1. Introduction
Pteropus (Mammalia: Chiroptera), commonly known as flying foxes, fulfill important ecosystem roles as seed dispersers and pollinators [
1,
2,
3,
4,
5,
6,
7,
8], but are threatened by intensive hunting and habitat conversion [
9,
10,
11,
12]. Many species are still poorly known because their distributions are largely restricted to small, remote islands [
13,
14], which is correlated with heightened extinction risk [
15]. Lack of information creates a significant impediment to the conservation of this highly threatened bat taxa, but logistically this may often be a difficult knowledge gap to fill. Most flying fox species occur in remote, marginal habitats and require dedicated effort to seek out compared to other bats, which discourages research. In the Old World Fruit Bat Action Plan [
16], a majority of flying fox species do not have enough data to contribute to species threat assessments and require greater survey and research efforts to understand
Pteropus natural history and the threats species face. Without this information, assessment of the effectiveness of conservation action and where to prioritize resources is not possible.
One such species is
Pteropus griseus (the gray flying fox), which is listed by the International Union for Conservation of Nature (IUCN) Red List as Data Deficient [
17]. Data Deficient species are those defined by the IUCN as those that have “inadequate information to make a direct, or indirect, assessment of its risk of extinction based on its distribution and/or population status” [
18]. This definition encapsulates how little is known about this species.
Pteropus griseus is endemic to Indonesia, and found in the Lesser Sundas, the Banda Islands, and Sulawesi. Their distribution on relatively remote islands presents logistical challenges to researchers that discourage the collection of data for threat assessments. Unfortunately, flying foxes are pressured intensely by hunting for the bushmeat trade in North Sulawesi [
12], and lack of information on the hunting system and flying fox populations can result in a lack of conservation effort. To fuel the demand for flying foxes from the bushmeat market in North Sulawesi province, hunters have started sourcing bats from other provinces [
12]. Without adequate fundamental natural history knowledge, such as habitat preferences or roosting ecology, and population trends of
P. griseus, the immediate threat of hunting may extirpate the species before an IUCN threat status can even be conferred.
Pteropus griseus is found on small islands in the Lesser Sundas and Sulawesi [
13,
17], but the only data available on their natural history originate from the work of Robert Goodwin in Timor in 1979 [
19]. From the Goodwin (1979) study, we know that
P. griseus prefers lowland forest and are commonly found near sea level (<30 m) but will forage at higher altitudes. Goodwin found individuals foraging on the fruits of
Ficus (figs),
Muntingia (the introduced Jamaica cherry), and presumably also the fruit of
Borassus (fan palms) where some individuals were found. Given the significantly different environments, biogeographic history, and faunal and floral assemblages between Sulawesi [
20] and the Lesser Sundas [
21], the subspecies
P. g. mimus (Sulawesi) and
P. g. griseus (Timor) likely utilize food and spatial resources differently in their native landscapes. The Lesser Sundas has the driest and most seasonal climate of the Indonesian archipelago [
21], contrasting with the climate of Sulawesi, where there is no real dry season [
20]. It is unlikely that the
P. griseus of Timor have the same roosting ecology as those of Sulawesi because of the different landscapes of these two islands. Goodwin [
19] only recorded solitary individuals hidden under leaves of
Borassus and
Corypha fan palms in Timor. No common day roost was found, though there were no mentions of specific efforts to determine whether the species roosted as larger groups. Additionally, the higher diversity of larger pteropodid species on Sulawesi (14 species of Pteropodinae compared to six on Timor) presents potentially higher competition for limited spatial resources (i.e., roost sites). Previous field expeditions by both of authors indicated that day roosts for large colonies are primarily located in mangroves. However, mangroves are one of the most threatened landscapes in the world [
22] and data from other parts of Indonesia indicate a loss of at least 80% of mangroves [
23,
24]. These preferred roost sites then become a limiting resource for flying fox species that may push competition.
In this study, we describe the only known contemporary common day roost (simplified here to “roost”) of
P. griseus in Sulawesi. The roost was initially found while surveying Central Sulawesi for flying foxes during a separate pollination study by one of the authors for her thesis work (Sheherazade, unpublished data). The individuals of interest have obvious morphological differences from the two, more common species (
P. alecto and
A. celebensis), found in Central Sulawesi. However, historically, Sulawesi satellite islands also had closely allied species such as
P. hypomelanus macassaricus and
P. speciosus, which look similar to
P. griseus. The only specimens of
P. griseus from Central Sulawesi were previously collected by J. J. Menden in 1938 on the nearby island of Peleng [
13]. However, no other contemporary records exist for the species on Sulawesi. We aim to confirm the identity of the captured bats as
P. griseus to ensure appropriate conservation action is taken. Without presenting data to clarify that this is different from the two more commonly hunted species (
P. alecto and
A. celebensis), protected status cannot be conferred to
P. griseus in Indonesia and long-term conservation management cannot occur. We present data to confirm the species identification and include notes on its distribution and threats. The implications for our findings regarding hunting threats are considered in greater detail as it pertains to the persistence of the population and protection of the roost site.
2. Materials and Methods
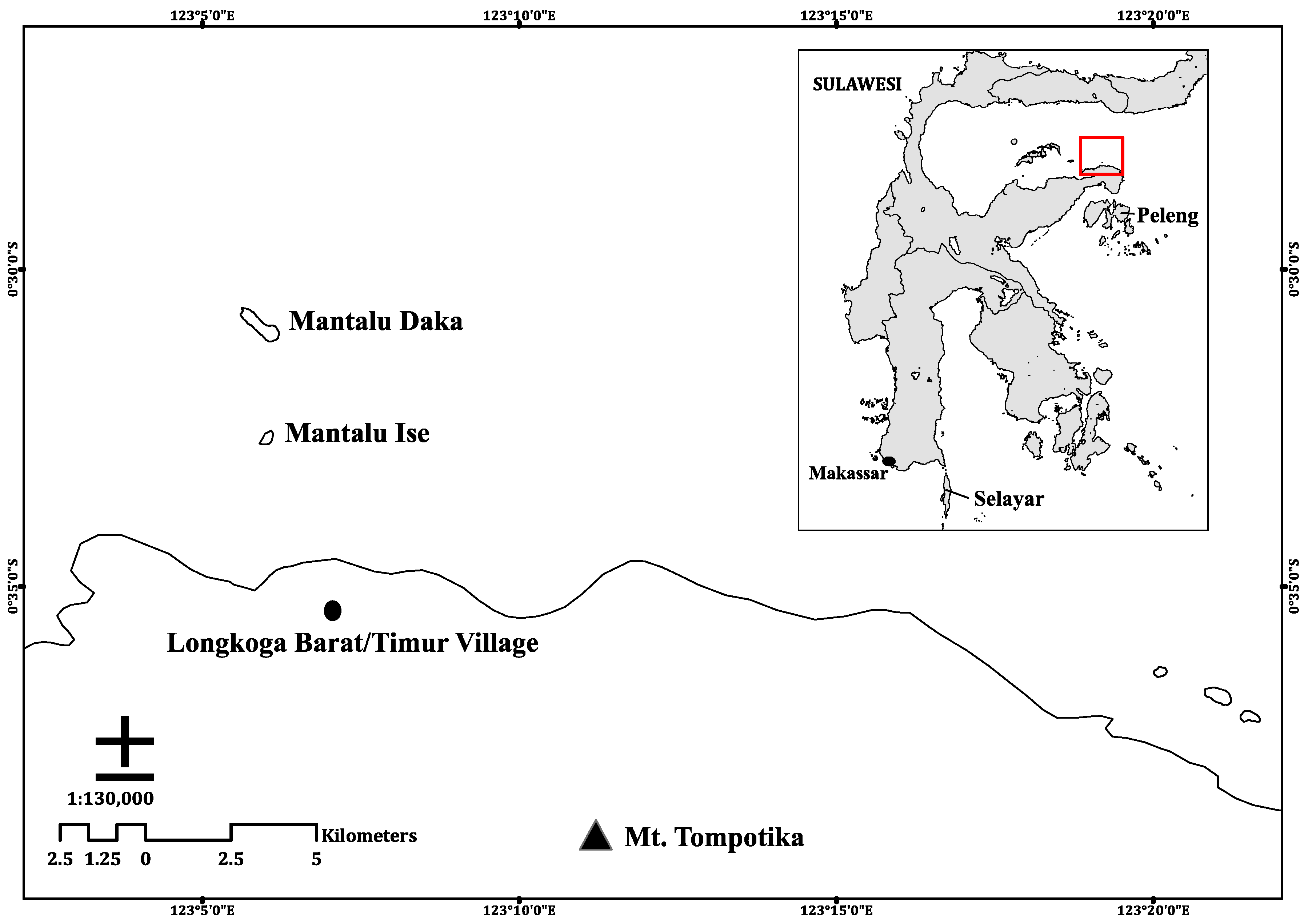
The study was conducted on the offshore island of Mantalu Daka, Sulawesi, Indonesia in June and November 2017 (
Figure 1). This island is located within the administrative area of Longkoga Barat/Timur Village in Bualemo subdistrict, Banggai, Central Sulawesi. Mantalu Daka is Saluan, one of the local dialects, for Big Island (Bahasa Indonesian:
Pulau Besar), and is ~1.5 km in length. A second island north of the village is called Mantalu Ise, Saluan for Small Island (Bahasa Indonesian:
Pulau Kecil), and is ~300 m in length. Plant species on the island were identified using regional and country-specific reference guides for mangroves [
23,
25,
26].
The outer part of the island is comprised of typical coastal vegetation dominated by mangroves (Excoecaria agallocha, Pemphis acidula, and Xylocarpus rumphii) and other secondary vegetation (Casuarina equisetifolia, Scaevola taccada, Inocarpus fagifer, Streblus asper, Trema sp., Premna corymbosa, and spiny forests). Crop plants, such as coconut, papaya, mango, and corn, planted by the local people were also found on the island. The fishermen and farmers commute between nearby villages on the mainland of Sulawesi and Mantalu Daka, but there are no permanent inhabitants on the island due to the lack of freshwater and high prevalence of mosquitoes. The inner core of the island consisted of dense spiny forest with quicksand pits, gradually deepening into a mangrove swamp.
We captured flying foxes following procedures described in Methods of Capturing and Handling Bats [
27], with some modifications to minimize disturbance caused by capturing activities (S. Wiantoro, pers. comm., 2018). All protocols were approved by the University of Florida under Institutional Animal Care and Use Committee (IACUC) protocol #201709800. We stacked two mist nets (Avinet 2.6 × 9 m, 38 mm mesh size, 4 shelves) using a double pulley system on a pair of 10 m aluminum poles placed at the edge of the island near a known flyway far from the day roost. The roost was found while one of the authors (Sheherazade) was surveying Central Sulawesi for sites to conduct a pollination experiment and determined there were three, not two species, at that roost when capturing the bats to swab their fur. We opened the mist nets from 2:00 a.m. to 6:00 a.m. to capture flying foxes returning to their roost. We previously conducted reconnaissance on the island to determine all possible flyways returning to the roost. We adjusted the position of the mist nets at least 100 m away from the previous position around the perimeter of the island every two or three nights to prevent permanent shifts in the flight paths used by the bats and to reduce predictability of mist net position. The island is not very large (area under 0.5 km
2) and we were limited in how far apart we could place the mist nets since bats were returning primarily from the southeast, where Mt. Tompotika is located.
We immediately extracted each flying fox from the net upon capture, recorded the sex and age class (adult, subadult, or juvenile determined by joint fusion [
28]), and measured external characteristics (forearm length, ear length, hind foot length, greatest skull length, and mass) using digital calipers and Pesola scales. We placed a small dot of non-toxic nail polish on one of the hind foot claws to indicate capture and prevent resampling of the same individual prior to release.
We compared species identities and measurements to flying foxes caught in 2012 from Central Sulawesi by one of the authors (Susan M. Tsang) and Sigit Wiantoro. These specimens have previously been verified by a combination of genetic analyses and comparison to reference material and natural history collection notes [
29,
30]. This includes comparative material to
P. hypomelanus macassaricus, which has an overlapping distribution with some
P. griseus subspecies in Sulawesi.
Along with some local community members, we conducted a bi-weekly preliminary census via an emergence count in June and November 2017 starting at dusk when the bats first leave the roost until all had completed their exit. Anecdotal evidence about roost characteristics and flying fox hunting were also collected from hunters and fisherman in nearby villages, and evidence and equipment from past hunting events were collected.
3. Results
The P. griseus roost was located at the center of the mangrove swamp on Mantalu Daka. We were unable to identify the mangrove tree species in the central swamp due to the lack of visible roots and flowers. The inner core of spiny forest and quicksand pits make the inner core of the mangrove, where the bats primarily roost, difficult to traverse.
Three species of flying foxes were identified at the roost during a reconnaissance survey in December 2016—
Pteropus griseus,
P. alecto (black flying fox), and
Acerodon celebensis (Sulawesi flying fox). The latter two species are easily identified by sight given their significantly larger size and distinct coloration—
P. alecto is entirely dark brown to black with a forearm (FA) range of 140 to 175 mm, and
A. celebensis is blonde with a brown face with a FA range 125 to 142 mm. In contrast,
P. griseus is smaller (FA range of 113 to 130 mm) and has a lighter gray head with a body in various shades of brown [
13]. In total, 52 flying foxes were captured (33 individuals in June and 19 individuals in November) after a total of 72 mist net hours. All captures of adults were
P. griseus. Of the total number of
P. griseus captured, only 17 were adults, with the rest a mix of juveniles and subadults. We captured only one juvenile of
A. celebensis in June. Both
P. alecto and
A. celebensis flew much higher than
P. griseus and would require higher canopy nets to capture, which was not possible given the coastal terrain.
Pteropus griseus was the smallest species found on the island (
Table 1, and see
Supplementary Table S1 for all data along with measurements of historical specimens at Museum Zoologicum Bogoriense, Indonesian Institute of Sciences (MZB-LIPI)). One individual was excluded from the morphological analyses because its greatest skull length (GSL) was anomalously large (99.2 mm), although all other features conformed to
P. griseus. The skull measurements would be outside the range of any of the potential species and would require further investigation to confirm its species identity. We ruled out the possibility of our captured individuals being
P. hypomelanus macassaricus since our captures had significantly smaller forearm lengths (
t = −2.01,
p < 0.05). Additionally, we ruled out
P. speciosus (at one point considered a subspecies of
P. griseus) since our captures were significantly larger. The range of morphological measurements from this study also generally agree with that of Bergmans and Rozendaal’s examination of Sulawesi pteropodids [
31], which gives a forearm range of 118 to 128 mm for
P. griseus. While it was difficult to confirm the subspecies identities of the individuals in this study based on scant external characteristics data from reference material, the pelage coloration was rather distinct in those captured in Mantalu Daka.
Pteropus griseus on this island had a brown-orange mantle, a tawny (orange-brown or yellow-brown) underbelly, mixing with light grayish hairs as the ventral fur neared the tibia. The head and face were distinctly light gray, darkening somewhat near the crown, and fur was scant around the eyes and muzzle (
Figure 2). There were a couple of individuals noted in photographs that differed in the head coloration (a darker gray-brown rostrum surrounded by brown-orange), but none of these were captured in hand for comparison. Andersen [
30] describes
P. g. mimus as having a brown and tawny coloration,
P. g. pallidus as dark brown with flecks of pale gray hairs, and
P. g. griseus as similar to
P. g. mimus but considerably lighter in color. This pelage coloration would lend support for identification as
P. g. griseus, but more study over the course of the year is needed to determine whether the pelage observed in this study represents only a single phase of coloration. For nightly foraging,
P. griseus were observed going to the dense, more species-rich rainforest of Mt. Tompotika on mainland Sulawesi to forage.
During the first capture season in June 2017, a large, mixed colony of flying foxes was recorded emerging at dusk every day, with a steady stream of bats that lasted approximately 25 min. The bats were primarily of larger individuals, likely
P. alecto or
A. celebensis. The identity of the bats as
P. alecto or
A. celebensis was corroborated by what hunters were taking to the market. However, the colony size shrank by November and emergence took only 10 min and the flow of bats was largely reduced. Only small to medium individuals were seen, presumably
P. griseus. Based on the preliminary census, there were around 300 to 500 bats. According to the local people, the higher level of hunting was initiated by the arrival of middlemen traders of bat bushmeat from North Sulawesi in 2017 requesting that locals hunt the bats. Hunting occurs throughout the year, with peaks in June and November.
Pteropus alecto and
A. celebensis were intensely hunted and comprised a majority of the hunters’ take because they roosted on the outer part of the island, making them considerably easier to catch and more accessible.
Pteropus griseus roosted in the inner part of the island surrounded by swamps and mosquito swarms, which makes they more difficult to catch but not exempt from hunting pressure. The middlemen did not appear during the second capture season (November 2017), and there were no longer any groups of
P. alecto and
A. celebensis readily visible at the outer edge of the island. However, given that our captures were only of
P. griseus and the only bats seen were small, we do not think a substantial group of
P. alecto or
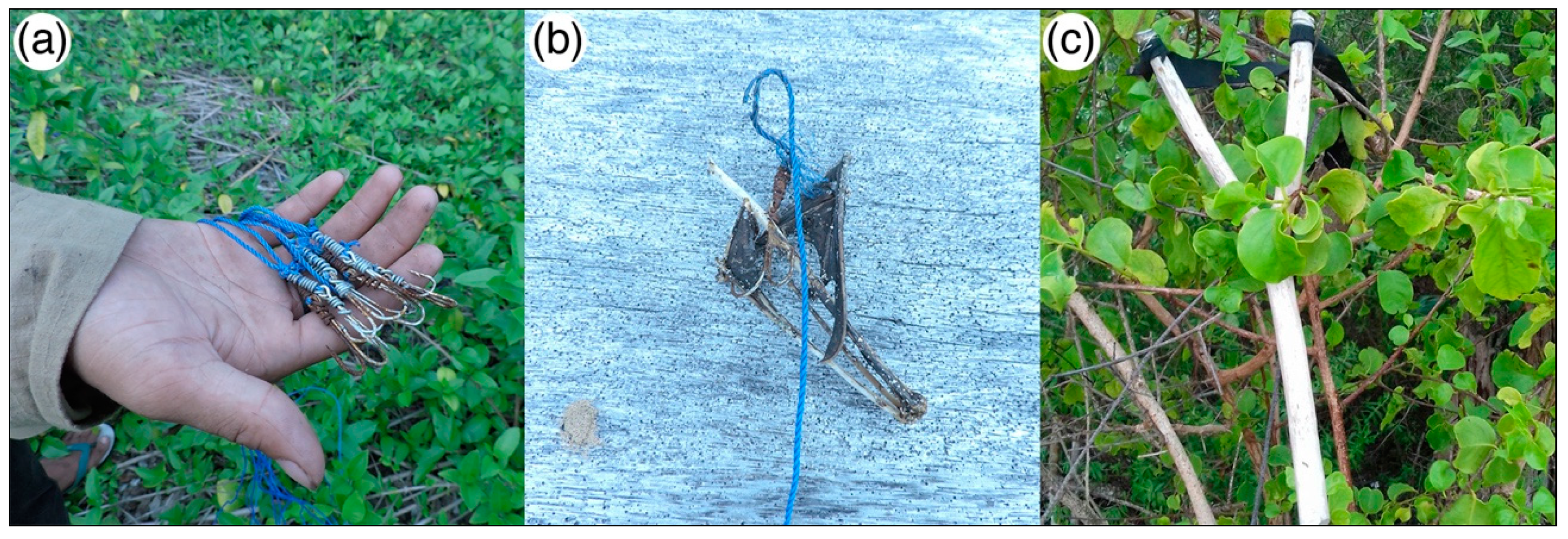
A. celebensis are found any longer on this island. There were no other reports of any sizeable groups of bats suddenly appearing elsewhere either. We ruled out migration of flying foxes to follow food resources as there were no fruit masting events in Sulawesi in 2017 that would cause a large colony to relocate to another site, and there was no subsequent return after a year had passed reported by the villagers or local conservationists. Fishing hooks and ropes were found along the flight path used by
P. griseus (
Figure 3). This equipment was commonly used to hunt flying foxes in Central Sulawesi. Tens of fishing hooks were hung along a rope that spans between two tall trees in the bat flight path. The wings would then be torn by the hooks, causing the bats to fall to the ground or to be stuck on the hooks. Hunters would leave the equipment overnight and collect the flying foxes the next morning. Some hunters also used large slingshots to shoot the bats directly (
Figure 3). Using these methods, a few hunters could gather tens to hundreds of flying foxes per day according to the estimates given to us by the local villagers of Longkoga Barat/Timur.
According to the people of Longkoga Barat/Timur village, flying fox hunting on the island is driven by demand for bushmeat from markets in North Sulawesi. As noted from our previous study, the extirpation of flying fox colonies from North Sulawesi province has driven hunters to attempt to source bats from other provinces instead [
12]. The villagers reported intense hunting occurred between the two capture seasons in this study, resulting in a noticeable decrease in the observable colony size due to hunting-related loss of bats or relocation to another island. During the beginning of this study, the three flying fox species were always seen on the island, but only
P. griseus was found during the second capture season. A middleman trader came to Longkoga Barat/Timur village and asked the villagers to hunt the flying foxes for them. Each flying fox was worth Rp 5000, or USD 0.36. This was an order of magnitude lower for the bats compared to the market price in North Sulawesi (Rp 50,000 or USD 3.63 per individual).
4. Discussion
The discovery of a day roost of
P. griseus overturns previous misconceptions that they only occur as individuals or small groups [
32], and their colonial roosting habits should spur on further action to document roost sites. Additionally, the discovery of a roost of
P. griseus within the reach of hunters and middlemen from the North Sulawesi market should spur more conservation action, as this is the only known existing roost of
P. griseus.
In their review of specimens collected from Sulawesi and its satellite islands, Bergmans and Rozendaal [
31], notes that
P. g. mimus was found on Selayar, in South Sulawesi, and its type locality is Ujung Padang (present day Makassar), also in South Sulawesi. There are no modern (post-1970s) records of
P. griseus near Makassar. Despite Bergmans and Rozedaal [
31] publishing on
P. griseus in 1988, they only examined historical specimens from 1908 and did not conduct any new fieldwork Type specimens of
P. griseus from Sulawesi date back to the late 19th century. The elevated level of development around Makassar, the largest city on Sulawesi, makes it rather unlikely that
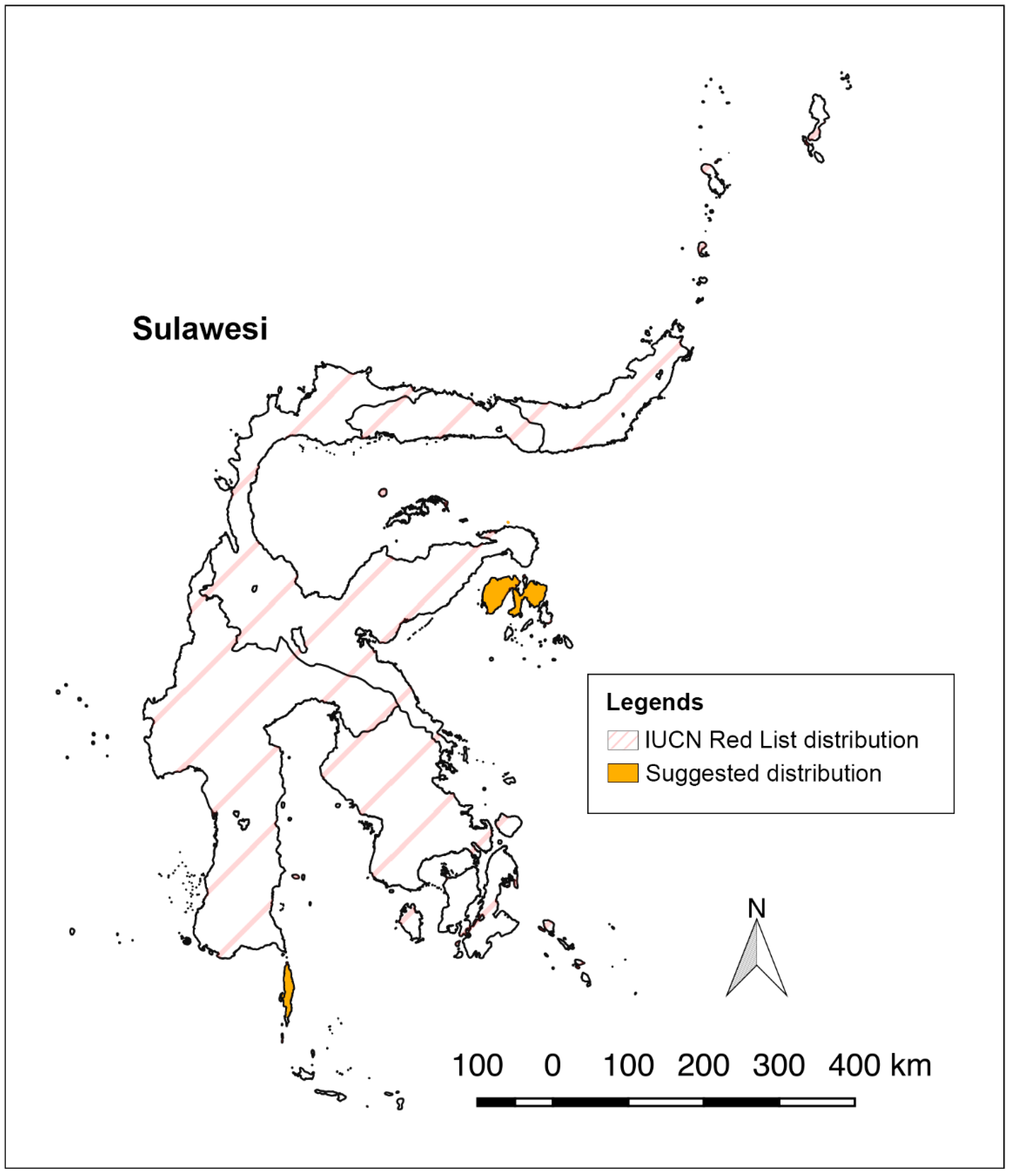
P. griseus are found near there anymore. The historical distribution and the newly discovered roost in this study point to a species distribution that is largely restricted to the satellite islands of Sulawesi, not the Sulawesi mainland. Here, we observed the species primarily flying to foraging areas on mainland Sulawesi closest to their roosts and then returns to their roost at dawn. This is a distance of about 20 to 25 km each way only. However, the current IUCN range map has all of Sulawesi shaded as part of the distribution of
P. griseus, which is likely an overestimation (
Figure 4). Additionally, one of the authors (Susan M. Tsang) and her collaborators have conducted multiple expeditions starting in 2012 to the present in Sulawesi, with concentrated efforts in Central Sulawesi, and have not discovered any groups of
P. griseus. The IUCN Red List map [
17] needs to be updated to reflect the restricted distribution of
P. griseus, as its limited distribution on small islands means that it faces heightened extinction risk.
The signs of disappearances of
P. alecto and
A. celebensis suggest an alarming population trend in flying fox species that will likely be mirrored in
P. griseus should no conservation action be taken. As the other species become rarer, it is likely that hunters will enter further into the swamp and locate the
P. griseus roost. Hunting at the roost poses a direct threat to the survivorship of individuals. Hunting activity can also indirectly decrease survivorship due to frequent roost disturbances leading to injuries, higher stress, or large-scale infant mortality from when fleeing mothers drop pups [
11]. The small colony size of
P. griseus makes the species especially vulnerable to hunting. While the preliminary census requires more repeated counts to obtain a more precise measure of the colony size, the estimate of 300 to 500 individuals only in November 2017 is few enough bats that a hunter could wipe out the colony after a single season of hunting events. Given the current observed rate of hunting, the roost would likely face extirpation should this be allowed to continue. A change in the IUCN Red List status to Endangered may be warranted for the species, as this is the only known roost currently, it faces a direct threat from intensive hunting, the roost does not exist in a protected area and no action is currently being taken, and there are only several hundred individuals. Hunting of flying foxes in Sulawesi has already extirpated colonies of flying foxes closer to the North Sulawesi markets and the expansion of the North Sulawesi hunting network to other Sulawesi provinces was noted by hunters in a previous study conducted by the authors in 2013 [
12], and now recorded first-hand in this study. We have begun to monitor the population more closely and are exploring options for conservation action to promote the persistence of
P. griseus on this island. This colony may represent one of the only opportunities to study the roosting ecology and diet of the species, and its protection should be considered a high conservation priority.
Flying fox conservation should be addressed by action at both the markets and at the roost. While hunting can occur at the roost or at foraging sites, roost protection is one of the most important actions to ensure persistence of a flying fox species, particularly when roost sites are known and threats are acute and can be contained [
11]. Working in collaboration with the local village, such as Longkoga Barat/Timur in this study, roost protection and environmental education about the importance of flying foxes to the ecosystem and human livelihoods can act as successful efforts to rebuff hunting [
10,
11]. As the authors have previously suggested in their market study [
12], it is important to recognize that bushmeat consumption is tightly linked to religious holidays and reducing demand with conservation non-governmental organizations (NGOs), relevant government agencies, and churches in North Sulawesi is important to the overall goal of flying fox conservation in all of Sulawesi. The loss of flying foxes may have profound impacts on native ecosystems and human well-being due to loss of their ecological services important to ecologically and economically important plants [
33,
34,
35,
36]. In particular, flying foxes in Sulawesi have significant roles as durian pollinators, which are of great economic significance at the local and national level (Sheherazade, pers. obs.).
This study highlights how little fundamental knowledge we have of flying foxes and where they are and is a call to action to address the most basic recommendation expressed in the Old World Fruit Bat Action Plan in 1992—surveying to collect distribution, habitat, or ecological information. Surveys are especially important in poorly studied areas with high pteropodid species richness. Compared to other pteropodid species, Pteropus species require specific, dedicated efforts to find, monitor, and study, making them particularly vulnerable to lack of data being an impediment to creating conservation management plans. Based on the current global flying fox distribution data, remote islands likely provide an important refuge for Pteropus species, but which islands and what kind of landscapes has not been clarified due to lack of research efforts. However, island landscapes are some of the most threatened by climate change due to eustatic sea level rise. Consequently, these islands may become rarer in the future and result in flying fox colonies roosting at suboptimal sites, i.e., closer to villages in more accessible forests. This presents more opportunities for hunting to occur, as it will become easier and knowledge of flying fox colony locations will increase among the public. To discourage hunting, engagement with more local communities would not only serve to help with local conservation action but also with locating roost sites in remote areas where researchers have yet to explore. This engagement creates critical opportunities for perception changes that can benefit conservation, as local communities can become a part of the discovery and management process.