bot. 1989, 76, 1602–1614. [Google Scholar] [CrossRef]
- Bingham, R.A.; Orthner, A.R. Efficient pollination of alpine plants. Nature 1998, 391, 238–239. [Google Scholar] [CrossRef]
- Lawton, R.O. Wind stress and elfin stature in a montane rain forest tree: An adaptive explanation. Am. J. Bot. 1982, 69, 1224–1230. [Google Scholar] [CrossRef]
- Ridley, H. The Dispersal of Plants throughout the World; L. Reeve & Co. Ltd.: Ashford, Kent, UK, 1930; ISBN 20057003410. [Google Scholar]
- Collins, S.L.; Uno, G.E. Seed predation, seed dispersal, and disturbance in grasslands: A comment. Am. Nat. 1985, 125, 866–872. [Google Scholar] [CrossRef]
- Tackenberg, O.; Stöcklin, J. Wind dispersal of alpine plant species: A comparison with lowland species. J. Veg. Sci. 2008, 19, 109–118. [Google Scholar] [CrossRef]
- Brooker, R.W.; Callaghan, T.V. The balance between positive and negative plant interactions and its relationship to environmental gradients: A model. Oikos 1998, 81, 196–207. [Google Scholar] [CrossRef]
- Gentili, R.; Armiraglio, S.; Sgorbati, S.; Baroni, C. Geomorphological disturbance affects ecological driving forces and plant turnover along an altitudinal stress gradient on alpine slopes. Plant Ecol. 2013, 214, 571–586. [Google Scholar] [CrossRef]
- Willson, M.F.; Whelan, C.J. Variation in postdispersal survival of vertebrate-dispersed seeds: Effects of density, habitat, location, season, and species. Oikos 1990, 57, 191–198. [Google Scholar] [CrossRef]
- Almeida-Neto, M.; Campassi, F.; Galetti, M.; Jordano, P.; Oliveira-Filho, A. Vertebrate dispersal syndromes along the atlantic forest: Broad-scale patterns and macroecological correlates. Glob. Ecol. Biogeogr. 2008, 17, 503–513. [Google Scholar] [CrossRef]
- Van der Pijl, L. Principles of Dispersal; Springer: Berlin, Germany, 1982; ISBN 978-3-642-87925-8. [Google Scholar]
- Morgan, J.; Venn, S. Alpine plant species have limited capacity for long-distance seed dispersal. Plant Ecol. 2017, 218, 813–819. [Google Scholar] [CrossRef]
- Riibak, K.; Reitalu, T.; Tamme, R.; Helm, A.; Gerhold, P.; Znamenskiy, S.; Bengtsson, K.; Rosén, E.; Prentice, H.C.; Pärtel, M.; et al. Dark diversity in dry calcareous grasslands is determined by dispersal ability and stress-tolerance. Ecography 2015, 38, 713–721. [Google Scholar] [CrossRef]
- Navarro, T.; El Oualidi, J.; Taleb, M.S.; Pascual, V.; Cabezudo, B. Dispersal traits and dispersal patterns in an oro-mediterranean thorn cushion plant formation of the eastern high Atlas, Morocco. Flora Morphol. Distrib. Funct. Ecol. Plants 2009, 204, 658–672. [Google Scholar] [CrossRef]
- Wipf, S.; Stöckli, V.; Herz, K.; Rixen, C. The oldest monitoring site of the Alps revisited: Accelerated increase in plant species richness on Piz Linard summit since 1835. Plant Ecol. Divers. 2013, 6, 447–455. [Google Scholar] [CrossRef]
- Rogora, M.; Frate, L.; Carranza, M.; Freppaz, M.; Stanisci, A.; Bertani, I.; Bottarin, R.; Brambilla, A.; Canullo, R.; Carbognani, M.; et al. Assessment of climate change effects on mountain ecosystems through a cross-site analysis in the Alps and Apennines. Sci. Total Environ. 2018, 624, 1429–1442. [Google Scholar] [CrossRef] [PubMed]
- Rogora, M.; Mosello, R.; Arisci, S.; Brizzio, M.C.; Barbieri, A.; Balestrini, R.; Waldner, P.; Schmitt, M.; Stähli, M.; Thimonier, A.; et al. An overview of atmospheric deposition chemistry over the Alps: Present status and long-term trends. Hydrobiologia 2006, 562, 17–40. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).