(Benzo[h]quinoline-κ2C,N)-[2,2′-bis(diphenylphosphino)-1,1′-binaphthalene-κ2P,P′]-platinum(II) Hexafluorophosphate
Abstract
1. Introduction
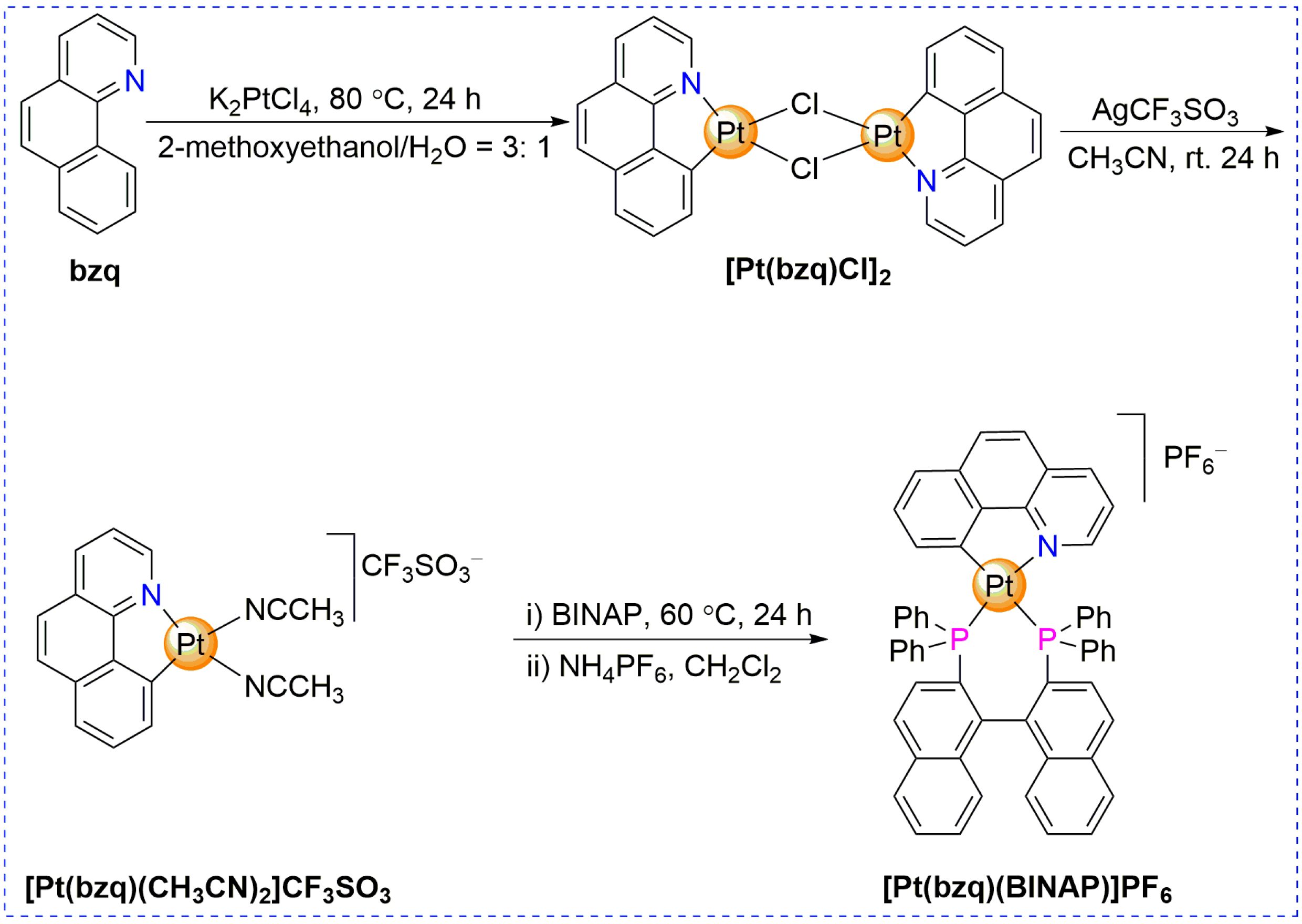
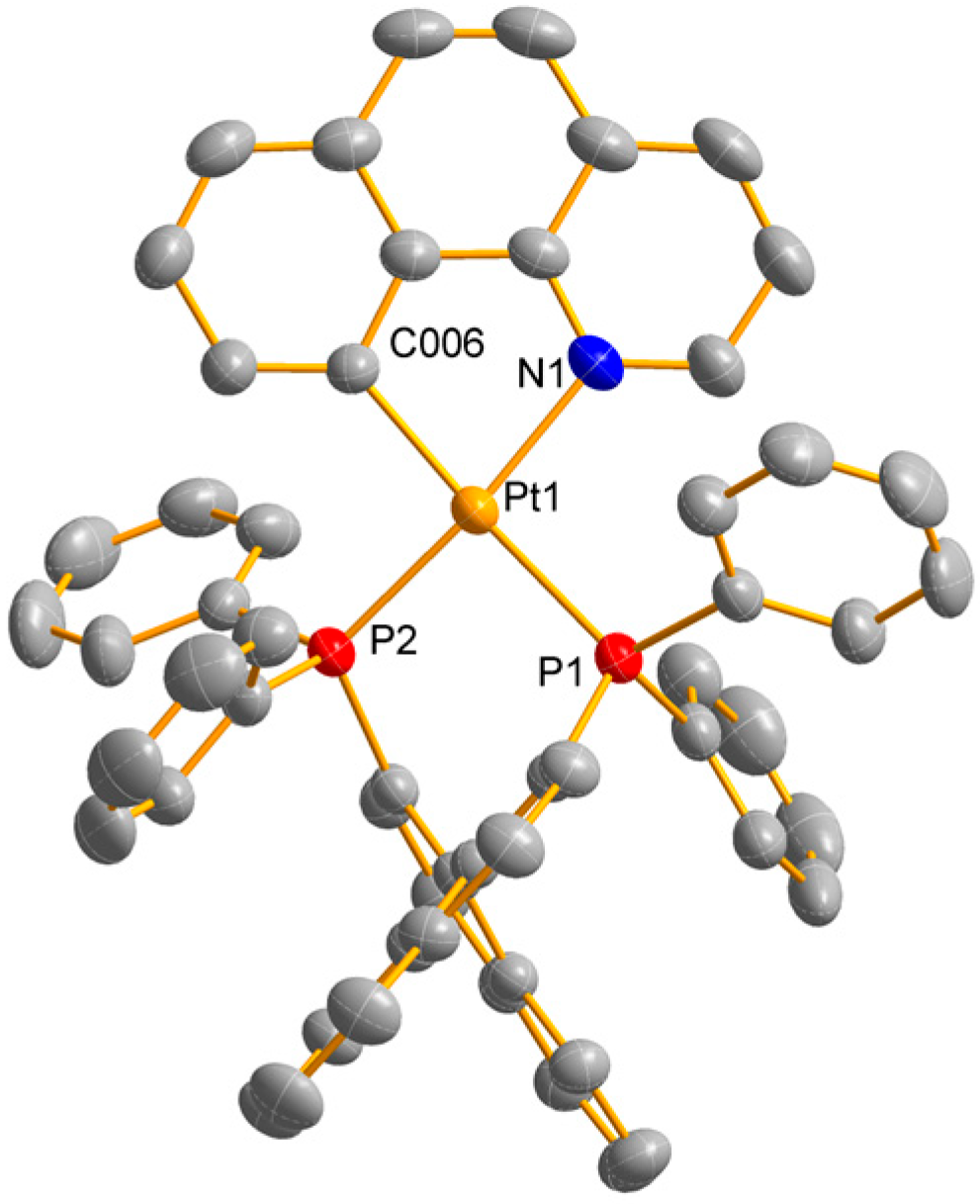
2. Results and Discussion
3. Materials and Instrumentation
3.1. General Methods and Physical Measurements
3.2. Synthesis of [Pt(bzq)(BINAP)]PF6
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BINAP | 2,2′-bis(diphenylphosphino)-1,1′-binaphthalene |
| bzq | benzo[h]quinoline |
| OLEDs | organic light-emitting diodes |
| dppb | 1,2-bis(diphenylphosphino)benzene |
| ppy | 2-phenylpyridine |
References
- Mjos, K.D.; Orvig, C. Metallodrugs in medicinal inorganic chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef]
- Lee, L.C.-C.; Lo, K.K.-W. Shining new light on biological systems: Luminescent transition metal complexes for bioimaging and biosensing applications. Chem. Rev. 2024, 124, 8825–9014. [Google Scholar] [CrossRef]
- Pragti; Kundu, B.K.; Mukhopadhyay, S. Target based chemotherapeutic advancement of ruthenium complexes. Coord. Chem. Rev. 2021, 448, 214169. [Google Scholar] [CrossRef]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal complexes in cancer therapy—An update from drug design perspective. Drug Des. Dev. Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef]
- Leung, C.-H.; Zhong, H.-J.; Chan, D.S.-H.; Ma, D.-L. Bioactive iridium and rhodium complexes as therapeutic agents. Coord. Chem. Rev. 2013, 257, 1764–1776. [Google Scholar] [CrossRef]
- Abdolmaleki, S.; Aliabadi, A.; Khaksar, S. Bridging the gap between theory and treatment: Transition metal complexes as successful candidates in medicine. Coord. Chem. Rev. 2025, 531, 216477. [Google Scholar] [CrossRef]
- Sun, W.; Kong, W.; Du, Q.; Zhang, S.; Guo, L.; Liu, Z. 2,4-Bis[(2,6-diisopropylphenyl)imino]-3-methylpentan-3-ol. Molbank 2018, 2018, M995. [Google Scholar] [CrossRef]
- Gill, M.R.; Vallis, K.A. Transition metal compounds as cancer radiosensitizers. Chem. Soc. Rev. 2019, 48, 540–557. [Google Scholar] [CrossRef]
- Liu, B.; Lystrom, L.; Kilina, S.; Sun, W. Effects of varying the benzannulation site and π conjugation of the cyclometalating ligand on the photophysics and reverse saturable absorption of monocationic iridium(III) complexes. Inorg. Chem. 2018, 58, 476–488. [Google Scholar] [CrossRef]
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.L.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs are unique: Opportunities and challenges of discovery and development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef]
- Haque, A.; Xu, L.L.; Al-Balushi, R.A.; Al-Suti, M.K.; Ilmi, R.; Guo, Z.L.; Khan, M.S.; Wong, W.-Y.; Raithby, P.R. Cyclometallated tridentate platinum(II) arylacetylide complexes: Old wine in new bottles. Chem. Soc. Rev. 2019, 48, 5547–5563. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.; Chan, M.H.-Y.; Pan, M.; Li, Y.; Yam, V.W.-W. Supramolecular assembly of organoplatinum(II) complexes for subcellular distribution and cell viability monitoring with differentiated imaging. Angew. Chem. Int. Ed. 2022, 134, e202210703. [Google Scholar] [CrossRef]
- Zhou, X.-Q.; Mytiliniou, M.; Hilgendorf, J.; Zeng, Y.; Papadopoulou, P.; Shao, Y.; Dominguez, M.P.; Zhang, L.; Hesselberth, M.B.S.; Bos, E.; et al. Intracellular dynamic assembly of deep-red emitting supramolecular nanostructures based on the Pt…Pt metallophilic interaction. Adv. Mater. 2021, 33, 2008613. [Google Scholar] [CrossRef]
- Carrara, S.; Aliprandi, A.; Hogan, C.F.; De Cola, L. Aggregation-induced electrochemiluminescence of platinum(II) complexes. J. Am. Chem. Soc. 2017, 139, 14605–14610. [Google Scholar] [CrossRef]
- Li, K.; Tong, G.S.M.; Yuan, J.; Ma, C.; Du, L.; Yang, C.; Kwok, W.-M.; Phillips, D.L.; Che, C.-M. Excitation-wavelength-dependent and auxiliary-ligand-tuned intersystem-crossing efficiency in cyclometalated platinum(II) complexes: Spectroscopic and theoretical studies. Inorg. Chem. 2020, 59, 14654–14665. [Google Scholar] [CrossRef]
- Li, J.; Tian, M.; Tian, Z.; Zhang, S.; Yan, C.; Shao, C.; Liu, Z. Half-sandwich iridium(III) and ruthenium(II) complexes containing PˆP-chelating ligands: A new class of potent anticancer agents with unusual redox features. Inorg. Chem. 2018, 57, 1705–1716. [Google Scholar] [CrossRef]
- Aghakhanpour, R.B.; Nabavizadeh, S.M.; Rashidi, M.; Kubicki, M. Luminescence properties of some monomeric and dimeric cycloplatinated(II) complexes containing biphosphine ligands. Dalton Trans. 2015, 44, 15829–15842. [Google Scholar] [CrossRef]
- Solomatina, A.I.; Su, S.-H.; Lukina, M.M.; Dudenkova, V.V.; Shcheslavskiy, V.I.; Wu, C.-H.; Chelushkin, P.S.; Chou, P.-T.; Koshevoy, I.O.; Tunik, S.P. Water-soluble cyclometalated platinum(II) and iridium(III) complexes: Synthesis, tuning of the photophysical properties, and in vitro and in vivo phosphorescence lifetime imaging. RSC Adv. 2018, 8, 17224–17236. [Google Scholar] [CrossRef]
- Waddell, P.G.; Slawin, A.M.Z.; Woollins, J.D. Correlating Pt–P bond lengths and Pt–P coupling constants. Dalton Trans. 2010, 39, 8620–8625. [Google Scholar] [CrossRef]
- Ghedini, M.; Pugliese, T.; La Deda, M.; Godbert, N.; Aiello, I.; Amati, M.; Belviso, S.; Lelj, F.; Accorsi, G.; Barigelletti, F. Spectroscopy and electrochemical properties of a homologous series of acetylacetonato and hexafluoroacetylacetonato cyclopalladated and cycloplatinated complexes. Dalton Trans. 2008, 32, 4303–4318. [Google Scholar] [CrossRef]
- Brooks, J.; Babayan, Y.; Lamansky, S.; Djurovich, P.I.; Tsyba, I.; Bau, R.; Thompson, M.E. Synthesis and characterization of phosphorescent cyclometalated platinum complexes. Inorg. Chem. 2002, 41, 3055–3066. [Google Scholar] [CrossRef] [PubMed]
- Tritto, E.; Chico, R.; Ortega, J.; Folcia, C.L.; Etxebarria, J.; Coco, S.; Espinet, P. Synergistic π–π and Pt–Pt interactions in luminescent hybrid inorganic/organic dual columnar liquid crystals. J. Mater. Chem. C 2015, 3, 9385–9392. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- McArdle, P. Oscail, a program package for small-molecule single-crystal crystallography with crystal morphology prediction and molecular modelling. J. Appl. Crystallogr. 2017, 50, 320–326. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Wang, H.; Zhang, M.; Wu, J.; Zhang, J.; Meng, X.; Yang, Y. (Benzo[h]quinoline-κ2C,N)-[2,2′-bis(diphenylphosphino)-1,1′-binaphthalene-κ2P,P′]-platinum(II) Hexafluorophosphate. Molbank 2026, 2026, M2120. https://doi.org/10.3390/M2120
Wang H, Zhang M, Wu J, Zhang J, Meng X, Yang Y. (Benzo[h]quinoline-κ2C,N)-[2,2′-bis(diphenylphosphino)-1,1′-binaphthalene-κ2P,P′]-platinum(II) Hexafluorophosphate. Molbank. 2026; 2026(1):M2120. https://doi.org/10.3390/M2120
Chicago/Turabian StyleWang, Haoni, Meiting Zhang, Jianwei Wu, Junqi Zhang, Xianglong Meng, and Yuliang Yang. 2026. "(Benzo[h]quinoline-κ2C,N)-[2,2′-bis(diphenylphosphino)-1,1′-binaphthalene-κ2P,P′]-platinum(II) Hexafluorophosphate" Molbank 2026, no. 1: M2120. https://doi.org/10.3390/M2120
APA StyleWang, H., Zhang, M., Wu, J., Zhang, J., Meng, X., & Yang, Y. (2026). (Benzo[h]quinoline-κ2C,N)-[2,2′-bis(diphenylphosphino)-1,1′-binaphthalene-κ2P,P′]-platinum(II) Hexafluorophosphate. Molbank, 2026(1), M2120. https://doi.org/10.3390/M2120




